Abstract
PURPOSE
To review current concepts regarding the pathogenesis of Graves ophthalmopathy (GO). We have presented this information in the context of potential target sites for novel disease therapies.
DESIGN
Review of recent literature.
METHODS
Synthesis of recent literature.
RESULTS
Enlargement of the extraocular muscle bodies and expansion of the orbital fatty connective tissues is apparent in patients with GO. These changes result from abnormal hyaluronic acid accumulation and edema within these tissues and expanded volume of the orbital adipose tissues. Recent studies have suggested that the increase in orbital fat volume is caused by stimulation of adipogenesis within these tissues. The orbital fibroblast appears to be the major target cell of the autoimmune process in GO. A subset of these cells is capable of producing hyaluronic acid and differentiating into mature adipocytes, given appropriate stimulation. In addition, orbital fibroblasts from patients with GO have been shown to display immunoregulatory molecules and to express both thyrotropin receptors (TSHRs) and insulin-like growth factor 1 receptors (IGF-1Rs). Increased TSHR expression in the GO orbit appears to be the result of stimulated adipocyte differentiation. The activation of IGF-1R on orbital fibroblasts by immunoglobulins from GO patients results in increased production of both hyaluronic acid and molecules that stimulate the infiltration of activated T cells into areas of inflammation.
CONCLUSIONS
Potential targets for novel therapeutic agents to be used in GO include blocking T-cell costimulation, depleting B cells, inhibiting cytokine action, targeting the IGF-1R or the TSHR, and preventing connective tissue remodeling.
Graves disease is a common disorder with an annual incidence in women of one per 1,000 population. In addition to hyperthyroidism, clinical involvement of the eyes develops in 25% to 50% of individuals with Graves disease.1 The annual incidence of Graves ophthalmopathy (GO) in women is approximately 16 in 100,000 and in men three in 100,000. Although some patients with GO experience only mild ocular discomfort, approximately 5% have severe ophthalmopathy, including excessive chemosis, proptosis, or even loss of vision.
The clinical symptoms and signs of GO can be explained mechanically by the discrepancy between the increased volume of the swollen orbital tissues and the fixed volume of the bony orbit.1 The expanded orbital tissues displace the globe forward and impede venous outflow from the orbit. These changes, combined with the local production of cytokines and other mediators of inflammation, result in pain, proptosis, periorbital edema, conjunctival injection, and chemosis.
Computed tomographic scans show that most patients with GO have enlargement of both the orbital fat compartment and the extraocular muscles (Figure 1) and that others appear to have involvement of only adipose tissue (Figure 2) or extraocular muscle (Figure 3). The extraocular muscle cells are intact in early, active stages of the disease, suggesting that they themselves are not the targets of autoimmune attack. Rather, the enlargement of the extraocular muscle bodies results from an accumulation within the perimysial connective tissues of hydrophilic glycosaminoglycans, especially hyaluronic acid, with attendant edema.1 In later stages of the disease, the resolving inflammatory process within the muscles may leave them fibrotic and with ocular misalignment.
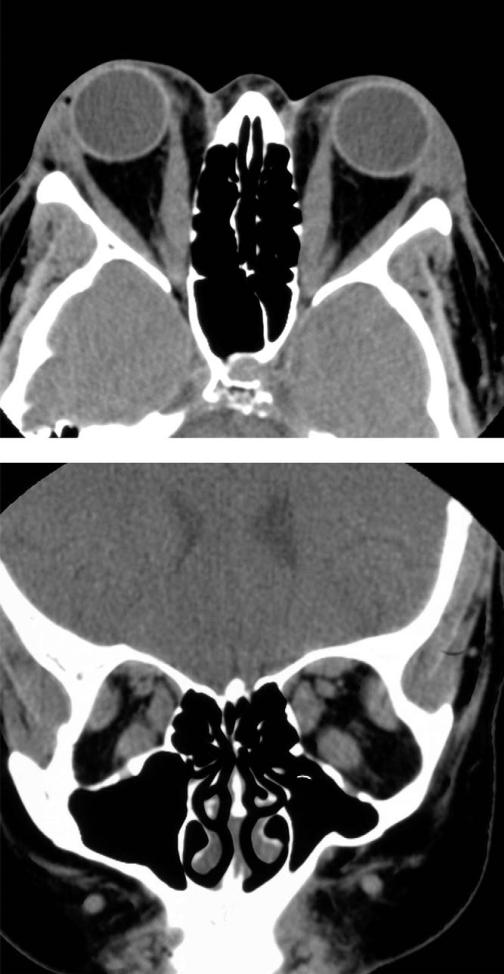
FIGURE 1.
