Abstract
Sexual motivation is a fundamental behavior in human. For a long time, this behavior has been somehow ignored from psychological and neuroscientific research. In this article – reflecting the collaboration of a clinical psychologist and a neuroscientist – we show that in the current period, sexual affiliation is one of the most promising affiliation context to articulate a debate, a dialog and convergence points between psychoanalysis and neuroscience. Recent data on healthy sexual behavior and its compulsive variant are discussed under the prism of neuroscience and psychoanalysis.
Keywords: brain, fMRI, sexual affiliation
The modern western society in which we live is slowly but surely conditioning women and men – children or adults – to a world where whatever we desire must be available for immediate acquisition. With a profusion of an end to waiting and frustration ‘unlimited’ consumer products are on offer. In this context, one that would rather ignore the feelings of frustration associated with abstinence, sexual behavior has also known an evolution in which the laws of supply and demand have come to reign, along with the rules of free competition, giving to ‘sex’ objects the same status as any other product. In just a few decades, access to pornography has not only been developed but also became banal. We are far from the censure of the early 20th century when kissing scenes were simply cut from cinematographic reels. Consumer studies show that on Google, the world’s number one search motor, the terms ‘sex’, ‘love’, ‘porn’ arrive at the fore of all requests by both type and nature. Sexuality has become recreational, and even imperative. In effect, it was as if the slogan of the new societal Super-ego had become: ‘Unfettered and unlimited pleasure is a must!’
In these dynamics, in which the power of images is constantly growing, where screens (television, internet, telephone, etc.) have replaced books and notebooks, the challenge of actually having to elaborate a thought process is in no way facilitated. One can question the ever-more important role of pornography as a new educational tool of sexuality, its effects on the psychic apparatus in its capacity to imagine or fantasize the sexual. The danger being that external images come little by little to replace fantasies (produced from within). The subject, thus, passes from an active role (implied in fantasmatic production or in the imagination) to passivity, in the act of consuming images (magazines, films). Progressively, sexual excitement is increasingly distanced from the loving feelings associated with a relationship. The fact of loving implies both an encounter and sharing, loving someone else has the particular power of being able to tear a person away from a regressive position of narcissic omnipotence in which he believes himself to be self-sufficient, capable of everything, all alone. If ‘I can do everything all on my own’, I don’t need anyone else. Yet, when I am attracted by another, I recognize that I am lacking, and in a certain passive manner, in expectation of and dependent on another. Loving, thus, implies an acceptation – by the other – of something of one’s own deficiencies. And that other, come to that, can never fully satisfy these deficiencies … And yet, in the affective splitting of the sex-addict, not being completely satisfied is equal to not being satisfied at all. Here, there are no half-measure. As soon as there is any frustration, one must, therefore, do away with this ‘other’, to be replaced by a new one, and so on … If desire, which can be fantasized without necessarily being accomplished, is also born from the frustration associated with absence and a sense of missing, it would seem that this regresses to the quality of a need in addictive sexuality.
The concept of addictive sexuality
In the history of psychiatry, the terms of ‘hypersexuality’ (Jim Orford, 1985), of ‘satyriasis’, of ‘donjuanism’ in men, and of ‘nymphomania’ in women have been used. From the end of the 19th century, Ernst Kretschmer isolated the category of ‘frenetic masturbaters’. Recently, the terms of ‘compulsive sexuality’, ‘sexual intoxication’ (Claude Crépault, 1993), ‘atypical impulsion disorder’ (Barth & Kinder, 1987), ‘neo-sexualities’, and ‘addictive sexuality’ (Mac Dougall, 1978) have appeared. The dependency here is not a dependence on a toxic object but rather a behavioral dependency in which sexual activity, or the seeking of it, is experienced in an obsessive and compulsive fashion. As early as 1978, the British psychoanalyst Joyce Mac Dougall introduced the term of ‘addictive sexuality’ in her book ‘Plaidoyer pour une certaine anormalité’. She was designating subjects obsessed by sex, ‘slaves of quantity’. It was an American psychologist (Carnes, 1983, 1989a, 1989b, 1991, 2001) who specifically developed the concept of sexual dependency in his best-seller ‘Out of the Shadows’. On the medical level and in the scientific literature, the notion of sexual dependency was developed by Goodman (1990, 1993), a psychiatrist of psychoanalytic orientation.
In the DSM-IV (DSM-IV, 1996) the classification manual of psychiatric disorders, addictive sexuality does not appear as such but is listed rather in the vague category of ‘unspecified sexual disorders’. The given definition also remains somewhat vague: ‘The disarray resulting from a mode of repetitive sexual relations implying a succession of sexual partners that the individual only perceives as objects to be used.’
In order to better detect subjects suffering from addictive sexuality, Patrick Carnes and Robert Weiss put together a questionnaire – The Sexual Addiction Screening Test – in two versions (one for the gay and bisexual population, another for the heterosexual population). If responses to the 25 questions result in 13 positive answers or more, the subject has a problem with addictive sexuality. The 1980s, marked by the appearance of the AIDS virus, were propitious to new research on addictive and compulsive sexual behavior (CSB) within which habitual promiscuity with multiple partners appears as an important risk factor in the transmission of the HIV virus. In 1987, the National Council on Sex Addiction was created to inform the public and warn about the risks related to addictive sexuality.
In 1988, the North American psychiatrists Reed and Blaine (1978) proposed a nosological description of addictive sexuality and described a process in four phases in which both compulsive and impulsive aspects can be found:
a phase of obsession, during which in response to existential difficulties the subject is totally absorbed by sexual preoccupations;
a phase of ritualization, that is to say the execution of rituals that precede sexual behavior;
a phase of sexual activity, which leads to a temporary and provisory relief; and
a phase of despair with a sentiment of the incapacity to control one’s own behavior.
Like other forms of dependency, sexual addiction builds up through a process in which several stages can be distinguished: experimentation, occasional (festive) use, regular use, but which do not yet constitute sufficient reason to seek help. It is when this use becomes a response to existential difficulties that an addictive problematic, that is to say a dependency, and which has become a source of suffering, begins to settle in. Of these practices, the subject may say ‘It’s stronger than me/I can’t help myself.’ From a symptomatic viewpoint, one can observe a polymorphy of manifestations. The starting point is a devouring sexual need that can have various manifestations: compulsive masturbation, addiction to pornography, cybersex on the Internet, promiscuity, increase in the number of sexual adventures, addiction to chat rooms, an excessive frequenting of sex-clubs, or pick-up venues (toilets, parks, motorway lay-bys) that become the only ‘pass-time’, recourse to prostitution, picking-up, and so on. This dependency on objects or situations presenting a sexual connotation (images, places, clubs, online pick-up sites, films, etc.) invades the subject’s everyday schedule. Coleman (1992) proposed an evaluation grid to detect sexual addiction. The subjects who present at least two of the following characteristics can recognize themselves as being sex-addicts:
compulsive and unsatisfying pick-ups implying a constant and insatiable search for multiple partners;
an unsatisfying compulsive sexuality in which the other is reduced to the state of partial object;
masturbation that is compulsive (or frenetic, with a frequency of 5–15 times a day, sometimes leading to injury, irritation of the skin, an intense feeling of fatigue, or even social and/or professional difficulties);
compulsive fixation on one or several inaccessible partner(s), an idealization of the love object; and
multiple compulsive love relationships (constantly seeking intensity of feelings through new adventures).
