Abstract
Background
Patients with bipolar disorder exhibit consistent deficits in facial affect identification at both behavioral and neural levels. However, little is known about which stages of facial affect processing are dysfunctional.
Methods
Event-related potentials (ERPs), including amplitude and latency, were used to evaluate two stages of facial affect processing: N170 to examine structural encoding of facial features and N250 to examine decoding of facial features in 57 bipolar disorder patients, 30 schizophrenia patients, and 30 healthy controls. Three conditions were administered: participants were asked to identify the emotion of a face, gender of a face, or whether a building was 1 or 2 stories tall.
Results
Schizophrenia patients’ emotion identification accuracy was lower than that of bipolar patients and healthy controls. N170 amplitude was significantly smaller in schizophrenia patients compared to bipolar patients and healthy controls, which did not differ from each other. Both patient groups had significantly longer N170 latency compared to healthy controls. For N250, both patient groups showed significantly smaller amplitudes compared with controls, but did not differ from each other. Bipolar patients showed longer N250 latency than healthy controls; patient groups did not differ from each other.
Conclusions
Bipolar disorder patients have relatively intact structural encoding of faces (N170) but are impaired when decoding facial features for complex judgments about faces (N250 latency and amplitude), such as identifying emotion or gender.
Introduction
One of the most studied aspects of social cognition in clinical and non-clinical samples is the ability to identify the emotion in a picture, or movie, or a face. This ability is easily measured and relates to success in daily functioning. There is evidence of facial affect processing deficits in patients with bipolar disorder at the behavioral, anatomical and functional levels. Several studies have noted that bipolar disorder patients are impaired compared to controls in labeling basic facial emotions (e.g., angry, disgusted, fearful, happy, sad and surprised) (Bozikas et al., 2006, Getz et al., 2003, Kohler et al., 2011, Vederman et al., 2011). Abnormalities in facial affect processing are also evident when using structural and functional MRI (fMRI) methodologies. Structural studies have revealed decreased cortical volume (Moorhead et al., 2007) and cortical thinning (Lyoo et al., 2006) in the fusiform gyrus, an area of the brain thought to be responsible for facial processing (Kanwisher et al., 1997). A recent meta-analysis of fMRI studies of emotion processing (e.g., facial affect processing) in bipolar disorder patients revealed increased activation in ventral-limbic areas, including the parahippocampal gyrus and the amygdala (Houenou et al., 2011). In the current study, we aimed to examine differences in facial affect processing between patients with bipolar disorder and schizophrenia using event-related potentials (ERPs).
ERPs have been highly useful for examining the time course of facial affect processing given its millisecond precision. Two ERP waveforms in particular, the N170 and the N250, have been closely linked to facial processing. The N170 response is a negative wave peaking at approximately 170 ms in bilateral parieto-occipital regions and is larger when viewing faces as compared to viewing non-faces (Bentin et al., 1996, Eimer, 2000). The N170 is thought to reflect the structural encoding of facial features, i.e. the arrangement of facial features with respect to other facial features (Bruce and Young, 1986, Eimer, 2000). The N170 is sometimes larger during facial affect processing (Miyohshi et al., 2004), but sometimes is not (Streit et al., 2001, Wynn et al., 2008). Source localization and simultaneous ERP-fMRI recordings have identified generators in the facial fusiform area and superior temporal sulcus as the main sources of the N170 (Itier and Taylor, 2004, Sadeh et al., 2010). The N250 is a negative wave peaking at approximately 250 ms in fronto-central midline regions and is thought to reflect the decoding of facial information, such as the recognition of complex features of the face that are associated with particular emotions or gender (Streit et al., 2001, Streit et al., 1999).
Only two studies to date have used ERPs to examine facial affect processing in bipolar disorder (Degabriele et al., 2011, Sokhadze et al., 2011). Both these studies found N170 in bipolar patients to be of smaller amplitude compared to healthy controls, though neither study examined N250. Both of these studies were conducted in the context of a cognitive manipulation (oddball or go/no-go paradigm) leaving uncertain what the results would be in an affect perception task lacking a cognitive manipulation. Also, the sample sizes were relatively low (bipolar patient n’s of 9 and 18 for the two studies), which precluded the ability to examine subgroups of bipolar patients.
