Abstract
Background
The prenatal migration of gonadotropin-releasing hormone (GnRH) neurosecretory neurons allows nutrients and human pheromones to alter GnRH pulsatility, which modulates the concurrent maturation of the neuroendocrine, reproductive, and central nervous systems, thus influencing the development of ingestive behavior, reproductive sexual behavior, and other behaviors.
Methods
This model details how chemical ecology drives adaptive evolution via: (1) ecological niche construction, (2) social niche construction, (3) neurogenic niche construction, and (4) socio-cognitive niche construction. This model exemplifies the epigenetic effects of olfactory/pheromonal conditioning, which alters genetically predisposed, nutrient-dependent, hormone-driven mammalian behavior and choices for pheromones that control reproduction via their effects on luteinizing hormone (LH) and systems biology.
Results
Nutrients are metabolized to pheromones that condition behavior in the same way that food odors condition behavior associated with food preferences. The epigenetic effects of olfactory/pheromonal input calibrate and standardize molecular mechanisms for genetically predisposed receptor-mediated changes in intracellular signaling and stochastic gene expression in GnRH neurosecretory neurons of brain tissue. For example, glucose and pheromones alter the hypothalamic secretion of GnRH and LH. A form of GnRH associated with sexual orientation in yeasts links control of the feedback loops and developmental processes required for nutrient acquisition, movement, reproduction, and the diversification of species from microbes to man.
Conclusion
An environmental drive evolved from that of nutrient ingestion in unicellular organisms to that of pheromone-controlled socialization in insects. In mammals, food odors and pheromones cause changes in hormones such as LH, which has developmental affects on pheromone-controlled sexual behavior in nutrient-dependent reproductively fit individuals across species of vertebrates.
Keywords: nutrition, metabolism, odors, adaptation, evolve
… behavioral epigenetics has yet to connect all its levels of analysis. It needs, and doesn’t yet have, at least one slam-dunk demonstration of all the links in a chain from behavior to neural activity to gene expression and back out again. (Berreby, 2011)
In the honey bee, the outputs of gene regulatory networks stemming from near identical genomes are altered by differing nutritional intakes which can be considered to be alternate trajectories along an epigenetic landscape. Differential nutrition results in different morphologies, different physiologies, different nervous systems and very different behaviors, all arising from different developmental trajectories that end in queen and worker. (Gabor Miklos & Maleszka, 2011, p. 403)
A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it. (Max Planck, 1858–1947)
Epigenetics: An essential mechanism for pruning down the wide range of possible behaviors permitted by genes, selecting those that fit an individual’s environment (Berreby, 2011).
Introduction
Members of the Society for Integrative and Comparative Biology (SICB) recently organized and held an ecological epigenetics symposium (January, 2013). Clearly, a new generation is familiar with the concept of ecologically driven epigenetic effects, which can be caused by sensory input that effects hormones, which affect behavior. To a lesser extent, a new generation may be familiar with the concept of human pheromones that effect hormones, which affect behavior. This review links the concept of human pheromones to a model for the epigenetic effects of pheromones on adaptive evolution via extension of ecological epigenetics to what is neuroscientifically known about ecological, social, neurogenic, and socio-cognitive niche construction. The need for this model of systems biology is clarified by the history of the model.
History of the model
Hormones and pheromones.
Socialization and behavior.
Innate versus learned (an ‘across-species’ problem).
Hormones and pheromones
Hormones are chemical messengers that transport signals between cells in multicellular organisms. Like other hormones, androgens organize and activate genetically predisposed vertebrate behavior.
The affects on mammalian behavior of androgen organization and activation were first reported by Phoenix, Goy, Gerall, and Young (1959). In the same year, a new class of biologically active substances was defined.
Pheromones are … substances which are secreted to the outside by an individual and received by a second individual of the same species, in which they release a specific reaction, for example, a definite behavior, or a developmental process. (Karlson & Luscher, 1959, p. 55).
Transport of chemical signals among organisms differentiates pheromones from hormones, which transport chemical signals within multicellular organisms. The definition of pheromones infers that they alter behavior by altering levels of hormones, which organize and activate developmental processes. In essence, pheromones are species-specific chemicals (e.g. social odors) that effect hormones, which cause behavioral affects.
Social odors and behavior
The importance of socialization to invertebrate and vertebrate behavioral affects became clear via experimental manipulations that took place outside the context of pheromone input during stimulus-dependent maturation (Fox, 1966). Unlike food odors, which are commonly linked from nutrient chemicals to innate hormone-driven behaviors (see, e.g. Charra, Datiche, Gigot, Schaal, and Coureaud, 2013), mammalian pheromones were typically not linked to innate hormone-driven behaviors. Instead, the association of pheromones with hormone-driven behaviors of conspecifics was largely ignored.
The history of dichotomous innate effects of food odors on hormones compared to learned affects of pheromones on behavior prevailed (see, e.g. Logan et al., 2012). Initial attempts failed to link the common features of genetically predisposed pheromone-driven invertebrate behavior and hormone-organized and hormone-activated vertebrate behavior. The innate vs. learned dichotomy has prevailed for at least 118 years despite the fact that it was addressed 25 years ago in the context of epigenetics (Johnston, 1987; Weismann, 1894; Woodson, 2012).
Innate vs. learned (the ‘across-species’ problem)
Innate invertebrate behaviors were somehow organized and they were somehow subsequently activated by food odors and pheromones. That vague concept for the development of species-specific invertebrate behaviors made it unlikely that details of the epigenetic effects of mammalian pheromones on adaptively evolved hormone-organized and hormone-activated behavior would be well-received.
The across-species continuum of adaptive evolution was missing. Fortunately, as the requirement to address levels of epigenetically effected biological organization and activation of sex differences in behavior via the gene, cell, tissue, organ, organ-system pathway became known (Naftolin, 1981), it also became clearer that mammalian pheromones linked sensory input directly to effects on hormones and their affects on sex differences in brain development and behavior (Kohl, unpublished).
Current concepts
Epigenetic effects.
Hormones and pheromones in vertebrates and invertebrates.
Olfaction, pheromones, hormones, and behavior across species.
Epigenetic effects
The epigenetic effects of mammalian pheromones on the gene, cell, tissue, organ, organ-system pathway, and on hormone-organized and hormone-activated mammalian behavior extended what was known about sexual differentiation in other vertebrate species to humans (Diamond, Binstock, & Kohl, 1996). Until recently, however, little evidence suggested that human pheromones caused a response that could be compared to responses from invertebrates in which pheromones affect a definite behavior or effect a developmental process (Karlson & Luscher, 1959). The first attempts to link pheromones to developmental processes involving hormone-organized and hormone-activated behavior across species (Kohl & Francoeur, 1995/2002) failed to interest those whose opinions about cause and effect did not incorporate emerging evidence from molecular epigenetics (Diamond et al., 1996) or evidence of putative human pheromones (Kohl, Atzmueller, Fink, & Grammer, 2001).
