Abstract
Background/Purpose
Mid-gestational (E14.5) fetal wounds heal regeneratively with attenuated inflammation and high levels of hyaluronan (HA) in their extracellular matrix (ECM), whereas late-gestational (E18.5) fetal wounds heal with scarring. IL-10 plays an essential role in the fetal regenerative phenotype and is shown to recapitulate scarless wound healing postnatally. We hypothesize a novel role of IL-10 as a regulator of HA in the ECM.
Methods
Murine fetal fibroblasts (FFb) from C57Bl/6 and IL-10−/− mice were evaluated in vitro. Pericellular matrix (PCM) and HA synthesis were quantified using a particle exclusion assay and ELISA. The effects of hyaluronidase and hyaluronan synthase (HAS) inhibitor (4-methylumbelliferone [4-MU]) were evaluated. An ex vivo fetal forearm culture incisional wound model comparing mid-gestation and late-gestation fetuses was used to evaluate IL-10’s effect on HA-rich ECM production with pentachrome and immunohistochemistry.
Results
FFb produce a robust HA-rich PCM which is IL-10 dependent and attenuated with hyaluronidase and HAS inhibition. Mid-gestation fetal wounds produce more ground substance and HA than late-gestation fetal wounds. IL-10 in late-gestation fetal wounds results in elevated ground substance levels and HA staining.
Conclusions
Our data demonstrate that IL-10 regulates an HA-rich ECM deposition, suggesting a novel non-immunoregulatory mechanism of IL-10 in mediating regenerative wound healing.
Keywords: Fetal, Wound healing, IL-10, Hyaluronan, Extracellular matrix
The mid-gestation fetus is capable of regenerative tissue repair with cutaneous wound healing indistinguishable from surrounding skin. In contrast, late-gestation fetal wounds heal with scar consistent with post-natal wound healing. The underlying mechanisms of fetal regenerative healing have not been fully elucidated.
The fetal extracellular matrix (ECM) has a distinct profile and is composed of high levels of high molecular weight hyaluronan [1,2]. The cellular source of hyaluronan synthesis is likely fetal fibroblasts, the main effectors cells of fetal wound healing. These cells, in vitro, have the ability to generate a large hyaluronan-rich pericellular matrix (PCM) [3]. Hyaluronan (HA) synthesis is regulated by the three isoforms of hyaluronan synthase (HAS1-3). HAS1 and HAS2 have been shown to produce high molecular weight HA and dominate the fetal phenotype [4].
Fetal wound healing is also characterized by an attenuated inflammatory response with decreased cellular infiltrate and an anti-inflammatory cytokine milieu [5]. Our laboratory has focused on the role of the anti-inflammatory cytokine, interleukin-10 (IL-10), and has demonstrated its importance in fetal wound healing including 1) elevated levels of IL-10 in fetal skin compared to post-natal skin [6], 2) supplementation of IL-10 in adult fibroblasts (AFb) recapitulates the large HA-rich PCM, 3) wounds created in fetal skin from transgenic IL-10 knockout mice heal with a scar at a gestational age that should heal scarlessly [7] and 4) the ability of IL-10 overexpression in post-natal skin to recapitulate the fetal regenerative phenotype and result in wound healing indistinguishable from the surrounding skin [6,8]. The mechanism, in part, for IL-10’s effects is likely through attenuation of the inflammatory response [8]. Another potential mechanism may be a direct effect of IL-10 on the extracellular matrix. To date, there are minimal data on the role of IL-10 in regulating extracellular matrix deposition, specifically on the regulation of HA synthesis.
Taken together, we hypothesize a novel role for IL-10 as a regulator of hyaluronan in the fetal extracellular matrix. To test this hypothesis, the role of IL-10 in regulating hyaluronan synthesis was first evaluated in vitro. We then examined its role in fetal wound healing using an ex vivo forearm organ culture model. To further confirm that HA is the major constituent of IL-10 mediated ECM deposition, we performed a series of experiments using hyaluronidase and a HAS synthase inhibitor, 4-methylumbelliferone (4-MU).
