Abstract
Riboswitches are RNA elements that undergo a shift in structure in response to binding of a regulatory molecule. These elements are encoded within the transcript they regulate, and act in cis to control expression of the coding sequence(s) within that transcript; their function is therefore distinct from that of small regulatory RNAs (sRNAs) that act in trans to regulate the activity of other RNA transcripts. Riboswitch RNAs control a broad range of genes in bacterial species, including those involved in metabolism or uptake of amino acids, cofactors, nucleotides, and metal ions. Regulation occurs as a consequence of direct binding of an effector molecule, or through sensing of a physical parameter such as temperature. Here we review the global role of riboswitch RNAs in bacterial cell metabolism.
Keywords: RNA structure, gene expression, regulation, transcription attenuation, translation
A wide variety of mechanisms that regulate genes involved in response to environmental changes, including availability of cellular metabolites, have been uncovered in bacteria. Historically, analysis of gene regulation has focused primarily on DNA-binding regulatory proteins that affect the interaction of RNA polymerase (RNAP) with the promoter region of the regulated genes. The DNA-binding proteins may act to increase or decrease transcription of their target promoters, and the activity of the regulatory protein is often modulated by interaction with an effector molecule that activates or represses DNA binding (e.g., lac repressor binding is decreased by lactose, trp repressor binding is activated by tryptophan). Collaboration of multiple regulatory proteins at a single promoter can be used to integrate multiple signals. Use of DNA-binding regulatory proteins requires that the cell encode and express these proteins, often constitutively, so that the proteins are in place to sense the physiological signal and promote the appropriate genetic response. Regulation at the level of transcription initiation is efficient, however, in that transcript synthesis occurs only under the appropriate condition. In some cases, regulatory proteins (such as MerR), and even RNAP, may be prebound to the promoter site, allowing a very rapid response to changing environmental conditions.
Gene regulation in bacteria can also use RNA-binding proteins that interact with the RNA transcript to control its fate. Regulatory outcomes include effects on attenuation of transcription (in transcriptional units that contain a termination signal, designated an attenuator, early in the transcript), mRNA stability, and translation initiation. Like DNA-binding proteins, RNA-binding proteins that function in gene regulation are often expressed constitutively, and their activity is modulated in response to the environmental signal (e.g., Bacillus subtilis TRAP protein is activated by tryptophan). Constitutive synthesis of a regulatory protein is potentially wasteful of cellular resources, but allows the cell to be poised to respond rapidly to physiological changes. RNA-binding proteins can also regulate their own expression, via direct binding to their own mRNA (e.g., ribosomal protein autoregulation).
Recent studies have revealed an important role for RNA as a regulatory molecule in bacteria. Small untranslated regulatory RNAs (sRNAs) can be encoded on the opposite strand of the DNA from their regulatory target (cis-encoded antisense sRNAs) or in other regions of the chromosome (trans-encoded sRNAs) (Wagner et al. 2002; Gottesman et al. 2006; Storz et al. 2006; Brantl 2007). Both cis- and trans-encoded sRNAs usually act by base-pairing (perfect or imperfect, respectively) with the target mRNA, often by affecting accessibility of the translation initiation region of the target transcript or by affecting mRNA stability. Trans-encoded sRNAs can also act by other mechanisms, including titration of a regulatory RNA-binding protein, as in the CsrA system (Babitzke and Romeo 2007) or direct interaction with RNAP (Wassarman 2007). Both cis-encoded and trans-encoded sRNAs can diffuse throughout the cytoplasm to modulate expression of targets encoded elsewhere in the genome. In systems of this type, the abundance of the sRNA usually changes in response to an environmental signal, therefore affecting the fate of its mRNA targets.
RNA segments can also act to regulate the expression of the transcript in which they are encoded. These RNA segments (which are both cis-encoded and cis-acting) can serve as targets for RNA-binding proteins or sRNAs, as described above, or can directly monitor a regulatory signal without the assistance of any additional regulatory factor. Regardless of the involvement of a separate regulatory factor that transmits the signal to the regulatory element in the transcript, changes in the structure of the target RNA can affect gene expression. This effect is most commonly mediated through controlling the formation of the helix of an intrinsic transcription terminator (which therefore determines whether transcription terminates early) or through controlling formation of a helix that sequesters the translation initiation region of the downstream transcript (Grundy and Henkin 2006). Effects on mRNA stability can also occur, either through direct modulation of cleavage by specific ribonucleases or by secondary outcomes of primary effects on translational efficiency (as nontranslated mRNAs are often more susceptible to degradation). RNAs that directly monitor a physiological signal, in the absence of any other cellular factor (such as a regulatory protein or translating ribosome), and exhibit structural shifts in response to that signal have been termed riboswitches. These RNAs fall into several different groups that differ in their overall architecture and the type of signal they recognize. The recognized signals include various small molecules (e.g., cofactors, amino acids, nucleotides, and metal ions), cellular components (e.g., specific tRNAs), and temperature. Overall, riboswitch RNAs can have a major impact on cell physiology because of the large number of gene families that they regulate.