Researchers have since learned about the epigenetic effects of nutrient chemicals and food odors on energy homeostasis that is required for individual survival (see for review Schneider, Klingerman, & Abdulhay, 2012). Many have also learned about the link from nutrient-dependent pheromone production to glucose-facilitated gonadotropin-releasing hormone (GnRH) pulse frequency (Roland & Moenter, 2011), which controls the secretion of pituitary hormones that control hypothalamic–pituitary–gonadal (HPG) axis (Wen, Ai, Alim, & Boehm, 2010) and HP–adrenal (HPA) axis steroidogenesis (Kotitschke, Sadie-Van Gijsen, Avenant, Fernandes, & Hapgood, 2009) during prenatal and postnatal brain development in mammals (Majdic & Tobet, 2011; Makris et al., 2013; Peper & Koolschijn, 2012). HPA axis and HPG axis steroidogenesis links ecological epigenetics via nutrient-dependent production and distribution of species-specific pheromones to their epigenetic effects, which in mammals appear to extend to effects of socialization on myelination (Liu et al., 2012; Makinodan, Rosen, Ito, & Corfas, 2012).
The model of ecological epigenetics and systems biology detailed here incorporates what is known about similarities in the epigenetic effects of nutrients associated with food odors and the epigenetic effects of pheromones associated with social odors on the development of hormone-organized and hormone-activated behavior across invertebrate and vertebrate species.
Hormones and pheromones in vertebrates and invertebrates
Until recently, there was no direct evidence that human pheromones caused a definite behavior. In contrast, there was sufficient evidence to extend the mammalian model for epigenetic effects of nutrients and pheromones on GnRH and on hormonal organization and activation of behavior (Diamond et al., 1996) to invertebrates (Elekonich & Robinson, 2000). In that context, ‘From fertilization to adult sexual behavior’ (Diamond et al., 1996, p. 333) was extended to invertebrates with details from ‘egg to adult’ (Elekonich & Robinson, 2000, p. 1514).
That extension of the vertebrate/mammalian model to invertebrates occurred as researchers integrated what was known about human pheromones and their effects on neuroendocrine systems during the development of human behavior (Kohl et al., 2001). Invertebrate research rapidly progressed to include the epigenetic effects of nutrients and pheromones on gene expression and on hormone-organized and hormone-activated behavioral transitions throughout life (Elekonich & Roberts, 2005), which is consistent with their epigenetic effects throughout the life of vertebrates, including mammals.
The honeybee model organism exemplifies what has been learned from the study of invertebrates (De Loof, Lindemans, Liu, De Groef, & Schoofs, 2012). This includes what has been learned about the epigenetic effects of nutrients and pheromones on juvenile hormone (JH). However, the molecular biology of cause and effect that is common to all species was left behind, and the epigenetic effects of human pheromones have not typically been considered in the same context as epigenetic effects of food odors and insect pheromones or the epigenetic effects of pheromones on other mammals.
The epigenetic effects of nutrients and pheromones extend across the life history of organisms, but from 1996 to 2012 the concept of molecular epigenetics and epigenetic effects on hormone-driven adaptive evolution of the human brain and behavior seems to have gone missing. Evolutionary psychologists and other social scientists, for example, refused to tether their hypotheses to a new discipline called ‘neuroevolutionary psychobiology’, to neurogenetics (Zoghbi & Warren, 2010), or to any biologically based discipline whatsoever (see for review Panksepp, Moskal, Panksepp, & Kroes, 2002). More than five decades of progress that directly links molecular epigenetics to behavior has been virtually ignored (Shapiro, 2012), but see Ledón-Rettig, Richards, and Martin (2012). Many evolutionary theorists have also ignored the fact that ‘reproductive isolation evidently can arise with little or no morphological differentiation’ (Dobzhansky, 1972, p. 665). That makes sense if nutrients and pheromones are intricately involved. At the same time, some theorists correctly claim that ‘nothing in biology makes any sense in the absence of evolution’ (Dobzhansky, 1973). If Dobzhansky was correct, claims of choice for mutations represented in morphology, which are commonly voiced in the context of random mutations theory, make no sense. For contrast, claims of choice for nutrient-dependent differences in pheromone production have always made sense across the evolutionary continuum of species diversity.
The problem is that many scientists continue to ignore the requirements of genetically predisposed hormone-dependent adaptive evolution, like the evolutionary theorists do. The first requirement is for nutrient-dependent ecological niche construction. The next requirement is for pheromone-controlled social niche construction. But few theorists have incorporated accurate claims about the epigenetic effects of sensory stimuli like food odors or pheromones on rewards associated with food and socialization that show up in ecological and social niche construction (Atasoy, Betley, Su, & Sternson, 2012).
This cause-and-effect problem remains despite the fact that the proximate cause of ecological and social niche construction must first be linked to brain imagery before correlates of hormone release (e.g. oxytocin) and brain imagery can be meaningfully interpreted (see for review Jack, Connelly, & Morris, 2012). Sensory input is the proximate cause of hormone release, which means that hormone release correlated with brain imagery is typically interpreted out of context. Meanwhile, others have accepted the use of alternative terms for pheromones, such as social odors, body odors, signature scents, signaling peptides, chemosignals, or semiochemicals. Arguably, these newer and historically less well-defined terms help some researchers to cautiously avoid any connection from the stereotypical effects of insect pheromones on hormones and behavior to their adaptively evolved epigenetic effects on what appears to be hormone-organized neurogenetically controlled (Swarup, Huang, Mackay, & Anholt, 2013) neurogenic niche construction in the human hypothalamus (Lee et al., 2012; Migaud et al., 2010; Mittag et al., 2013; Nepomnaschy, Vitzthum, & Flinn, 2009). Clearly, however, the epigenetic effects of food odors and pheromones are involved in neurogenic niche construction as exemplified in nematodes (Bumbarger, Riebesell, Rödelsperger, & Sommer, 2013), and in flies (Swarup et al., 2013).
The newer and often redefined terms for pheromones limit the use of what is now known about their epigenetic effects, which are also associated with social stress on adaptively evolved socio-cognitive niche construction (Flinn, Nepomnaschy, Muehlenbein, & Ponzi, 2011; O’Connell & Hofmann, 2011, 2012; Whiten & Erdal, 2012). For example, social stress (see for review McEwen, 2012, 2013) associated with the absence of pheromones (Niwa et al., 2013) or with their presence and aggression (Barik et al., 2013) is as likely as nutrient-dependent stress associated with food acquisition to alter hormones and behavior during development. That is because the epigenetic effects of nutrient stress associated with food odors and the epigenetic effects of social stress associated with pheromones occur via the GnRH-controlled pathway, which includes both HPG axis and HPA axis regulation.