1. Methods
1.1. Cell culture
All protocols were approved by the Cincinnati Children’s Hospital IACUC committee (9D10087). Primary dermal fibroblasts were isolated from mid-gestation age fetuses (day 14.5) from control C57Bl/6 (Jackson Laboratories, Strain 000664) and transgenic IL-10−/− mice (Jackson Laboratories, Strain 002251) per Hiramatsu et al. 2011 [9]. Fibroblasts were maintained in culture in Dulbecco’s modified Eagle’s Media (DMEM) supplemented with 10% bovine growth serum (BGS) at 37 °C with 5% CO2. All experiments were conducted between passage 5 and 15.
1.2. Pericellular matrix
Particle exclusion assay was performed to evaluate PCM formation. Fibroblasts were plated at 1 × 105 cells per well in a 6-well plate and allowed to settle overnight. Cells were serum starved in DMEM with 2% BGS for 24 hours. Treatment to inhibit HAS1-3 (4-methylumbelliferone, 0.3 mM/ml) or to digest hyaluronan (hyaluronidase, 10.0 U/ml) were added at the initiation of serum starvation. 500 μl of suspended glutaraldehyde stabilized sheep erythrocytes (1 × 108 cells/ml) was added and allowed to settle. Randomly selected individual fibroblasts were photomicrographed and evaluated with computer-assisted morphometric analysis. The PCM ratio is expressed as the ratio of PCM area to cell body area.
1.3. HA ELISA
Fibroblasts were plated at 1 × 106 cells and allowed to settle overnight. Cells were serum starved, as above, for 24 hours. Media were collected and HA ELISA was performed according to manufacturers protocol (Corgenix). HA ELISA was standardized to total protein content evaluated by Coomassie blue assay per manufacturers protocol (Pierce).
1.4. Ex vivo culture
All protocols were approved by the Cincinnati Children’s Hospital IACUC committee (9D10087). Methods of killing animals were in accordance with recommendations of the Panel on Euthanasia of the American Veterinrary Medical Association. Forelimb organ culture was performed as previously described by Iocono et al [10]. Briefly, time-date pregnant C57Bl/6 mice (term, 21 days) were killed at mid-gestational age day 14.5 (E14.5) and late-gestational age day 18.5 (E18.5) and fetuses were harvested via laparotomy (n = 5 per group). An incisional wound was made in each forelimb with a 1-mm microscalpel. The wound was closed with a single 9–0 nylon suture. The forelimbs were amputated at the level of the shoulder and placed in the center-well of organ culture dishes on top of a stainless steel mesh (BD Falcon 353037) with serum free BGJb media for three days. After 3 days, the forelimbs were washed and fixed in 10% neutral buffered formalin and mechanically processed and paraffin embedded.
1.5. Pentachrome
5 μm wound sections were deparaffinized, rehydrated and stained according to the modified Movat’s pentachrome protocol according to manufacturer’s protocol (Poly Scientific, Bayshore, NY). In brief, sections were serially stained in Alcian blue, Verheoff’s hematoxyllin, Scarlet-acid fuchsin and saffron.
1.6. Immunohistochemistry
Fixed sections were labeled for hyaluronan binding protein, HABP. 5 μm wound sections were deparaffinized and rehydrated. Antigen retrieval was performed using target retrieval solution (Dako, Carpathia, CA) at 70 °C overnight (E18.5) or 95 °C for ten minutes (E14.5). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution and samples were blocked using 2% bovine serum albumin (BSA, Jackson Immunoresearch, West Grove, PA) in phosphate buffered saline (PBS). Samples were incubated with a primary biotinylated antibody for HABP (EMD Biosciences, Billerica, MA) at 1 pg/μl (E18.5) or 0.6 pg/μl (E14.5) overnight in 2% BSA. Following PBS washes, samples were incubated with peroxidase-conjugated avidin-biotin-complex (Vestastain Elite ABC Kit, Vector Laboratories, Burlingame, CA). Slides were developed using 3′-3′-diaminobenzidine (Vector Laboratories, Burlingame, CA) and counterstained with hematoxylin (Dako, Carpathia, CA). Following staining, slides were dehydrated and mounted.
1.7. Statistical analysis
Data are expressed as the mean ± S.D. values with at least three samples for each group. Statistical analysis of data was performed using analysis of variance (ANOVA), followed by post hoc tests (Tukey) and Student’s t test when appropriate using Excel (Microsoft). A p-value of <0.05 was considered statistically significant.