Computed tomographic scan of a 50-year-old woman with congestive Graves ophthalmopathy (GO) shows mild enlargement of extraocular muscles and expansion of orbital fat compartment. (Top) Axial view. (Bottom) Coronal view.
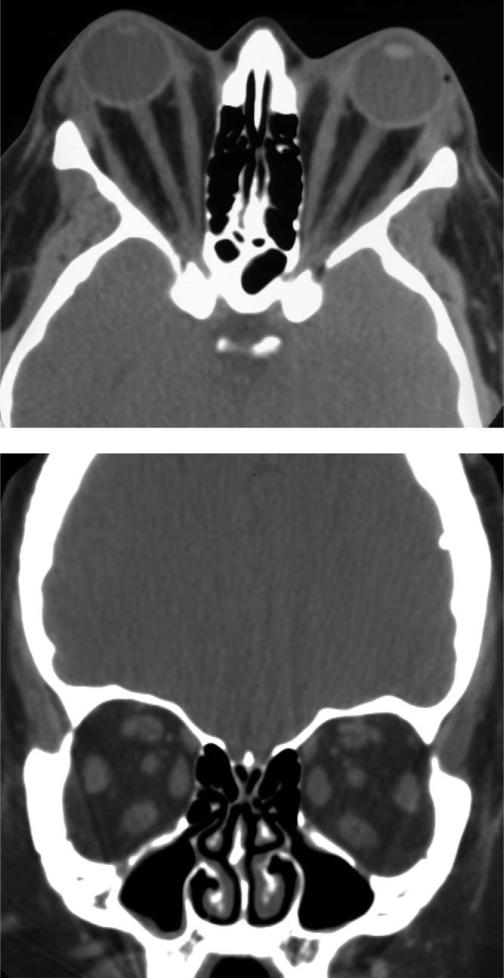
FIGURE 2.
Computed tomographic scan of a 43-year-old woman with Graves ophthalmopathy (GO) and marked proptosis related to expansion of the retrobulbar fat compartment shows minimal enlargement of the extraocular muscles. Each optic nerve is “straightened.” (Top) Axial view. (Bottom) Coronal view.
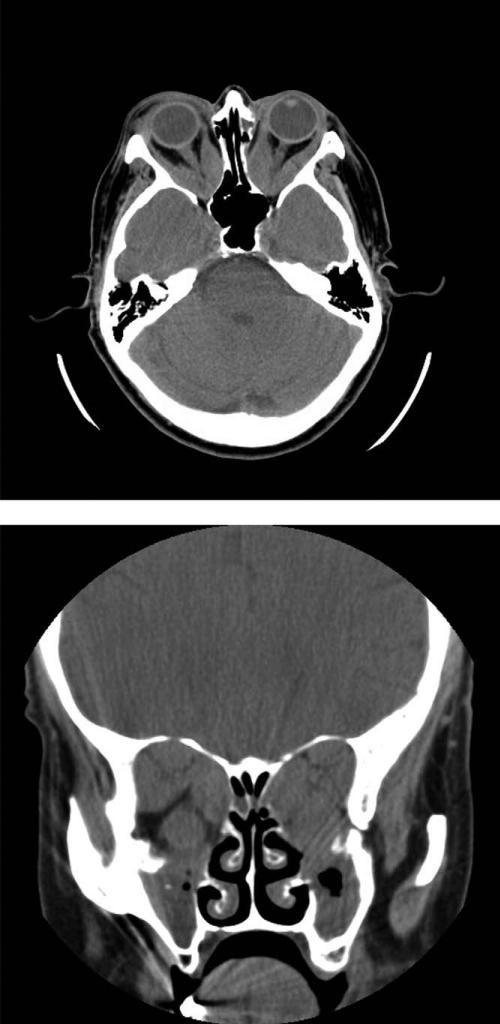
FIGURE 3.