Nevertheless, 15 years after the first edition of a book about human pheromones: The Scent of Eros: Mysteries of Odor in Human Sexuality (Kohl & Francoeur, 1995/2002) appeared on the bookshelves, we were told in a summary of The Great Pheromone Myth (Doty, 2010):
For more than 50 years researchers – including many prominent scientists – have identified pheromones as the triggers for a wide range of mammalian behaviors and endocrine [hormone-organized and hormone-activated] responses. In this provocative treatise, renowned olfaction expert Richard L. Doty rejects this idea and states bluntly that – in contrast to insects – pheromones in mammals do not exist.
The epigenetic effects of nutrients on evolved differences in the diet and starch digestion of dogs and wolves (Axelsson et al., 2013) were detailed at the same time differences in the socialization of these subspecies were attributed to explorations involving only chemosensory input in 3 to 4-week-old wolf pups. For comparison, differences in starch digestion and exploration involving multisensory input in dogs begin a mere 2 weeks later (Lord, 2013). The differences in nutrient-dependent pheromone-controlled socialization, however, extend across a life-time of more aggressive behavior in wolves that have not been domesticated because less digested starch from their diet genetically predisposes infants to first respond to olfactory/pheromonal cues as they initially explore their postnatal environment.
If only the differences between wolves and dogs were considered, it would be clear that mammalian pheromones exist, which may explain why they continue to be discussed in terms of olfactory/pheromonal control of adaptively evolved hormone-organized and hormone-activated behavior (Kohl, 2012). Indeed, mammalian pheromones are included in discussions that are essential in understanding the role of molecular epigenetics and the ecological epigenetics of nutrient-dependent pheromone-controlled reproduction in species from microbes to man. Furthermore, the concept of nutrient-dependent pheromone-controlled reproduction is important to neuroscientific progress. For example, it is essential for a better understanding of evolutionary endocrinology (Zafon, 2012) and its role in evolutionary medicine (Stearns, 2012).
Olfaction, pheromones, hormones, and behavior across species
Olfactory/pheromonal input is obviously important to the nutrient-dependent, hormone-organized and hormone-activated pheromone-controlled development of the invertebrate brain and behavior (Dickman, Kucharski, Maleszka, & Hurd, 2013; Lyko et al., 2010; Lyko & Maleszka, 2011). The claim that mammalian pheromones do not exist is an academically irresponsible misrepresentation. It is like saying that food odors do not exist for some species. If another species could think about such a claim, its answer to the question of whether food odors exist would probably be the same as its answer is to the question of whether pheromones exist. All organisms show us quite clearly that their survival is nutrient-dependent and that pheromones control their reproduction.
Researchers, who deny the existence of pheromones in mammals, including the human pheromone-deniers, short-circuit interdisciplinary discussion of the nutrient-driven ecological epigenetics and the pheromone-controlled adaptive evolution of human behavior. For contrast, the honeybee has emerged as a model organism for understanding the epigenetic link from food odors and pheromones to neural networks of the mammalian brain, which ultimately determine human behavior (Kohl, 2012). That fact can now be discussed in the context of the mammalian model of ecological epigenetics and systems biology that is represented here.
Epigenetic effects on hormones that affect behavior
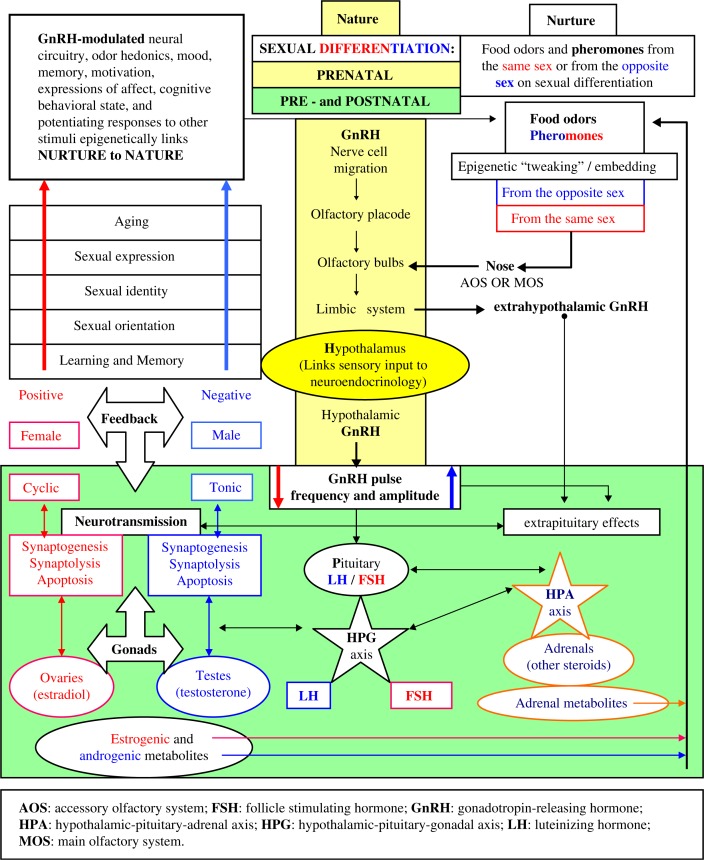
The mammalian model (see Fig. 1).
‘Biological embedding’/adaptive evolution.
Ecological epigenetics from rats and mice to men.
That’s not funny.
An epigenetic continuum of nutrient-dependent pheromone-controlled adaptive evolution.
Mathematical models vs. biological facts.
Fig. 1.
The mammalian model.
The mammalian model
Nature: The genetically predisposed embryonic migration of GnRH neurons to the hypothalamus is controlled by in utero nutrient stress and social stress (see Fig. 1). Stress alters fine-tuning, which is required for the homeostasis that supports the optimal epistatic outcome of gestation.
Nurture: Genetic predispositions, nutrient stress, and social stress continue to impact prenatal and postnatal development of the brain during sexual differentiation (Makris et al., 2013). Food odors and sex differences in the effects of pheromones on neurosecretory neurons in brain tissue of the hypothalamus alter postnatal brain development via their epigenetic effects on GnRH pulse frequency.
Prenatal and postnatal development: Pulsatile GnRH secretion alters the levels of other hormones and their interactive feedback on the HPG and the HPA axes. These alterations occur during synaptogenesis, synaptolysis, and apoptosis that cause changes in neurotransmission during the development of hormone-organized and hormone-activated behaviors.
The complexity of the mammalian model can be reduced: nutrients and pheromones epigenetically effect hormones that organize and activate behaviors, which are associated with other sensory input during the development of adaptively evolved behavior.
