Abstract
There is accumulating evidence which demonstrates that chronic cerebral ischaemia can induce white matter lesions (WMLs), and microglia-activation-mediated cytokines and proteases releasing during the ischaemia might play a vital role in pathogenesis. In addition, hypoxia-induced upregulated expression of fractalkine promotes the activation of microglia and their migration to the lesions through interaction with its receptor CX3CR1. However, the specific mechanisms involved in fractalkine/CX3CR1-mediated microglial activation have not been fully identified. In the present study, we constructed lentivirus encoding shRNA against CX3CR1 and transduced into microglial cells in under hypoxic conditions. Moreover, we analysed the proliferation, cytokine secretion and signal-pathway activation of the microglia. We found that CX3CR1 RNAi-mediated gene downregulation could attenuate hypoxic-induced microglial proliferation, cytokine secretion [including tumuor necrosis factor-α (TNF-α), interleukin-1β (IL-1β)] and matrix metalloproteinase-2 (MMP-2) synthesis. These effects were shown to be nediated through p38MAPK/PKC activation. Therefore, our results reveal a novel mechanism of fractalkine/CX3CR1 involvement in activation of microglia. Thus CX3CR1 RNAi might provide a therapeutic strategy which could be useful in chronic cerebral ischaemia.
Keywords: CX3CR1, hypoxi, microglia, p38MAPK/PKC, RNAi
Activation of microglia is a natural response to CNS infection to eliminate cell debris after injury and to support tissue repair (Harrigan et al. 2008; Rotshenker 2009; Szretter et al. 2009). Microglia can release soluble factors such as cytokines, heat-shock proteins and acute-phase proteins that propagate and perpetuate the cerebral immune response (Rasley et al. 2006; Magliozzi et al. 2007; Lin & Levison 2009). However, an excessive and sustained activation of microglial cells is detrimental to neurons and oligodendrocytes (Basu et al. 2005; Dilger & Johnson 2008; Kawashita et al. 2009).
Fractalkine is one of the few constitutive chemokines that is expressed in brain tissue and is widely distributed in the central nervous system, with notably high expression in neurons (Latchney et al. 2004; Thomas et al. 2008; Heinisch & Kirby 2009). It interacts with its specific receptor, CX3CR1, and thereby mediates inflammation and participates in wound healing by improving the adhesion of CX3CR1-positive cells and promoting cell migration (Pachot et al. 2008; Bourd-Boittin et al. 2009; Fevang et al. 2009). In addition, p38MAPK/PKC has been implicated in the regulation of proinflammatory cytokines and apoptosis in the microglial response to stress (Tian et al. 2000).
However, the specific mechanism involved in fractalkine/CX3CR1-mediated microglial activation in hypoxia remains elusive. To investigate it, we silenced CX3CR1 expression in order to study the effect, and to analyse further the molecular mechanism that are involved in the activation of microglia.
Materials and methods
Cell culture
The mouse microglial cell line, N9, was cultured in Dulbecco's modified Eagle's medium (DMEM, Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% foetal calf serum, antibiotics and L-glutamine.
Ethical approval
All experimental procedures involving animals were performed in accordance with the National Institutes of Health Guide for Care and Use of Animals and with the approval of our institutional ethics committee.
Lentivirus encoding CX3CR1 shRNA construction
A CX3CR1 small hairpin RNA (shRNA)-expressing lentivirus gene transfer vector was constructed by Genechem Co., Ltd, Shanghai, China. The target sequence of the shRNA was 5-GCCTGTCTCTT CCATATGA-3. The identity of the vector was confirmed by PCR and sequencing analysis. The recombinant CX3CR1 shRNA-expressing lentivirus (Lv-rCX3CR1) and the control lentivirus (Lv-CON) were prepared at a titre of 109 TU (transfection unit)/ml.
Lentivirus transduction and detection assay N9 cells were transduced with lentiviral vectors at a multiplicity of infection (MOI) of 20 in complete medium containing polybrene (5 μg/ml) at 37 °C and 5% CO2 for 18–24 h. The cells were then washed and cultured in fresh medium containing 10% foetal bovine serum (FBS). Cells were harvested 3 days following transduction.
Hypoxic exposure
Hypoxia was induced by exposing cells to a gas mixture of 5% CO2, 94% N2 and 1% O2 in an airtight chamber in the presence of Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS. The chamber containing the cell cultures was incubated for various periods up to 6 h at 37 °C. Using this method, the PO2 of the medium was verified to be 10 mm Hg with an electronic gas analyser.