Computed tomographic scan of a 58-year-old woman with profound visual loss from optic neuropathy in association with Graves ophthalmopathy (GO) shows massive enlargement of all extraocular muscles and apical compression of the optic nerves. Minimal fat is visible. (Top) Axial view. (Bottom) Coronal view.
Severity of proptosis appears to be more closely related to the orbital adipose and connective tissue volume than to muscle volume.2 This expanded adipose tissue volume results from both hyaluronic acid–related edema and the emergence of a population of newly differentiated fat cells within these tissues.3
In GO, the characteristic histologic changes within the orbital tissues outlined above suggest that the orbital fibroblast constitutes the target cell. However, rather than being a homogeneous population of cells, the fibroblasts exhibit remarkable phenotypic heterogeneity. One sub-population of these cells can produce hyaluronic acid and inflammatory prostanoids; other cells (termed “preadipocyte fibroblasts” or “preadipocytes”) can differentiate into mature adipocytes. The former subpopulation is found in connective tissues investing the extraocular muscles, and the latter, the preadipocytes, is found primarily in the orbital fat compartment. These phenotypic differences between fibroblasts within the orbit may help to explain why some patients with GO have predominant eye muscle disease (albeit with occasional evidence of fat accumulation within the muscles) and others have expansion of the adipose tissue compartment as the major disease feature.4
Fibroblasts also possess a wide array of tissue-specific phenotypes, which likely affect the apparently selective involvement of the skin of the anterior lower legs, termed thyroid (“pretibial”) dermopathy. This condition is evident in approximately 15% of Graves patients having severe GO and is much less common in Graves hyperthyroidism overall. Indeed, this finding is a clinical marker of severe ophthalmopathy.5 The histologic changes in the subdermal connective tissues in thyroid dermopathy appear similar to those within the GO orbit but without the increase in adipose tissue volume.
Early studies of orbital fibroblasts focused on cytokines, their effects on orbital fibroblasts biology, and phenotypic differences between fibroblasts from the orbit and the skin.1 For instance, orbital fibroblasts treated with interferon-γ or leukoregulin synthesize high levels of hyaluronic acid, but similarly treated dermal fibroblasts produce only small amounts.6 More recent studies have centered on the particular sensitivity of orbital fibroblasts to the induction of CD40 by interferon-γ treatment. This receptor is an important B-lymphocyte activator that is bound by CD154, a receptor expressed at high levels by T lymphocytes. CD40/CD154 ligation causes fibroblasts to produce several mediators of inflammation, including interleukin (IL)-1, IL-6, and IL-8, and to synthesize high levels of hyaluronic acid.7
Preadipocyte fibroblasts also show regional differences in the expression of adipocyte-specific genes and vary in their adipogenic potential; peroxisome proliferator-activated receptor (PPAR)-γ agonists enhance differentiation of preadipocyte fibroblasts from subcutaneous sites, but those from omental sites are refractory to these agents.8 The study of such depot-specific differences in fibroblast phenotype may help to explain why patients with GO have expanded orbital adipose tissues, without evidence of involvement of other adipose tissue depots, and why the lower legs are more commonly affected than are other skin regions.
In addition to these phenotypic differences between fibroblasts, unique anatomic features of the orbit and lower extremities appear to be clinically important in Graves disease.9 The unyielding bony orbit predisposes to compression of low-pressure venous channels, increasing retrobulbar pressure and periorbital edema. Similarly, prolonged standing contributes to compromise of channels in the lower extremities, most likely contributing to the dependent edema seen in thyroid dermopathy. Moreover, individual anatomic variability, such as the shape of the orbits or variations in venous or lymphatic vessels, may place some individuals with Graves disease at special risk for the development of severe GO or dermopathy.
The close clinical relationship between Graves hyperthyroidism and GO10 and the correlation between thyroid-stimulating autoantibody levels and clinical activity of GO11 suggest that immunoreactivity against thyrotropin receptor (TSHR) may underlie both conditions. The concept that TSHR-expressing orbital adipose tissue may be targeted in GO evolved from early studies showing thyrotropin (TSH) binding to guinea pig adipose and retro-orbital tissues, or to porcine orbital connective tissue membranes. The expression of this receptor in human fat tissue was first suggested by studies showing regulation of lipolysis by physiologic levels of TSH in human fetal and newborn adipocytes but not in adult adipocytes.12 These results implicated TSH and its receptor in the normal regulation of thermogenesis in early postnatal life.