In the current study, we examined N170 and N250 in a sample of patients with bipolar disorder. We compared them to healthy controls, and to patients with schizophrenia, who have well-established deficits in facial affect perception (Kohler et al., 2010) and anatomical abnormalities in brain regions associated with facial affect processing, such as decreased left fusiform gray matter volume (Goghari et al., 2011). A final aim of our study was to examine the potential impact of antipsychotic medication and bipolar subtype (I vs. II) on the ERP measures given the relatively large sample size of bipolar patients we recruited.
Method
Participants
The sample consisted of 58 bipolar patients, 32 schizophrenia patients and 30 healthy comparison subjects. Of the bipolar patients, 39 had Bipolar I (11 of those with a history of psychosis) and 19 with Bipolar II. Bipolar patients were recruited from bipolar outpatient clinics at the University of California, Los Angeles (UCLA) and the Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHS). Schizophrenia patients were recruited from outpatient treatment clinics at the VAGLAHS and from board-and-care residences in the community through staff presentations and referral. Diagnosis was based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al., 1997). Bipolar and schizophrenia patients with no medication changes in the past six weeks, no inpatient hospitalization in the past 3 months, and no changes in housing were recruited.
Healthy controls were recruited through newspaper and internet advertisements and were screened with the SCID-I and were screened for select personality disorders using the SCID-II (First et al., 1996). They were excluded if they met criteria for any lifetime psychotic disorder, bipolar mood disorder, recurrent depression, substance dependence, paranoid, schizotypal, or schizoid personality disorder, or if they reported a history of psychotic disorder among their first-degree relatives.
Additional exclusion criteria for all three groups included being younger than 18 or older than 60 years, having an IQ below 70 based on chart review, actively using substances in the past 6 months, having an identifiable neurological disorder, seizures, history of head injury resulting in loss of consciousness for more than one hour, or being insufficiently fluent in English. All participants gave written informed consent after receiving a detailed explanation of study procedures in accordance with procedures approved by the Institutional Review Boards at UCLA and the VAGLAHS.
Twenty-two schizophrenia patients were receiving second-generation antipsychotic medications, 4 were receiving first-generation antipsychotic medications, 2 were receiving both types of antipsychotics, and 2 were not taking an antipsychotic medication at the time of testing. Most bipolar patients (n = 50) were receiving at least one type of psychoactive drug: 27 were on anticonvulsants, 12 were taking lithium, 32 were taking an antipsychotic medication, and 29 were taking antidepressants.
Clinical Ratings
Diagnosis was determined at a consensus case-review meeting with senior diagnosticians based on the information obtained in the interview. Bipolar and schizophrenia patients’ psychiatric symptoms were evaluated using the expanded 24-item UCLA version of the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993b). Bipolar patients’ depression and mania ratings were evaluated using the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) as well as the Young Mania Rating Scale (YMRS; Young et al., 1978). For the BPRS, we report the total score, as well as the means for positive, negative, depression/anxiety, and agitation/mania factors (Kopelowicz et al., 2008). All the clinical assessments and diagnostic interviews were conducted by interviewers trained to reliability through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) based on established procedures (Ventura et al., 1993a, Ventura et al., 1998). Training was conducted within the MIRECC and involved viewing videotapes and conducting live interviews to establish inter-rater reliability to a minimum kappa of 0.75.
ERP Paradigm
All participants performed 3 different classification tasks while viewing black and white pictures of either faces or buildings, based on a procedure we previously developed (Wynn et al., 2008). Participants were asked to identify the gender of a face, the emotion of a face, or whether a building was 1 or 2 stories in separate blocks. Face pictures were drawn from Ekman and Friesen’s Pictures of Facial Affect Set (Ekman and Friesen, 1976). A total of 36 faces (18 male, 18 female) were used. Faces depicted 1 of 6 different emotions: afraid (7 pictures), angry (6 pictures), ashamed (4 pictures), happy (11 pictures), sad (4 pictures), and surprised (4 pictures). Thirty-six pictures of buildings (18 1-story and 18 of 2-story) were downloaded from an internet website (images.google.com). Face and building pictures were black and white photographs, and all were resized to the same dimension (20 X 25 cm). Stimuli were not normalized on other visual properties such as contrast or brightness levels. All stimuli were presented on a cathode-ray tube monitor placed 1 m in front of the subject. Subjects received 3 blocks of 72 pictures per block, in a fixed block order (building identification, gender identification, and emotion identification). Pictures within each block were presented in a random order for each subject, with each picture presented twice during the block.