This sexuality disorder seems to be stronger in men, in that 80% of subjects treated for sexual addictions are men. But these statistics remain empirical in the measure that there have been no epidemiological studies on the question. This neo-pathology apparently concerns roughly 3%–6% of the overall population of the United States, where it is now possible, for men as well as women, to frequent the sex addicts anonymous (SAA), an antidote to the distress of solitary sex-maniacs. In France, research in clinical psychopathology took some time to take an interest in the phenomenon. In the field of psychoanalysis, it was the works of Joyce Mac Dougall that as early as 1978 took an interest in ‘the psychic economy of addiction’. Since then, work in the field of addictology has progressively developed research, reflections, and observations on these new behaviors of dependency. Addictive sexuality is considered as an ‘addiction without drugs’, and the term of ‘compulsive sexuality’ is often employed to designate it in a larger sense. Important research poles of clinical psychiatry for the different ‘addictive practices’ today also accommodate ‘sex addict.’
Elements of psychopathology
Phenomenological psychopathology of addictive sexuality
From the phase of searching up until the actual sexual activity, the subjects concerned may feel some benefits: reassurance, well-being, an almost manic excitement, a narcissistic shoring-up, and sensations of pleasure. But as soon as the sexual act has taken place, certain subjects can be gripped by sadness, remorse, as well as by feelings of shame, and guilt. One fine clinical psychopathology study for that matter shows that – unlike in the schema proposed by Carnes – these reactions vary largely depending on whether the subject functions according to a neurotic, borderline, psychotic, or perverse structure. Reactions of shame, disgust, and guilt being much less frequent in the borderline, personality organization often present in these subjects. One may rather note reactions of intense depression (of a narcissistic type) when the object or situation targeted by the idealization has not been found or satisfied. The subject then feels empty and will have a tendency to identify himself with rubbish or waste matter. The negative effects are then experienced: a feeling of going round in circles, risk taking, low self-esteem, depressive elements, and associated pathologies (alcoholism, poly toxicomania, etc.). The inability to be happy in a couple relationship, repetition compulsion, giving the impression of a loss of control, and the centering of an existence that only focuses on a single preoccupation; these elements – associated with a painful sense of failure – are often at the origin of a request for treatment. It is often the sense of marginalization and loss of meaning that forces recognition of the dependency.
Time immobilized by the repetition compulsion
From the point of view of temporality, the sex-addict suffers from a repetition compulsion, often giving him an impression of a stagnant temporality that is not open toward the future. Kernberg (2008) described the characteristics of a specific type of subjective temporality within the narcissistic pathological personality organization. He distinguished the subjective temporality of neurotic subjects (tending toward the investment of a complete object) and that of subjects reduced to a narcissistic libido. It is as if the latter made great efforts to shrink or destroy time to reassure themselves with an illusion of permanence and eternity. In this configuration, it is difficult to integrate new experiences; the repetition compulsion shows that time does not pass and that it is frozen. A number of narcissistic patients ‘wake up’ at the age of 40, 50, or 60 with a desperate feeling of years lost: life has gone by without leaving a trace. The experience of shrinkage of time in these cases can lead to an intense and growing fear of death, a sense of injustice at the briefness of life as they experience it. This fear is also related to the infantile fears of abandonment and solitude. A deep sense of the pointlessness of life predominates when there is an absence of investment in love, work, ideals, children, or values. In a certain way, the non-establishment of a relationship of trust in others does not allow the development of a trusting relationship to time.
How to live without belief?
To believe, one must be able to place one’s trust not only in others but also in oneself. The etymology of the word ‘confidant’, cum-fides (with faith), says precisely this. Sex-addicts experience great difficulties in believing that they will find a response to their desires in others (Estellon, 2002, 2005a, 2005b, 2009, 2011, 2012). Believing requires recognition of not only the power of invisible phenomena but also hope. Some of these beliefs are vital: belief in the natural functioning of one’s own body; in the continuity of the self; in the reality of the outside world; in the consciousness of others. Without that, it will be difficult to fully inhabit one’s own life. And as Freud reminds us, to love other people, one must first be able to love oneself. Subject to narcissistic fragilities and affective deficiencies that he in general would rather ignore, the sex-addict uses other people’s bodies for a fix and to forget that he no longer believes in anything much, including himself. Unlike the collector who watches over his objects by keeping them close to hand, the sex-addict ‘zaps’ from one anonymous body to another, without holding onto anything. In these practices, the sexual partner cannot exist in his identitary or historical dimension: he is an anonymous body who constitutes a palliative for the body in urgent demand of erogenous stimulation.
Relationship zapping and the anonymity of encounters
The verb ‘to zap’ makes reference to several dimensions: that of forgetting of an impatience with waiting, a feeling of boredom preceding a breaking away. The temporality of zapping is interesting in that it proceeds from a current present that is open onto an immediate future. In this posture, it is a matter of contenting oneself with fragments as they appear without worrying about the whole, of switching to something else. The notions of duration, accomplishment, of the associative link, are quite simply wiped out. Zapping functions partly through the vision. It fills the mind with images and sensations without leaving any time for actual elaboration. This temporality is characterized by an impatient present tense that wants nothing to do with waiting. Defensive strategies often lead the sex-addict to experience any durable construction – implying stability – as being mediocre or as yet another constraint. Hence, the need for constant change in order to feel free … up until the day when the subject can no longer bear his addiction to change. Engaging in a therapeutic undertaking can be the occasion for the subject to maintain a relationship in a stable rhythm as a couple, with certain overall constants. Within this complex undertaking, the aspect of the framework’s stability associated with a certain flexibility is determinant.
The space of the backrooms
If addictive sexuality is a disorder that is easier to live with in big cities than in the countryside, this is in part due to the strong urban implantation of various venues dedicated to sexual encounters: clubs, bars, baths and saunas, cruising areas, prostitution zones, sex shops, and so on. The backroom – literally ‘the room at the back’ is an isolated place in a private establishment (bar, discothèque, sex-club, sauna) that is organized in such a way as to permit the interested clients to isolate themselves to have sexual relationships in the half-dark (Mac Dougall, 1978, 1991, 1993, 1996; Estellon 2005a, 2005b, 2012). After having spotted objects of interest in the soft zones (at the bar or on the dance floor), one can head toward the hard zone to get comfortable and share sex … If they have today spread to the libertine world, backrooms were born in the American gay milieu in the 1970s. Strangely, they developed just as homosexuality started to come out of the shadows, as if to pay a funereal homage to the dark, hidden, clandestine, and shameful places long frequented – for lack of choice – by homosexuals repressed by the police. Somber, damp, and strong smelling, they replaced the former urinals where sexual activity took place inter faeces et urinas. Often fitted out with minor conveniences (slings, glory holes, cages reminiscent of the prison world, labyrinths …), they can be play areas that are appreciated by sex-addicts. In these spaces, there is no social identity, no faces, but body parts revealed by hands touching, caressing, feeling, entering. If the AIDS years led to the closing down of most of these places in the United States, it is in the major European cities that they flourish today.
Personality traits, defense mechanisms
According to different authors (Carnes, Earle, Estellon, Goodman, and Poudat) one finds certain common personality traits in sex-addicts such as:
affective insecurity;
intolerance of frustration;
a tendency to action and to acting out;
a ‘belief’ in an operative and mechanical sexual model;
the presence of a second state during the crisis;
the compulsive system;
emotional overflow;
difficulty with conjugal stability;
rational anxiety;
affective and social isolation; and
masked depression.
Certain characteristics – the invasive presence of obsessions and the prevalence of compulsive systems – have led some authors to make a link between these clinical elements and obsessive compulsive disorders. Except that to the opposite of the sex-addict, the obsessional does not take action: he fantasizes more than he takes act. The strategies of taking act and of externalization of conflict are rather reminiscent of a ‘borderline’ psychic functioning in which an escape through behavior little elaborated by a thought process is often privileged: alienated from the outside world, the ego of these subjects is easily exposed to an overflowing of emotions, breaking down, and states of distress.