2. Results
2.1. In vitro – IL-10 is essential to HA-rich PCM formation
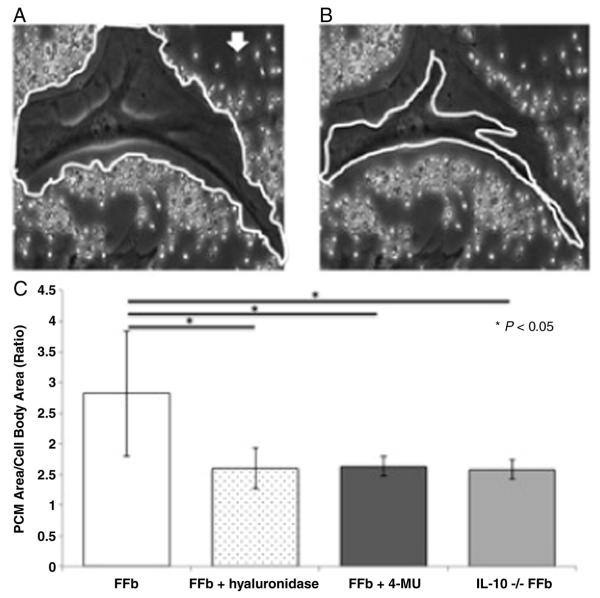
Mid-gestation fetal fibroblasts (FFb) produce a large PCM matrix (2.82 ± 1.02) (Fig. 1). To confirm the PCM is composed of HA, FFb were cultured with hyaluronidase for 24 hours. Treatment with hyaluronidase results in significantly smaller PCM than control (FFb 2.82 ± 1.02 vs FFb + hyaluronidase 1.60 ± 0.33, p = 0.003). Similarly, inhibition of HAS1-3 with 4-MU results in decreased PCM formation (FFb 2.82 ± 1.02 vs FFb + 4-MU 1.63 ± 0.16, p = 0.00008). To evaluate the role of IL-10, transgenic IL-10−/− fibroblasts at the same gestational age (IL10−/− FFb) were evaluated and found to have a significantly attenuated PCM ratio when compared to control FFb at the same gestational age (FFb 2.82 ± 1.02 vs IL10−/− FFb 1.58 ± 0.16, p = 0.000001).
Fig. 1.
Pericellular matrix (PCM) ratio calculated as PCM area (A) over cell body area (B). Arrow indicates an example sheep erythrocyte. Fetal fibroblasts (FFb, E14.5) produce a robust hyaluronan (HA) rich PCM. PCM is significantly attenuated with hyaluronidase and with inhibition of HA synthase with 4-methylumbelliferone. PCM production is also significantly decreased in IL-10−/− fibroblasts (IL10 −/− FFb) of the same gestational age.
2.2. In vitro – IL-10 is essential to HA production
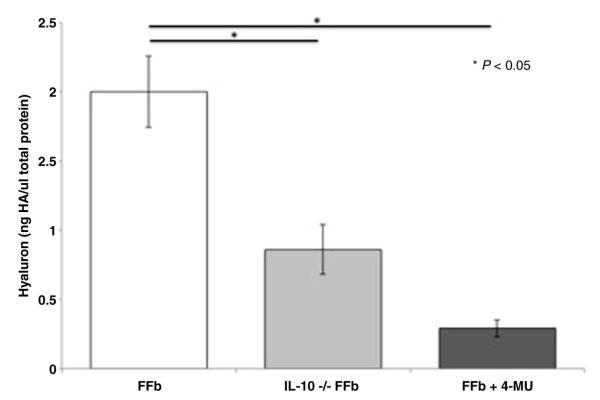
Mid-gestation fetal fibroblasts produce significantly more hyaluronan than transgenic IL-10−/− FFb (FFb 2.0 ± 0.26 ng HA/μg total protein vs IL-10−/− 0.86 ± 0.18 ng HA/μg total protein, p = 0.003) (Fig. 2). Inhibition of HA synthesis by HAS1-3 with 4-MU attenuates HA synthesis (FFb 2.00 ± 0.26 ng HA/μg total protein vs FFb + 4MU 0.29 ± 0.06 ng HA/μg total protein, p = 0.003).