A trial began with a fixation cross presented for 400 ms, a blank screen for 500 ms, a picture for 500 ms, and a blank screen for 1000 ms. At the end of each trial a screen appeared which prompted the participant to identify the picture depending on which block was presented. For the emotion identification task, the six possible emotions were presented; for gender identification, the two possible choices (male or female) were presented; and for building identification, the two possible choices (1 or 2 stories) were presented. Thus, chance level of performance was 0.16 for the emotion identification task and 0.50 for the other two tasks. Participants responded verbally and their response was entered by the tester, at which point the next trial began. Behavioral data were collected simultaneously during the EEG recording. The total number of correct responses (out of 72 per condition) was analyzed.
EEG Recording and Analysis
EEG recordings were acquired with a 64-channel BioSemi ActiveTwo amplifier (Biosemi B. V., Amsterdam, Netherlands). Data were sampled at 1024 Hz with a bandpass of 0 to 100 Hz. Additional electrodes were placed above and below the left eye and at the outer canthi of both eyes to monitor blinks and eye movements. Each active electrode was measured online with respect to a common mode sense electrode during data collection, forming a monopolar channel. An additional electrode was placed at the nose tip and all EEG data were re-referenced offline to this electrode.
Offline data processing was performed using BrainVision Analyzer 2 software (Brain Products, Gilching, Germany). Bad electrodes were removed from the recording and a spline interpolation was used to recreate the electrode. Interpolation was performed only on a subset of participants in each group: the mean number of interpolated electrodes was 1.67 in 3 schizophrenia patients, 3.33 in 15 bipolar patients, and 1.8 in 5 healthy controls; the remainder of the participants in each group did not have any interpolated electrodes. Eyeblinks were removed from the data using a standard regression-based algorithm (Gratton et al., 1983). Data were then epoched at -100 to 700 ms relative to stimulus onset and were baseline corrected to the prestimulus interval. A high-pass filter of 1 Hz and a low-pass filter of 20 Hz (zero-phase shift with a 24 dB/octave rolloff) was then applied. Epochs containing activity exceeding ± 75 μV at all scalp electrode sites were automatically rejected. The mean (standard deviation) number of acceptable trials across the three conditions (out of 216) was 186 (27.3) for schizophrenia patients, 186 (25.8) for bipolar patients, and 193 (24.2) for healthy controls; there were no statistical differences between the groups in the number of trials accepted. Two bipolar patients and 1 schizophrenia patient had more than 50% of trials rejected and were not included in the analysis. Thus, the final sample size for each group used in all the analyses was 57 bipolar patients, 30 schizophrenia patients, and 30 healthy controls.
ERPs in the building condition were subtracted from ERPs in the gender and emotion identification conditions to obtain a waveform that is mostly reflecting activity due to processing of faces. We then examined two separate ERP components: the N170 and N250. A time window of activity was defined separately for each group and each ERP component based on the peak activity observed by inspection of the mean global field power averaged across the gender and emotion identification tasks. The width of the time window was selected to ensure coverage of each component with the mean activity within each window serving as the main dependent measure. The peak latency for each ERP component defined within those windows was also examined. For healthy controls, the time windows were 136–156 ms for the N170 and 210–250 ms for the N250; for bipolar patients, 145–165 for the N170 and 250–290 for the N250; and for schizophrenia patients, 149–169 for the N170 and 240–280 for the N250. The N170 was examined in six parieto-occipital electrode sites where the response was largest based on visual inspection of the topographical maps (P7/8, P9/10, PO7/8); activity was examined separately for each hemisphere by averaging the three left and the three right electrodes. The N250 was examined as the average of activity at electrodes Fz and FCz.
Data analysis
One-way ANOVAs and chi-square tests were used to assess group differences for continuous and categorical demographic variables, respectively. To investigate group differences in ERPs, a repeated measures analysis of variance (ANOVA) was performed separately for each component; only the difference wave ERPs for the emotion and gender identification tasks were analyzed. Separate analyses in the BD patients were analyzed to examine subgroups: BD I vs. BD II (n = 19 and 38, respectively), and bipolar disorder patients on vs. off antipsychotic medications (n = 32 and 25, respectively). Greenhouse-Geisser corrections (ε) were used in the repeated measures ANOVAs that contained more than one degree of freedom to correct for violations of sphericity. We report the uncorrected degrees of freedom, ε, and the corrected p values. Significant interactions were decomposed using a series of one-way ANOVAs and Least Significant Difference (LSD) comparisons.