Cell proliferation under hypoxia
Cell proliferation was measured by a colorimetric assay using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT). In brief, N9 cells were seeded into 96-well plates in triplicate at 5 × 103 cells/well and incubated in culture medium overnight. Then, the cells were treated with CX3CR1 siRNA (1 × 109 pfu) or controls in a total volume of 0.2 ml in each well for 4 h under hypoxia. Thereafter, 20 μl of indicator dye MTT solution (5 mg/ml) was added to each well and cultures were continued for 48 h at 37 °C, 5% CO2. After centrifugation, the supernatant was removed from each well. The coloured formazan crystal produced from MTT was dissolved with 0.15 ml of dimethyl sulphoxide (DMSO), and then the optical density (OD) value A490 was measured by multiscanner autoreader (Dynatech MR 5000; Dynatech Laboratories, Chantilly, VA, USA). In addition, the N9 cells were also counted using microscope after 0–8 h hypoxia.
Examination of CX3CR1 in N9 cells by Western blot analysis
At 3 days after transduction, CX3CR1 shRNA-transduced N9 cells were harvested by trypsinization and analysed. Non-transduced cells and control-transduced cells were used as controls. Total cellular extracts were prepared by lysing the cells in RIPA buffer for 30 min on ice. Equivalent amounts of protein, as determined by the use of the BCA protein assay kit (Pierce, IL, USA), were loaded onto 10% sodium dodecyl sulphate (SDS) PAGE gels. CX3CR1 protein was detected by the use of rabbit anti-CX3CR1 polyclonal antibody (Abcam, MA, USA) at a dilution of 1:1000, and GAPDH, which served as a loading control, was detected by the use of rabbit anti-GAPDH polyclonal antibody (Santa Cruz Biotechnology, CA, USA) at a dilution of 1:2000. After incubation with the appropriate secondary antibody for 1 h at room temperature, an enhanced chemiluminescence kit (Pierce) was used for the chemiluminescent detection of the proteins.
Flow cytometric analysis of apoptosis
An annexin V–fluorescein isothiocyanate kit (Oncogene) was used to detect apoptosis. Briefly, the cells were infected with CX3CR1 siRNA (109 pfu) or controls for 2 days. The cells were seeded into 100-ml bottles and incubated to 80–85% confluence. Then, the cells were harvested, washed with ice-cold PBS twice and resuspended in binding buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4, 150 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2, 4% bovine serum albumin]. Annexin V–fluorescein isothiocyanate (0.5 mg/ml) and propidium iodide (PI) (0.5 mg/ml) were then added to a 250-ml aliquot (5 × 106 cells) of this cell suspension. After 15-min incubation in the dark at room temperature, stained cells were immediately analysed by flow cytometry (Coulter Biosciences). Apoptosis cells were determined by annexin V-positive and PI-negative cells. All of the samples were assayed in triplicate, and the cell apoptosis rate was calculated as follows: apoptosis rate = (apoptotic cell number/total cell number) × 100%.
Enzyme-linked immunosorbent assay (ELISA) of cytokine secretion Cell cultures were centrifuged, and supernatant was analysed with ELISA. tumuor necrosis factor-α, IL-1β and MMP-2 levels were measured with a cytokine-specific ELISA (eBioscience, San Diego, CA, USA). The absorbance of each well was read at 405 nm using a Labsystems Multiskan ELISA reader.
Statistical analysis
Data are presented as the mean ± SD. For evaluation of statistical significance, Student's t-test was performed. *P < 0.05 was considered significant.
Results
CX3CR1 expression of N9 cells under hypoxia
To identify the effect of hypoxia on CX3CR1 expression of N9 cells, N9 cells were harvested after 0–8 h hypoxia and CX3CR1 expression was measured by Western blot analysis. As shown in Figure 1, low levels of CX3CR1 protein were observed in N9 cells under normoxia; however, hypoxia increased CX3CR1 protein expression as early as 1 h, with levels peaking at 4 h.
Figure 1.
