Abstract
Purpose
Higher SNR and improved contrast have been demonstrated at Ultra-high magnetic fields (≥7T) in multiple targets, often with multi-channel transmit B1+ methods to address the deleterious impact on tissue contrast due to spatial variations in B1+ profiles.
When imaging the heart at 7T, however, respiratory and cardiac motion, as well as B0 inhomogeneity, greatly increase the methodological challenge. In this study we compare 2-spoke parallel transmit (pTX) RF pulses with static B1+ shimming in cardiac imaging at 7T.
Methods
Using a 16-channel pTX system, slice-selective 2-spoke pTX pulses and static B1+ shimming were applied in cardiac CINE imaging. B1+ and B0 mapping required modified cardiac triggered sequences. Excitation homogeneity and RF energy were compared in different imaging orientations.
Results
2-spoke pulses provide higher excitation homogeneity than B1+ shimming, especially in the more challenging posterior region of the heart. The peak value of channel-wise RF energy was reduced, allowing for higher flip angle, hence increased tissue contrast. Image quality with 2-spoke excitation proved to be stable throughout the entire cardiac cycle.
Conclusion
2-spoke pTX excitation has been successfully demonstrated in the human heart at 7T, with improved image quality and reduced RF pulse energy when compared to B1+ shimming.
Introduction
Recent advances in ultra-high field (UHF) MRI operating at field strengths of ≥7T have demonstrated significant improvements in contrast and image quality for a variety of applications at 7T (1), in particular for vascular targets (2, 3) that directly benefit from higher SNR and longer T1 relaxation times at UHF (4, 5).
Cutting edge UHF technology has already enabled initial studies of cardiac and coronary imaging at 7T (4, 6–13). The heart, however, is a challenging organ for MRI due to cardiac and respiratory motion, requiring cardiac gating and/or acquisitions performed under breath hold. Additionally, at UHF the shorter RF wavelength compared to lower field strength leads to strong spatial heterogeneities of the transmit B1+ magnitude (14, 15), resulting in contrast variations and potentially severe signal losses in the human heart at 7T (6, 9).
To address this problem, multi-channel transmit (TX) coils in combination with B1+ shimming (16–18) have been successfully applied in multiple targets (19, 20), including the heart (4, 6–8, 21). In this study we follow the often used definition of B1+ shimming (also labeled “static B1+ shimming”) as an approach where each of the individual transmit channels of a coil array is fed by a common RF pulse shape with a complex, channel specific factor controlling RF phase and/or amplitude, resulting in spatially more uniform flip angles. In general, excitation non-uniformity has also been addressed with parallel transmission (pTX) (22, 23) which in contrast to B1+ shimming allows the complex, channel-specific factors to vary temporally during the course of an RF pulse, providing additional degrees of freedom to improve excitation homogeneity and/or reduce total RF energy, as shown in the brain or liver (24–27). And a simulation study (28) has indeed shown that cardiac imaging is also expected to directly benefit from multi-spoke pTX RF pulses at 7T.
However, performing cardiac pTX acquisitions at 7T is experimentally fairly challenging due to heart motion, respiratory motion and blood flow. Heart motion requires cardiac triggered acquisition for both B0/B1+ calibration maps as well as for the pTX sequence. However, ECG recording at 7T is significantly impacted by the magneto-hydrodynamic effect as demonstrated by Suttie et al. (7), and a careful placement and potential relocation of the ECG leads is required in order to obtain a suitable ECG signal. Using pTX resources also requires precise synchronization between the individual TX channels and sequence execution for cardiac-gated MR sequences. Furthermore, for the pTX application the question arises as to whether a single B1+ solution can be suitable for CINE imaging over the entire cardiac cycle. To avoid respiratory motion artifacts in calibration data and pTX acquisition it is desirable to perform all acquisitions under breath-hold, which means that a robust multi-channel B1+ calibration should ideally be completed within a mere period of about 20s, a challenge even more demanding as the number of transmit channels increases. Limiting the impact of blood flow effects also requires designing pTX RF pulses as short as possible.
In this work we investigate the feasibility of applying slice selective 2-spoke pTX RF pulses in cardiac CINE imaging at 7T using a 16-channel pTX system, in the context of the aforementioned constraints. We acquire B1+ calibration maps, using a very fast, multi-channel B1+ calibration approach, as well as off-resonance frequency maps (ΔB0 maps), required for the RF pulse design, both collected under breath-hold and with ECG triggering. We compare the resulting flip angle homogeneity and RF energy of the 2-spoke excitation with B1+ shimming results.
Methods
Principle and Optimization
In the small tip angle approximation, the transverse magnetization m(r) generated by an RF excitation can be written by the Fourier integral of the excitation k-space trajectory weighted by the sum of the spatial sensitivities (r) of the K individual TX channels and the RF pulses bi(t) applied to each channel:
| [1] |
In the discrete case this equation can be represented by a matrix formulation, which is the basis for the spatial domain method for RF pulse design as demonstrated by Grissom et al (29):
| [2] |
In this formulation, the vector b with length K×L contains the RF waveforms of the K TX channels for L discrete time points, and the system matrix A incorporates the excitation trajectory, spatial maps of magnetic field deviations ΔB0 and the transmit B1+ sensitivity maps.
In this work we aim to obtain slice selective cardiac CINE images with improved in-plane excitation homogeneity. This can be achieved using 3D-tailored RF pulses (24, 30, 31), so called ‘spoke pulses’, which deposit RF energy in excitation k-space along line segments (spokes) in kz direction while the individual spokes are separated by shifts in kx and ky direction. Due to its intrinsic slice selectivity, the technique requires only 2D sensitivity maps and 2D ΔB0 maps for the slice of interest (instead of 3D maps), which is advantageous for B0/B1+ mapping requirements and also reduces significantly the optimization complexity.
The goal of the optimization process is to find a suitable vector b that produces a transverse magnetization m which matches closely a desired target magnetization mt. To solve this problem, we utilize in this work the magnitude least squares optimization (32):
| [3] |
The algorithm minimizes the difference between the magnitudes of desired and actual excitation pattern within a predefined ROI (see e.g. Figure 1+2) while limiting the total RF energy by the regularization term ‖b‖2, and the tradeoff is controlled by the regularization parameter λ. Pixels located outside the ROI are ignored by the algorithm through the binary matrix w. In this work the entries of vector mt are set to the real value 1 and according to the linearity of the small tip angle approximation the RF voltages of the K TX channels are scaled to achieve flip angles of about 10° in the experiment. Within the optimization the individual spokes are treated as hard pulses, thus the vector b in equation 3 has the length K×S and contains the complex weighting for the K channels and S spokes. In practice, equation 3 is solved iteratively using the least squares optimization , while updating the phases of the target magnetization mt as described in (32).