Riboswitch architecture
Several classes of riboswitch RNA structural arrangements have emerged. The simplest class of riboswitches are the RNA thermosensors. These are RNA structural elements that affect expression of the downstream genes, usually by sequestration of the Shine-Dalgarno (SD) sequence (Narberhaus et al. 2006). The regulatory response occurs in response to a change in temperature rather than binding of an effector molecule. In most cases, the RNA is in an inactive state (e.g., with the SD inaccessible to ribosome binding) under normal growth conditions, but an increase in temperature results in the melting of the RNA helix and release of the SD sequence into a single-stranded state (Fig. 1). RNA thermosensors differ from other riboswitches in that no effector recognition domain is required, with the regulatory response instead tuned to the melting temperature of the inhibitory helix. Small changes in the stability of the helix can therefore result in major changes in gene expression and in temperature response. These regulatory elements are often used to control genes that allow the cell to respond to sudden changes in temperature, including heat-shock genes (Morita et al. 1999); this type of system is also used in pathogenic organisms to induce virulence gene expression in response to entry of the bacterium into a mammalian host (Johansson et al. 2002).
Figure 1.
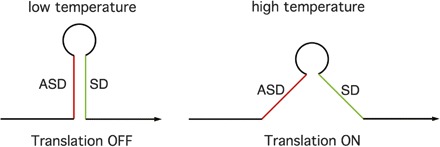
RNA thermosensors. (Left) At low temperature, the helical structure is stable, resulting in sequestration of the SD sequence by pairing with the ASD sequence (SD); this results in inhibition of translation initiation. (Right) At higher temperature, the ASD–SD helix is disrupted and the SD sequence is available for translation initiation.
In contrast to the single-domain thermosensor RNAs, a standard small molecule-binding riboswitch RNA is comprised of two separate elements, a ligand-binding domain and a gene expression domain (Fig. 2). The ligand-binding domain is responsible for specific recognition of the ligand and discrimination between the correct ligand and related compounds. Binding of the ligand promotes a structural shift in this domain, usually resulting in sequestration of a segment of RNA that would otherwise interact with another part of the RNA to affect gene expression. The most common riboswitch arrangement is one in which binding of the ligand causes the RNA to fold into a structure that inhibits gene expression (e.g., by formation of the helix of an intrinsic terminator or a helix that sequesters the translation initiation region of the mRNA). In systems where binding of the effector turns gene expression off, the ligand is often the end product of the biosynthetic pathway in which the regulated genes are involved, resulting in a form of feedback control. A rarer class involves RNA elements in which the mRNA in the absence of ligand is in a conformation that inhibits gene expression; in systems of this type (e.g., the glycine-binding gcv element) (Mandal et al. 2004), binding of a substrate of the pathway promotes an RNA structural rearrangement that shifts the transcript into an “on” state where gene expression can occur (e.g., by favoring formation of an antiterminator structure that competes with the terminator helix or by forming a helix that sequesters a sequence that would otherwise occlude the translation initiation region), resulting in a form of “feed-forward” control.
Figure 2.
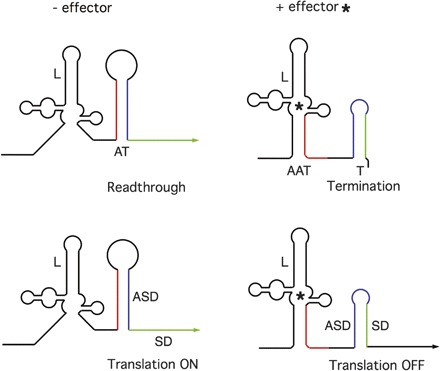
Basic model of a standard metabolite-binding riboswitch RNA. (Left) In the absence of the effector, the ligand-binding domain (L) is unoccupied, and the RNA is in a conformation that allows expression of the downstream coding sequence, either through formation of an antiterminator element (AT) that prevents formation of the terminator helix and therefore allows transcription to continue (top), or through capture of the ASD into a structure that liberates the SD sequence and allows translation to initiate (bottom). (Right) In the presence of the effector (*), the ligand-binding domain is occupied, resulting in a structural shift in the downstream region of the RNA. This allows the terminator (T) to form, which results in premature termination of transcription (top) or sequestration of the SD sequence in an ASD–SD helix that prevents translation initiation (bottom). Variations from this basic model are discussed in the text.
Physical separation of the ligand-binding and gene expression domains allows use of the same ligand-binding domain for regulation at multiple levels of gene expression by combination with different expression domains. For example, the Thi-box element that binds thiamine pyrophosphate (TPP) to regulate genes involved in thiamine metabolism can be found in all three kingdoms and can regulate at the levels of transcription termination, translation initiation, and mRNA splicing (Miranda-Rios et al. 2001; Mironov et al. 2002; Winkler et al. 2002b; Kubodera et al. 2003; Sudarsan et al. 2003a; Cheah et al. 2007). The TPP-binding domain is structurally conserved in all Thi-box riboswitches (Edwards and Ferre-D’Amare 2006; Serganov et al. 2006; Thore et al. 2006), but the arrangement of this domain relative to the RNA segment involved in gene expression (the terminator helix, SD–ASD [anti-Shine-Dalgarno] helix, or splicing elements) varies.