Fig. 2.
Hyaluronan in media normalized to total protein content. Fetal fibroblasts (FFb, E14.5) produce significantly more hyaluronan (HA) than IL-10 −/− fibroblasts (IL-10 −/− FFb) of the same gestational age. Inhibition of HA synthase 1–3 by 4-methyumbelliferone significantly attenuates fetal production of HA.
2.3. Ex vivo – fetal forelimb organ cultures remain intact and viable at day 3
All specimens were morphologically intact at the time of fixation with no evidence of contamination or tissue degeneration. Mid-gestation and late-gestation fetal wounds maintain normal tissue architecture throughout the section. No disruption of epithelial or mesenchymal structures was noted at 3 days following wounding. Inhibition of HA synthesis in mid-gestation fetal wounds results in a disorganized wound bed and delayed re-epithelialization. Treatment of last-gestation fetal wounds with IL-10 has no disruption of epithelial or mesenchymal structures.
2.4. Ex vivo – IL-10 increases ground substance in fetal wounds
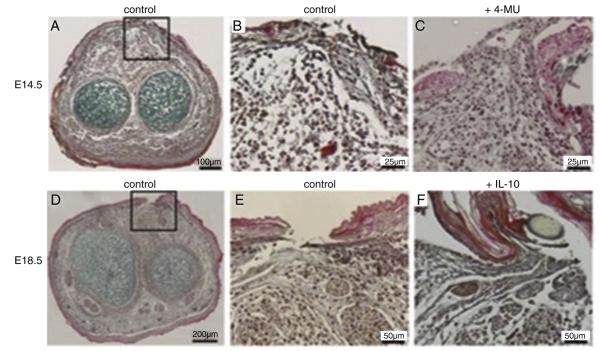
Mid-gestation fetal wounds have high levels of ground substance three days following wounding (Fig. 3A, B). To confirm the ground substance in the fetal wounds is primarily composed of hyaluronan, a loss-of-function experiment was performed by inhibiting HAS1-3 with 4-MU. HAS1-3 inhibition results in decreased ground substance in mid-gestation fetal wounds at day 3 following wounding (Fig. 3C). Late-gestation fetal wounds demonstrate less ground substance than mid-gestation fetal wounds at 3 days following wounding (Fig. 3D) with more collagen formation at the wound site (Fig. 3E). Supplementation of recombinant IL-10 in late-gestation fetal wound environment results in an increase in ground substance similar to mid-gestation wounds with a decrease in collagen around the wound (Fig. 3F).
Fig. 3.
Fetal forelimb incisions cultured ex vivo 3 days. Mid-gestation (E14.5) fetal forelimb shows increased ground substance (blue) throughout the mesenchyme (A entire section, B wound site). (C) Inhibition of hyaluronan synthesis decreases ground substance with a disorganized extracellular matrix. Late-gestation fetal forelimb incision has decreased ground substance throughout the section (D) and increased collagen (yellow) at the wound site (E). (F) Supplementation with recombinant IL-10 (200 ng/ml) results in increase ground substance at the wound site. (Pentachrome staining, magnification ×20).
2.5. Ex vivo – IL-10 increases hyaluronan in fetal wounds
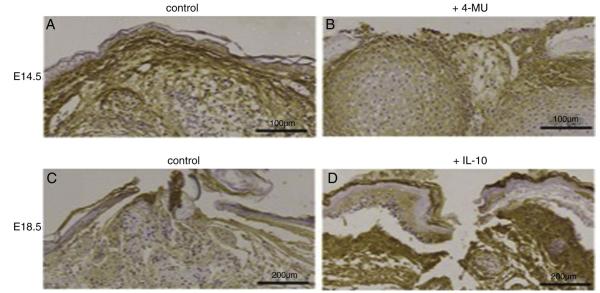
Mid-gestation fetal wounds (Fig. 4A) have substantial hyaluronan staining throughout the section at three days following wounding compared to late-gestation fetal wounds (Fig. 4C). HA staining persists with inhibition of HA synthesis with disorganized wound matrix and attenuated re-epithelialization (Fig. 4B). Supplementation of late-gestation fetal wounds with IL-10 increases hyaluronan staining, recapitulating the mid-gestation pattern (Fig. 4D).