The impact of epigenetic effects of chemical ecology on behavioral affects is now relatively well-known (Ledón-Rettig et al., 2012). Effects on hormones and their affects on behavior have been detailed in the honeybee model organism. ‘The concept that is extended is the epigenetic tweaking of immense gene networks in “superorganisms” that “solve problems through the exchange and the selective cancellation and modification of signals”’ (references in Kohl, 2012). An environmental drive appears to have evolved from that of food ingestion in unicellular organisms to that of socialization in insects. In mammals, food odors and pheromones cause changes in hormones such as luteinizing hormone (LH), which has developmental affects on sexual behavior in nutrient-dependent, reproductively fit individuals across species of vertebrates (see for review and citations Kohl, 2012). These developmental affects cause the ‘biological embedding’ of responses to the signals that are most important to the survival of individuals and species (i.e. the signals from nutrients that metabolize to species-specific pheromones).
‘Biological embedding’/adaptive evolution
The concept of ‘biological embedding’ (Hertzman, 2012) is consistent with what is known about classical conditioning of responses to sensory input and the requirements for biologically relevant sensory input to alter adaptive evolution. This ‘classical’ conditioning is also called Pavlovian or ‘respondent’ conditioning because organisms automatically respond to the most relevant sensory input, which was exemplified in ‘Pavlov’s dog’. Responses are automatic (e.g. ‘innate’) because food odors and pheromones are the most relevant of all sensory signals to survival. These genetically predisposed innate responses have also been linked via molecular epigenetics to de novo protein synthesis and memory formation in the amygdala of rats (Duvarci, Nader, & LeDoux, 2008) and in the amygdala of mice (Griggs, Young, Rumbaugh, & Miller, 2013).
Stories that make mutations responsible for adaptive evolution or that make operant conditioning associated with visual or auditory input responsible for adaptive evolution lack details of the molecular mechanisms or scientific support that links them to the de novo protein synthesis required for adaptive evolution. Those stories seem to somehow allow either randomness to cause adaptively evolved behavior or to allow the consequences of behavior to cause it. The story lines are missing the known role of ecological epigenetics and instead seem to suggest that behavior is caused by unknown epigenetic effects of visual or auditory stimuli on gene expression in hormone-secreting nerve cells of brain tissue.
For decades, misrepresentations of cause and effect from outside the context of ecological epigenetics have muddied the waters of clear thought for behaviorists and their minions (e.g. some other social scientists). Now, the honeybee model organism links what is known about molecular epigenetics to nutrient-dependent, pheromone-controlled, classically conditioned behaviors in species from microbes to man (Kohl, 2012).
Quorum sensing in brainless microbes, for example, is a form of pheromone-controlled reproduction that helps to ensure unicellular species do not out-reproduce their supply of nutrient chemicals (Ng & Bassler, 2009). Thus, microbes also exemplify a primitive form of controlled cell-to-cell communication. The conservation of controlled cell-to-cell communication is exemplified in the hormone-controlled genetically predisposed behavior of multicellular organisms.
Clearly, the behavior of unicellular and multicellular organisms is nutrient-dependent and pheromone-controlled. This control is exemplified in all adaptively evolved individuals of all species. Across the continuum of adaptively evolved behaviors, pheromones are to microbes what hormones are to the other species that produce them. It is also clear that no matter what they are called, nutrient-dependent ‘phylogenetic signals’ (Shapiro et al., 2012) are pheromones that, like hormones, control communication between cells and among species. Should we expect any species to be an evolutionary outlier?
Ecological epigenetics from rats and mice to men
Food odors and pheromones link the honeybee model organism and other invertebrate model organisms to olfaction and odor receptors across species. This link provides ‘… a clear evolutionary trail that can be followed from unicellular organisms to insects to humans’ (Kohl, 2012).
The recently detailed mouse model (Li et al., 2013) builds on what is known about olfactory/pheromonal communication in species from microbes to man and incorporates works from mammals that elucidate the molecular mechanisms that are clearly involved. Sex-dependent production of a mouse ‘chemosignal’ with incentive salience appears to have arisen de novo via coincident adaptive evolution that involves an obvious two-step synergy between commensal bacteria and a sex-dependent liver enzyme that metabolizes the nutrient chemical choline.
The result of this synergy is (1) a liver enzyme that oxidizes trimethylamine to (2) an odor that causes (3) species-specific behaviors. Thus, the complex systems that biology required to get from nutrient acquisition and nutrient metabolism to species-specific odor-controlled behavior is exemplified by adaptive evolution of an attractive odor to mice that repels rats (see for review Li et al., 2013).
The mouse odor also repels humans. High excretion rates of trimethylamine-associated odor in humans cause ‘fish odor syndrome’. The aversive body odor has been attributed to a mutation (Dolphin, Janmohamed, Smith, Shephard, & Phillips, 1997). This attribution is not consistent with the portrayal of synergy in the mouse model, which enables both the production of the odor and the response to the odor.
This synergy requires at least two things to happen simultaneously: for example, (1) natural selection for nutrient chemicals and (2) sexual selection for odor production. Sexual selection for nutrient-dependent odor production is not likely to be achieved via one mutation involved in nutrient acquisition and another mutation that is involved in odor production because two mutations are not likely to simultaneously occur.
For contrast, the epigenetic tweaking of immense gene networks by nutrient intake (e.g. glucose uptake in cells) exemplifies a vastly more complex synergy. Glucose causes changes in GnRH pulse frequency and amplitude in mammals (Roland & Moenter, 2011). The GnRH pulse controls nutrient-dependent and sex steroid hormone-dependent body odor production in a manner similar to the nutrient-dependent and hormone-dependent production of pheromones in the honeybee model organism and nutrient-dependent pheromone production in microbes. For example, the diet of the honeybee queen determines her pheromone production, which controls interactions among colony members (Gabor Miklos & Maleszka, 2011; Kohl, 2012).
In the mouse model, the diet of the mice determines their nutrient-dependent pheromone production and social interactions with other mice. The mouse model also reveals something that was not revealed in the context of dogs and wolves (Axelsson et al., 2013; Lord, 2013). The aversive human body odor associated with fish odor syndrome can be epigenetically controlled by reducing dietary choline intake. It can also be controlled through antibiotic use (citations in Li et al., 2013). This may be important in the context of chemical ecology and epigenetic effects of genetically predisposed nutrient-dependent pheromone-controlled human interactions (Martin et al., 2010; Preti & Leyden, 2010).
The recommended daily intake of choline was recently doubled for pregnant women because choline is essential for in utero human brain development (Yan et al., 2012). Maternal choline intake also alters the epigenetic state of human stress-associated fetal cortisol-regulating genes (Jiang et al., 2012), which are believed to be important for stress attenuation via the HPA axis (Korosi et al., 2010). Presumably, this links stress to memory formation and consolidation in the amygdala via protein biosynthesis (Duvarci, Nader, & LeDoux, 2008; Griggs et al., 2013; Li et al., 2013). Compared to any theory of mutation-caused adaptive evolution, however, the difference in this link from olfactory/pheromonal input to genetically predisposed memory via protein biosynthesis includes the molecular mechanisms that link epigenetic effects on genes to behavior and back via learning and memory.