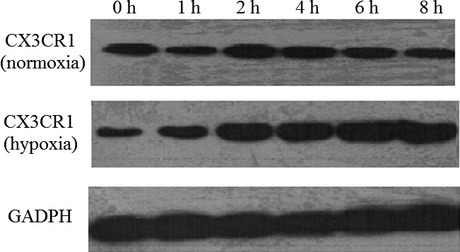
Hypoxia stimulates CX3CR1 expression in N9 cells. N9 cells were subjected to hypoxia for 0–8 h, and CX3CR1 levels were determined using Western blot analysis. Data are averages from three independent triplicate experiments. Compared with control groups, *P < 0.05.
N9 cell growth under hypoxia
We first detected N9 cell growth after 0–8 h hypoxia. Cell proliferation was measured by a colorimetric assay using MTT. As shown in Figure 2, N9 cell growth increased at 4 h. However, CX3CR1 shRNA could inhibit N9 cell growth under hypoxia.
Figure 2.
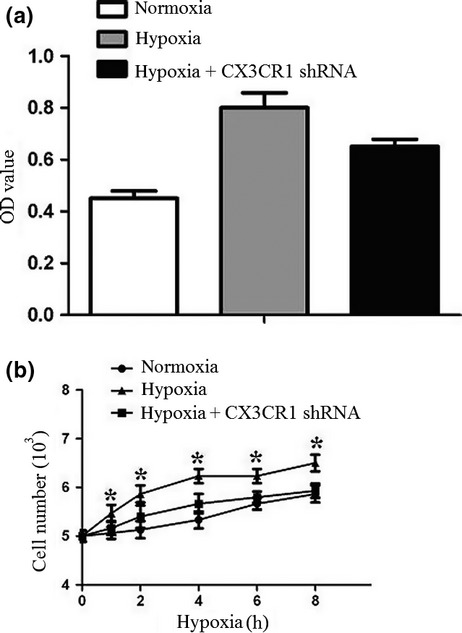
Cell proliferation under hypoxia. N9 cells or CX3CR1 shRNA-transduced N9 cells were subjected to hypoxia for 8 h. Cell proliferation was measured by MTT assay. The cell proliferation of the CX3CR1 RNAi group was notably inhibited. Data are averages from three independent triplicate experiments compared with control groups, *P < 0.05.
N9 cell apoptosis under hypoxia
To detect whether hypoxia could induce apoptosis of N9 cells, the cell apoptosis ratio was detected by flow cytometry. Apoptosis cells were determined by annexin V-positive and PI-negative cells. As shown in Figure 3, there was no difference in the N9 apoptosis rate between the three groups.
Figure 3.
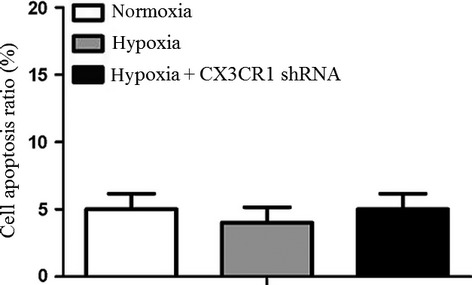
Analysis of cell apoptosis by flow cytometry assay. N9 cells or CX3CR1 shRNA-transduced N9 cells were subjected to hypoxia for 4 h. The apoptosis cells were determined by annexin V-positive and propidium iodide-negative cells. CX3CR1 shRNA could not promote N9 cell apoptosis under hypoxia. Data are averages from three independent triplicate experiments compared with controls, *P < 0.05.
Hypoxia-induced cytokine secretion of N9 cells
To detect cytokine secretion by N9 cells, we analysed the tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and matrix metalloproteinase 2 (MMP-2) expression of N9 cells under hypoxia. As shown in Figure 4, hypoxia induced a significant increase in TNF-α, IL-1β and MMP-2 expression. However, CX3CR1 shRNA could inhibit TNF-α, IL-1β and MMP-2 expression of N9 cells under hypoxia.
Figure 4.