Results
Demographic and Clinical Characteristics
Table 1 presents the demographic and clinical information for the three groups. The schizophrenia patients had significantly lower levels of personal education compared to the healthy controls and bipolar disorder patients. However, we attempted to match the groups on parental, not personal, education and there was no statistical difference between the groups on parental education. There were no significant differences in age or gender distribution between the three groups. Schizophrenia patients had significantly higher scores for total BPRS (t85 = 3.82, p < 0.001), BPRS positive symptoms (t85 = 6.74, p < 0.001), and BPRS negative symptoms (t85 = 3.87, p < 0.001) compared to patients with bipolar disorder.
Table 1.
Demographics, Clinical Characteristics, and Behavioral Performance
| Healthy Controls (N = 30) | Bipolar Patients (N = 57) | Schizophrenia Patients (N = 30) | |
|---|---|---|---|
| Age (Mean/SD) | 40.6 (10.1) | 44.9 (10.4) | 45.3 (9.4) |
| Gender (% Male) | 63.3% | 56.9% | 65.6% |
| Personal Education (Mean/SD)* | 14.8 (1.6) | 14.1 (2.1) | 12.7 (3.4) |
| Parental Education (Mean/SD) | 13.6 (2.7) | 13.4 (3.0) | 12.8 (3.6) |
| BPRS Total (Mean/SD)+ | --------- | 33.6 (7.3) | 40.5 (9.2) |
| Positive+ | --------- | 1.13 (0.20) | 2.02 (0.96) |
| Negative+ | --------- | 1.22 (0.45) | 1.79 (0.93) |
| Depression/Anxiety | --------- | 2.11 (0.89) | 1.90 (0.63) |
| Agitation/Mania | --------- | 1.20 (0.36) | 1.14 (0.30) |
| HDRS Total (Mean/SD) | --------- | 9.1 (7.5) | --------- |
| YMRS Total (Mean/SD) | --------- | 3.5 (3.9) | --------- |
| Emotion Identification** | 59.8 (5.4) | 57.4 (5.9) | 53.0 (7.6) |
| Gender Identification | 71.2 (1.0) | 71.3 (1.2) | 70.7 (1.6) |
| Building Identification | 65.4 (4.3) | 66.7 (2.4) | 65.0 (5.1) |
BPRS = Brief Psychiatric Rating Scale; HDRS = Hamilton Depression Rating Scale; YMRS = Young Mania Rating Scale.
Schizophrenia patients significantly lower in personal education compared to healthy controls and bipolar patients, p < 0.05.
Schizophrenia patients significantly higher than bipolar patients, p < 0.05.
Schizophrenia patients significantly lower in emotion identification performance compared to healthy controls and bipolar patients.
Identification Task Performance
A 3 (group) x 3 (task) repeated measures ANOVA on the behavioral data revealed significant main effects of task (F2, 222 = 322.5, p < 0.001, ε = 0.71) and group (F2, 111 = 7.86, p < 0.001), as well as a group X task interaction (F4, 222 = 6.44, p < 0.001, ε = 0.71). The group X task interaction was due to the schizophrenia patients performing significantly worse than healthy controls and bipolar patients on the emotion identification task, with no differences in performance between groups on the other identification tasks. The behavioral performance can be seen in Table 1.
N170 Mean Amplitude and Latency Analyses
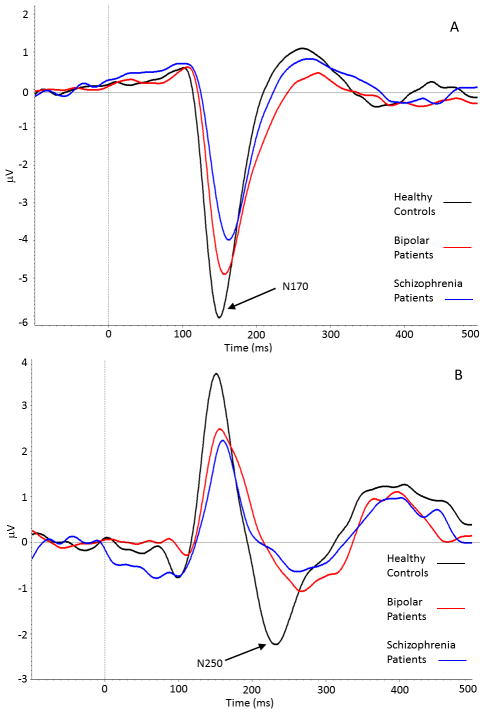
The grand average N170 difference waveform for each group, collapsed across task and hemisphere, can be seen in Figure 1, Panel A. A 3 (group) X 2 (task) X 2 (hemisphere) repeated measures ANOVA on N170 amplitude revealed significant main effects of task (F1, 114 = 11.27, p < 0.001) and hemisphere (F1, 114 = 9.66, p < 0.005), and a marginal main effect for group (F2, 114 = 2.60, p < 0.08). The group X task interaction (F2, 114 = 1.60, p < 0.21) and other interactions were not significant. Mean N170 amplitudes and latencies, collapsed across the two identification tasks, can be seen in Table 3.