The psychoanalytic clinical data of sex-addict subjects (Estellon, 2005; Mac Dougall, 1978) gives a glimpse of a characteristic psychic functioning – quite close to a borderline functioning with recourse to certain common defensive strategies.
The love/sex split
Splitting is the essential defensive operation used by sex-addicts. Its main aim is to avoid the confrontation of the subject, in the face of his affective ambivalence, with depressive suffering. Here, splitting radically separates affectionate impulses from sexual impulses. Sexual partners will be little invested affectively, whereas other people in the entourage (friends, family, inaccessible partner) are highly invested.
Denial
Complementary to splitting and shored up by it, denial permits the exclusion of the field of consciousness and facilitates the isolation of those representations or affects that are not in keeping with the ideal self-image. Everything that might fragilize the psychic life by its contradictory or ambiguous character is evacuated. A loss, a grieving process, a separation, for example, might in such subjects provoke reactions in which there is no apparent suffering. Denial shoring up the splitting, the sex-addict can, with complete indifference, leave any partner who has been imprudent enough to become tenderly attached to them. In the same way, when the sex-addict feels strangely affectionate toward a partner, reactions of distress or violence can appear.
Idealization, omnipotence, and devalorization
The mechanism of idealization functions in a way that is complementary to splitting. A new person can be strongly idealized: presenting no failings, doted with all possible qualities, they are apprehended as being ‘perfect’. Splitting allows, when the deceptions or frustrations have spoiled the perfection of this object, to denigrate them, to disdain them, and deinvest them as easily as the touch of a finger is able with a remote control to zap a boring TV program instantaneously. I am thinking of a certain patient who regularly changed sexual partners: as soon as they became frustrating, they were ‘rubbed out’ according to her expression. This is the phenomenon of devalorization. A corollary of omnipotence, it allows one, without suffering, to get rid of an object when it does not bring the expected or desired satisfaction. Splitting thus ensures that a part of the Ego remains idealized (the grandiose self) in such a way that the feelings of suffering, frustration, deception, desire, or hatred, when they are experienced, can always be imputed to the action of an ill behaving other. These charicatural reactions permit the Ego – whose frontiers are unsure – not to collapse. This defense mechanism constitutes a veritable motor for the zapping mode of functioning described above.
Anaclitic anxieties and their avoidance
From a relationship point of view, addictive sexuality can be understood as a phobic strategy that allows one to avoid any real encounter with others. One often finds at the basis of these compulsive practices two types of relationship anxiety: an anxiety of intrusion coupled with an anxiety of abandonment. Haunted by these anxieties, the problematic of attachment becomes unbearable. In such a configuration, the certainty of a breakup is preferable to the torments of its uncertainty. And, so as ‘not to lose one’s mind’ one ‘zaps’ from one body to another. The fact of systematically leaving the other, anonymous person allows one not to suffer the anxiety of being invaded and even less the worries of abandonment. Thus, one avoids the depressive suffering linked to deceptions caused by the other in whom one might have believed and to whom one might have become attached.
Seen in this optic, one better understands how the other person comes to be ‘consumed’, instrumentalized as a pleasure object. Curiously, what takes place is a reversal of the latent desires and the pragmatic and compulsive behavior: if what is subconsciously sought is love, what is requested in reality is only a matter of sex. This sexual frenzy often covers up a strong and denied affective deficiency.
Comorbid disorders and risk taking
Other dependencies
During sexual activity, drugs may be used by the more dependent subjects: poppers, GHB, or Gamma Butyrolactone (GBL), cocaine, crystal, hashish, and so on. Currently, the most common are poppers, cocaine, and GHB, we will here describe poppers and GHB as they are more specifically used during sexual activity.
Poppers are vasodilators originally used in medicine to cure certain cardiac illnesses, containing butyl and pentyl nitrates; sniffed in a non-medical use, their effects are almost immediate: a brief, vertiginous, and stimulating high, a heightening of the internal pressure of the eye. The user feels a strong sensation of inner heat and sensuality is exacerbated. The effect of the sniff lasts about 2 min.
GHB is a very powerful anesthetic. In a liquid form, it can be drunk pure or mixed with non-alcoholic drinks. Sometimes called the ‘rapist’s drug’, GHB provokes overall relaxation, euphoria, and heightens sexual excitement. The effects depend largely on the dosage but vary from euphoria, relaxation, somnolence, deep (comatose) sleep, and unconsciousness to the loss of inhibitions, an intensification of perception, a need to talk, or slight vertigo. For lack of means, GBL (Gamma Butyrolactone, the precursor of GHB, used in industry as paint dissolver, epoxy, or nail varnish) may be consumed. In the organism, GBL turns into GHB; more dangerous; being more difficult to dose, it can provoke potentially fatal comas.
Risk taking (barebacking, HIV, STD, etc.)
Latin etymology reminds us that risk (resecare, literally ‘to cut’) is foremost a cutting-off point. Insolent and seductive, risk taking allows one to leave behind the securized world in which one is immersed. One of the dangers linked to the chronicization of addictive sexuality comes from the risk of no longer being able to find satisfaction with the usual sensations and searching more ‘pure’ or ‘stronger’ ones: one can, for example, do away with condoms that get in the way of pure sensation, consume various drugs which, if heightening the sensations experienced, no longer allow one to evaluate certain vital risks. Barebacking (unprotected sex despite the risk of transmission of the HIV virus) is more and more frequent among sex-addicts in the gay world. On top of the risks of HIV, this practice increases the risks of contamination of various other sexually transmitted diseases that can prove dangerous if they are not detected in time (Syphilis, Hepatitis B). When he becomes interested in searching for stronger sensations, the sex-addict can direct his sexuality toward more hard-core practices (fist-fucking, bondage, SM, urophilia, scatophilia, etc.). It is in these aspects that addictive sexuality can become a source of suffering, of isolation, and marginalization. The results of the studies by Peretti-Watel et al. (2006) show that risk taking is that much higher in subjects who are depressed or vulnerable.
Masked depression
Depression often constitutes the backdrop on which sexually addictive behavior is constituted and chronicized. Whether in works on psychoanalytic psychopathology (Estellon, 2002, 2009; Mac Dougall, 1978; Pirlot, 2009) or on behavior (Bancroft & Vukadinovic, 2004), current research makes the link between the recourse to sexual addictions and elements of narcissistic depression such as the loss of self-esteem. The loss of belief in the positive effects of a relationship, the loss of hope in others, progressively makes one lose sight of the meaning of a life that has – despite the erogenous sensations that exhaust themselves in compulsive repetition – become sad and empty.
Research in neurobiology
In this chapter, it is interesting to summarize some recent studies issued from cognitive neuroscience that investigated human male sexual behavior in general and, for some of them, its compulsive practice. Although using very different theoretical and experimental settings, sexual affiliation is for us one of the most promising context to articulate a dialog between neuroscience and psychoanalysis. Today, what do we know on the neural circuits involved in human male sexual arousal? Is that possible to identify variations within these neural processes when sexual practice is becoming compulsive ? Here are the two main questions of this section.