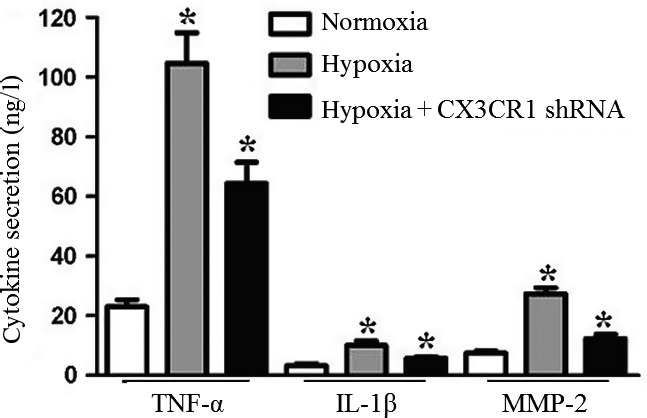
Detection of cytokine secretion of N9 cells under hypoxia. N9 cells or CX3CR1 shRNA-transduced N9 cells were subjected to hypoxia for 4 h. Supernatants were removed and further analysed for cytokine production (tumuor necrosis factor-α, IL-1β and MMP-2) with enzyme-linked immunosorbent assay. Experiments performed in triplicate showed consistent results. Compared with controls, *P < 0.05.
Hypoxia-induced CX3CR1 expression through p38MAPK/PKC pathway
Previous evidence demonstrated that neuronal p38MAPK/PKC signalling is an emerging regulator of cell fate and function in the nervous system. In this study, we also evaluated p38MAPK/PKC activation 4 h after hypoxia. As shown in Figure 5, hypoxia induced a significant increase of p38MAPK/PKC total protein. However, CX3CR1 shRNA could inhibit p38MAPK/PKC total protein expression. The results demonstrated that hypoxia induced CX3CR1 expression was mediated through the p38MAPK/PKC pathway.
Figure 5.
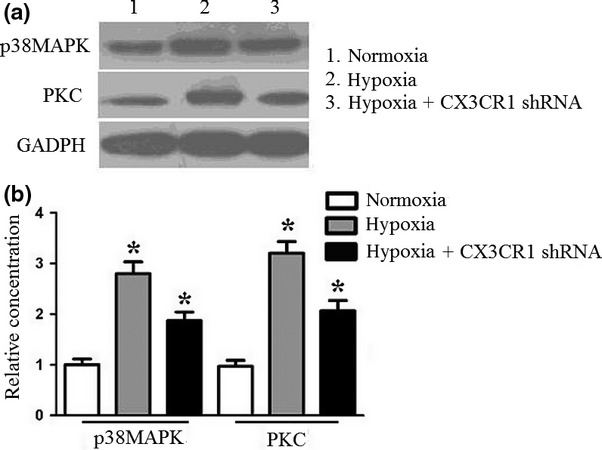
Western blot analysis of p38MAPK/PKC pathway under hypoxia. N9 cells or CX3CR1 shRNA-transduced N9 cells were subjected to hypoxia for 4 h. Cells were treated with lysis buffer to further analyse the p38MAPK/PKC expression using Western blot assay. The protein expression was calculated using densitometry data. Experiments performed in triplicate showed consistent results. Compared with controls, *P < 0.05.
Discussion
In the present study, we demonstrated for the first time that CX3CR1 RNAi could attenuate hypoxic-induced microglial proliferation, cytokine secretion including factor-α (TNF-α), and interleukin-1β (IL-1β) and the protease matrix metalloproteinase-2 (MMP-2) through p38MAPK/PKC activation, which has not been reported in previously.
Chronic cerebral ischaemia is thought to induce white matter lesions (WMLs), which contribute to subcortical vascular dementia (Brown et al. 2009; Kim et al. 2009; Miki et al. 2009). Several reports have shown that participation of activated microglial cells is crucial in WML pathology (Boer et al. 2008; Deng et al. 2008; Wu et al. 2008). As is the case in several other neurodegenerative and neuroinflammatory diseases, these immunocompetent cells of the central nervous system have been found to be activated in WMLs, releasing cytokines and proteases, including MMP-2, and causing progression of the disease process (Acarin et al. 2000; Rezaie & Dean 2002; Simpson et al. 2007).