Figure 1.
Panel A, Grand average N170 difference waveform, collapsed across facial identification task and hemisphere, for healthy controls (black line), bipolar disorder patients (red line), and schizophrenia patients (blue line). The N170 was significantly larger in healthy controls and bipolar disorder patients compared to schizophrenia patients. Panel B, Grand average N250 difference waveform, collapsed across facial identification task, for healthy controls (black line), bipolar disorder patients (red line), and schizophrenia patients (blue line). The N250 was significantly different between all three groups.
Table 3.
Mean (standard deviation) N170 and N250 amplitude and latency for bipolar patients only, collapsed across task.
| Bipolar Type Diagnosis | Antipsychotic Medication Status | |||
|---|---|---|---|---|
| Bipolar 1 (n = 38) | Bipolar 2 (n = 19) | Not Taking Antipsychotics (n = 25) | Taking Antipsychotics (n = 32) | |
| N170 Amplitude (μV) | −4.74 (2.99) | −4.62 (2.94) | −4.70 (2.71) | −4.70 (3.16) |
| N170 Latency (ms) | 159.6 (12.5) | 159.7 (10.6) | 159.3 (10.6) | 160.0 (12.8) |
| N250 Amplitude (μV) | −0.86 (1.54) | −1.05 (2.80) | −0.70 (1.67) | −1.10 (2.27) |
| N250 Latency (ms) | 250.0 (23.8) | 263.9 (23.9)* | 249.2 (24.2) | 258.9 (24.3) |
Bipolar 2 > Bipolar 1 for N250 latency, F (1, 55) = 4.34, p < 0.05.
The marginal group effect was followed up with LSD contrasts and revealed that schizophrenia patients had significantly lower N170 amplitudes compared to healthy controls but not bipolar patients; there was no statistical difference between healthy controls and bipolar disorder patients (see Table 2). The task main effect revealed that N170 amplitudes were larger during the gender identification task compared to the emotion identification task, -4.86 (3.05) μV vs. −4.48 (3.18) μV, respectively. The significant hemisphere main effect was due to greater activity in the right compared to left hemisphere, −5.10 (3.73) vs. −4.24 (3.04) μV, respectively.
Table 2.
Mean (standard deviation) N170 and N250 amplitude and latency for each group, collapsed across task.
| Group | ||||
|---|---|---|---|---|
| Healthy Controls | Schizophrenia Patients | Bipolar Patients | Group Main Effect Statistics | |
| N170 Amplitude (μV) | −5.51 (3.33) | −3.79 (2.92) | −4.70 (2.95) | F (2, 114) = 11.33, p < 0.001 HC = BD < SZ, p < 0.05 |
| N170 Latency (ms) | 151.1 (8.7) | 161.8 (11.4) | 159.7 (11.8) | F (2, 114) = 8.46, p < 0.001 HC < SZ = BD, p < 0.05 |
| N250 Amplitude (μV) | −1.81 (1.82) | −0.58 (1.81) | −0.92 (2.02) | F (2, 114) = 6.99, p < 0.001 HC < BD = SZ, p < 0.05 |
| N250 Latency (ms) | 236.3 (21.4) | 243.4 (21.8) | 254.6 (24.5) | F (2, 114) = 6.73, p < 0.01 HC = SZ < BD, p < 0.05 |
HC = Healthy Controls
BD = Bipolar Disorder Patients
SZ = Schizophrenia Patients
For latency, a 3 (group) X 2 (task) X 2 (hemisphere) repeated measures ANOVA revealed a significant main effect of group, F2, 114 = 8.46, p < 0.001, and a significant main effect of task, F1, 114 = 5.12, p < 0.03. The group X task interaction (F2, 114 = 0.84, p < 0.50) and other interactions were not significant. The significant main effect of group was due to healthy controls having a significantly faster latency compared to bipolar patients and schizophrenia patients (see Table 2). The significant main effect of task was due to a significantly faster latency during emotion identification, 157.0 (12.0) ms, compared to gender identification, 159.1 (12.8) ms.