Recent studies have also revealed riboswitch architectures in which the gene expression domain participates directly in ligand recognition. The SAM-binding SMK box is an example of this type of riboswitch. In this case, binding of the effector stabilizes pairing of a sequence that overlaps the SD recognition sequence for the 30S ribosomal subunit with a complementary ASD sequence located near the 5′ end of the RNA, thereby preventing translation initiation (Fuchs et al. 2006, 2007). Residues within the SD–ASD pairing make specific contacts with the SAM molecule that are crucial for SAM recognition (Lu et al. 2008).
The T-box system represents a special class of riboswitch RNAs in which binding of a specific uncharged tRNA to the 5′ region of the transcript, without any accessory factor, promotes expression of the downstream genes, which usually encode the cognate aminoacyl-tRNA synthetase, amino acid biosynthesis or transporter protein (Grundy and Henkin 1993; Vitreschak et al. 2008; A. Gutierrez-Precado, T.M. Henkin, F.J. Grundy, C. Yanofsky, and E. Merino, in prep.). Discrimination of the cognate tRNA from other tRNAs in the cellular pool employs base-pairing at both the anti-codon loop and acceptor end of the tRNA, as well as recognition of other features of the tRNA three-dimensional structure (Fig. 3; Grundy and Henkin 1993; Grundy et al. 2002; Henkin and Grundy 2006). Accumulation of a specific uncharged tRNA signals the requirement for increased expression of genes involved in generation of the aminoacylated form of that tRNA. Unlike other riboswitches that respond to a single effector, the T-box RNA interacts with both uncharged and charged tRNA. The aminoacylated tRNA acts as a competitive inhibitor of binding of uncharged tRNA, so that the system monitors the substrate:product ratio rather than the absolute amount of the substrate (Yousef et al. 2005). This makes physiological sense as the total tRNA concentration remains fairly constant under normal growth conditions, and the aminoacylation status of the tRNA pool is the relevant parameter.
Figure 3.
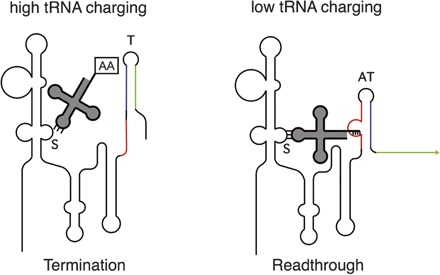
The T-box mechanism. (Left) When a high proportion of a tRNA with a specific anti-codon is charged with the cognate amino acid, the tRNA anti-codon loop can interact with the matching codon (the Specifier Sequence, S) in an internal loop in the RNA. The presence of the amino acid at the 3′ end of the tRNA blocks interaction with the antiterminator element (AT). In the absence of this second interaction, the more stable terminator helix (T) will form, and transcription terminates. (Right) When the tRNA is poorly charged, the uncharged tRNA interacts both at the Specifier Sequence and at the antiterminator bulge. This second interaction stabilizes the antiterminator and prevents formation of the terminator helix, which results in readthrough of the termination site and transcription of the full-length mRNA. Each gene in the T-box family responds specifically to the cognate tRNA, with specificity determined primarily by the Specifier Sequence–anti-codon pairing. Discrimination between charged and uncharged tRNA occurs at the antiterminator.
Why RNA?
The plasticity of RNA as a cis-acting regulatory molecule is dependent on several factors. First is the extreme structural flexibility of RNA, enabling highly specific recognition of a wide range of regulatory signals. Recognition can use the base-pairing properties of RNA nucleotides (as in the T-box system and the purine-responsive riboswitches) as well as a variety of other chemical and shape interactions that allow discrimination of the true effector from related molecules. A second factor is the ability of RNA to undergo conformational changes, often involving alternate base-pairing within the regulatory RNA, in response to a signal. This allows structural changes in one domain of the RNA to be transmitted to other domains, resulting in large-scale structural modulation in response to binding of molecules that in many cases are tiny relative to the size of the riboswitch RNA itself. A third factor is the intrinsic role of RNA in the gene expression machinery, which allows changes in RNA structure to directly impact gene regulation. All of these factors are exploited effectively by riboswitch RNAs.
Identification of riboswitch systems
Most riboswitch RNAs were initially recognized based on the identification of conserved RNA sequence and structural features in the 5′ region of sets of bacterial genes known (or predicted) to respond in concert to a specific physiological signal. Identification of these patterns was initially done manually (e.g., Grundy and Henkin 1993, 1998) and gradually employed bioinformatics tools of increasing complexity (e.g., Gelfand et al. 1999; Miranda-Rios et al. 2001; Vitreschak et al. 2002, 2003). Global searches for new RNA patterns that could potentially act as riboswitches (e.g., Bengert and Dandekar 2004; Abreu-Goodger and Merino 2005; Weinberg et al. 2007) have expanded the list of riboswitch candidates and revealed variations on the pattern for known riboswitch RNAs. Most searches have focused on the 5′-untranslated region (UTR) in bacterial genomes; the identification of Thi-box elements in the 3′-UTR of plant genes (Sudarsan et al. 2003a; Cheah et al. 2007) illustrates the need for expanded searches, especially in eukaryotic genomes. The physical linkage of riboswitches to their target coding sequences greatly facilitates their characterization; for trans-acting regulatory RNAs, identification of the target gene(s) can present a major challenge (Vogel and Wagner 2007).