Figure 1.
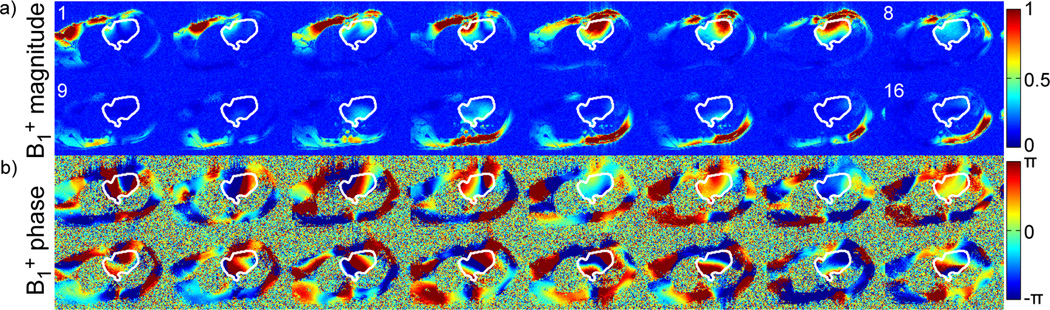
B1+ magnitude (a) and phase (b) sensitivity profiles of the 16 channel transceiver coil in four-chamber view. The white contours delimit the target ROI.
Figure 2.
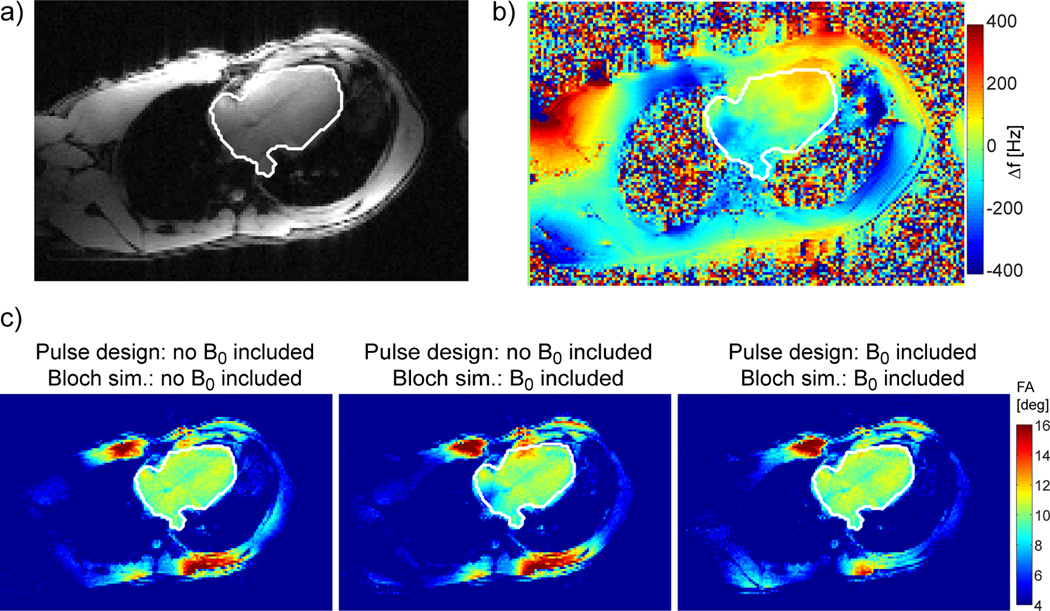
a) Sum of the B1+ magnitude profiles shown in Figure 1a in four-chamber view, with the same target ROI covering myocardium, lumen and aorta. b) Off-resonance frequency map, expressed in Hz, for same subject and same view as shown in a). c) Bloch simulations for 2-spoke RF pulses with/without including ΔB0 in the RF pulse design and/or Bloch simulation. Left: ΔB0 is assumed to be 0 for RF pulse design and for the Bloch simulation. Middle: ΔB0 is assumed to be 0 for the RF pulse design but the actual ΔB0 map shown in b) is incorporated in the Bloch simulation. Right: ΔB0 map from b) is used in both, RF pulse design and Bloch simulation.
In this work we compare the performance of B1+ shimming and 2-spoke excitation for cardiac imaging at 7T. Due to technical limitations no more than 2-spokes will be used for the pulse design. A single spoke excitation (S=1), which is commonly performed along the kz axis with kx=ky=0, is identical to B1+ shimming as discussed in the introduction, i.e. a single RF pulse shape applied with channel-specific phase (20, 33, 34) or phase and magnitude (17, 19) modulation. (Throughout this work ‘B1+ shimming’ denotes a 1-spoke excitation). All 2-spoke pulses designed in this study were based on a collection of 2-spoke locations sharing a point symmetry about kx=ky=0, with a radius kr/2π=(kx2+ky2)0.5/2π ranging from 0 to 10m−1 in steps of 1m−1 and an azimuthal angle ranging from 0° to 180° in 10° steps.
For all spoke excitations a same RF sub-pulse shape was used, consisting of a Hanning filtered SINC-shaped RF pulse with bandwidth-time-product of 4. In order to reduce artifacts due to blood flow during the excitation we chose the RF duration to be as short as possible within the given SAR limits (see paragraph SAR computation and power monitoring); for the same reason, the gradients for the 2-spoke pulses were designed in a bipolar manner. RF pulse durations (without gradient ramps) were set to 1.6 ms for B1+ shimming excitation and to 0.8 ms for each sub-pulse of 2-spoke pulses, so that the total RF pulse duration for B1+ shimming and 2-spoke excitation was kept constant. Taking the gradient switching into account the 2-spoke RF pulse excitation is 0.4 ms longer.