Should we anticipate a problem with brain-directed social behavior in children of mother’s who adopt the new dietary choline recommendation? Could additional choline epigenetically alter brain development and cause too much prenatal synaptogenesis, too little synaptolysis, or altered apoptosis? Could sex differences in the mother–infant bond which are correlated with nutrient intake and pheromone production (Nguyen, Gesquiere, Alberts, & Altmann, 2012) and with oxytocin secretion (Donaldson & Young, 2008) be altered by a child whose mother ingested too much choline? What might happen if she epigenetically caused her own production of a fishy odor or a genetically predisposed ‘fishy’ body odor in her infant?
Could the child’s interactions with conspecifics be altered due to an aversive odor? What if maternal choline intake causes prenatal and postnatal variations in myelination and neurotransmission associated with brain imagery in autism spectrum disorders? What if choline interacts with glucose and lipid metabolism to cause neurodegenerative diseases later in life, like Alzheimer’s? Such speculations may be warranted until there is a comparable model of nutrient-dependent pheromone-controlled species diversity in ecological, social, neurogenic, and socio-cognitive niche construction.
That’s not funny!
One clown asked another: ‘Do I smell funny to you?’ Adults with ‘fish odor syndrome’ are not likely to think that question is funny. Mice do not think about whether their brain development or their natural odor production is determined by diet. Yet, without thought, nutrient-dependent and pheromone-controlled chromatin remodeling is responsible for transgenerational epigenetic inheritance involved in species diversity (Kim et al., 2008; MacDonald & Roskams, 2009; Nadeau, 2009; Riccio, 2011; Tammen, Friso, & Choi, 2013). This suggests that questions about the adaptive evolution of species diversity (Papasaikas & Valcarcel, 2012) are not likely to be answered outside the context of the molecular biology common to all species (Shapiro, 2010).
An epigenetic continuum of nutrient-dependent / pheromone-controlled adaptive evolution
Nematodes
Differences in the behavior of nematodes are determined by nutrient-dependent rewiring of their primitive nervous system (Bumbarger et al., 2013). Species incompatibilities in nematodes are associated with cysteine-to-alanine substitutions (Wilson et al., 2011), which may alter nutrient-dependent pheromone production.
Insects
The honeybee is currently an accepted model organism of nutrient-dependent pheromone-controlled adaptive evolution of the brain and behavior that is consistent with what is known about neurogenic niche construction in nematodes (Bumbarger et al., 2013). In flies, ecological and social niche construction can be linked to molecular-level cause and effect at the cellular and organismal levels via nutrient-dependent changes in mitochondrial tRNA and a nuclear-encoded tRNA synthetase. The enzyme enables attachment of an appropriate amino acid, which facilitates the reaction required for efficient and accurate protein synthesis (Meiklejohn et al., 2013). In wasps, manipulation of the genetics of evolved species-specific pheromones characterized the change in a pre-existing signaling molecule triggered by a glucose-dependent (Yadav, Joshi, & Gurjar, 1987) stereochemical inversion (Niehuis et al., 2013). In Ostrinia moth species, substitution of a critical amino acid is sufficient to create a new pheromone blend (Lassance et al., 2013). In the ‘peppered moth’ example of rapid response to human-induced environmental changes, which were heretofore considered to be driven by selective predation, some evidence now suggests the migration pattern of 2 km per evening is consistent with the male moth’s ability to detect the nutrient-dependent pheromones of the female from 2 km upwind (see for review Cook & Saccheri, 2013).
Mammals
Current concepts now limit attempts to explain selection for nutrient-dependent changes in coat color and kinked tails in mice via mutations theory, since mutations theory does not address pleiotropy and epistasis (see for review Feinberg & Irizarry, 2010). Until recently, the association of the nutrient choline in humans and its metabolism to trimethylamine odor in different species of mice was the best example of how a change in diet becomes associated with the presence of mammalian conspecifics whose androgen estrogen ratio-associated odor distinguishes them sexually, and also as nutrient-dependent physically fit mates (Stensmyr & Maderspacher, 2013). The mouse model makes it clearer that glucose uptake changes cellular thermodynamic equilibrium and differential pathway regulation that results in adaptively evolved fitness in species from microbes (Kondrashov, 2012) to mammals. Species-specific health and reproductive fitness is associated with nutrient-dependent amino acid substitutions and with pheromone-controlled reproduction. Disease is associated with mutations exemplified in cancer where perturbations of the glucose-dependent thermodynamic/thermoregulatory equilibrium are equally clear (Locasale, 2012).
Humans
Two additional recent reports link substitution of the amino acid alanine for the amino acid valine (Grossman et al., 2013) to nutrient-dependent pheromone-controlled adaptive evolution. The alanine substitution for valine does not appear to be under any selection pressure in mice. The cause-and-effect relationship was established in mice by comparing the effects of the alanine, which is under selection pressure in humans, via its substitution for valine in mice (Kamberov et al., 2013).
These two reports (Grossman et al., 2013; Kamberov et al., 2013) tell a new short story of adaptive evolution. The story begins with what was probably a nutrient-dependent variant allele that arose in central China approximately 30,000 years ago. The effect of the allele is adaptive and it is manifested in the context of an effect on sweat, skin, hair, and teeth. In other mammals, like the mouse, the effect on sweat, skin, hair, and teeth is due to an epigenetic effect of nutrients on hormones responsible for the tweaking of immense gene networks that metabolize nutrients to pheromones. The pheromones control the nutrient-dependent hormone-dependent organization and activation of reproductive sexual behavior in mammals such as mice and humans, but also in invertebrates as previously indicated. That means the adaptive evolution of the human population, which is detailed in these two reports, is also likely to be nutrient-dependent and pheromone-controlled, since there is no other model for that.
Mathematical models vs. biological facts
Random mutations that somehow cause one or more amino acid substitutions are not likely to simultaneously cause adaptive evolution from the bottom up via the thermodynamics of chromatin remodeling and control of adaptive evolution from the top down via organism-level thermoregulation. However the nutrient-dependent substitution of alanine for valine (Grossman et al., 2013; Kamberov et al., 2013) appears to result in species-specific organism-level changes in skin, glands, and hair, through pheromone-controlled reproduction.
Increased apocine gland secretions associated with the amino acid substitution in mice links smaller human breasts capable of more milk production and more pheromone production to sex differences in imprinting on the species-specific odor of the mother and classical conditioning of associated hormone-organized and hormone-activated behaviors throughout life. For example, sex differences in preferences for breast size are probably classically conditioned via associations with the unconscious effect of odor from human female breasts that differentially alters hormone-dependent neurogenesis from birth. Fos protein expression from pubertally born neurons that are integrated into region-specific areas of the brain activated by socio-sexual behavior in the Syrian hamster (Mohr & Sisk, 2013), more strongly suggests that the role of nutrient-dependent pheromone-controlled regulation of GnRH pulse frequency and amplitude is the biological core of mammalian reproductive sexual behavior.