Fig. 4.
Fetal forelimb incisions cultured ex vivo 3 days. (A) Mid-gestation (E14.5) fetal forelimb have more hyaluronan throughout the mesenchyme. (B) Inhibition of hyaluronan synthesis results in decreased HA staining. (C) Late-gestation fetal forelimbs have decrease HA throughout the mesenchyme. (D) Supplementation of recombinant IL-10 (200 ng/ml) results in increased local HA. (Immunohistochemistry for hyaluronan binding protein, HABP, magnification ×20).
3. Discussion
We have demonstrated a novel-role for IL-10 as a regulator of hyaluronan synthesis in the fetal extracellular matrix. IL-10 is essential to the fetal fibroblasts ability to produce its characteristic robust HA-rich extracellular matrix as evidenced by fibroblasts from IL-10−/− mice producing scant pericellular matrices. Adult fibroblasts produce a similarly attenuated PCM, but are capable of recapitulating the fetal phenotype with IL-10 supplementation. The importance of IL-10 in the regeneration fetal hyaluronan-rich ECM was then confirmed in an ex vivo incisional wound model. Mid-gestation fetal forelimbs capable of regenerative healing have more ground substance and HA staining than late-gestation scar forming counterparts. Supplementation of IL-10 to late-gestation forelimb recapitulated the regenerative phenotype with increase ground substance and HA staining.
IL-10 has been identified to play an essential role in regenerative fetal wound healing, which is characterized by minimal inflammation and decreased cellular infiltrate [8]. A 35-kDa homodimeric cytokine, IL-10 is a potent anti-inflammatory cytokine produced by Th2 cells, B cells and macrophages [11]. The importance of IL-10 in fetal development is speculated to be in the prevention of a maternal immune response to the fetus, the placenta and their ‘foreign’ antigens [12,13]. Early-gestation fetal skin, serum and amniotic fluid have elevated levels of IL-10. Conversely, IL-10 is minimally expressed in neonatal skin [14]. Moreover, in a transplant model, transgenic IL-10 knockout skin demonstrates scar formation at a gestation age which heals scarlessly in wild-type fetal mice [7]. Our study supports the importance of IL-10 in fetal development and wound healing, demonstrating its ability to recapitulate the mid-gestation phenotype in late-gestation forelimbs.
In additional to an anti-inflammatory milieu, fetal wounds are characterized by increased levels and prolonged elevation of hyaluronan following injury compared to post-natal wounds [15]. Iocono et al. demonstrate that chronic elevation of HA in the wound microenvironment promotes the deposition of a more organized matrix, similar to the dermis of uninjured skin [10]. Our in vitro and ex vivo data suggest this prolonged HA level can be mediated by IL-10, and is capable of recapitulating the mid-gestation phenotype in late-gestation wounds. Regenerative mid-gestation fetal wounds are known to produce predominantly high molecular weight HA, which has been shown to be anti-inflammatory in nature [4]. Further study is needed to determine the molecular weight of IL-10 mediated hyaluronan production in fetal wound healing.
Our data, through two independent models, demonstrate the importance of IL-10 in mediating hyaluronan production. Through an in vitro model, we show the essential role of IL-10 in the production of an HA-rich PCM in mid-gestational fibroblasts, as demonstrated by lack of characteristic fetal PCM in IL-10−/− fibroblasts, and the ability of Il-10 to recapitulate the fetal phenotype in late-gestational fibroblasts. This hyaluronan enhanced ECM may provide a latticework for cells to migrate more efficiently throughout wound healing, thereby minimizing the time for the fibroblast to produce excess collagen characteristic of scar formation [16]. These findings were further translated to an ex vivo model, demonstrating production of an HA-rich extracellular matrix throughout the mid-gestation tissue. This ECM is locally recapitulated with addition of IL-10 in the late-gestation wound bed. The ex vivo model permits histological evaluation of fetal wounds with inhibition of HAS1-3 and supplementation of IL-10. This model permits manipulation and study of physiology that are impossible in vitro, for example the constitutive HAS2 knockout has been shown to be lethal [17]. Forelimbs in ex vivo organ culture have been shown to continue to mature and develop, providing the opportunity for modulation of the local wound microenvironment in fetal development. In utero studies are necessary to elucidate the role of IL-10 on HA production and ECM regulation in vivo. These future studies will be helpful in elucidating the contribution of systemic circulation, bone marrow including endothelial progenitor cells, and an intact immune system in mitigating the effects of IL-10 in the wound environment.