N250 Mean Amplitude and Latency Analyses
Mean N250 amplitudes and latencies, collapsed across identification tasks, can be seen in Table 2. The grand average N250 difference waveform for each group, collapsed across task, can be seen in Figure 1, Panel B. A 3 (group) X 2 (task) repeated measures ANOVA on N250 amplitude revealed a significant main effect of group, F2, 114 = 5.02, p < 0.01. The main effect of task (F1, 114 = 0.12, p < 0.73) and the group X task interaction (F2, 114 = 2.00, p < 0.15) were not significant. The group main effect was due to significant differences in amplitude between the healthy controls and the two patient groups, with activity in bipolar patients intermediate between the healthy controls and schizophrenia patients (see Table 2).
For latency, a 3 (group) X 2 (task) repeated measures ANOVA on N250 latency revealed a significant main effect of group, F2, 114 = 6.73, p < 0.01. The main effect of task (F1, 114 = 0.11, p < 0.74) and the group X task interaction (F2, 114 = 0.75, p < 0.50) were not significant. The group effect was due to bipolar patients having a significantly longer latency compared to healthy controls and schizophrenia patients (see Table 2). There was no significant difference in latency between healthy controls and schizophrenia patients.
Subgroup Analyses
We examined amplitude and latency differences between BD I vs. BD II patients and between bipolar disorder patients taking vs. not taking antipsychotic medication, shown in Table 3. For the N170, there were no significant effects on amplitude or latency for either subgroup comparison. For the N250, there was a significant effect of BD I vs. BD II on latency, with those diagnosed with bipolar II having slower latencies compared to those diagnosed with bipolar I. There were no effects of antipsychotic medication on N250 amplitude.
Discussion
The results of the current study revealed a complex pattern of ERP deficits associated with facial affect processing in patients with bipolar disorder. While bipolar patients had normal N170 amplitude, the wave took significantly longer to reach peak. For the N250, both amplitude and latency deficits were seen compared to healthy controls. These results do not appear to be influenced by antipsychotic medication, based on a subgroup analysis of bipolar patients, and are comparable for BD I and BD II. In contrast, schizophrenia patients exhibited amplitude deficits for both N170 and N250, but a latency deficit only for the N250. Taken together, these results point to deficits in the later stage of facial affect decoding in bipolar disorder; structural encoding remains intact, though taking longer to peak.
The intact N170 amplitude in bipolar patients is not consistent with two previously published papers that reported reduced N170 (Degabriele et al., 2011, Sokhadze et al., 2011). However, there are several methodological differences between our study and the studies that found N170 deficits. A key methodological difference was that the previous studies used a cognitive oddball task with faces as standard and rare stimuli, whereas we utilized a much simpler facial emotion identification task. It is possible that the more demanding cognitive nature of their task resulted in a reduced N170. Despite normal N170 amplitude, bipolar patients showed significantly longer latency compared to healthy controls. This result suggests that bipolar patients’ ability to structurally encode faces is intact, though they are delayed in generating the full response. The abnormal N170 response in the schizophrenia patients is largely consistent with the literature (Campanella et al., 2006, Herrmann et al., 2004, Turetsky et al., 2007), although other studies, including a previous study from our laboratory (Wynn et al., 2008), have failed to detect an N170 deficit. There are several possible methodological reasons for this discrepancy. In our previous study in schizophrenia, we used a rather large time range (80 ms) to examine the mean activity of the N170, while in the current study we used a more narrow time range (20 ms). Further, in our previous study we used a single time window for both groups to identify mean activity, while in the current study the time window was defined separately for each group. The methods used in the current study, we believe, reflect a more accurate assessment of the N170 and may have been more sensitive at detecting group differences in the N170 response compared to our previous study.