Increased numbers of hormone-regulated eccrine sweat glands in mice (Grossman et al., 2013; Kamberov et al., 2013) have similar thermoregulatory effects that help to control the core temperature of the human body. At the same time, the increased secretion of watery sweat serves to alter pheromone production, which results from the microbial metabolism of nutrient-dependent substances in apocrine and eccrine sweat. The effect of the alanine substitution on increased hair thickness links it to trapped pheromones and a visual signal of sexually dimorphic nutrient-dependent reproductive fitness in cultures where females have longer head hair than males. It may be that in all cultures the appearance of scalp hair is an indicator of the androgen estrogen ratio, especially in women. That does not necessarily mean the alanine substitution is responsible for pheromone-controlled reproduction in this human population. It does suggest an explanation of cause and effect across species that should be compared to another model for adaptive evolution.
Conclusion
Nutrient-dependent pheromone-controlled reproduction underlies what is common (Locasale, 2012) to all models of natural selection, sexual selection, and species diversity (Frady, Palmer, & Kristan, 2012). Animal models are often used to model human physical and mental disorders. The honeybee already serves as a model organism for studying human immunity, disease resistance, allergic reaction, circadian rhythms, antibiotic resistance, the development of the brain and behavior, mental health, longevity, diseases of the X chromosome, learning and memory, as well as conditioned responses to sensory stimuli (Kohl, 2012).
The mammalian model (Diamond et al., 1996) detailed here allows what we have learned from the study of invertebrates (Elekonich & Roberts, 2005; Elekonich & Robinson, 2000) to be used in attempts to understand the development of human behavior and in attempts to understand human physical disease. Those who adopted the position that mammalian pheromones do not exist, or who continue to insist that human pheromones do not exist, have for more than two decades made it seem that there was something ‘fishy’ or ‘funny’ about this model. For comparison to their claims, this model of systems biology exemplifies experimental results. For instance, ‘our experiments suggest that excitatory odor responses are transiently suppressed (in terms of overall firing rates), but more complex temporal shaping of responses may occur because of interplay of intrinsic properties, sensory drive, and the feedback activity’ (Markopoulos, Rokni, Gire, & Murthy, 2012, p. 1186). In context, this was suggested in ‘Feedback loops link odor and pheromone signaling with reproduction’ (Boehm, Zou, & Buck, 2005).
If this genes-to-behavior-and-back model of systems biology is correct, it shows what has gone missing from cause and effect in the context of adaptive evolution of the human brain and behavior. What is missing is the complex interplay of intrinsic properties, sensory drive, and the feedback activity, which requires the acknowledgement that mammalian pheromones, including human pheromones, obviously exist. That fact should be as obvious as the fact that the ecological epigenetics of food odors exist.
Moving forward
Evidence from genome-wide analysis suggests that polymorphisms cause alterations in neural connections and signaling in olfactory pathways, which contribute to natural variation in olfactory perception in flies (Swarup et al., 2013). That evidence links olfactory/pheromonal input to genetically predisposed species-specific behavior via previously unmodeled epistatic interactions that must occur throughout the lifecycle transitions of all organisms. Thus, the epigenetic ‘tweaking’ of the immense gene networks that occurs via exposure to nutrient chemicals and pheromones can now be modeled in the context of the microRNA/messenger RNA balance, receptor-mediated intracellular signaling, and the stochastic gene expression required for nutrient-dependent pheromone-controlled adaptive evolution. The role of the microRNA/messenger RNA balance (Breen, Kemena, Vlasov, Notredame, & Kondrashov, 2012; Duvarci, Nader, & LeDoux, 2008; Griggs et al., 2013; Monahan & Lomvardas, 2012) in adaptive evolution will certainly be discussed in published works that will follow.
Issues for further consideration
Highly conserved ligand–receptor signaling mechanisms are the biochemical mechanisms of ecological epigenetics. They enable nutrient-dependent pheromone-controlled reproduction in all organisms. These molecular mechanisms allow life to recognize the difference between self and non-self chemical cues and to respond to novelty. The behavioral responses to novelty appear to be the basis for diversified life (Monahan & Lomvardas, 2012).
Unconscious affects that are manifested during the development of diversified life and human behavior are, by their very nature, part of life that few people think about (Kohl et al., 2001). Therefore, the largest contributor to the development of our personal preferences may be the unconscious epigenetic effects of food odors and pheromones on hormones that organize and activate behavior. If so, the model represented here is consistent with what is known about the epigenetic effects of ecologically important nutrients and pheromones on the adaptively evolved behavior of species from microbes to man. Minimally, this model can be compared to any other factual representations of epigenesis and epistasis for determination of the best scientific ‘fit’.
Conflict of interest and funding
The author has not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Atasoy D, Betley J. N, Su H. H, Sternson S. M. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson E, Ratnakumar A, Arendt M.-L, Maqbool K, Webster M. T, Perloski M, et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- Barik J, Marti F, Morel C, Fernandez S. P, Lanteri C, Godeheu G, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339(6117):332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- Berreby D. Environmental impact. The Scientist – Magazine of the Life Sciences. 2011;25(3):40. [Google Scholar]
- Boehm U, Zou Z, Buck L. B. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123(4):683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Breen M. S, Kemena C, Vlasov P. K, Notredame C, Kondrashov F. A. Epistasis as the primary factor in molecular evolution. Nature. 2012;490(7421):535–538. doi: 10.1038/nature11510. [DOI] [PubMed] [Google Scholar]
- Bumbarger D. J, Riebesell M, Rödelsperger C, Sommer R. J. System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Cell. 2013;152(1):109–119. doi: 10.1016/j.cell.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Charra R, Datiche F, Gigot V, Schaal B, Coureaud G. Pheromone-induced odour learning modifies Fos expression in the newborn rabbit brain. Behavioural Brain Research. 2013;237:129–140. doi: 10.1016/j.bbr.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Cook L. M, Saccheri I. J. The peppered moth and industrial melanism: Evolution of a natural selection case study. Heredity. 2013;110(3):207–212. doi: 10.1038/hdy.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loof A, Lindemans M, Liu F, De Groef B, Schoofs L. Endocrine archeology: do insects retain ancestrally inherited counterparts of the vertebrate releasing hormones GnRH, GHRH, TRH, and CRF? General and Comparative Endocrinology. 2012;177(1):18–27. doi: 10.1016/j.ygcen.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Diamond M, Binstock T, Kohl J. V. From fertilization to adult sexual behavior. Hormones and Behavior. 1996;30(4):333–353. doi: 10.1006/hbeh.1996.0040. [DOI] [PubMed] [Google Scholar]