Recent advances from neuroimaging studies
From now several decades, the growing development of neuroimaging techniques shed light on brain processes with a new angle. It is now possible to record brain activity in a wide variety of perceptual, cognitive, or motor paradigms. In addition to a considerable increased knowledge of the brain circuits involved in general cognitive processes, two fields meet an exponential development: the so-called affective neuroscience (Pankseep, 2003), interested in the identification of brain networks involved in emotional and motivational informations processing and the so-called social neuroscience (Insel and Fernald, 2004) crystallized around the cerebral correlates of the processing of social information. These two approaches converge on questions such as the investigation of brain processing of emotional informations emitted by conspecifics. In this regard, recent studies have helped to highlight the connections between cognitive and neural systems involved in the production of the action and the perception of the action by a third party. As a good example, famous studies identified a specific group of neurons within the premotor cortex that were increasing their firing rate as well as during the production of an action as during the perception of the same action produced by a conspecific (Rizzolatti & Arbib, 1998).
Two emerging fields within cognitive neuroscience: affective and social neurosciences
The above-developed perspective is now extending to the topic of emotions. At the moment, empathy is one of the most studied affiliation contexts, in particular through the perception–action model coupling mechanisms it assumes (Preston, de Waal, & Frans, 2002). To feel an emotion when observing the same one in another person involves a physiological synchrony between the observer and the observed one (Levenson & Ruef, 1992). For example, to be exposed to a disgusting flavor activates the same neural structures than to observe a disgust facial expression in another person (see the example of the insula in Phillips et al., 1998). Insular lesions prevent both the experience of disgust and the recognition of social cues conveying disgust (Calder et al., 2000). Comparably, the neural circuits of nociception are activated both by real pain and the representation of the affective state of someone in pain (Morrison et al., 2004; Singer et al., 2004). These results suggest that neural structures involved in emotional information processing also participate in the intersubjectivity of interacting people. However, the recovery between activated structures is not total. This suggests a certain amount of dissociation between regions involved in self-perception and those involved in other perception. This should avoid a confusion between a ‘self’ and an ‘other’ representation and therefore, the expression of an excessive empathy, potentially maladaptive (Batson et al., 1997). Although a functional context such as empathy encompasses an intrinsic social component, relationships between individuals first obey to an intrinsic interattraction motivational component (that can be either positive or negative). However, this interattraction component can be studied within different functional contexts. Among these contexts, sexual behavior and its associated neural circuits has been more and more studied during last years, shedding light on interesting new results for socioaffective neuroscience. Until the end of the 90s, the investigation of mental and neural representations associated to sexual behavior was poorly studied but is now exponentially emerging. However, it has to be noted that this is mostly investigated in human males.
Sexual affiliation: a functional context between affective and social neurosciences
At the moment, an exponential number of scientific studies investigates the neural correlates of human male sexual arousal. For the purpose of this paper, we won’t establish an exhaustive list of activated areas within these different studies. Here, we would like to underline some recent results issued from socioaffective neuroscience studies that used sexual affiliation to study motivated social interactions and provided more general results.
Here, we will first focus on results obtained in healthy human males and secondly on very preliminary data from patients that could be involved in CSB occurrence.
Contemporary data on healthy human male sexual function
To understand the present assumptions of neuroscience on the neural networks that could be involved in sexual addiction, it is necessary to present some interesting recent results on the role of the brain on healthy human male sexual motivation. First, it has to be noted that functional magnetic resonance imaging (fMRI), that is, the possibility to use MR imaging to identify brain activations thanks to local blood flow variations recording, was discovered in 1993. However, the first study that used functional neuroimaging to investigate the neural correlates of human male sexual motivation used another technique (Positron Emission Tomography) and was published in 1999 (Stoléru et al., 1999).
In this article, we present the main results from studies with two objectives: (1) when they were published, a preliminary exploration of the neural circuits involved in sexual visual information processing and (2) to use the high specificity of this behavior as a working model to identify the neural circuits involved generally in social relations with a motivational component. Mainly, to explore the neural correlates of healthy human male sexual motivation, these studies used fMRI. Among other neuroimaging techniques, fMRI has a relatively good spatial resolution, but a quite poor temporal resolution (around 1 second instead of milliseconds regarding the classical temporal unit of the cell functions). For this last reason, fMRI is sometimes criticized regarding its ‘localisationist’ approach. Currently, an attempt that can be offered to try to overcome this pitfall is to try to unify the corresponding psychological and neural components in a neurobehavioral model.
For healthy human male, a neurobehavioral model has been proposed (Redoute et al., 2000; Stoléru et al., 1999) including: (1) a cognitive component, associated to the identification and categorization of stimuli; (2) an emotional component, related to the pleasure sensation that is linked to the pleasure associated to sexual arousal increase; (3) a motivational component, linked to the processes involved in directing behavior toward a sexually relevant target; (4) a physiological component, related to all physiological variations (i.e. genital, respiratory, and cardiovascular responses). Therefore, this is important to bring together a psychological process and a cerebral activation and to explain how these processes articulate with the other components of the model. Here, we sum up some data related to cognitive and physiological components of the neurobehavioral model.
With several decades of studies of the neural correlates of healthy human male sexual arousal, the feasibility of this scientific approach has been widely demonstrated. In other words, the presentation of decontextualized visual sexual stimuli within a scanner was valid. For example, in one of our first studies, participants reported significantly higher ratings in response to sexual visual stimuli than for neutral stimuli for the dimensions ‘beauty of the depicted character’, ‘perceived desire to engage a relation’, ‘perceived intensity of erectile response’, ‘intensity of pleasure’, and ‘interest for pictures’. Although the experimental setting and procedures were not ecological to study sexual arousal, behavioral results indicate a possible induction of sexual attitudes.
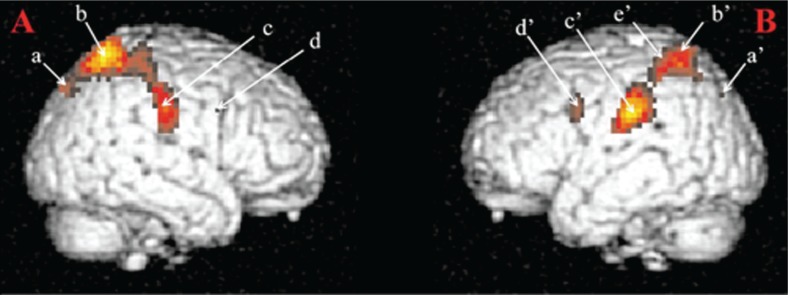
Regarding brain processes involved in visual sexual material processing, a good example is illustrated in Fig. 1. In accordance with the hypotheses of the study, the recorded brain activations were interpreted as involved in the cognitive processes linked to motivational information processing (Mouras et al., 2003). For example, activations were identified in the inferior parietal lobules (IPLs; Fig. 1, c). Interestingly, these areas were known to be activated in monkeys during visual fixation episodes occurring during environmental exploration (Lynch et al., 1977) and in human in cocaine users exposed to scenes where cocaine was used (Garavan et al., 2000). For this region, an early activation was thought, for example, as being involved in enhancing attentional mechanisms toward motivationally relevant targets. Through a more precise study of temporal activations for this part of the brain, we were able to demonstrate activations within the superior parietal lobules. In this study (one of the first fMRI studies), these activations were nonetheless sustained not only during all visual sexual stimuli presentation period but also in the early as for IPLs. These results were in accordance with a very short categorization time for sexual stimuli (Pizzagalli et al., 2002). Therefore, these activations were interpreted as the anatomical support of an early amplification of attentional processes directed toward visual sexual stimuli.
Fig. 1.