A prerequisite for involvement of TSHR as an autoantigen in GO is that this protein be expressed in affected orbital tissues. Studies aimed at identifying TSHR in the orbital tissues have been performed by several laboratories using many different approaches. Results of these studies were in general agreement and demonstrated the presence of TSHR mRNA and protein in orbital adipose tissue specimens and derivative cultures from patients with GO and from patients without GO.13–15 However, additional studies showed that levels of TSHR are in fact higher in orbital adipose tissue from patients with GO than from patients without GO, suggesting that increased TSHR expression in the orbit might be involved in disease development.16 This concept is further supported by the finding of a positive correlation between GO patients’ TSHR mRNA levels in orbital adipose tissues excised during decompression surgery and the patients’ clinical activity score.17 Similarly, TSHR appears to be more abundant in pretibial dermopathy than in normal pretibial skin.9
A relationship between adipogenesis and TSHR expression appears also to be present in cultures of orbital preadipocyte fibroblasts undergoing in vitro differentiation. Levels of TSHR mRNA, as well as leptin and adiponectin mRNA (encoding genes expressed exclusively by mature adipocytes, used here as markers of differentiation), are approximately tenfold higher in cultures containing mature adipocytes than in undifferentiated cultures.18,19 Similarly, expression of these genes is increased in orbital adipose tissue specimens from GO patients, compared with normal tissue specimens, and significant positive correlations exist between mRNA levels corresponding to TSHR and those encoding leptin and adiponectin.20 Taken together, these results suggest that adipogenesis is enhanced in the orbits of patients with GO and that increased TSHR expression is a consequence of this process.
The only animal model of Graves disease in which ocular changes suggestive of GO have been reported was developed by Many and associates.21 They transferred into mice T cells from syngeneic animals that had been immunized with a TSHR fusion protein or vaccinated with TSHR cDNA. Thyroiditis and anti-TSHR antibodies were reported in these animals, but hyperthyroidism did not occur and thyroid-stimulating autoantibodies were not produced, limiting the study's usefulness as an animal model of Graves disease. However, the authors described tissue edema, dissociation of muscle fibers, minimal lymphocytic infiltration, and the presence of TSHR immunoreactivity within the orbital adipose tissues in the majority of immunized animals. Unfortunately, although the histopathologic findings appeared promising, none of the characteristic clinical signs of GO developed in the mice. Of particular interest is a recent article by these authors in which they questioned the validity of the thyroid and ocular changes reported in their previous study.22 Nevertheless, the partial success of this model suggests that transfer of TSHR-primed T cells may hold potential for the induction of ocular disease and supports the concept that TSHR may be an important orbital autoantigen.
Recent studies by Pritchard and associates23 demonstrated that fibroblasts from patients with Graves disease are activated by immunoglobulins (IgG) from these same donors to synthesize molecules that stimulate the infiltration of activated T cells into areas of inflammation. This process is mediated through the insulin-like growth factor 1 receptor (IGF-1R), suggesting that patients with GO have circulating autoantibodies directed against this receptor.24 The IgG-stimulated activation of this receptor does not, however, appear to be restricted to fibroblasts from the orbit and pretibial skin because fibroblasts obtained from diverse sites in these Graves patients behaved similarly. These findings suggest that IGF-1R may represent a second autoantigen in Graves disease, with an important role in lymphocyte trafficking. The relatively restricted involvement of the orbit and pretibial skin in the extrathyroidal manifestations of Graves disease may be explained, in part, by the particular sensitivity of fibroblasts from these sites to stimulation by cytokines and other immune factors.