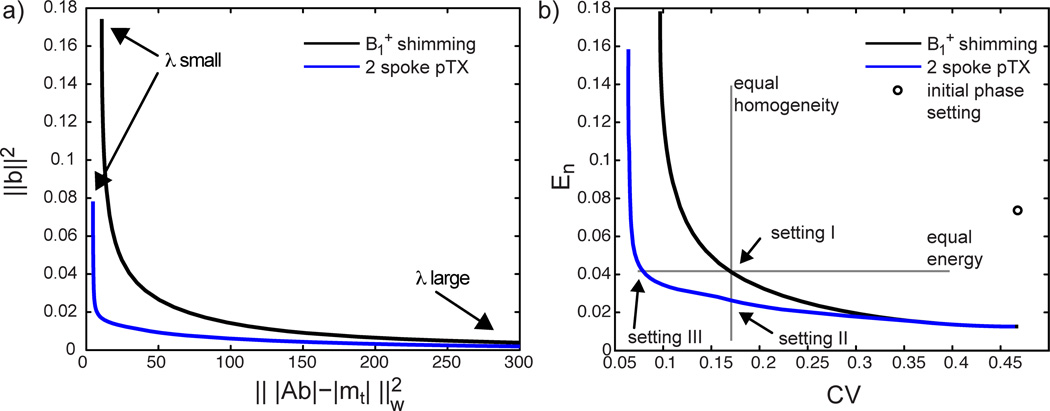
In order to determine optimum tradeoffs between the residual error and the energy, L-curves were built by solving Eq. 3 while varying the value of λ, both for B1+ shimming and 2-spoke excitation. For each solution, the coefficient of variation (CV) of the transverse magnetization, defined as
| [4] |
, was calculated within the target region defined by w. Note that minimizing the quadratic residual error in equation 3 can provide solutions where meanw|Ab|<1; CV however is inherently normalized by the mean value of |Ab| within the ROI defined by w. Additionally, the resulting total normalized energy En required to achieve meanw|Ab|=|mt| with |mt| = 1 was calculated by
| [5] |
where the factor S takes into account the pulse duration for B1+ shimming (S=1) and 2-spoke pTX (S=2) (see Figure 3). Three different excitation settings were compared in-vivo:
B1+ shimming
2-spoke solution yielding same CV as I (vertical line in Figure 3)
2-spoke solution yielding same total energy En as I (horizontal line in Figure 3)
Figure 3.
a) L-curve plot of the two regularization terms ‖b‖2 and for B1+ shimming and 2-spoke excitation with different values of the regularization parameter λ ranging over 8 orders of magnitude. B0 variations were neglected for both plots. b) L-curve plot of normalized total energy En (Equ. 5) and CV (Equ. 4), derived from a). Except for CV, all quantities are given in arbitrary units. Compared to a) in plot b) both CV and En are normalized quantities taking the mean value of |Ab| into account in order to achieve the target of mt=1. Additionall, the RF pulse duration is taken into account for the calculation of En. The gray lines define points of same energy (horizontal line) and same homogeneity (vertical line) which were used to define the 3 experimental settings for this study.
For each solution, additionally to CV and En, the energy per individual transmit channel, Ei (i=1…K), and the maximum value of the individual energies, Ei,max, were calculated with
| [6] |
All computations were performed in Matlab (The Mathworks, Nattick, MA, USA).
Hardware and experimental setting
Five volunteers, who signed a consent form approved by a local Institutional Review Board, were imaged on a 7 Tesla whole body MR system (Magnetom 7T, Siemens AG, Erlangen, Germany) equipped with a prototype 16-channel pTX system (Siemens AG, Erlangen, Germany). The 16-element RF coil consisted of two 8-transceiver arrays (35), positioned under the back and over the chest of the subject laying in supine position. Three electrodes were positioned on the chest skin for ECG recording. MR sequences provided by the vendor were modified to synchronize 16 transmit RF pulses simultaneously with ECG triggering. Calibrations were run to characterize potential time shifts between RF pulses and gradient waveforms; any measured temporal delay was incorporated into subsequent RF calculation.
SAR computation and power monitoring
Safe RF power operating levels were determined based on IEC (International Standard IEC 60601-2-33 2010) guidelines for SAR limits in normal and first level operation mode. For this purpose, electromagnetic simulation of the 16 channel transceiver body coil loaded with a whole body model was performed using a finite difference time domain solver (REMCOM, Pittsburgh, PA). A set of 160 B1+ shimming and 2-spoke solutions including those used for the experiments were computed for different orientations and corresponding local 10g average SAR values. The worst case of these solutions indicated that the IEC maximum local 10g average SAR limits over a 6 minute averaging window, i.e. 10W/kg in normal mode or 20W/kg in first level operation, would be reached when applying 1.49W or 2.97W, in normal mode and first level, respectively, on each of the 16 channels. In this study, a conservative setting was applied by limiting the maximum power per channel, continuously monitored by the vendor’s multi-channel power monitoring system, to a maximum value of 2W on a 6 minutes averaging window. Following the IEC recommendation, the maximum limit was set to twice this value (i.e. 4W) on the 10s averaging window.
B0 and B1+ mapping
B0 and B1+ mapping is challenging due to heart motion and breathing motion. Thus, ECG triggering and a fast acquisition, i.e. within a 20 seconds breath-hold, is required to obtain clean maps. In addition, to reduce the impact of cardiac motion resulting from ventricle contraction during the systolic phase, both B0 and B1+ maps were acquired with a 300 ms delay relative to the R-wave of the ECG.
ΔB0-maps, necessary to define matrix A in equation 3 for the 2-spoke RF pulse design, were obtained in a single slice for each view using a cardiac-triggered gradient echo sequence at two different TEs with following acquisition parameters: resolution=2.8×2.8×5mm3, FOV=450×292×5mm3, BW=801Hz/Px, TE1/TE2=2.3ms/3.3ms, TR1/TR2=4.3ms/5.3ms, corresponding to 550ms acquisition time per cardiac cycle. Four images were averaged for each TE. Note that the B0 shimming routine of our system could not operate in pTX mode during this study, thus all experiments were acquired with the system tune-up shim.
To map B1+ of the individual channels we used a fast, multi-channel B1+ estimation method for transceiver arrays (36) that has demonstrated satisfactory B1+ map quality for multi-channel transmit methods at 7T (26, 33, 34, 37). Briefly, 16 complex small tip angle GRE images are acquired in single shot mode with cardiac gating, with a single TX channel active at a time, while all RX channels are used for reception, providing 256 images of distinct pairs of one transmit and one receive channels using the following acquisition parameters: resolution=2.8×2.8×5 mm3, FOV=450×292×5mm3, TE/TR=2.6/4.7ms, BW=801Hz/Px. B0 and B1+ calibration maps were obtained in all subjects in transversal and pseudo 4-chamber views; additional maps were collected in short axis view for 3 subjects. All acquisitions were performed under breath hold on exhale; neither partial Fourier nor Parallel Imaging was used. In two subjects an absolute flip angle map was estimated, using the AFI (38) sequence, under breathold and without cardiac gating, with a 2-spoke excitation RF pulse.