Brain areas activated in response to visual sexual stimuli as compared to neutral stimuli. Notes: (A) Right hemisphere: (a) parietooccipital sulcus; (b) superior parietal lobule; (c) postcentral gyrus; (d) precentral gyrus. (B) Left hemisphere: (a) superior occipital gyrus; (b) superior parietal lobule; (c) inferior parietal lobule; (d) precentral gyrus; (e) intraparietal sulcus. These activations were obtained for a statistical threshold of p < 0.05, corrected for multiple comparisons. Figure from Mouras et al. (2003).
Source: Adapted with permission from Mouras et al. (2008).
Through published studies, a recurrent question is to know if the identified psychological and cerebral processes are specific to the functional context of sexual behavior. Importantly, the male sexual function is characterized by a highly specific physiological response, that is, the genital response. For this purpose, a specific MR-compatible device able to record volumetric penile plethysmography concurrently to cerebral BOLD responses was manufactured. Our objective was to perform correlational analyses between the genital and cerebral response’s magnitude. This was applied in two recent studies (Mouras et al., 2003; Virginie Moulier et al., 2006). It allowed to precise the involvement of a specific neuronal system in the brain – the mirror neuron system – for visual sexual stimuli processing, which means visual stimuli with a high motivational relevance. Importantly, the mirror neuron system was first discovered in monkey as a specific category of motor neurons with an increasing firing rate either during the observation of an action or the mere realization of this action. Today, the question of the involvement of this system within socioaffective information cerebral processing is one of the major debates for contemporary cognitive neurosciences.
A recent study (Carr et al., 2003) demonstrated the involvement of the mirror neuron system in empathy, whereas these neurons are classically known to respond to the observation of movements performed by others. Consequently, it was important here to assess the degree of implication of these mirror neurons in the observation of sexual exchange. Therefore, our hypotheses were that (1) in response to the presentation of sexual visual stimuli, the magnitude of activation of the brain networks involved in movement observation and motor imagery would predict the intensity of genital response and (2) the level of the erectile response would predict the amplitude of the BOLD response in primary and secondary somatosensory areas corresponding to the projection of the penis. Three results are developed here (see Fig. 2 and 3) illustrating how their generalization was possible for neuroscience of social interactions.
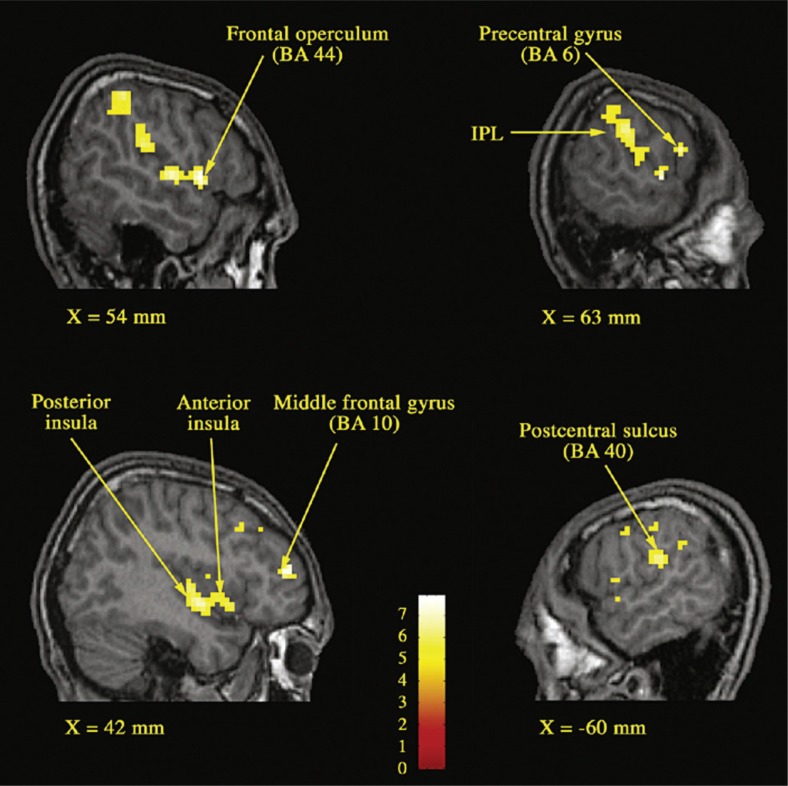
Fig. 2.
Parasagittal sections showing brain areas where the fMRI signal was correlated with the concurrent penile volumetric response.
Notes: X refers to the distance in millimeters from the sagittal plane. Height threshold: pb.05, corrected for multiple comparisons.
Abbreviations: BA, Brodmann area; IPL, inferior parietal lobule from Mouras et al. (2008).
Source: Adapted with permission from Mouras et al. (2008).
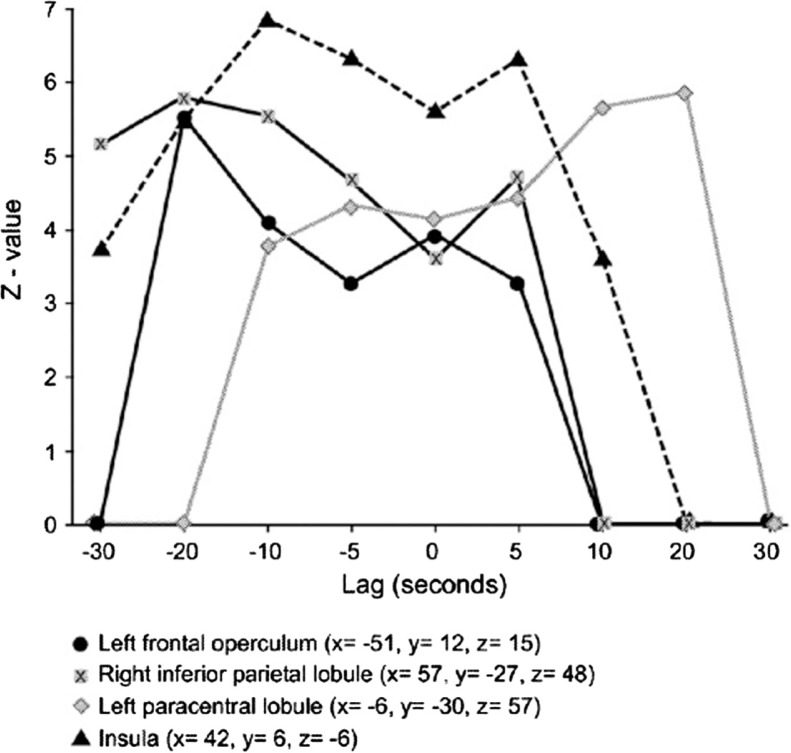
Fig. 3.
Correlation profiles between BOLD and plethysmographic signals as a function of the lag between the two signals.
Notes: The vertical axis represents the Z statistic associated with the correlation coefficient between the two signals, and the horizontal axis the lag, which is the time interval (seconds) by which the correlated values are separated. Positive lags are those where the plethysmographic response was correlated with subsequent values of the BOLD signal from Mouras et al. (2008).
Source: Adapted with permission from Mouras et al. (2008).
The first region in which the variations in BOLD signal were correlated to those of genital response was the left frontal operculum. This region is part of the mirror neuron system that encodes the correspondence between observed events and internally generated actions promoting a link between observer and the actor (Rizzolatti & Arbib, 1998). In the functional context of sexual affiliation, two interpretations are possible regarding the role of this neural system: preparing to perform an activity similar to that observed and/or support of an internal representation of movement (Decety et al., 1994). On the one hand, these neurons may play an important role in action’s preparation (in these experiments, participants often report episodes of motor imagery usually corresponding to imagined sexual acts). On the other hand, the behavioral response was also recorded by physiological (the erectile response) and some of the efferences of the frontal operculum to the insula could be the corresponding anatomical support. Therefore, these results place the physiological response at the center of the processes involved in social relations with a strong motivational component. In addition to mirror neurons areas, the correlational analyses identified several regions pertaining to the network of motor imagery and action’s observation (Decety et al., 2006; Grezes et al., 2006) including the inferior parietal lobules (Decety et al., 1994; Stephan et al., 1995) and the left supramarginal gyrus (Buccino et al., 2001; Decety, 2001; Grezes et al., 2001; Hanakawa et al., 2003; Stephan et al., 1995). These results are in accordance with those showing a possible anticipatory activation of this network, that is, before the observation of motor scenes (Kilner et al., 2004).