We found larger N170 amplitudes during the gender identification task vs. the emotion identification task, though the effect size was relatively small (Cohen’s d = 0.15). This pattern may have to do with the nature of the task demands. For example, several studies have suggested that neural responding, particularly in the amygdala, is stronger during implicit tasks compared to explicit tasks (i.e. emotion identification using emotional faces) (Chen et al., 2006, Critchley et al., 2000). The gender identification using emotional faces can be considered an implicit task of emotion processing, in contrast to the explicit task of emotion identification.
On the N250, bipolar patients’ amplitude was intermediate between that of healthy controls and schizophrenia patients, with activity significantly smaller compared to healthy controls. These results indicate that both bipolar disorder and schizophrenia patients have deficits in the decoding of facial features, though the deficit is not as pronounced in bipolar disorder. Similar to the N170 results, bipolar disorder patients also exhibited a significantly longer N250 latency compared to healthy controls. However, this effect is driven in large part by BD II patients 2, as they had significantly longer latencies than BD I patients. These results imply that bipolar disorder, and particularly BD II, is associated with neural delays associated with decoding of facial affect. Although BD I patients are, by definition, more functionally impaired they are not always as clinically impaired as BD II patients (e.g., Undurraga et al., in press). In fact, the BD II patients in the current study had more self-reported depressive episodes (average of 22.9 episodes versus 14.5 for BD I patients).
Our results in bipolar patients (who had an adequate number of patients on, and off, antipsychotic medications for meaningful comparisons) revealed no significant ERP effects of antipsychotic medications. These results strongly suggest that the amplitude and latency differences between patients and controls are not due to antipsychotic medications.
We did not detect a group X task (emotion vs. gender identification) interaction for the N170 or N250, which raises the question as to whether our between-group effects are due to face processing in general versus facial emotion process in particular. That question cannot be resolved with the current study because there were no emotionally neutral faces. Hence, subjects may have been processing emotion implicitly in the gender condition.
We examined the N170 and N250 with difference waves in which we subtracted activity in response to a control condition (i.e., houses). The use of difference waves was chosen as a way to control for visual processing that is not specific to faces, but it obscures the separate ERP responses to the components of the difference wave. Hence, we have presented the values for each condition separately in a supplemental online table.
The study had several limitations. First, we intentionally selected bipolar patients who were outside of a mood episode, making it unclear whether ERP deficits would be present or exacerbated during a mood episode. Second, the two patient groups were not matched for type of psychoactive medication. For example, all of the bipolar patients taking an antipsychotic were on a second-generation medication, whereas a number of the schizophrenia patients were on first-generation or mixed antipsychotics. Third, we did not exclude healthy controls with a first-degree relative with a mood disorder, and that could produce smaller differences between controls (if they were at risk for mood disorder) and bipolar patients. However, this selection feature is unlikely to have affected the results as only one control reported a first-degree relative with a mood disorder. Fourth, the tasks varied in terms of their difficulty, potentially influencing the results. The range in difficulty across tasks likely explains both the task effect and task by group effect for behavior (as all groups were at ceiling on the building and gender identification tasks, but not the emotion identification task). However, the range in task difficulty does not help explain the EEG results due to the lack of a group by task interaction. Finally, because we did not have an equal number of faces depicting each emotion, we could not examine the effects of specific emotions (e.g., happiness, disgust) on ERPs. Future studies with more complete coverage of emotion could determine if specific emotions affect the N170 or N250 differentially in bipolar and schizophrenia patients.
While bipolar disorder and schizophrenia patients share several features (genetics, some clinical features, etc.), they exhibit distinctly different patterns of deficits across stages of facial affect processing based on ERPs. Schizophrenia patients show deficits at multiple stages of facial affect processing whereas the deficits in bipolar disorder appear to be less severe and appear at the later stage of affect decoding. These results are consistent with findings from a recent meta-analysis showing that facial affect processing deficits are present in mood disorders, including bipolar disorder, but are not as severe as in schizophrenia (Kohler et al., 2011).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH089634 to MFG). The authors wish to thank Crystal Gibson, Cory Tripp, Katie Weiner, Mark McGee, Christen Waldon and Amanda Bender for their assistance with recruitment and testing.
Footnotes
The authors do not have any commercial or other relationships that could constitute a conflict of interest.