In accord with the fact that most riboswitches were identified initially by investigation of individual sets of genes or pathways, initial studies focused on analysis of the conditions to which each set of genes responds and the regulatory response (e.g., Grundy and Henkin 1993, 1998; Nou and Kadner 2000; Miranda-Rios et al. 2001). Demonstration that these systems operate by direct measurement of the physiological signal, without participation of a regulatory protein or other cellular factor, required in vitro analyses (see Table 1; Grundy et al. 2002; Mironov et al. 2002; Nahvi et al. 2002; Winkler et al. 2002a, b).
Table 1.
Classes of metabolite-binding riboswitch RNAs
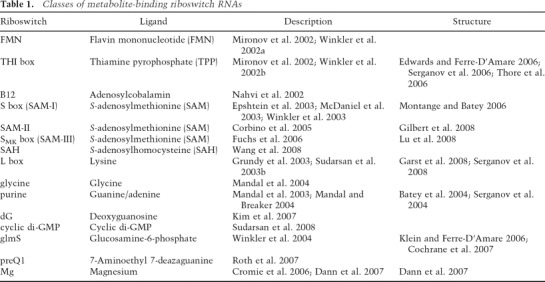
The recent identification of subclasses of elements that are present only in small groups of organisms (e.g., SMK box and SAM-II) (Corbino et al. 2005; Fuchs et al. 2006) or that exhibit extensive structural diversity and low conservation (e.g., SMK box) (Fuchs et al. 2006) suggest that additional riboswitch elements remain to be uncovered. The rapidly growing data set of genomic sequences and improved bioinformatics techniques provide the necessary tools for their identification.
What can RNA sense?
As noted above, the simplest riboswitches are the RNA thermosensors, as these have no requirement for specific ligand recognition and require only the presence of an RNA helix with an appropriate melting temperature, positioned at an appropriate position within the transcript (e.g., within the translation initiation region) to exert its effect on gene expression. Riboswitches that bind small molecules contain ligand-binding domains of varying complexity, ranging from the ∼40-nucleotide (nt) preQ1-binding domain (Roth et al. 2007), to the ∼200-nt lysine-binding domain of the L-box riboswitch (Grundy et al. 2003). T-box RNAs are even larger (∼200–300 nt), due to the need to accommodate recognition of the full tRNA structure. Even a relatively small effector-binding domain, such as the SAM-binding domain of the Enterococcus faecalis SMK box (which can be reduced to ∼50 nt), can sometimes be found in much larger forms in nature, with insertions of one or two 200-base-pair (bp) helical elements at positions that are not directly involved with SAM binding (Fuchs et al. 2006).
A list of the metabolite-binding riboswitches that have been characterized is shown in Table 1. The ligands range in complexity from metal ions to complex cofactors. Three-dimensional structures are available for a growing subset of these RNAs. In most cases, the structure is known only for the ligand-binding domain in complex with its cognate ligand, although a few structures are also available for complexes with noncognate but structurally related ligands. Exceptions are the SAM-II- and SMK-box RNAs, in which the region of the RNA responsible for gene expression control (the SD, in both cases) is an intrinsic component of the SAM-binding domain (Gilbert et al. 2008; Lu et al. 2008). In most cases, little information is available about the structure of the RNA in the absence of ligand and the transitions that occur upon ligand binding.
Calibration of riboswitch RNAs to effector concentration
One of the crucial parameters for riboswitch function is the ability of the riboswitch to be tuned to a physiologically relevant concentration of the effector molecule. For example, SAM-responsive S-box riboswitch RNAs generally respond to low micromolar concentrations, whereas the lysine-responsive L-box RNA requires low millimolar concentrations for a similar effect on gene expression (Grundy et al. 2003; McDaniel et al. 2003); these regulatory concentrations are correlated with the range of effector concentration within the cell, under appropriate growth conditions.
As noted above, most riboswitch RNAs contain a ligand-binding domain that in isolation can recognize the effector molecule. The resulting structural shift in the ligand-binding domain results in structural changes in a downstream portion of the RNA that impacts gene expression, usually through effects on transcription termination and translation initiation. Sensitivity to the effector is dependent not only on the affinity of the ligand-binding domain of the riboswitch for the cognate effector, but also on the probability that the RNA will carry out the ligand-dependent structural shift. The magnitude of the regulatory response is also affected by the probability that the RNA will be in the ligand-bound state even in the absence of the ligand. This dual effect can result in varying measurements of ligand affinity depending on how affinity is monitored and the RNA element tested.