For somatosensory areas, important results have been obtained. For some areas (lower part of Brodmann 2), activation preceded the genital response itself, suggesting that these areas ‘flew’ the physiological response, thereby pointing to the concept of somatosensory imagery (Porro et al., 1996). Everything happens as if the observation of sexual explicit scenes induced activation only in motor areas corresponding to the hand, but also in areas of the somatosensory cortex corresponding to the representation of the hand. Such processes may play a role in recognizing emotions in others by the observer (Adolphs et al., 2000) or in preserving the sense of self during action’s observation (Avikainen et al., 2002; Ruby et al., 2001). Finally, for other regions, such as primary and secondary somatosensory areas, some of them seemed to ‘drive’ the physiological response, whereas others are ‘driven’ by it, suggesting the existence of positive feedback loops during genital response occurrence.
Exploratory data on CSB
The exploration of the neural networks involved in healthy human sexual behavior through modern neuroimaging techniques remains recent, centered on healthy human male sexual motivation. Even if the number of studies on this topic is growing exponentially, there are still a lot of scientific questions to solve. As shown by this article, the question of the neural circuits involved in addiction is an important topic. In the field of sexual addiction, different kinds of data are now available. Today, modern neuroimaging techniques such as MRI allow using different modalities to record during the same experimental session either data regarding the anatomical properties of the brain or its functional properties through neural activations recorded in any kind of behavior. However, from one study to another, the evaluation method to assess and evaluate compulsive sexual behavior have been different.
Recently, (Miner et al., 2009) compared 16 healthy human males, that is, eight control participants and eight patients with a sexual addiction. To recruit patients with a certain homogeneity, authors used as inclusion criterion the CSB syndrome. In 2000, Coleman et al. (2000) listed definition criteria for this syndrome: occurrence of recurrent and intense fantasies, imperious sexual needs inducing a personal distress during at least 6 months. As explained by authors, this syndrome shares criteria with other addictions such as bulimic behavior and pathological gambling.
Although this study was the first to present data on patients with a CSB, important hypotheses have been proposed through the report of single cases. The first one is the disruption of frontal brain areas that usually have an inhibitory effect on sexual behavior and that could induce an hypersexuality. Here, the recorded informations were only anatomical. With highly specific settings, diffusion tensor imaging (DTI) gives very precise information on cerebral white matter organization and integrity. Therefore using DTI, authors compared cerebral microarchitecture in a group of patients with CSB and a group of appealed control participants. In parallel, informations were recorded from both groups through standardized questionnaires dealing with (1) intensity of symptoms of the CSB; (2) intensity of different traits related to impulsivity; and (3) skills regarding emotional regulation. Moreover, control participants and patients were involved in a ‘Go-noGo’ behavioral task dedicated to evaluate the magnitude of impulsivity of participants by testing their inhibitory skills. In this task, they were asked to push a button when a given letter appeared on a screen, hereafter called the target letter. In a first version of the task, they were asked to click the left mouse button for each occurrence of a letter that was different from the target one (83% of the trials) and to inhibit this click in response to the target letter. This first version of the task was dedicated to evaluate the intensity of impulsivity. In a second version of the task, they were asked to left click only in response to the target letter’s occurrence (17% of the trials). This second version of the task was dedicated to measure inattention of participants by measuring omission errors when they omit to left click on the mouse for a target letter occurrence.
The results of this study were very interesting. Regarding impulsivity, patients with higher impulsivity scores as measured by standardized questionnaires were more sensitive to negative emotions. For the ‘Go-noGo’ behavioral task that indexed impulsivity, patients made more commission (t 14=3.09, P<0.008) and omission (t 14=2.69, P<0.018) errors during the condition where the target letter was presented frequently and, globally, made more errors than the control group for both conditions (commission errors: t 14=2.98, P<0.01; omission errors: t 14=2.76, P<0.014).
Regarding anatomical data, two different types of analyses have been performed. Globally, the patients demonstrated a ‘‘low diffusivity’’ (one of the indexes calculated by DTI and indexing the white matter integrity) that was lower in a particular zone of the frontal lobe, the superior frontal cortex. These results were changing a little bit when correlational analyses were performed between anatomical and cerebral data: a negative correlation was demonstrated between anatomical parameters and either impulsivity or negative emotion sensitivity for the inferior frontal cortex. How to interpret these results?
Firstly, these results demonstrate that CSB shares a lot of properties with other impulsivity control disorders such as kleptomania, pathological gambling, and alimentation disorders. Thanks to the Go-noGo behavioral task, a higher level of impulsivity was reported in patients with CSB than in control participants. These results are in accordance with those demonstrated in patients with compulsive obsessional troubles as reported in a recent study using the same behavioral task in a group of patients depicting trichotillomania as compared to controlled participants (Chamberlain et al., 2007).
The cerebral side was also explored in terms of anatomy and processes. The anatomical data did not support the first hypotheses of the authors. Indexes calculated by DTI did not show any significant difference between patients with CSB and control participants. These results were different than those of previous studies reporting a disorganization of the inferior frontal cortex in other categories of impulsivity disorders (Grant et al., 2006; Hoptman et al., 2002; Rüsch et al., 2007). Although no significant results were reported, some preliminary tendencies regarding anatomical data appeared. DTI indexes variations were reported for the superior frontal lobe between patients and control participants that supports an alteration of axons within this region. For neuroimaging, a very interesting approach is to perform correlational analyses between neuroimaging and behavioral data. For this specific study, authors demonstrated a correlation between impulsivity measures and white matter troubles for the inferior frontal gyrus. Similar results were demonstrated in studies dealing with compulsive obsessional disorders.
As explained above and as postulated by Eli Coleman (1992), CSB would be a partial response to negative affects such as depression or anxiety. Such an hypothesis is in accordance with responses reported in patients with higher ratings on a negative emotional scale and with behavioral and anatomical data recorded more generally in anxious troubles. As mentioned above, anatomical data would support CSB more as an obsessional compulsive behavior than an impulsivity control disorder.
Therapeutic
Clinical and therapeutic difficulties
Clinically, this type of addiction raises the problem of its screening. Indeed, to consult a therapist because of a too intense sexual like is not trivial. Moreover, the denial of the problems related to this kind of behavior, avoidance of suffering of depressive symptoms associated with a personal questioning do not help much these patients to consult a therapist. When a therapeutic process begins, it will promote a flexible but robust therapeutic setting so that the patient gradually learns to keep a rhythm that is to say, also invest in the therapeutic relationship rather than to go from on therapist to zap from one therapist to another one as soon as frustration is felt. Emotional reactions on edge, acts, or acting out often make it difficult to develop a good psychic elaboration.