If one could predict the patients with Graves hyperthyroidism in whom significant ocular complications would develop, these patients could be offered early treatment with agents not appropriate for patients at lower risk. In the future, skin biopsy specimens from patients with Graves disease might provide useful information concerning the activity in patients’ orbital tissues. However, for this predictive test to be developed, characteristics must be identified in these tissues or derivative cell cultures that distinguish Graves patients who later develop GO from those who do not. Several in vitro responses of dermal fibroblasts from Graves patients have in fact been shown to differ from normal, including the elaboration of immunomodulatory molecules in response to treatment with Graves IgG.23 However, no feature described to date distinguishes subgroups of Graves patients with or without GO. This supports the concept that environmental or mechanical factors, such as tissue trauma or orbital shape and volume, may be important in ocular disease development.9
The advice to stop smoking forms the centerpiece of patient counseling for prevention of GO. Current or former smokers constitute 64% of Graves patients with GO, 48% of those without overt GO, and only 28% of healthy individuals.25 In addition, smoking is highly associated with the development of more severe GO,26 with the failure of immunosuppressive therapy,27 and with aggravation of GO after radioiodine therapy for thyrotoxicosis. These effects do not appear to be related to behavioral changes associated with thyrotoxicosis or to differences in the age, gender, or educational background of smokers compared with nonsmokers.26 Although mechanisms underlying this association remain unclear, contributors may include effects of orbital hypoxia and the effect of free radicals in tobacco smoke on orbital fibroblast proliferation.28 Smokers have lower levels of IL-1 receptor antagonists than do nonsmokers, which could adversely affect the orbital disease process.29 Clearly, the strong association between smoking and GO holds important clues to the pathogenesis of this disorder.
The use of prophylactic corticosteroids concurrently with radioiodine therapy for Graves hyperthyroidism has also been reported to prevent the progression of GO in patients with preexisting eye disease.10 The theoretical underpinning of this concept is that the destruction of thyroid tissue by radioiodine may result in the release of TSHR, which might augment the immune response directed against this antigen on orbital cells. Indeed, levels of TSHR-directed autoantibodies in the circulation have been shown to increase immediately after radioiodine therapy.30 Bartalena and associates10 compared eye changes in hyperthyroid patients randomly assigned to receive radioiodine, methimazole alone, or both radioiodine and concurrent prophylactic prednisone. These investigators found that eye disease worsened within six months in 15% of the patients receiving radioiodine therapy, in 2.7% of the patients receiving only antithyroid drugs, and in none of the patients receiving both radioiodine and prednisone. Among the patients whose eye status worsened after radioiodine therapy, 74% had preexisting GO, and the majority were smokers. Although the eye changes were largely mild and returned to baseline within two to three months in 65% of the cases, eight patients (5%) in the radioiodine group required additional therapy for ocular disease. The authors concluded that any worsening of GO after radioiodine therapy can be prevented by concurrent treatment with corticosteroids and that this should be considered in patients with preexisting GO, especially if they are smokers.
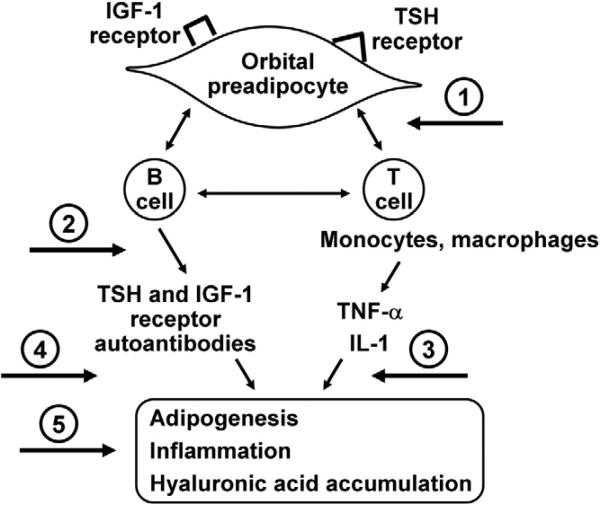
Several novel approaches to treatment of GO follow logically from the current understanding of pathogenesis (Figure 4). Studies from several laboratories underline the pathogenic role of both Th-1–type and macrophage-derived cytokines in early disease pathogenesis.31 Therefore, monoclonal antibodies that target proinflammatory cytokines and chemokines might hold particular promise. Specifically, biologic agents that block tumor necrosis factor (TNF)-α (infliximab, adalimumab, or etanercept) or IL-1 receptor (anakinra) are attractive theoretical choices. These agents are effective in rheumatoid arthritis and Crohn disease therapy, and they are under investigation for the treatment of such diverse conditions as uveitis, sarcoidosis, interstitial lung disease, graft-vs-host disease, and Sjögren syndrome. However, although these drugs have revolutionized the treatment of several immune-mediated inflammatory diseases, there is growing evidence that TNF inhibition is associated with serious side effects. Of particular concern are several case reports of serious infections, including the reactivation of Mycobacterium tuberculosis.32 In addition, lymphoma, demyelinating disorders, hepatotoxicity, aplastic anemia, and a lupus-like syndrome have been described in association with TNF-α antagonists. In the future, the application of knowledge concerning genetic variability and TNF/TNF receptor polymorphisms might aid in the targeting of anti–TNF-α therapy to patients most likely to benefit and least likely to have adverse effects.