Chemokines form a new superfamily of small glycoproteins. The basic function of a chemokine is to induce the directional chemotaxis of cells with corresponding receptors. Fractalkine is one of the few chemokines that is expressed in the central nervous system. Studies have shown upregulated expression of fractalkine in selected central nervous system lesions, such as those caused by inflammation, ischaemia and hypoxia (Xu et al. 2007; Yang et al. 2007; Ryu et al. 2008). CX3CL1 chemokine whose specific-receptor CX3CR1 is expressed by microglia. Through interaction with its receptor, CX3CR1, it promotes the activation of microglial cells and their migration to the lesions, increases the number of mononuclear cells, natural killer cells and T lymphocytes in the blood, plays a critical role in the clearance of necrotic tissue and promotes neurofunction repair (Denes et al. 2008; Abbadie et al. 2009; Lyons et al. 2009). In this study, we explore the specific mechanism involved in fractalkine/CX3CR1-mediated microglial activation in an experimental model representative of transient hypoxia,
Firstly, we detected the effect of hypoxia on CX3CR1 expression of N9 cells. N9 cells were harvested after 0–8 h hypoxia, and CX3CR1 expression was measured by Western blot analysis. The results demonstrated that CX3CR1expression was increased as early as 1 h, with levels peaking at 4 h. The results suggested that hypoxia could promote CX3CR1 expression.
Secondly, we detected N9 cell growth after 0–8 h hypoxia. The results demonstrated that N9 cell growth increased as early as 1 h, with levels peaking at 4 h. However, CX3CR1 shRNA could inhibit N9 cell growth under hypoxia. Additionally, we also detected apoptosis of N9 cells. However, the results demonstrated that CX3CR1 shRNA could not promote N9 cell apoptosis under hypoxia. The results suggested that fractalkine/CX3CR1 could promote N9 cell growth but not inhibit cell apoptosis under hypoxia.
Thirdly, to detect the cytokine secretion of N9 cells, we analysed the TNF-α, IL-1β and MMP-2 expression of N9 cells under hypoxia. The results demonstrated that hypoxia induced a significant increase in TNF-α, IL-1β and MMP-2 expression. However, CX3CR1 shRNA could inhibit TNF-α, IL-1β and MMP-2 expression by N9 cells under hypoxia.
Lastly, to further explore the mechanism of hypoxia-induced CX3CR1 expression, we analysed the p38MAPK/PKC pathway. The results demonstrated that hypoxia induced a significant increase in p38MAPK/PKC total protein level. However, CX3CR1 shRNA could inhibit p38MAPK/PKC total protein expression. The results demonstrated that hypoxia induced CX3CR1 expression through the p38MAPK/PKC pathway.
Taken together, the results demonstrated that hypoxia induced microglial proliferation and cytokine secretion through p38MAPK/PKC activation. In addition, our results also revealed a novel mechanism of fractalkine/CX3CR1 involved in the activation of microglia, and thus CX3CR1 RNAi might represent a promising strategy for chronic cerebral ischaemia therapy.
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC No. 81070930, No. 81200902 and No. 81200908).
References
- Abbadie C, Bhangoo S, Koninck De Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res. Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acarin L, Gonzalez B, Castellano B. Neuronal, astroglial and microglial cytokine expression after an excitotoxic lesion in the immature rat brain. Eur. J. Neurosci. 2000;12:3505–3520. doi: 10.1046/j.1460-9568.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- Basu A, Lazovic J, Krady JK, et al. Interleukin-1 and the interleukin-1 type 1 receptor are essential for the progressive neurodegeneration that ensues subsequent to a mild hypoxic/ischemic injury. J. Cereb. Blood Flow Metab. 2005;25:17–29. doi: 10.1038/sj.jcbfm.9600002. [DOI] [PubMed] [Google Scholar]
- Boer K, Troost D, Jansen F, et al. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology. 2008;28:577–590. doi: 10.1111/j.1440-1789.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- Bourd-Boittin K, Basset L, Bonnier D, L'Helgoualc'h A, Samson M, Theret N. CX3CL1/fractalkine shedding by human hepatic stellate cells: contribution to chronic inflammation in the liver. J. Cell Mol. Med. 2009;13:1526–1535. doi: 10.1111/j.1582-4934.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. J. Neurol. Sci. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J. Cereb. Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Deng Y, Lu J, Sivakumar V, Ling EA, Kaur C. Amoeboid microglia in the periventricular white matter induce oligodendrocyte damage through expression of proinflammatory cytokines via MAP kinase signaling pathway in hypoxic neonatal rats. Brain Pathol. 