Behavioral individual or group SAA therapies
Carnes (1989a, 1989b) was the first to develop a behavioral method based on the 12 steps of Anonymous Alcoholics that aims to rehabilitate these individuals. The method is quite simple: it offers targeted groups complete abstinence regarding sexual behavior. Other authors such as Earl (1995) developed care programs in the same direction. Schneider (1991) highlighted the difficulty of these individuals to stop their behavior: the individual suffers from the same withdrawal symptoms as the one who continues to consume alcohol and drugs such as anxiety, insomnia, tremors, headache, and a depressive syndrome. This simple idea has some merit to attract patients, who, desperate and alone, then have a new challenge to overcome. Indeed, abstinence will be able to afford to give, eventually, relief to a later love affair, which would – before – not resist to the temptation to change automatically (because the other, that is, the partner, did not exist as such), but is the internal psychic structure really modified by this conditional abstinence?
Issues of psychodynamic therapies
The challenge for an analytical therapy is not to focus on the symptom. Thus, the final objective won’t be abstinence per se but rather a solution that suits the subject, found by him, giving him the opportunity to break free of his shackles. This type of care in the sense that such treatment will involve to be confronted to difficult life episodes, scarrying when it comes to open conflict and painful memories for consciousness. Moreover, as for some borderline states, the analytical cure for sex-addicts often reveals a great difficulty in remembering the past, which does not facilitate the elaboration processes. Behaviorally, which is implicitly aimed in the cure is learning a relationship with the other. Within the model of the therapeutic relationship (stable, rhythmic, challenging), the subject will learn a relational kind of consistency that he will be able to appreciate and incorporate the side of his emotional and sexual life. These psychotherapies may, depending on the intensity of pain experienced, be coupled with a medical care.
Pharmacological aspects
On the pharmacological side, treatments are various and depend largely on their effect on the patient: anticonvulsant molecules, hormonal therapy-reducing libido, serotoninergic antidepressants (clomipramine or selective inhibitors of serotonin reuptake), mood stabilizers and anxiolytics can be prescribed. Inhibitory hormonal therapy (often designed as castrating) is usually reserved for a forensic use. A recent study by Gulsun et al. (2007) shows the positive effects that clominpramine has on the status of patients with compulsive sexuality.
Conclusion
On the neurobiological side, as shown by the data discussed in this article, the investigation of the role of the brain in emotions and motivations remained for a long time out of the range of cognitive neuroscience. A fortiori, it took a decade after the discovery of an imaging technique such as functional MRI to see the first studies on male sexual behavior appear. Although developing exponentially, the field remains largely unexplored for healthy sexual behavior. For sexual addiction, neuroscientists still have little data. However, these data are encouraging and suggest that the disorder observed on the behavioral side resonates with that observed on the neural side. The sexual affiliation model and its associated troubles could become one of the most second working model for the convergence and the dialog between psychoanalysis and neuroscience. Regarding therapy, these new addictions called ‘drug free addictions’ just began to be recognized and treated. Large clusters of research centers in clinical psychiatry are at the forefront of current research on these different ‘addictive practices’ and now host ‘sex addicts.’ These new clinical configurations are an opportunity for clinical psychopathology invited to rethink some of its theoretical and technical cares. Clinical and scientific advances in this topic are of great interest for other fields (sociology, anthropology, epidemiology, and public health) in terms of their impact in reducing risk for HIV prevention.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to write this review.
References
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neurosciences. 2000;20(7):2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avikainen S, Forss N, Hari R. Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage. 2002;15(3):640–646. doi: 10.1006/nimg.2001.1029. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. Journal of Sex Research. 2004:217–234. doi: 10.1080/00224490409552230. [DOI] [PubMed] [Google Scholar]
- Barth R, Kinder B. The mislabeling of sexual impulsivity. Journal of Sex and Marital Therapy. 1987;13:15–23. doi: 10.1080/00926238708403875. Brunner/Mazel inc. [DOI] [PubMed] [Google Scholar]
- Batson C.D, Sager K, Garst E, Kang M, Rubchinsky K, Dawson K. Is empathy-induced helping due to selfother merging? Journal Personality Social Psychology. 1997;73:495–509. [Google Scholar]
- Buccino G, Binkofski F, Fink G.R, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neurosciences. 2001;13(2):400–404. [PubMed] [Google Scholar]
- Calder A.J, Keane J, Manes F, Antoun N, Young A.W. Impaired recognition and experience of disgust following brain injury. Nature Neuroscience. 2000;3(11):1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Carnes P. The sexual addiction. Minneapolis: CompCare Publications; 1983. [Google Scholar]
- Carnes P. Contrary to love: Helping the sexual addict. Center City, MN: Hazelden; 1989a. [Google Scholar]
- Carnes P. Contrary to love: Helping the sexual addict. Minesota: CompCare Publishers; 1989b. [Google Scholar]
- Carnes P. Don’t call it love: Recovery from sexual addiction. New York: Bantam; 1991. [Google Scholar]
- Carnes P. Out of the shadows: Understanding sexual addiction. Center City, MN: Hazelden; 2001. [Google Scholar]
- Carr L, Iacoboni M, Dubeau M.C, Mazziotta J.C, Lenzi G.L. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of National Academic Science USA. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain S. R, Fineberg N. A, Blackwell A. D, Clark L, Robiins T. W, Shahkian B. J. A neuropsychological comparison of obsessive-compulsive disorder and trichotillomania. Neuropsychologia. 2007;45:654–662. doi: 10.1016/j.neuropsychologia.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Coleman E. Is your patient suffering from compulsive behavior? Paraphilia and related disorders. Psychiatry Annals. 1992;22:320–325. [Google Scholar]
- Coleman E, Gratzer T, Nesvacil L, Raymond N. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: A retrospective study. Journal of Clinical Psychiatry. 2000;61:282–284. [PubMed] [Google Scholar]
- Crépault C. Une classification des désordres psychosexuels, Contracept. Ferst. Sex. 1993;21(2):177–183. [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, Bettinardi V, Tadary B, Woods R, et al. Mapping motor representations with positron emission tomography. Nature. 1994;371(6498):600–602. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J. The power of simulation: Imagining one’s own and other’s behavior. Brain Research. 2006;1079(1):4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- DSM-IV. Critères diagnostiques. Paris: Masson; 1996. (cf. chapitre sur les troubles du contrôle des impulsions) [Google Scholar]
- Earl R. Sex addiction: Case studies and management. New York: Brunnel Mazel, inc; 1995. [Google Scholar]
- Estellon V. Cliniques méditerranéennes. ‘Les homosexualités aujourd’hui: un défi pour la psychanalyse? Ramonville Saint-Agne, Érès; 2002. De l’angoisse à l’orgasme: la métaphore autoérotique en défaut dans la sexualité addictive; p. 65. [Google Scholar]
- Estellon V. Psychiatrie française. Paris: AFP; 2003. Sexualité autocalmante et effacement de l’autre. Déc. 2003, ‘Les violences de l’humain ‘. [Google Scholar]
- Estellon V. Cliniques méditerranéennes, 72. 2005. Sexualités précaires et précarité sexuelle. Ramonville Saint-Agne, Érès, 2005. [Google Scholar]
- Estellon V. Cliniques méditerranéennes, Précarités, exclusion, abandon. 2005a. Sexualités précaires et précarité sexuelle, in revue. Ramonville St-Agne, Eres. [Google Scholar]