FIGURE 4.
Proposed targets for agents of potential benefit in the treatment of Graves ophthalmopathy (GO) include (1) blocking T-cell costimulation, (2) depleting B cells, (3) inhibiting cytokine action, (4) targeting the insulin-like growth factor 1 (IGF-1) receptor or the thyrotropin (TSH) receptor, and (5) preventing connective tissue remodeling. IL-1, interleukin 1; TNF-α, tumor necrosis factor α.
Mounting evidence for the participation of autoantibodies directed against TSHR and IGF-1R in GO11,24 suggests that blocking early phases of B-cell maturation involving CD20 ligation might be beneficial. A currently available biologic agent that might be studied in this regard is the anti–B cell agent rituximab. This agent, widely used in the treatment of non-Hodgkin lymphoma, is a chimeric murine/human monoclonal antibody directed against the CD20 antigen found on the surface of normal and malignant B lymphocytes. It is thought to act through induction of B-cell apoptosis and complement-mediated and antibody-dependent cell-mediated cytotoxicity. In a recent trial of rituximab plus cyclophosphamide with or without prednisolone for treatment-resistant, erosive rheumatoid arthritis, the combination of rituximab and cyclophosphamide was well tolerated and optimally effective.33 Other compounds that hold promise include costimulation inhibitors, such as CTLA4-Ig or alefacept, that block the “second signal” necessary for T-cell activation.34 By targeting this early step in the immune response, these agents hold the theoretical advantage of blocking both the production of autoantibodies and the secretion of inflammatory cytokines.
Pharmacologic agents that block IGF-1 binding to its receptor or that target IGF-1R activation are potential candidates for GO therapy because they could block the effects of circulating IGF-1 receptor autoantibodies on orbital cells.23 These drugs are under active development because IGF can promote carcinogenesis. They include anti–IGF-1R antibodies, small-molecule inhibitors of the IGF-1R tyrosine kinase, and fragments of antisense RNA. Other related approaches to GO therapy might include targeting early phases of adipogenesis in orbital preadipocytes. If the differentiation of orbital preadipocytes could be blocked, disease manifestations resulting from increased adipose tissue volume within the orbit might be prevented.20 PPAR-γ ligation is important in the initiation of adipogenesis, and PPAR-γ agonists have been shown to stimulate both adipogenesis and TSHR expression in cultured orbital preadiopocytes.19 Therefore, agents that might be developed to specifically block PPAR-γ ligation would hold therapeutic promise for GO. Of related interest is a report of a patient with GO in whom increased proptosis developed after treatment of diabetes mellitus type 2 with the PPAR-γ agonist rosiglitazone.35 Such thiazolidinedione drugs that sensitize tissues to insulin through engagement of the PPAR-γ receptor may thus be relatively contraindicated in patients with GO.
Useful information on the efficacy of any drug (or combinations of therapeutic agents) in the treatment or prevention of GO can be gained only through prospective, randomized double-masked trials. Such trials will likely need to be large and multicentered if prevention of GO is an end point, and they should be designed to ascertain specific therapeutic benefits in relation to side effects and costs. Equally important will be the continued study of GO pathogenesis and the immune system dysregulation initiating the orbital disease process. From this information, new approaches to prediction, prevention, or treatment of GO will be developed. The promise of basic science research is that current treatment strategies involving surgery and immunosuppressive medications with serious side effects will be made obsolete.