Cardiac imaging protocol
CINE Gradient echo images were acquired in transversal, pseudo 4-chamber and short axis views using a modified cardiac triggered pTX sequence. For excitation setting I, the following acquisition parameters were used: resolution (non interpolated) =2.3×2.3×5mm3, FOV=450×292×5mm3, TE/TR=2.9/5.6ms, BW=554Hz/Px, segments=8. Reconstruction parameters included 2D interpolation (factor 2) and edge enhancement filter. All acquisitions were performed under breath hold on exhale. For setting II and III, as the total RF pulse module duration is 0.4ms longer due to additional gradient switching compared to setting I, TE was about 0.2ms longer, and the time window that determines the temporal resolution increased from 41.7ms to 44.8ms. Up to 32 (B1+ shimming) or 30 (2-spokes) images were acquired per cardiac cycle. All other parameters were kept identical in setting II and III.
For excitation settings II and III, the voltage was adjusted to achieve the same flip-angle as obtained with setting 1, which actually always resulted in lower associated power values, as will be detailed in results and discussion sections.
Results
Figure 1 shows the estimated magnitude (a) and relative phase (b) of the 16 B1+ maps, along a 4-chamber view, obtained in one representative subject (#3). Anterior and posterior coil elements are numbered 1–8 and 9–16, respectively, and the white ROI delimits the spatial target for B1+ shimming. It should be noted that these maps, well defined and free from significant artifact, were obtained without applying any spatial smoothing filter, which indirectly confirms the robustness of the fast, multi-channel mapping approach utilized in this study (36). One can also appreciate that the partial proton density bias, inherently observed with this fast estimation technique, has only a limited impact on the final B1+ maps. This residual bias, easier to identify when summing up the magnitude image of all individual B1+ maps as shown in Figure 2a, is assumed to be responsible for the apparent |B1+| variations between lumen (higher) and myocardium (lower). In Figure 2b the corresponding ΔB0 map, acquired with tune-up shim in the same slice, shows resonance offset frequency variations between −250 Hz and 150 Hz through the ROI. This cardiac-gated map, measured during diastole, was also free of any significant artifact, which is critical for the subsequent design of 2-spoke pTX RF. The necessity of including ΔB0 into the 2-spoke RF pulse design is demonstrated in Figure 3c which shows simulations of the Bloch equation using a 2-spoke excitation for 3 different scenarios: i) no ΔB0 off-resonance is present (left image); ii) off-resonance shown in Fig. 2b is present but not included in the pulse design (middle); iii) off-resonance in Fig. 2b is present and included in the design (right). As seen in the middle image, at the location of strong ΔB0 with off-resonance frequencies beyond −200Hz, the flip angle is reduced by more than 50% without correction whereas including ΔB0 in the pulse design effectively restitutes a satisfactory excitation profile.
In Figure 3a, the regularization term ‖b‖2, representing the total (non-normalized) RF energy, is plotted against the residual error of the excitation target for B1+ shimming and 2-spoke excitation, with λ values ranging over 8 orders of magnitude, resulting in both cases in an L-shaped curve. In Figure 3b, these data point have been normalized to obtain a same average target flip angle, with the resulting RF energy En (see Eq. 5) plotted against the CV value. One additional point (circle) in Figure 3b also shows the normalized result for the non-optimized starting B1+ phases with equal magnitude for the 16 TX channels, indicating a very inefficient B1+ shimming setting with poor homogeneity and high RF energy. These L-curves clearly show that with B1+ shimming as well as 2-spoke pTX excitation different tradeoffs can be chosen, setting a higher priority to reduce either CV or RF energy. In Figure 3b, the two curves converge on the right end, corresponding to the fact that, when a strong penalty is imposed upon RF energy, the optimal k-space locations for the 2 spokes are close, or equal, to the location of a single B1+ shimming spoke with kx=ky=0.