In a number of cases, the affinity for the ligand of the isolated ligand-binding domain of the RNA is much higher than the concentration required for a regulatory response. For example, the L-box lysine-binding domain (from the B. subtilis lysC gene) has a much higher affinity for lysine than the concentration of lysine required for lysine-dependent transcription termination in a purified in vitro transcription system (Grundy et al. 2003; Sudarsan et al. 2003b). The in vitro transcription assays better reflect the lysine pools in the cell (2–4 mM), which suggests that functional assays may give a more representative value of the biologically relevant effector concentration to which the system responds in vivo. The differences in affinity sometimes observed in vivo vs. in vitro may be due in part to differences in RNA folding, as nascent RNAs fold as they are synthesized in a 5′-to-3′ direction, while in vitro binding assays often use full-length aptamer RNAs that are denatured and refolded in the presence of the ligand. These differences may also reflect the requirement for the ligand to bind in the restricted time frame of leader RNA transcription, especially for RNAs regulated at the level of transcriptional attenuation.
Another important consideration for understanding the sensitivity of a riboswitch to its effector is the fact that different genes regulated by the same riboswitch may exhibit a differential response. The B. subtilis genome contains 11 transcriptional units preceded by SAM-responsive S-box riboswitches (Grundy and Henkin 1998). Comparative analysis revealed a 200-fold variance in affinity for SAM of the isolated SAM-binding element, and 100-fold variability in sensitivity to SAM in a purified in vitro transcription system (Tomsic et al. 2008). It is interesting to note that genes that are involved in central pathways leading to methionine and SAM are highly sensitive to SAM, so that they are repressed even when cellular pools are relatively low, whereas genes encoding a methionine transport system are relatively insensitive to repression by SAM, so that expression of the transporter will be induced when SAM pools are only slightly reduced. The consequence of this differential response is that the cell can respond to an initial reduction in SAM by first inducing expression of a transport system that would allow scavenging of any available extracellular SAM; only if this attempt to increase SAM pools is unsuccessful does the cell induce expression of the more complex (and energetically costly) biosynthetic pathway.
Combinatorial regulation
Like regulatory proteins, which can act in concert to integrate multiple signal inputs at a single promoter site, regulatory RNAs can also act together with other systems to fine-tune the response of a single gene to different physiological conditions. For example, the B. subtilis ilv-leu operon, which includes genes involved in branched chain amino acid biosynthesis, is regulated at the level of transcription attenuation by the T-box mechanism in response to decreased charging of tRNALeu (Garrity and Zahler 1994). The activity of the promoter of this operon is also repressed by the CodY DNA-binding protein in response to high levels of isoleucine and GTP (Shivers and Sonenshein 2005). This dual response allows the cell to independently monitor both leucine and isoleucine, using two distinct regulatory mechanisms operating at two different levels of gene expression. Multiple riboswitch RNAs can also be used to modulate the overall regulatory effect. Tandem copies of the same riboswitch, as is found in certain T-box genes (e.g., the B. subtilis thrZ gene), result in decreased sensitivity to the effector (in this case, uncharged tRNAThr) as independent binding at each riboswitch motif is required to allow readthrough of the termination site and final expression of the downstream coding sequence (Gendron et al. 1994). For thrZ, a requirement for high levels of uncharged tRNAThr allows the cell to preferentially express the primary ThrRS, encoded by the thrS gene, which is regulated by a single tRNAThr-responsive T-box riboswitch. Tandem Thi-box riboswitches result in a similar decrease in sensitivity to TPP concentrations in Bacillus anthracis (Welz and Breaker 2007). Combination of multiple riboswitches of different classes can allow measurement of two different signals, as is observed in the Bacillus clausii metE gene, which encodes a B12-independent form of methionine synthase. Expression of metE is repressed both by SAM (which signals that methionine pools are high and is monitored by an S-box riboswitch) and by B12 (which signals the cell to switch to the more efficient B12-dependent form of methionine synthase, encoded by the metH gene; Sudarsan et al. 2006). Similarly, a complex array of an S box, a T box, and an antisense RNA is used to regulate a cysteine biosynthesis operon in Clostridium acetobutylicum (Andre et al. 2008).
Perspectives
In the past several years, our recognition of the role of riboswitch RNA elements in gene regulation in bacteria has expanded dramatically. There are currently nearly 200 listings in the PubMed database for the term “riboswitch,” which was coined only in 2002; there is an extensive literature for the field that predates the use of that term. The identification of the first few riboswitch elements in eukaryotic systems suggests that this type of regulatory mechanism is used even more widely than is currently appreciated. Important questions remain about the evolutionary history of various riboswitch classes, their mechanistic and structural features, and their physiological roles in the cell. The riboswitch field is likely to remain extremely active for a long time to come.
Acknowledgments
This work was supported by grants from the National Institutes of Health NIGMS R01GM47823 and R01GM63615.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1747308.