Biography
 James A. Garrity, MD, completed his Ophthalmology training at Mayo Clinic before spending a fellowship year (orbital diseases and neuro-ophthalmology) in Pittsburgh, Pennsylvania, with John Kennerdell, MD. Dr Garrity returned to Mayo Clinic in 1986 to join the staff. Currently, Dr Garrity's practice interests are heavily biased toward orbital diseases and Graves ophthalmopathy.
James A. Garrity, MD, completed his Ophthalmology training at Mayo Clinic before spending a fellowship year (orbital diseases and neuro-ophthalmology) in Pittsburgh, Pennsylvania, with John Kennerdell, MD. Dr Garrity returned to Mayo Clinic in 1986 to join the staff. Currently, Dr Garrity's practice interests are heavily biased toward orbital diseases and Graves ophthalmopathy.
 Rebecca S. Bahn, MD, obtained her MD from the Mayo Medical School and completed postgraduate training in internal medicine and endocrinology at Mayo Clinic. She is currently a Professor of Medicine at Mayo Clinic College of Medicine, Associate Chair of Internal Medicine and Associate Director of Research for Career Development, and Director of the American Thyroid Association. Dr Bahn has an active clinical thyroidology practice and directs a research laboratory studying the pathogenesis of Graves hyperthyroidism and ophthalmopathy.
Rebecca S. Bahn, MD, obtained her MD from the Mayo Medical School and completed postgraduate training in internal medicine and endocrinology at Mayo Clinic. She is currently a Professor of Medicine at Mayo Clinic College of Medicine, Associate Chair of Internal Medicine and Associate Director of Research for Career Development, and Director of the American Thyroid Association. Dr Bahn has an active clinical thyroidology practice and directs a research laboratory studying the pathogenesis of Graves hyperthyroidism and ophthalmopathy.
REFERENCES
- 1.Bahn RS, Heufelder AE. Pathogenesis of Graves ophthalmopathy. N Engl J Med. 1993;329:1468–1475. doi: 10.1056/NEJM199311113292007. [DOI] [PubMed] [Google Scholar]
- 2.Peyster RG, Ginsberg F, Silber JH, Adler LP. Exophthalmos caused by excessive fat: CT volumetric analysis and differential diagnosis. AJR Am J Roentgenol. 1986;146:459–464. doi: 10.2214/ajr.146.3.459. [DOI] [PubMed] [Google Scholar]
- 3.Bahn RS. Clinical review 157: pathophysiology of Graves ophthalmopathy: The cycle of disease. J Clin Endocrinol Metab. 2003;88:1939–1946. doi: 10.1210/jc.2002-030010. [DOI] [PubMed] [Google Scholar]
- 4.Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 5.Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, Powell CC, Ahmed DD, Garrity JA. Graves dermopathy and acropachy are markers of severe Graves ophthalmopathy. Thyroid. 2003;13:1141–1144. doi: 10.1089/10507250360731541. [DOI] [PubMed] [Google Scholar]
- 6.Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268:C382–C388. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 7.Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998;274:C707–C714. doi: 10.1152/ajpcell.1998.274.3.C707. [DOI] [PubMed] [Google Scholar]
- 8.Adams M, Montague CT, Prins JB, et al. Activators of peroxisome proliferator-activated receptor γ have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapoport B, Alsabeh R, Aftergood D, McLachlan SM. Elephantiasic pretibial myxedema: insight into and a hypothesis regarding the pathogenesis of the extrathyroidal manifestations of Graves disease. Thyroid. 2000;10:685–692. doi: 10.1089/10507250050137761. [DOI] [PubMed] [Google Scholar]
- 10.Bartalena L, Marcocci C, Bogazzi F, et al. Relation between therapy for hyperthyroidism and the course of Graves ophthalmopathy. N Engl J Med. 1998;338:73–78. doi: 10.1056/NEJM199801083380201. [DOI] [PubMed] [Google Scholar]