A major finding in this study is the significant advantage of using 2-spoke excitation: a same level of residual excitation errors (i.e. same CV) can be obtained with substantially less energy (vertical gray line, setting II in Figure 3b) or, alternatively, for a same energy level, excitation errors can be substantially reduced (horizontal gray line, setting III).
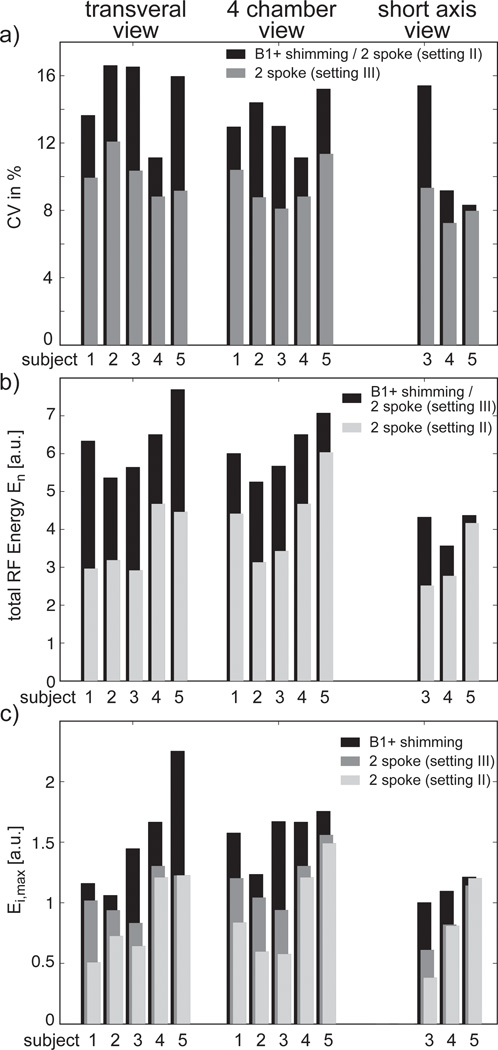
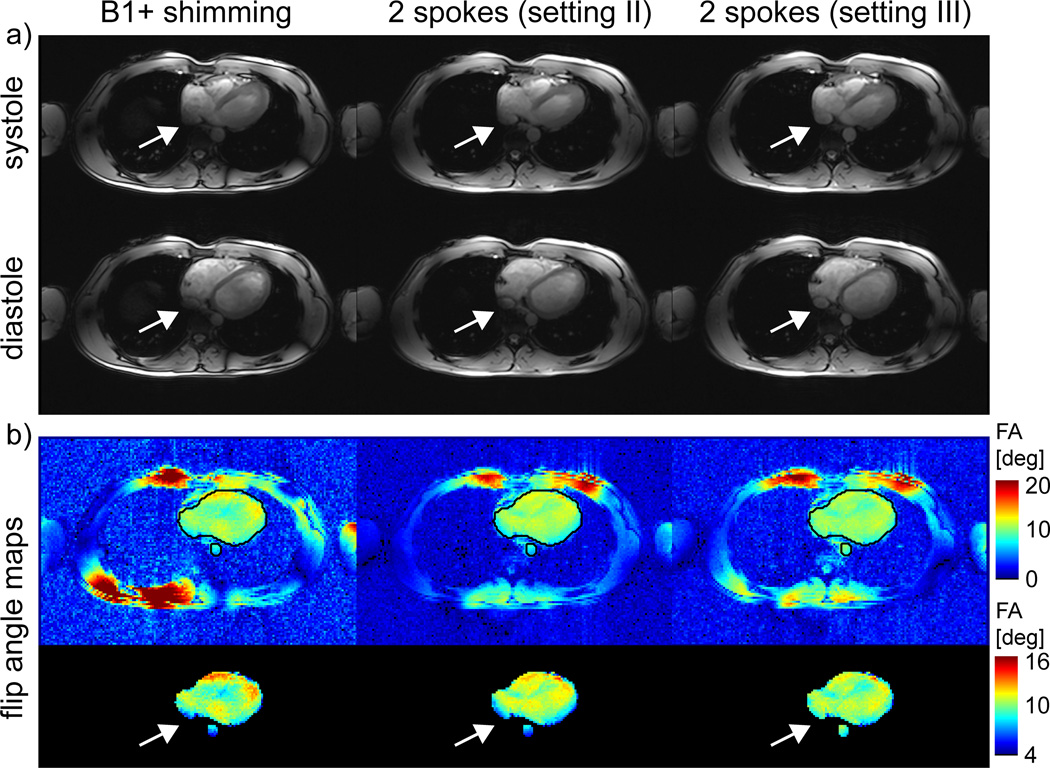
This effect is quantitatively summarized in Figure 4 for all the volunteers where CV (Figure 4a), normalized total energy En (Figure 4b) and maximum energy per channel Ei,max (Figure 4c) are displayed for all volunteers, settings and orientation. For example, in 4-chamber view, CV values vary between 11.1% and 15.2% for B1+ shimming and setting II (with identical values by design definition). However, the same CV and flip angle values were obtained, for all subjects, with a smaller total energy En for setting II which was reduced by a factor of 1.45±0.19 (mean ± std) (see Figure 4b). Similar energy reductions were obtained in transversal (1.77±0.25) and short axis views (1.35±0.28). Alternatively, when a same En was preserved for B1+ shimming and setting III, the CV value was reduced on average by a factor 1.42±0.17 compared to B1+ shimming in the four-chamber view and by a factor 1.47±0.17 and 1.31±0.30 for the transversal and short axis view, as shown in Figure 4c. Interestingly, in 2 out of 3 subjects, the energy reduction for the short axis view was less pronounced than for the four-chamber and transversal views, possibly the consequence of a smaller ROI where relatively low CV values could already be achieved with B1+ shimming. It was consistently observed that using 2-spoke pulses to reduce CV predominantly benefitted the posterior region of the heart where |B1+| was typically low with B1+ shimming. This can be seen in a representative subject in Figure 5a showing transverse cardiac views acquired during systole and diastole for the 3 different settings, as well as corresponding simulated flip angle maps in Figure 5b. Using 2-spokes with setting III, the CV value was reduced from 16.6% to 12.1% and the contrast in the posterior heart section as well as the aorta could be recovered compared to B1+ shimming and setting II. Similar results are demonstrated in the 4 chamber view in Figs. 6a+b for B1+ shimming and setting III. Even though, by design, settings III and B1+ shimming had identical En, with CV much improved in setting III, the distribution of RF energy through individual channels was favorable for 2-spokes since Ei,max was smaller in all orientations and all subjects, with an average reduction factor of 1.37 (see Figure 4). An even higher reduction factor of 1.81 on average was observed for setting II. The reduced Ei,max value with 2-spokes has practical consequences, since a conservative maximum power limit was imposed on a per-channel basis.
Figure 4.
Quantitative summary of a) CV, b) En and c) Ei,max for all volunteers and all views. In a) the CV for setting I and II is identical due to the study design whereas in b) En for setting I and III is kept the same. Short axis view data was only acquired in 3 out of 5 subjects.
Figure 5.
a) transversal view during systole (top row) and diastole (bottom row) obtained from CINE images acquired with excitation settings I-III. b) corresponding flip angle maps using simulations of the Bloch simulations for a target flip angle of 10° for the entire slice (top) and for the optimization ROI only (bottom). The flip angle is significantly reduced in the posterior region of the heart (see arrows) for settings I and II. Note the different color scales between both rows.
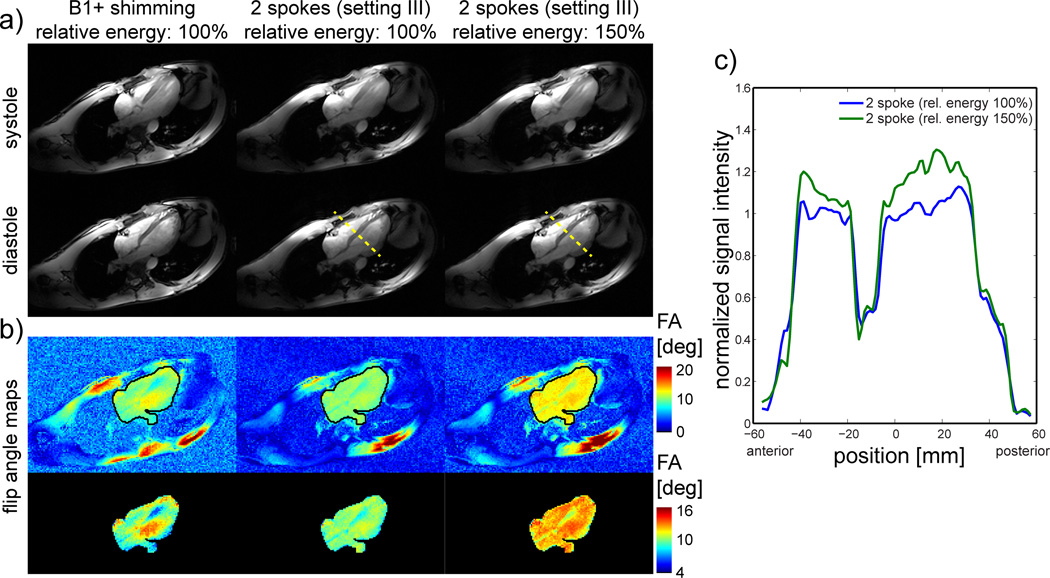
Figure 6.
a) pseudo 4-chamber view during systole (top row) and diastole (bottom row) obtained from CINE images acquired with excitation settings I and III only. Since Ei,max was lower by a factor >1.5 with setting III (middle column) compare to setting I, the transmitter voltage/RF pulse energy could be increased by 22%/50% to obtain higher contrast values (right column). b) Corresponding flip angle maps for the entire slice (top) and for the optimization ROI only (bottom). Note the different color scales for the upper and lower row. c) Plots along the yellow dashed lines in a) to visualize the enhanced contrast by the increased transmitter voltage. The plots were corrected for the coil’s receive profile.