2008;18:387–400. doi: 10.1111/j.1750-3639.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevang B, Yndestad A, Damas JK, et al. Chemokines and common variable immunodeficiency; possible contribution of the fractalkine system (CX3CL1/CX3CR1) to chronic inflammation. Clin. Immunol. 2009;130:151–161. doi: 10.1016/j.clim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd'heuil D, Mongin AA. Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J. Neurochem. 2008;106:2449–2462. doi: 10.1111/j.1471-4159.2008.05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinisch S, Kirby LG. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience. 2009;164:1210–1223. doi: 10.1016/j.neuroscience.2009.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashita E, Tsuji D, Kawashima N, Nakayama K, Matsuno H, Itoh K. Abnormal production of macrophage inflammatory protein-1alpha by microglial cell lines derived from neonatal brains of Sandhoff disease model mice. J. Neurochem. 2009;109:1215–1224. doi: 10.1111/j.1471-4159.2009.06041.x. [DOI] [PubMed] [Google Scholar]
- Kim SK, Cho KO, Kim SY. The plasticity of posterior communicating artery influences on the outcome of white matter injury induced by chronic cerebral hypoperfusion in rats. Neurol. Res. 2009;31:245–250. doi: 10.1179/174313209X382278. [DOI] [PubMed] [Google Scholar]
- Latchney LR, Fallon MA, Culp DJ, Gelbard HA, Dewhurst S. Immunohistochemical assessment of fractalkine, inflammatory cells, and human herpesvirus 7 in human salivary glands. J. Histochem. Cytochem. 2004;52:671–681. doi: 10.1177/002215540405200511. [DOI] [PubMed] [Google Scholar]
- Lin HW, Levison SW. Context-dependent IL-6 potentiation of interferon-gamma-induced IL-12 secretion and CD40 expression in murine microglia. J. Neurochem. 2009;111:808–818. doi: 10.1111/j.1471-4159.2009.06366.x. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, et al. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J. Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- Miki K, Ishibashi S, Sun L, et al. Intensity of chronic cerebral hypoperfusion determines white/gray matter injury and cognitive/motor dysfunction in mice. J. Neurosci. Res. 2009;87:1270–1281. doi: 10.1002/jnr.21925. [DOI] [PubMed] [Google Scholar]
- Pachot A, Cazalis MA, Venet F, et al. Decreased expression of the fractalkine receptor CX3CR1 on circulating monocytes as new feature of sepsis-induced immunosuppression. J. Immunol. 2008;180:6421–6429. doi: 10.4049/jimmunol.180.9.6421. [DOI] [PubMed] [Google Scholar]
- Rasley A, Tranguch SL, Rati DM, Marriott I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J. Mol. Neurosci. 2009;39:99–103. doi: 10.1007/s12031-009-9186-7. [DOI] [PubMed] [Google Scholar]
- Ryu J, Lee CW, Hong KH, et al. Activation of fractalkine/CX3CR1 by vascular endothelial cells induces angiogenesis through VEGF-A/KDR and reverses hindlimb ischaemia. Cardiovasc. Res. 2008;78:333–340. doi: 10.1093/cvr/cvm067. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Ince PG, Higham CE, et al. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol. Appl. Neurobiol. 2007;33:670–683. doi: 10.1111/j.1365-2990.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- Szretter KJ, Samuel MA, Gilfillan S, Fuchs A, Colonna M, Diamond MS. The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis. J. Virol. 2009;83:9329–9338. doi: 10.1128/JVI.00836-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J. Neurochem. 2008;106:696–705. doi: 10.1111/j.1471-4159.2008.05421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Litvak V, Lev S. Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells. J. Neurosci. 2000;20:6478–6487. doi: 10.1523/JNEUROSCI.20-17-06478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ohlsson M, Warner EA, et al. Glial reactions and degeneration of myelinated processes in spinal cord gray matter in chronic experimental autoimmune encephalomyelitis. Neuroscience. 2008;156:586–596. doi: 10.1016/j.neuroscience.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang S, Jiang X, et al. Hypoxia-induced astrocytes promote the migration of neural progenitor cells via vascular endothelial factor, stem cell factor, stromal-derived factor-1alpha and monocyte chemoattractant protein-1 upregulation in vitro. Clin. Exp. Pharmacol. Physiol. 2007;34:624–631. doi: 10.1111/j.1440-1681.2007.04619.x. [DOI] [PubMed] [Google Scholar]
- Yang XP, Mattagajasingh S, Su S, et al. Fractalkine upregulates intercellular adhesion molecule-1 in endothelial cells through CX3CR1 and the Jak Stat5 pathway. Circ. Res. 2007;101:1001–1008. doi: 10.1161/CIRCRESAHA.107.160812. [DOI] [PubMed] [Google Scholar]