- 11.Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF. Association of thyrotrophin receptor antibodies with the clinical features of Graves ophthalmopathy. Clin Endocrinol (Oxf) 2000;52:267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 12.Marcus C, Ehren H, Bolme P, Arner P. Regulation of lipolysis during the neonatal period: importance of thyrotropin. J Clin Invest. 1988;82:1793–1797. doi: 10.1172/JCI113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heufelder AE, Dutton CM, Sarkar G, Donovan KA, Bahn RS. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297–300. doi: 10.1089/thy.1993.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Feliciello A, Porcellini A, Ciullo I, et al. Expression of thyrotropin-receptor mRNA in healthy and Graves disease retro-orbital tissue. Lancet. 1993;342:337–338. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 15.Starkey KJ, Janezic A, Jones G, et al. Adipose thyrotrophin receptor expression is elevated in Graves and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–380. doi: 10.1677/jme.0.0300369. [DOI] [PubMed] [Google Scholar]
- 16.Bahn RS, Dutton CM, Natt N, et al. Thyrotropin receptor expression in Graves orbital adipose/connective tissues: potential autoantigen in Graves ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 17.Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves ophthalmopathy patients. Clin Endocrinol (Oxf) 2003;58:280–287. doi: 10.1046/j.1365-2265.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 18.Valyasevi RW, Erickson DZ, Harteneck DA, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84:2557–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 19.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-γ (PPARγ) and thyrotropin receptor by PPARγ agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab. 2002;87:2352–2358. doi: 10.1210/jcem.87.5.8472. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for enhanced adipogenesis in the orbits of patients with Graves ophthalmopathy. J Clin Endocrinol Metab. 2004;89:930–935. doi: 10.1210/jc.2003-031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Many M-C, Costagliola S, Detrait M, Denef J-F, Vassart G, Ludgate M. Development of an animal model of autoimmune thyroid eye disease. J Immunol. 1999;162:4966–4974. [PubMed] [Google Scholar]
- 22.Baker G, Mazziotti G, von Ruhland C, Ludgate M. Reevaluating thyrotropin receptor-induced mouse models of Graves disease and ophthalmopathy. Endocrinology. 2005;146:835–844. doi: 10.1210/en.2004-1015. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 25.Bartalena L, Martino E, Marcocci C, et al. More on smoking habits and Graves ophthalmopathy. J Endocrinol Invest. 1989;12:733–737. doi: 10.1007/BF03350047. [DOI] [PubMed] [Google Scholar]
- 26.Prummel MF, Wiersinga WM. Smoking and risk of Graves disease. JAMA. 1993;269:479–482. [PubMed] [Google Scholar]
- 27.Eckstein A, Quadbeck B, Mueller G, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–776. doi: 10.1136/bjo.87.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimuates retroocular fibroblast proliferation in Graves ophthalmopathy. Exp Eye Res. 1997;65:311–316. doi: 10.1006/exer.1997.0353. [DOI] [PubMed] [Google Scholar]
- 29.Hofbauer LC, Muhlberg T, Konig A, Heufelder G, Schworm HD, Heufelder AE. Soluble interleukin-1 receptor agonist serum levels in smokers and nonsmokers with Graves ophthalmopathy undergoing orbital radiotherapy. J Clin Endocrinol Metab. 1997;82:2244–2247. doi: 10.1210/jcem.82.7.4068. [DOI] [PubMed] [Google Scholar]
- 30.Gamstedt A, Wadman B, Karlsson A. Methimazole, but not betamethasone, prevents 131I treatment-induced rises in thyrotropin receptor autoantibodies in hyperthyroid Graves disease. J Clin Endocrinol Metab. 1986;62:773–777. doi: 10.1210/jcem-62-4-773. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Bahn RS. Relative overexpression of macrophage-derived cytokines in orbital adipose tissue from patients with Graves ophthalmopathy. J Clin Endocrinol Metab. 2003;88:4246–4250. doi: 10.1210/jc.2003-030380. [DOI] [PubMed] [Google Scholar]
- 32.Ellerin T, Rubin RH, Weinblatt ME. Infections and anti-tumor necrosis factor α therapy. Arthritis Rheum. 2003;48:3013–3022. doi: 10.1002/art.11301. [DOI] [PubMed] [Google Scholar]
- 33.Leandro MJ, Edwards JC, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis. 2002;61:883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 35.Starkey K, Heufelder A, Baker G, et al. Peroxisome proliferator-activated receptor-γ in thyroid eye disease: contraindication for thiazolidinedione use? J Clin Endocrinol Metab. 2003;88:55–59. doi: 10.1210/jc.2002-020987. [DOI] [PubMed] [Google Scholar]