The images shown in Figure 6a+b with B1+ shimming (left column) were acquired using a voltage close to the maximum allowed for the corresponding Ei,max. By keeping same flip angle and total energy but using setting III, not only CV value and signal intensity in the posterior heart areas clearly improved, but also Ei,max was reduced by a factor >1.5. Further gain in tissue contrast when using a higher flip angle could only be achieved with setting III where, thanks to the reduction in Ei,max, the nominal voltage could be increased by 22% (i.e. energy increase of 50%). This resulted in a clear contrast improvement between lumen and myocardium as shown in Figure 6a (right column), which is better visualized on a normalized line plot (Figure 6c) following the dashed line in Figure 6a after removing the estimated receive coil profiles from the data.
Especially when applying 2-spoke pTX pulse to cardiac imaging, a question arises about the validity of using a unique set of calibration maps (B1+, B0), measured during diastole, to calculate B1+ solutions to be applied through the entire cardiac cycle. However, as can be seen in Figure 7 showing 8 phases out of 32 (or 30 for 2-spoke) of the cardiac cycle (CINE acquisition), no significant differences in overall signal magnitude profile were observed at different phases of the cardiac cycle. For this particular comparison, B1+ shimming and 2-spoke excitation settings were chosen to have similar energy En, flip angle and CV values.
Figure 7.
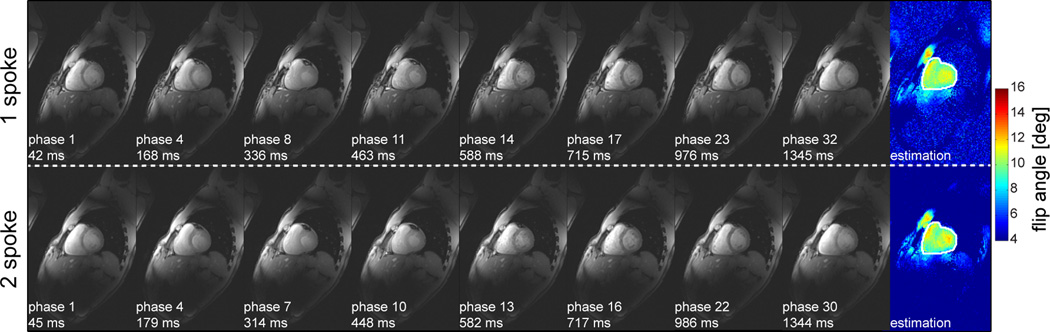
Cardiac images at 8 different phases of the cardiac cycle extracted from a 32-phase CINE acquisition, using either B1+ shimming (upper row) or 2-spoke (lower row) excitation, with corresponding flip angle maps obtained from Bloch simulations for a target flip angle of 10° in the rightmost column. Note the absence of noticeable difference in the overall profile of image signal magnitude between different phases of the cardiac cycle (from left to right) suggesting an absence of significant variation of excitation profiles. (B1+ shimming and 2-spoke settings were chosen so as to have similar energy En, flip angle and CV values).
Discussion
Recently several groups have shown very promising cardiac images at 7T using multi transmit channel coils, driven either by a single amplifier with fixed phases between the TX elements or by multiple amplifiers in combination with B1+ (phase) shimming (4, 6–12). On the other hand, multi-spoke pTX pulses have proven to outperform B1+ shimming, providing better flip angle homogeneity and/or reduced RF energy in several applications, but has not yet been fully demonstrated in the human heart. Thus, the aim of this paper was to investigate the feasibility of using 2-spoke pTX excitation in cardiac imaging at 7T and to compare these results with B1+ shimming in the context of multiple outstanding methodological challenges.
Motion and blood flow are two major obstacles in cardiac pTX. Because of respiratory motion, fast calibration of B1+ or B0 maps is typically restricted to single breath-hold modality (free breathing would require long calibration scans, incompatible with clinical settings). Cardiac motion requires ECG-based cardiac gating capability in MR sequences; in the case of pTX systems, this can present further hardware and software challenges as independent RF pulse design capability on each channel, with proper synchronization, is required on top of ECG triggering requirements. In the present work, these requirements necessitated to modify ECG-triggered MR sequences and to calibrate time shifts between RF pulses and gradient waveforms. With these modifications in place, robust multi-channel B1+ calibration maps, based on a small flip angle approach (36), could be acquired within one breath-hold (20s); cardiac diastole (300ms post ECG standard trigger) was found to provide the most efficient acquisition modality.
Blood flow may impact B1+ and B0 map calibration, as well as final image quality when using 2-spoke pTX pulses. A short RF sub-pulses duration (800µs) was used in 2-spoke pulses to reduce the latter impact, while providing a reasonable tradeoff between duration and limiting SAR. Flow compensation was not included in that fast calibration scans to preserve short TE and short acquisition times that were of greater importance in the overall success of cardiac imaging at 7T. In all subjects, B1+ maps free of significant artifact were obtained to the cost of a partial bias in proton density, inherent to the chosen method, that did not prevent considerable gains to be obtained with B1+ shimming and 2-spoke pulses, even though non-biased B1+ maps may ultimately provide further quality improvement.
Mapping the absolute flip angle in the heart at 7T is still fairly challenging with current mapping protocols due to aforementioned issues, and obtaining such maps accurately is sometimes forgone to the benefit of more qualitative comparisons (39). Nevertheless, we were able to measure absolute flip angle maps using a 3D AFI acquisition in a breath-hold without cardiac triggering (AFI mapping is not compatible with adapting TR to each cardiac cycle), using the same 2-spoke excitation pulses as for the CINE acquisition. Based on these results we determined that that the average flip angle in the heart when collecting images with our designed RF pulses was between 7° and 10°, consistent with our expectation. Naturally, the actual average flip angle may somewhat vary between subjects; however, using the same RF pulse design approach resulted in similar RF voltages in all subjects with similar contrast between myocardium and blood. In one particular case, taken here as an example, where the flip angle was 10°, the specific voltages required for a 90°, 0.5ms square pulse, varied between 40V and 352V through the 16 channels for B1+ shimming (voltages for 2-spoke excitation with same En 46V – 367V). Note, that the peak voltage for the 2-spoke excitation can be higher than for B1+ shimming although the maximum energy per channel Ei,max is lower.