References
- Abreu-Goodger C., Merino E. RibEX: A Web server for locating riboswitches and other conserved bacterial regulatory elements. Nucleic Acids Res. 2005;33:W690–W692. doi: 10.1093/nar/gki445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre G., Even S., Putzer H., Burquiere P., Croux C., Danchin A., Martin-Verstraete I., Soutourina O. S-box and T-box riboswitches and antisense RNA control a sulfur metabolic operon of Clostridium acetobutylicum. Nucleic Acids Res. 2008;36:5955–5969. doi: 10.1093/nar/gkn601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitzke P., Romeo T. CsrB sRNA family: Sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 2007;10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Batey R.T., Gilbert S.D., Montange R.K. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature. 2004;432:411–415. doi: 10.1038/nature03037. [DOI] [PubMed] [Google Scholar]
- Bengert P., Dandekar T. Riboswitch finder—A tool for identification of riboswitch RNAs. Nucleic Acids Res. 2004;32:W154–W159. doi: 10.1093/nar/gkh352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantl S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr. Opin. Microbiol. 2007;10:102–109. doi: 10.1016/j.mib.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Cheah M.T., Wachter A., Sudarsan N., Breaker R.R. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- Cochrane J.C., Lipchock S.V., Strobel S.A. Structural investigation of the glmS ribozyme bound to its catalytic cofactor. Chem. Biol. 2007;14:97–105. doi: 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbino K.A., Barrick J.E., Lim J., Welz R., Tucker B.J., Puskarz I., Mandal M., Rudnick N.D., Breaker R.R. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in α-proteobacteria. Genome Biol. 2005;6:R70. doi: 10.1186/gb-2005-6-8-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie M.H., Shi Y., Latifi T., Groisman E.A. An RNA sensor for intracellular Mg2+ Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Dann C.E., Wakeman C.A., Sieling C.L., Baker S.C., Irnov I., Winkler W.C. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:415–424. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- Edwards T.E., Ferre-D’Amare A.R. Crystal structures of the thi-box riboswitch bouund to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Epshtein V., Mironov A.S., Nudler E. The riboswitch-mediated control of sulfur metabolism in bacteria. Proc. Natl. Acad. Sci. 2003;100:5052–5056. doi: 10.1073/pnas.0531307100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R.T., Grundy F.J., Henkin T.M. The SMK box is a new SAM-binding RNA for translational regulation of SAM synthetase. Nat. Struct. Mol. Biol. 2006;13:226–233. doi: 10.1038/nsmb1059. [DOI] [PubMed] [Google Scholar]
- Fuchs R.T., Grundy F.J., Henkin T.M. S-adenosylmethionine directly inhibits binding of 30S ribosomal subunits to the SMK box translational riboswitch RNA. Proc. Natl. Acad. Sci. 2007;104:4876–4880. doi: 10.1073/pnas.0609956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity D.B., Zahler S.A. Mutations in the gene for a tRNA that functions as a regulator of a transcriptional attenuator in Bacillus subtilis. Genetics. 1994;137:627–636. doi: 10.1093/genetics/137.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garst A.D., Heroux A., Rambo R.P., Batey R.T. Crystal structure of the lysine riboswitch regulatory RNA element. J. Biol. Chem. 2008;283:22347–22351. doi: 10.1074/jbc.C800120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand M.S., Mironov A.A., Jomantas J., Kozlov Y.I., Perumov D.A. A conserved RNA structure element involved in the regulation of riboflavin biosynthesis genes. Trends Genet. 1999;15:439–442. doi: 10.1016/s0168-9525(99)01856-9. [DOI] [PubMed] [Google Scholar]
- Gendron N., Putzer H., Grunberg-Manago M. Expression of both Bacillus subtilis threonyl-tRNA synthetase genes is autogenously regulated. J. Bacteriol. 1994;176:486–494. doi: 10.1128/jb.176.2.486-494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S.D., Rambo R.P., Van Tyne D., Batey R.T. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat. Struct. Mol. Biol. 2008;15:761–772. doi: 10.1038/nsmb.1371. [DOI] [PubMed] [Google Scholar]
- Gottesman S., McCullen C.A., Guillier M., Vanderpool C.K., Majdalani N., Benhammou J., Thompson K.M., FitzGerald P.C., Sowa N.A., FitzGerald D.J. Small RNA regulators and the bacterial response to stress. Cold Spring Harb. Symp. Quant. Biol. 2006;71:1–11. doi: 10.1101/sqb.2006.71.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F.J., Henkin T.M. tRNA as a positive regulator of transcripton antitermination in B. subtilis. Cell. 1993;74:475–482. doi: 10.1016/0092-8674(93)80049-k. [DOI] [PubMed] [Google Scholar]
- Grundy F.J., Henkin T.M. The S box regulon: A new global transcripton termination control system for methionine and cysteine biosynthesis genes in Gram-positive bacteria. Mol. Microbiol. 1998;30:737–749. doi: 10.1046/j.1365-2958.1998.01105.x. [DOI] [PubMed] [Google Scholar]