Although we have known for over 30 years the fetus is capable of scarless wound healing, we now have the molecular tools to elucidate the underlying mechanism of the fetal regenerative response [4]. This study makes use of multiple models to demonstrate the essential role of IL-10 mediated hyaluronan synthesis by fibroblasts in fetal wound healing. Better understanding of fetal wound healing has implications beyond cosmetic benefit with potential to restore integrity to tissue and possibly to any pathology characterized by excessive fibrosis including pulmonary fibrosis, hepatic cirrhosis, intra-abdominal adhesions and hypertrophic scarring.
References
- [1].Longaker MT, Whitby DJ, Adzick NS, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25:63–8. doi: 10.1016/s0022-3468(05)80165-4. [discussion 68-69] [DOI] [PubMed] [Google Scholar]
- [2].Krummel TM, Nelson JM, Diegelmann RF, et al. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–4. doi: 10.1016/s0022-3468(87)80117-3. [DOI] [PubMed] [Google Scholar]
- [3].Gallivan EK, Crombleholme TM, Moriarty KP. Effect of fetal serum on fibroblast pericellular matrix formation. J Surg Res. 1996;64:128–31. doi: 10.1006/jsre.1996.0318. [DOI] [PubMed] [Google Scholar]
- [4].Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24:371–8. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Adzick NS, Harrison MR, Glick PL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–9. doi: 10.1016/s0022-3468(85)80210-4. [DOI] [PubMed] [Google Scholar]
- [6].Gordon A, Kozin ED, Keswani SG, et al. Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen. 2008;16:70–9. doi: 10.1111/j.1524-475X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- [7].Liechty KW, Kim HB, Adzick NS, et al. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–72. doi: 10.1053/jpsu.2000.6868. [discussion 872–863] [DOI] [PubMed] [Google Scholar]
- [8].Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–60. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- [9].Hiramatsu K, Sasagawa S, Outani H, et al. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. J Clin Invest. 2011;121:640–57. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iocono JA, Ehrlich HP, Keefer KA, et al. Hyaluronan induces scarless repair in mouse limb organ culture. J Pediatr Surg. 1998;33:564–7. doi: 10.1016/s0022-3468(98)90317-7. [DOI] [PubMed] [Google Scholar]
- [11].Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- [12].Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cadet P, Rady PL, Tyring SK, et al. Interleukin-10 messenger ribonucleic acid in human placenta: implications of a role for interleukin-10 in fetal allograft protection. Am J Obstet Gynecol. 1995;173:25–9. doi: 10.1016/0002-9378(95)90164-7. [DOI] [PubMed] [Google Scholar]
- [14].Heyborne KD, McGregor JA, Henry G, et al. Interleukin-10 in amniotic fluid at midtrimester: immune activation and suppression in relation to fetal growth. Am J Obstet Gynecol. 1994;171:55–9. doi: 10.1016/s0002-9378(94)70077-x. [DOI] [PubMed] [Google Scholar]
- [15].Estes JM, Adzick NS, Harrison MR, et al. Hyaluronate metabolism undergoes an ontogenic transition during fetal development: implications for scar-free wound healing. J Pediatr Surg. 1993;28:1227–31. doi: 10.1016/s0022-3468(05)80303-3. [DOI] [PubMed] [Google Scholar]
- [16].Alaish SM, Yager D, Diegelmann RF, et al. Biology of fetal wound healing: hyaluronate receptor expression in fetal fibroblasts. J Pediatr Surg. 1994;29:1040–3. doi: 10.1016/0022-3468(94)90275-5. [DOI] [PubMed] [Google Scholar]
- [17].Roughley PJ, Lamplugh L, Lee ER, et al. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine (Phila Pa 1976) 2011;36:E914–20. doi: 10.1097/BRS.0b013e3181f1e84f. [DOI] [PubMed] [Google Scholar]