In 2 subjects, a slight reduction of signal amplitude was observed within the left ventricle in 4-chamber view with 2-spoke pulses compared to B1+ shimming, possibly the result of blood flow between sub-pulses (data not shown). Even in these cases, however, the overall image pattern closely matched the predicted excitation profile. Cardiac motion itself is another potential source of excitation pattern fluctuation; however, as shown in Figure 8, image quality was preserved throughout the cardiac cycle for both B1+ shimming and 2-spoke pTX.
The higher excitation fidelity obtained with 2-spoke pTX pulses, with improved image quality particularly in the posterior part of the heart, suggests that targets extending beyond the heart muscle, such as coronary arteries (13, 37) may greatly benefit from pTX methods. Alternatively, for a given CV value, 2-spoke excitation could be used to reduce total RF energy, with, in the current study, an average reduction factor of 1.77/1.45/1.35, for transversal/four chamber/short axis view, respectively. With 2-spoke pulses, a consistent reduction of Ei,max was observed in all cases, which can have important consequences, especially when RF power limits are set on a conservative per-channel basis, since larger flip angle may be used with much improved tissue contrast.
It is of interest to note that several of the TX channels located on the bottom array do not contribute significantly to B1+ in the heart (see Figure 1), in agreement with previous simulation results (28). This suggests that with more optimal multi-channel coil arrangements, including coil element distribution along z-axis, B1+ methods could further enable cardiac imaging at 7T.
Conclusion
Despite major challenges such as motion and blood flow, 2-spoke pTX RF pulse excitation was successfully demonstrated for cardiac MR imaging at 7T. Compared to B1+ shimming, a better excitation fidelity or a reduced RF pulse energy could be achieved, opening the door to a broader range of applications, including uniform excitation for quantitative mapping approaches or coronary artery imaging at ultra-high field.
Acknowledgement
We want to acknowledge Josef Pfeuffer and Michael Hamm (Siemens AG, Healthcare Sector, Erlangen, Germany) for their help with the pTX configuration of our 7T system, and Jinfeng Tian (University of Minnesota, Minneapolis, MN) for calculating the RF coil electromagnetic model utilized in this study. This work was supported by NIH grants (P41 EB015894, R21 EB009138, R01 EB006835, R01 EB007327, S10 RR26783), Deutsche Forschungsgemeinschaft (DFG SCHM 2677-1/1) and by the WM KECK Foundation.
References
- 1.Moser E, Stahlberg F, Ladd ME, Trattnig S. 7-T MR--from research to clinical applications? NMR Biomed. 2012;25(5):695–716. doi: 10.1002/nbm.1794. [DOI] [PubMed] [Google Scholar]
- 2.Kang CK, Park CW, Han JY, Kim SH, Park CA, Kim KN, Hong SM, Kim YB, Lee KH, Cho ZH. Imaging and analysis of lenticulostriate arteries using 7.0-Tesla magnetic resonance angiography. Magn Reson Med. 2009;61(1):136–144. doi: 10.1002/mrm.21786. [DOI] [PubMed] [Google Scholar]
- 3.Schmitter S, Bock M, Johst S, Auerbach EJ, Ugurbil K, Van de Moortele PF. Contrast enhancement in TOF cerebral angiography at 7 T using saturation and MT pulses under SAR constraints: impact of VERSE and sparse pulses. Magn Reson Med. 2012;68(1):188–197. doi: 10.1002/mrm.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgers CT, Piechnik SK, Delabarre LJ, Van de Moortele PF, Snyder CJ, Neubauer S, Robson MD, Vaughan JT. Inversion recovery at 7 T in the human myocardium: Measurement of T(1), inversion efficiency and B(1) (+) Magn Reson Med. 2012 doi: 10.1002/mrm.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57(2):308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- 6.Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele PF, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med. 2009;61(3):517–524. doi: 10.1002/mrm.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suttie JJ, Delabarre L, Pitcher A, van de Moortele PF, Dass S, Snyder CJ, Francis JM, Metzger GJ, Weale P, Ugurbil K, Neubauer S, Robson M, Vaughan T. 7 Tesla (T) human cardiovascular magnetic resonance imaging using FLASH and SSFP to assess cardiac function: validation against 1.5 T and 3 T. NMR Biomed. 2012;25(1):27–34. doi: 10.1002/nbm.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole-body imaging at 7T: preliminary results. Magn Reson Med. 2009;61(1):244–248. doi: 10.1002/mrm.21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niendorf T, Graessl A, Thalhammer C, Dieringer MA, Kraus O, Santoro D, Fuchs K, Hezel F, Waiczies S, Ittermann B, Winter L. Progress and promises of human cardiac magnetic resonance at ultrahigh fields: A physics perspective. J Magn Reson. 2012 doi: 10.1016/j.jmr.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Niendorf T, Sodickson DK, Krombach GA, Schulz-Menger J. Toward cardiovascular MRI at 7 T: clinical needs, technical solutions and research promises. Eur Radiol. 2010;20(12):2806–2816. doi: 10.1007/s00330-010-1902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Knobelsdorff-Brenkenhoff F, Frauenrath T, Prothmann M, Dieringer MA, Hezel F, Renz W, Kretschel K, Niendorf T, Schulz-Menger J. Cardiac chamber quantification using magnetic resonance imaging at 7 Tesla--a pilot study. Eur Radiol. 2010;20(12):2844–2852. doi: 10.1007/s00330-010-1888-2. [DOI] [PubMed] [Google Scholar]
- 12.Winter L, Kellman P, Renz W, Grassl A, Hezel F, Thalhammer C, von Knobelsdorff-Brenkenhoff F, Tkachenko V, Schulz-Menger J, Niendorf T. Comparison of three multichannel transmit/receive radiofrequency coil configurations for anatomic and functional cardiac MRI at 7.0T: implications for clinical imaging. Eur Radiol. 2012;22(10):2211–2220. doi: 10.1007/s00330-012-2487-1. [DOI] [PubMed] [Google Scholar]
- 13.van Elderen SG, Versluis MJ, Webb AG, Westenberg JJ, Doornbos J, Smith NB, de Roos A, Stuber M. Initial results on in vivo human coronary MR angiography at 7 T. Magn Reson Med. 2009;62(6):1379–1384. doi: 10.1002/mrm.22168. [DOI] [PubMed] [Google Scholar]