- Grundy F.J., Henkin T.M. From ribosome to riboswitch: Control of gene expression in bacteria by RNA structural rearrangements. Crit. Rev. Biochem. Mol. Biol. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- Grundy F.J., Winkler W.C., Henkin T.M. tRNA-mediated transcription antitermination in vitro: Codon–anticodon pairing independent of the ribosome. Proc. Natl. Acad. Sci. 2002;99:11121–11126. doi: 10.1073/pnas.162366799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F.J., Lehman S.C., Henkin T.M. The L box regulon: Lysine sensing by leader RNAs of bacterial lysine biosynthesis genes. Proc. Natl. Acad. Sci. 2003;100:12057–12062. doi: 10.1073/pnas.2133705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin T.M., Grundy F.J. Sensing metabolic signals with nascent RNA transcripts: The T-box and S-box riboswitches as paradigms. Cold Spring Harb. Symp. Quant. Biol. 2006;71:231–237. doi: 10.1101/sqb.2006.71.020. [DOI] [PubMed] [Google Scholar]
- Johansson J., Mandin P., Renzoni A., Chiaruttini C., Springer M., Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110:551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Kim J.N., Roth A., Breaker R.R. Guanine riboswitch variants from Mesoplasma florum selectively recognize 2′-deoxyguanosine. Proc. Natl. Acad. Sci. 2007;104:16092–16097. doi: 10.1073/pnas.0705884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.J., Ferre-D’Amare A.R. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–1756. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]
- Kubodera T., Watanabe M., Yoshiuchi K., Yamashita N., Nishimura A., Nakai S., Gomi K., Hanamoto H. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 2003;555:516–520. doi: 10.1016/s0014-5793(03)01335-8. [DOI] [PubMed] [Google Scholar]
- Lu C., Smith A.M., Fuchs R.T., Ding F., Rajashankar K., Henkin T.M., Ke A. Crystal structures of the SAM-III/SMK riboswitch reveal the SAM-dependent translation inhibition mechanism. Nat. Struct. Mol. Biol. 2008;15:1076–1083. doi: 10.1038/nsmb.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M., Breaker R.R. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat. Struct. Mol. Biol. 2004;11:29–35. doi: 10.1038/nsmb710. [DOI] [PubMed] [Google Scholar]
- Mandal M., Boese B., Barrick J.E., Winkler W.C., Breaker R.R. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell. 2003;113:577–586. doi: 10.1016/s0092-8674(03)00391-x. [DOI] [PubMed] [Google Scholar]
- Mandal M., Lee M., Barrick J.E., Weinberg Z., Emilsson G.M., Ruzzo W.L., Breaker R.R. A glycine-dependent riboswtch that uses cooperative binding to control gene expression. Science. 2004;306:275–279. doi: 10.1126/science.1100829. [DOI] [PubMed] [Google Scholar]
- McDaniel B.A.M., Grundy F.J., Artsimovitch I., Henkin T.M. Transcription termination control of the S box system: Direct measurement of S-adenosylmethionine by the leader RNA. Proc. Natl. Acad. Sci. 2003;100:3083–3088. doi: 10.1073/pnas.0630422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Rios J., Navarro M., Soberon M. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthetic gene expression in bacteria. Proc. Natl. Acad. Sci. 2001;98:9736–9741. doi: 10.1073/pnas.161168098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov A.S., Gusarov I., Rafikov R., Lopez L.E., Shatalin K., Kreneva R.A., Perumov D.A., Nudler E. Sensing small molecules by nascent RNA: A mechanism to control transcription in bacteria. Cell. 2002;111:747–756. doi: 10.1016/s0092-8674(02)01134-0. [DOI] [PubMed] [Google Scholar]
- Montange R.K., Batey R.T. Structure of the S-adeosylmethionine riboswitch regulatory mRNA element. Nature. 2006;441:1172–1175. doi: 10.1038/nature04819. [DOI] [PubMed] [Google Scholar]
- Morita M.T., Tanaka Y., Kodama T.T., Kyogoku Y., Yanagi H., Yura T. Translational induction of heat shock transcription factor σ32: Evidence for a built-in RNA thermosensor. Genes & Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi A., Sudarsan N., Evert M.S., Zou X., Brown K.L., Breaker R.R. Genetic control by a metabolite binding mRNA. Chem. Biol. 2002;9:1043–1049. doi: 10.1016/s1074-5521(02)00224-7. [DOI] [PubMed] [Google Scholar]
- Narberhaus F., Waldminghaus T., Chowdbury S. RNA thermometers. FEMS Microbiol. Rev. 2006;30:3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- Nou X., Kadner R.J. Adenosylcobalamin inhibits ribosome binding to btuB RNA. Proc. Natl. Acad. Sci. 2000;97:7190–7195. doi: 10.1073/pnas.130013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A., Winkler W.C., Regulski E.E., Lee B.W., Lim J., Jona I., Barrick J.E., Ritwik A., Kim J.N., Welz R., et al. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nat. Struct. Mol. Biol. 2007;14:308–317. doi: 10.1038/nsmb1224. [DOI] [PubMed] [Google Scholar]