- Estellon V. Thérapie Psychomotrice et Recherches. 2005b. Déshumanités des sexualités et espoir de soins in revue. Monaco, Ed du S.N.U.P., 2006. [Google Scholar]
- Estellon V. 10 cas de Psychopathologie de l’adulte. Paris: sous la direction de François Marty; 2009. Fonctionnement limite et engendrement du dispositif thérapeutique. Inpress. [Google Scholar]
- Estellon V. Perspectives psychiatriques, dossier ‘Cliniques de lextrême. Paris: L’esprit du temps; 2011. Sexualite limites. [Google Scholar]
- Estellon V. Cliniques méditerranéennes. 2012. Le temps immobilisé, pensée magique et fonctionnement limite. ERES, Toulouse, 2011. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho J.K, Sperry L, Ross T.J, et al. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goodman A. Addiction: Définition and implications. British Journal of Addiction. 1990;85:14. doi: 10.1111/j.1360-0443.1990.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Goodman A. Diagnosis and treatment of sexual addiction. Journal of Sex and Marital Vjerapy. 1993;1S(5):225–251. doi: 10.1080/00926239308404908. [DOI] [PubMed] [Google Scholar]
- Grant J. E, Correaia S, Brennan-Krohn T. White matter integrity in kleptomania: A pilot study. Psychiatry Research: Neuroimaging. 2006;147:233–237. doi: 10.1016/j.pscychresns.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis . Human Brain Mapping. 2001;12(1):1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsun M, Gulcat Z, Aydin H. Treatment of compulsive sexual behaviour with clomipramine and valproic acid. Clinical Drug Investigation. 2007;27:219–223. doi: 10.2165/00044011-200727030-00005. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan M.A, Van Gelderen P, Hallett M. Functional properties of brain areas associated with motor execution and imagery. Journal of Neurophysiology. 2003;89(2):989–1002. doi: 10.1152/jn.00132.2002. [DOI] [PubMed] [Google Scholar]
- Hoptman M. J, Volavka J, Johnson G, Weiss E, Bilder R.M, Lim K. O. Frontal white matter microstructure, aggression, and impulsivity in men with schizo-phrenia: A preliminary study. Biological Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Insel T. R, Fernald R. D. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Kernberg O. F. The destruction of time in pathological narcissism. International Journal of Psychoanalysis. 2008;89:299–312. doi: 10.1111/j.1745-8315.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- Kilner J.M, Vargas C, Duval S, Blakemore S.J, Sirigu A. Motor activation prior to observation of a predicted movement. Nature. Neuroscience. 2004;7(12):1299–1301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Levenson R. W, Ruef A. M. Empathy: A physiological substrate. Journal of. Personality Social Psychology. 1992;63(2):234–246. [PubMed] [Google Scholar]
- Lynch J.C, Mountcastle V.B, Talbot W.H, Yin T.C. Parietal lobe mechanisms for directed visual attention. Journal of Neurophysiology. 1977;40(2):362–389. doi: 10.1152/jn.1977.40.2.362. [DOI] [PubMed] [Google Scholar]
- Mac Dougall J. Plaidoyer pour une certaine anormalité. Paris: Gallimard; 1978. [Google Scholar]
- Mac Dougall J. ‘De la sexualité addictive’. Psychiatrie française. 1991;22:29–51. [Google Scholar]
- Mac Dougall J. Les Troubles de la sexualité. Monographies de la RFP; 1993. L’addiction à l’autre: Réflexion sur les néo-sexualités et la sexualité addictive. [Google Scholar]
- Mac Dougall J. Éros aux mille et un visages. Paris: Gallimard; 1996. [Google Scholar]
- Mick T. M, Hollander E. Impulsive-compulsive sexual behavior. CNS Spectrums. 2006;11:944–955. doi: 10.1017/s1092852900015133. [DOI] [PubMed] [Google Scholar]
- Miner M. H, Raymond N, Mueller B. A, Lloyd M, Lim K. O. Preliminary investigation of the impulsive and neuroanatomical characteristics of compulsive sexual behavior. Psychiatry Research. 2009;174(2):146–151. doi: 10.1016/j.pscychresns.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cognitive. Affective and Behavioral Neuroscience. 2004;4(2):270–278. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B, et al. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. NeuroImage. 2006;33(2):689–699. doi: 10.1016/j.neuroimage.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Mouras H, Stoléru S, Bittoun J, Glutron D, Pélégrini-Issac M, Paradis A.L, Burnod Y. Brain processing of visual sexual stimuli in healthy men: A functional magnetic resonance imaging study. Neuroimage. 2003;20(2):855–869. doi: 10.1016/S1053-8119(03)00408-7. [DOI] [PubMed] [Google Scholar]
- Mouras H, Stoléru S, Moulier V, Pélégrini-Issac M, Rouxel R, Grandjean B, et al. Activation of mirror-neuron system by erotic video clips predicts degree of induced erection: an fMRI study. NeuroImage. 2008;42(3):1142–1150. doi: 10.1016/j.neuroimage.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Orford J. Excessive Appetites, a Psychological Views of Addictions. New York: John Wiley and Sons, Ltd; 1985. [Google Scholar]
- Pankseep J. At the interface of the affective, behavioral, and cognitive neurosciences: De-coding the emotional feelings of the brain. Brain Cognitive. 2003;52:4–14. doi: 10.1016/s0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Peretti-Watel P, Spire B, Schiltz M. A, Bouhnik A. D, Heard I, Lert F, et al. Vulnerability, unsafe sex and non-adherence to HAART: Evidence from a large sample of French HIV/AIDS outpatients. Social Science Medicine. 2006;62:2420–2433. doi: 10.1016/j.socscimed.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Phillips M.L, Young A.W, Scott S.K, Calder A.J, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of Biological Sciences. 1998;265(1408):1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirlot G. Psychanalyse des addictions. Paris: Armand Colin; 2009. [Google Scholar]
- Pizzagalli D.A, Lehmann D, Hendrick A.M, Regard M, Pascual-Marqui R.D, Davidson R.J. Affective judgments of faces modulate early activity (approximately 160 ms) within the fusiform gyri. NeuroImage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- Porro C.A, Francescato M.P, Cettolo V, Diamond M.E, Baraldi P, Zuiani C, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: A functional magnetic resonance imaging study. Journal of Neurosciences. 1996;16(23):7688–7698. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S. D, De Waal F. B. M. Empathy: Its ultimate and proximate bases. Behavior of Brain Sciences. 2002;25(1):1–20. doi: 10.1017/s0140525x02000018. discussion 20–71, février. [DOI] [PubMed] [Google Scholar]
- Redouté J, Stoléru S, Grégoire M.C, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11(3):162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. C, Blaine D. A. Sexual addictions dans Holistic Nursing. Practice. 1978;2:75–83. doi: 10.1097/00004650-198808000-00012. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib M.A. Language within our grasp. Trends Neuroscience. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: A PET investigation of agency. Nature Neuroscience. 2001;4(5):546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Rüsch N, Weber M, Il’yasov K. A, Lieb K, Ebert D, Hennig J, et al. Inferior frontal white matter microstructure and patterns of psychopathology in women with borderline personality disorder and comorbid attention-deficit hyperactivity disorder. Neuroimage. 2007;35:738–747. doi: 10.1016/j.neuroimage.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Schneider J. P. How to recognize the signs of sexual addiction. Postgraduate Medecine. 1991;90:171–182. doi: 10.1080/00325481.1991.11701111. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan R.J, Frith C.D. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Stephan K.M, Fink G.R, Passingham R.E, Silbersweig D, Ceballos-Baumann A.O, Frith C.D, Frackowiak R.S. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. Journal of Neurophysiology. 1995;73(1):373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Grégoire M.C, Gérard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Archived Sex Behavior. 1999;28(1):1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Wainberg M. L, Muench F, Morgenstern J, Hollander E, Irwin T. W, Parsons J. T, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. Journal of Clinical Psychiatry. 2006;67:1968–1973. doi: 10.4088/jcp.v67n1218. [DOI] [PubMed] [Google Scholar]