- 14.Van de Moortele PF, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Ugurbil K. B(1) destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med. 2005;54(6):1503–1518. doi: 10.1002/mrm.20708. [DOI] [PubMed] [Google Scholar]
- 15.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen P, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim TS, Lee R, Baertlein BA, Abduljalil AM, Zhu H, Robitaille PM. Effect of RF coil excitation on field inhomogeneity at ultra high fields: a field optimized TEM resonator. Magn Reson Imaging. 2001;19(10):1339–1347. doi: 10.1016/s0730-725x(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 17.Mao W, Smith MB, Collins CM. Exploring the limits of RF shimming for high-field MRI of the human head. Magn Reson Med. 2006;56(4):918–922. doi: 10.1002/mrm.21013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan JT, Hetherington HP, Otu JO, Pan JW, Pohost GM. High frequency volume coils for clinical NMR imaging and spectroscopy. Magn Reson Med. 1994;32(2):206–218. doi: 10.1002/mrm.1910320209. [DOI] [PubMed] [Google Scholar]
- 19.Hetherington HP, Avdievich NI, Kuznetsov AM, Pan JW. RF shimming for spectroscopic localization in the human brain at 7 T. Magn Reson Med. 2010;63(1):9–19. doi: 10.1002/mrm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marjanska M, Auerbach EJ, Valabregue R, Van de Moortele PF, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25(2):332–339. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan JT, Adriany G, Snyder CJ, Tian J, Thiel T, Bolinger L, Liu H, DelaBarre L, Ugurbil K. Efficient high-frequency body coil for high-field MRI. Magn Reson Med. 2004;52(4):851–859. doi: 10.1002/mrm.20177. [DOI] [PubMed] [Google Scholar]
- 22.Katscher U, Bornert P, Leussler C, van den Brink JS. Transmit SENSE. Magn Reson Med. 2003;49(1):144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med. 2004;51(4):775–784. doi: 10.1002/mrm.20011. [DOI] [PubMed] [Google Scholar]
- 24.Setsompop K, Alagappan V, Gagoski B, Witzel T, Polimeni J, Potthast A, Hebrank F, Fontius U, Schmitt F, Wald LL, Adalsteinsson E. Slice-selective RF pulses for in vivo B1+ inhomogeneity mitigation at 7 tesla using parallel RF excitation with a 16-element coil. Magn Reson Med. 2008;60(6):1422–1432. doi: 10.1002/mrm.21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, Amadon A. kT-points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012;67(1):72–80. doi: 10.1002/mrm.22978. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Schmitter S, Auerbach EJ, Pfeuffer J, Hamm M, Ugurbil K, Van de Moortele PF. Proceedings of the 19th Annual Meeting of ISMRM. Montreal, Canada: 2011. Parallel Transmission in Liver MRI at 7T: Initial Results. (Abstract 2940) [Google Scholar]
- 27.Wu X, Vaughan JT, Ugurbil K, Van de Moortele PF. Parallel excitation in the human brain at 9.4 T counteracting k-space errors with RF pulse design. Magn Reson Med. 2010;63(2):524–529. doi: 10.1002/mrm.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Schmitter S, Tian J, Vaughan JT, Ugurbil K, Van de Moortele PF. Proceedings of the 19th Annual Meeting of ISMRM. Montreal, Canada: 2011. SAR Analysis of Parallel Transmission in Cardiac Imaging at 7T. (Abstract 492) [Google Scholar]
- 29.Grissom W, Yip CY, Zhang Z, Stenger VA, Fessler JA, Noll DC. Spatial domain method for the design of RF pulses in multicoil parallel excitation. Magn Reson Med. 2006;56(3):620–629. doi: 10.1002/mrm.20978. [DOI] [PubMed] [Google Scholar]
- 30.Saekho S, Yip CY, Noll DC, Boada FE, Stenger VA. Fast-kz three-dimensional tailored radiofrequency pulse for reduced B1 inhomogeneity. Magn Reson Med. 2006;55(4):719–724. doi: 10.1002/mrm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zelinski AC, Wald LL, Setsompop K, Alagappan V, Gagoski BA, Goyal VK, Adalsteinsson E. Fast slice-selective radio-frequency excitation pulses for mitigating B+1 inhomogeneity in the human brain at 7 Tesla. Magn Reson Med. 2008;59(6):1355–1364. doi: 10.1002/mrm.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setsompop K, Wald LL, Alagappan V, Gagoski BA, Adalsteinsson E. Magnitude least squares optimization for parallel radio frequency excitation design demonstrated at 7 Tesla with eight channels. Magn Reson Med. 2008;59(4):908–915. doi: 10.1002/mrm.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellermann J, Goerke U, Morgan P, Ugurbil K, Tian J, Schmitter S, Vaughan T, Van De Moortele PF. Simultaneous bilateral hip joint imaging at 7 Tesla using fast transmit B(1) shimming methods and multichannel transmission - a feasibility study. NMR Biomed. 2012;25(10):1202–1208. doi: 10.1002/nbm.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metzger GJ, Auerbach EJ, Akgun C, Simonson J, Bi X, Ugurbil K, van de Moortele PF. Dynamically applied B1+ shimming solutions for non-contrast enhanced renal angiography at 7.0 Tesla. Magn Reson Med. 2013;69(1):114–126. doi: 10.1002/mrm.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder CJ, Delabarre L, Moeller S, Tian J, Akgun C, Van de Moortele PF, Bolan PJ, Ugurbil K, Vaughan JT, Metzger GJ. Comparison between eight- and sixteen-channel TEM transceive arrays for body imaging at 7 T. Magn Reson Med. 2012;67(4):954–964. doi: 10.1002/mrm.23070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Moortele PF, Ugurbil K. Proceedings of the 17th Annual Meeting of ISMRM. Honolulu, USA: 2009. Very Fast Multi Channel B1 Calibration at High Field in the Small Flip Angle Regime. (Abstract 367) [Google Scholar]
- 37.Metzger GJ, DelaBarre L, Bi X, Shah S, Zuehlsdorff S, Vaughan JT, Ugurbil K, Van de Moortele PF. Proceedings of the 19th Annual Meeting of ISMRM. Montreal, Canada: 2011. Left Coronary Artery Imaging at 7T: Initial Results using Multiple B1+ Shimming Algorithms and Targets. (Abstract 116) [Google Scholar]
- 38.Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007;57(1):192–200. doi: 10.1002/mrm.21120. [DOI] [PubMed] [Google Scholar]
- 39.Versluis MJ, Tsekos N, Smith NB, Webb AG. Simple RF design for human functional and morphological cardiac imaging at 7tesla. J Magn Reson. 2009;200(1):161–166. doi: 10.1016/j.jmr.2009.06.014. [DOI] [PubMed] [Google Scholar]