- Serganov A., Yuan Y.R., Pikofskaya O., Polonskala A., Malinina L., Phan A.T., Hobartner C., Micura R., Breaker R.R., Patel D.J. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem. Biol. 2004;11:1729–1741. doi: 10.1016/j.chembiol.2004.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A., Polonskaia A., Phan A.T., Breaker R.R., Patel D.J. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serganov A., Huang L., Patel D.J. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature. 2008;455:1263–1267. doi: 10.1038/nature07326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers R.P., Sonenshein A.L. Bacillus subtilis ilvB operon: An intersection of global regulons. Mol. Microbiol. 2005;56:1549–1559. doi: 10.1111/j.1365-2958.2005.04634.x. [DOI] [PubMed] [Google Scholar]
- Storz G., Opdyke J.A., Wassarman K.M. Regulating bacterial transcription with small RNAs. Cold Spring Harb. Symp. Quant. Biol. 2006;71:269–273. doi: 10.1101/sqb.2006.71.033. [DOI] [PubMed] [Google Scholar]
- Sudarsan N., Barrick J.E., Breaker R.R. Metabolite-binding RNA domains are present in the genes of eukaryotes. RNA. 2003a;9:644–647. doi: 10.1261/rna.5090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan N., Wickiser J.K., Nakamura S., Evert M.S., Breaker R.R. An mRNA structure in bacteria that controls gene expression by binding lysine. Genes & Dev. 2003b;17:2688–2697. doi: 10.1101/gad.1140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan N., Hammond M.C., Block K.F., Welz R., Barrick J.E., Roth A., Breaker R.R. Tandem riboswitch architectures exhibit complex gene control functions. Science. 2006;314:300–304. doi: 10.1126/science.1130716. [DOI] [PubMed] [Google Scholar]
- Sudarsan N., Lee E.R., Weinberg Z., Moy R.H., Kim J.N., Link K.H., Breaker R.R. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321:411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore S., Leibungut M., Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- Tomsic J., McDaniel B.A., Grundy F.J., Henkin T.M. Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S box elements in Bacillus subtilis exhibit differential sensitivity to SAM in vivo and in vitro. J. Bacteriol. 2008;190:823–833. doi: 10.1128/JB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitreschak A.G., Rodionov D.A., Mironov A.A., Gelfand M.S. Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res. 2002;30:3141–3151. doi: 10.1093/nar/gkf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitreschak A.G., Rodionov D.A., Mironov A.A., Gelfand M.S. Regulation of the vitamin B12 metabolism and transport in bacteria by a conserved RNA structural element. RNA. 2003;9:1084–1097. doi: 10.1261/rna.5710303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitreschak A.G., Mironov A.A., Lyubetsky V.A., Gelfand M.S. Comparative genomic analysis of T-box regulatory systems in bacteria. RNA. 2008;14:717–735. doi: 10.1261/rna.819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Wagner E.G. Target identification of small noncoding RNAs in bacteria. Curr. Opin. Microbiol. 2007;10:262–270. doi: 10.1016/j.mib.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wagner E.G., Altuvia S., Romby P. Antisense RNAs in bacteria and their genetic elements. Adv. Genet. 2002;46:361–398. doi: 10.1016/s0065-2660(02)46013-0. [DOI] [PubMed] [Google Scholar]
- Wang J.X., Lee E.R., Morales D.R., Lim J., Breaker R.R. Riboswitches that sense S-adenosylhomocysteine and activate genes involved in coenzyme recycling. Mol. Cell. 2008;29:691–702. doi: 10.1016/j.molcel.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman K.M. 6S RNA: A regulator of transcription. Mol. Microbiol. 2007;65:1425–1431. doi: 10.1111/j.1365-2958.2007.05894.x. [DOI] [PubMed] [Google Scholar]
- Weinberg Z., Barrick J.E., Yao Z., Roth A., Kim J.N., Gore J., Wang J.X., Lee E.R., Block K.F., Sudarsan N., et al. Identification of 22 candidate structured RNAs in bacteria using the Cmfinder comparative genomics pipeline. Nucleic Acids Res. 2007;35:4809–4819. doi: 10.1093/nar/gkm487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz R., Breaker R.R. Ligand binding and gene control characteristics of tandem riboswitches in Bacillus anthracis. RNA. 2007;13:573–582. doi: 10.1261/rna.407707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler W.C., Cohen-Chalamis S., Breaker R.R. An mRNA structure that controls gene expression by binding FMN. Proc. Natl. Acad. Sci. 2002a;99:15908–15913. doi: 10.1073/pnas.212628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler W.C., Nahvi A., Breaker R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002b;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- Winkler W.C., Nahvi A., Sudarsan N., Barrick J.E., Breaker R.R. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nat. Struct. Biol. 2003;10:701–707. doi: 10.1038/nsb967. [DOI] [PubMed] [Google Scholar]
- Winkler W.C., Nahvi A., Roth A., Collins J.A., Breaker R.R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- Yousef M.R., Grundy F.J., Henkin T.M. Structural transitions induced by the interaction between tRNAGly and the Bacillus subtilis glyQS T box leader RNA. J. Mol. Biol. 2005;349:273–287. doi: 10.1016/j.jmb.2005.03.061. [DOI] [PubMed] [Google Scholar]