References
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozikas VP, Tonia T, Fokas K, Karavatos A, Kosmidis MH. Impaired emotion processing in remitted patients with bipolar disorder. Journal of Affective Disorders. 2006;91:53–56. doi: 10.1016/j.jad.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77 :305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Campanella S, Montedoro C, Streel E, Verbanck P, Rosier V. Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: An event-related potentials study. Clinical Neurophysiology. 2006;36:71–78. doi: 10.1016/j.neucli.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: A functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degabriele R, Lagopoulos J, Malhi G. Neural correlates of emotional face processing in bipolar disorder: An event-related potential study. Journal of Affective Disorders. 2011;133:212–220. doi: 10.1016/j.jad.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Eimer M. The face-specific N170 component reflects late stages in the structural encoding of faces. Cognitive Neuroscience. 2000;11:2319–2324. doi: 10.1097/00001756-200007140-00050. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1996. Structured Clinical Interview for DSM-IV Avis II Personality Disorders. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1997. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. [Google Scholar]
- Getz GE, Shear PK, Strakowski SM. Facial affect recognition deficits in bipolar disorder. Journal of the International Neuropsychological Society. 2003;9:623–632. doi: 10.1017/S1355617703940021. [DOI] [PubMed] [Google Scholar]
- Goghari VM, MacDonald AW, III, Sponheim SR. Temporal lobe structures and facial emotion recognition in schizophrenia patients and nonpsychotic relatives. Schizophrenia Bulletin. 2011;37:1281–1294. doi: 10.1093/schbul/sbq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. American Journal of Psychiatry. 2004;161:915–917. doi: 10.1176/appi.ajp.161.5.915. [DOI] [PubMed] [Google Scholar]
- Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, Wessa M. Neuroimaging-based markers of bipolar disorder: Evidence from two meta-analyses. Journal of Affective Disorders. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. NeuroReport. 2004;15:1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Research. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: A meta-analytic review. Schizophrenia Bulletin. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RL, Mintz J. Consistency of brief psychiatric rating scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41:77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sungh YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Biopolar Disorders. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Miyohshi M, Katayama J, Morotomi T. Face-specific N170 component is modulated by facial expressional change. NeuroReport. 2004;15:911–914. doi: 10.1097/00001756-200404090-00035. [DOI] [PubMed] [Google Scholar]
- Moorhead TWJ, McKirdy J, Sussmann JED, Hall J, Lawrie SM, Johnstone EC, McIntosh AM. Progressive gray matter loss in patients with bipolar disorder. Biological Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: A simultaneous ERP-fMRI investigation. Human Brain Mapping. 2010;31:1490–1501. doi: 10.1002/hbm.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokhadze EM, Tasman A, Tamas R, El-Mallakh RS. Event-related potential study of the effects of emotional facial expressions on task performance in euthymic bipolar patients. Applied Psychophysiology and Biofeedback. 2011;36:1–13. doi: 10.1007/s10484-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides A, Sinneman T, Wölwer W, Dammers J, Zilles K, Gaebel W. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: A magnetoencephalographic study. American Journal of Psychiatry. 2001;158:1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides AA, Liu L, Wölwer W, Dammers J, Gross J, Gaebel W, Muller-Gartner HW. Neurophysiological correlates of the recognition of facial expressions of emotion as revealed by magnetoencephalography. Cognitive Brain Research. 1999;7:481–491. doi: 10.1016/s0926-6410(98)00048-2. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: When and why does it go awry? Schizophrenia Research. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ, Valenti M, Pacchiarotti I, Vieta E. Suicidal risk factors in bipolar I and II disorder patients. Journal of Clinical Psychiatry. doi: 10.4088/JCP.11m07041. (in press) [DOI] [PubMed] [Google Scholar]
- Vederman AC, Weisenbach SL, Rapport LJ, Leon HM, Haase BD, Franti LM, Schallmo MP, Saunders EF, Kamali MM, Zubieta JK, Langenecker SA, McInnis MG. Modality-specific alterations in the perception of emotional stimuli in Bipolar Disorder compared to Healthy Controls and Major Depressive Disorder. Cortex. 2011 doi: 10.1016/j.cortex.2011.03.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: 'The Drift Busters'. International Journal of Methods in Psychiatric Research. 1993a;3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A. Training and quality assurance with the Structured Clinical Interview for DSM-IV. Psychiatry Research. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. International Journal of Methods in Psychiatric Research. 1993b;3:227–243. [Google Scholar]
- Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophrenia Bulletin. 2008;34:679–687. doi: 10.1093/schbul/sbn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.