- Dickman M. J, Kucharski R, Maleszka R, Hurd P. J. Extensive histone post-translational modification in honey bees. Insect Biochemistry and Molecular Biology. 2013;43(2):125–137. doi: 10.1016/j.ibmb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Species of Drosophila. Science. 1972;177(4050):664–669. doi: 10.1126/science.177.4050.664. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. Nothing in biology makes any sense except in the light of evolution. American Biology Teacher. 1973;35:125–129. [Google Scholar]
- Dolphin C. T, Janmohamed A, Smith R. L, Shephard E. A, Phillips l. R. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nature Genetics. 1997;17(4):491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- Donaldson Z. R, Young L. J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–9004. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Doty R. L. The great pheromone myth. Baltimore: The Johns Hopkins University Press; 2010. [Google Scholar]
- Duvarci S, Nader K, LeDoux J. E. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learning & Memory. 2008;15(10):747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekonich M. M, Roberts S. P. Honey bees as a model for understanding mechanisms of life history transitions. Comparative Biochemistry and Physiology. Part A. Molecular & Integrative Physiology. 2005;141(4):362–371. doi: 10.1016/j.cbpb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Elekonich M. M, Robinson G. Organizational and activational effects of hormones on insect behavior. Journal of Insect Physiology. 2000;46(12):1509–1515. doi: 10.1016/s0022-1910(00)00101-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P, Irizarry R. A. Stochastic epigenetic variation as a driving force of development, evolutionary adaptation, and disease. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(Suppl. 1):1757–1764. doi: 10.1073/pnas.0906183107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn M. V, Nepomnaschy P. A, Muehlenbein M. P, Ponzi D. Evolutionary functions of early social modulation of hypothalamic–pituitary–adrenal axis development in humans. Neuroscience and Biobehavioral Reviews. 2011;35(7):1611–1629. doi: 10.1016/j.neubiorev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Fox M. W. Neuro-behavioral ontogeny: A synthesis of ethological and neurophysiological concepts. Brain Research. 1966;2(1):3–20. doi: 10.1016/0006-8993(66)90059-x. [DOI] [PubMed] [Google Scholar]
- Frady E. P, Palmer C. R, Kristan W. B., Jr Sexual attraction: Sex-specific wiring of neural circuitry. Current Biology. 2012;22(22):R953–R956. doi: 10.1016/j.cub.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor Miklos G. L, Maleszka R. Epigenomic communication systems in humans and honey bees: From molecules to behavior. Hormones and Behavior. 2011;59(3):399–406. doi: 10.1016/j.yhbeh.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Griggs E. M, Young E. J, Rumbaugh G, Miller C. A. MicroRNA-182 regulates amygdala-dependent memory formation. The Journal of Neuroscience. 2013;33(4):1734–1740. doi: 10.1523/JNEUROSCI.2873-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman S. R, Andersen K. G, Shlyakhter I, Tabrizi S, Winnicki S, Yen A, et al. Identifying recent adaptations in large-scale genomic data. Cell. 2013;152(4):703–713. doi: 10.1016/j.cell.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman C. Putting the concept of biological embedding in historical perspective. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl. 2):17160–17167. doi: 10.1073/pnas.1202203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A, Connelly J. J, Morris J. P. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in Human Neuroscience. 2012;6:280. doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Yan J, West A. A, Perry C. A, Malysheva O. V, Devapatla S, et al. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. The FASEB Journal. 2012;26(8):3563–3574. doi: 10.1096/fj.12-207894. [DOI] [PubMed] [Google Scholar]
- Johnston T. D. The persistence of dichotomies in the study of behavioral development. Developmental Review. 1987;7(2):149–182. [Google Scholar]
- Kamberov Y. G, Wang S, Tan J, Gerbault P, Wark A, Tan L, et al. Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell. 2013;152(4):691–702. doi: 10.1016/j.cell.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Luscher M. Pheromones: A new term for a class of biologically active substances. Nature. 1959;183(4653):55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kim H. G, Kurth I, Lan F, Meliciani I, Wenzel W, Eom S. H, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. American Journal of Human Genetics. 2008;83(4):511–519. doi: 10.1016/j.ajhg.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J. V. Human pheromones and food odors: Epigenetic influences on the socioaffective nature of evolved behaviors. Socioaffective Neuroscience & Psychology. 2012;2:17338. doi: 10.3402/snp.v2i0.17338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J. V, Atzmueller M, Fink B, Grammer K. Human pheromones: Integrating neuroendocrinology and ethology. Neuro Endocrinology Letters. 2001;22(5):309–321. [PubMed] [Google Scholar]
- Kohl J. V, Francoeur R. T. The scent of Eros: Mysteries of odor in human sexuality. 2nd ed. New York: Continuum Press; 1995/2002. [Google Scholar]
- Kondrashov F. A. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proceedings of Biological Sciences. 2012;279(1749):5048–5057. doi: 10.1098/rspb.2012.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu Z.-W, Borok E, Gao X.-B, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. The Journal of Neuroscience. 2010;30(2):703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotitschke A, Sadie-Van Gijsen H, Avenant C, Fernandes S, Hapgood J. P. Genomic and nongenomic cross talk between the gonadotropin-releasing hormone receptor and glucocorticoid receptor signaling pathways. Molecular Endocrinology. 2009;23(11):1726–1745. doi: 10.1210/me.2008-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassance J.-M, Liénard M. A, Antony B, Qian S, Fujii T, Tabata J, et al. Functional consequences of sequence variation in the pheromone biosynthetic gene pgFAR for Ostrinia moths. Proceedings of the National Academy of Sciences United States of America. 2013;110:3967–3972. doi: 10.1073/pnas.1208706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledón-Rettig C. C, Richards C. L, Martin L. B. Epigenetics for behavioral ecologists. Behavioral Ecology. 2012. Retrieved from: http://beheco.oxfordjournals.org/content/early/2012/09/21/beheco.ars145.abstract. [DOI]
- Lee D. A, Bedont J. L, Pak T, Wang H, Song J, Miranda-Angulo A, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nature Neuroscience. 2012;15(5):700–702. doi: 10.1038/nn.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Penzo M. A, Taniguchi H, Kopec C. D, Huang Z. J, Li B. Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience. 2013;16:332–339. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Korzan W. J, Ferrero D. M, Chang R. B, Roy D. S, Buchi M, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Current Biology. 2013;23(1):11–20. doi: 10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht J. M, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature Neuroscience. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale J. W. The consequences of enhanced cell-autonomous glucose metabolism. Trends in Endocrinology and Metabolism. 2012;23(11):545–551. doi: 10.1016/j.tem.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Logan D. W, Brunet L. J, Webb W. R, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current Biology. 2012;22(21):1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord K. A comparison of the sensory development of wolves (Canis lupus lupus) and dogs (Canis lupus familiaris) Ethology. 2013;119(2):110–120. [Google Scholar]
- Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. The honey bee epigenomes: Differential methylation of brain DNA in queens and workers. PLoS Biology. 2010;8(11):e1000506. doi: 10.1371/journal.pbio.1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko F, Maleszka R. Insects as innovative models for functional studies of DNA methylation. Trends in Genetics. 2011;27(4):127–131. doi: 10.1016/j.tig.2011.01.003. [DOI] [PubMed] [Google Scholar]
- MacDonald J. L, Roskams A. J. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Progress in Neurobiology. 2009;88(3):170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Majdic G, Tobet S. Cooperation of sex chromosomal genes and endocrine influences for hypothalamic sexual differentiation. Frontiers in Neuroendocrinology. 2011;32(2):137–145. doi: 10.1016/j.yfrne.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen K. M, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Swaab D. F, van der Kouwe A, Abbs B, Boriel D, Handa R, et al. Volumetric parcellation methodology of the human hypothalamus in neuroimaging: Normative data and sex differences. NeuroImage. 2013;69:1–10. doi: 10.1016/j.neuroimage.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markopoulos F, Rokni D, Gire D. H, Murthy V. N. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Saathoff M, Kuhn F, Max H, Terstegen L, Natsch A. A functional ABCC11 allele is essential in the biochemical formation of human axillary odor. The Journal of Investigative Dermatology. 2010;130(2):529–540. doi: 10.1038/jid.2009.254. [DOI] [PubMed] [Google Scholar]
- McEwen B. S. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl. 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. Hormones and the social brain. Science. 2013;339(6117):279–280. doi: 10.1126/science.1233713. [DOI] [PubMed] [Google Scholar]
- Meiklejohn C. D, Holmbeck M. A, Siddiq M. A, Abt D. N, Rand D. M, Montooth K. L. An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genetics. 2013;9(1):e1003238. doi: 10.1371/journal.pgen.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. The European Journal of Neuroscience. 2010;32(12):2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Mittag J, Lyons D. J, Sallstrom J, Vujovic M, Dudazy-Gralla S, Warner A, et al. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. The Journal of Clinical Investigation. 2013;123:509–516. doi: 10.1172/JCI65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr M. A, Sisk C. L. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4792–4797. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan K, Lomvardas S. How keeping active pays off in the olfactory system. eLife Sciences. 2012;1:e00326. doi: 10.7554/eLife.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J. H. Transgenerational genetic effects on phenotypic variation and disease risk. Human Molecular Genetics. 2009;18(R2):R202–R210. doi: 10.1093/hmg/ddp366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F. Understanding the bases of sex differences. Science. 1981;211(4488):1263–1264. doi: 10.1126/science.7209509. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy P. A, Vitzthum V. J, Flinn M. V. Evolutionary endocrinology: Integrating proximate mechanisms, ontogeny, and evolved function. American Journal of Human Biology. 2009;21(6):728–730. doi: 10.1002/ajhb.20924. [DOI] [PubMed] [Google Scholar]
- Ng W.-L, Bassler B. L. Bacterial quorum-sensing network architectures. Annual Review of Genetics. 2009;43(1):197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N, Gesquiere L, Alberts S. C, Altmann J. Sex differences in the mother-neonate relationship in wild baboons: Social, experiential and hormonal correlates. Animal Behaviour. 2012;83(4):891–903. [Google Scholar]
- Niehuis O, Buellesbach J, Gibson J. D, Pothmann D, Hanner C, Mutti N. S, et al. Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature. 2013;494:345–348. doi: 10.1038/nature11838. [DOI] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339(6117):335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell L. A, Hofmann H. A. Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Frontiers in Neuroendocrinology. 2011;32(3):320–335. doi: 10.1016/j.yfrne.2010.12.004. [DOI] [PubMed] [Google Scholar]
- O’Connell L. A, Hofmann H. A. Evolution of a vertebrate social decision-making network. Science. 2012;336(6085):1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Moskal J. R, Panksepp J. B, Kroes R. A. Comparative approaches in evolutionary psychology: Molecular neuroscience meets the mind. Neuro Endocrinology Letters. 2002;23(Suppl. 4):105–115. [PubMed] [Google Scholar]
- Papasaikas P, Valcarcel J. Splicing in 4D. Science. 2012;338(6114):1547–1548. doi: 10.1126/science.1233219. [DOI] [PubMed] [Google Scholar]
- Peper J. S, Koolschijn P. C. Sex steroids and the organization of the human brain. The Journal of Neuroscience. 2012;32(20):6745–6746. doi: 10.1523/JNEUROSCI.1012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix C. H, Goy R. W, Gerall A. A, Young W. C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65(3):369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Preti G, Leyden J. J. Genetic influences on human body odor: From genes to the axillae. The Journal of Investigative Dermatology. 2010;130(2):344–346. doi: 10.1038/jid.2009.396. [DOI] [PubMed] [Google Scholar]
- Riccio A. Dynamic epigenetic regulation in neurons: Enzymes, stimuli and signaling pathways. Nature Neuroscience. 2011;13(11):1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- Roland A. V, Moenter S. M. Regulation of gonadotropin-releasing hormone neurons by glucose. Trends in Endocrinology and Metabolism. 2011;22(11):443–449. doi: 10.1016/j.tem.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J, Klingerman C. M, Abdulhay A. A. Sense and nonsense in metabolic control of reproduction. Frontiers in Endocrinology. 2012;3:26. doi: 10.3389/fendo.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. J, Friedman J, Cordero O. X, Preheim S. P, Timberlake S. C, Szabo G, et al. Population genomics of early events in the ecological differentiation of bacteria. Science. 2012;336(6077):48–51. doi: 10.1126/science.1218198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. Mobile DNA and evolution in the 21st century. Mobile DNA. 2010;1(1):4. doi: 10.1186/1759-8753-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Rethinking the (im)possible in evolution. Progress in Biophysics and Molecular Biology. 2012. Retrieved from: http://dx.doi.org/10.1016/j.pbiomolbio.2012.08.016.
- Stearns S. C. Evolutionary medicine: Its scope, interest and potential. Proceedings of Biological Sciences. 2012;279(1746):4305–4321. doi: 10.1098/rspb.2012.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr M, Maderspacher F. Olfactory evolution: Mice rethink stink. Current Biology. 2013;23(2):R59–R61. doi: 10.1016/j.cub.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Swarup S, Huang W, Mackay T. F. C, Anholt R. R. H. Analysis of natural variation reveals neurogenetic networks for Drosophila olfactory behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(3):1017–1022. doi: 10.1073/pnas.1220168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammen S. A, Friso S, Choi S. W. Epigenetics: The link between nature and nurture. Molecular Aspects of Medicine. 2013;34(4):753–764. doi: 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann A. The effect of external influences upon development (The Romanes Lecture) London: Henry Frowde; 1894. [Google Scholar]
- Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16372–16377. doi: 10.1073/pnas.1000423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Erdal D. The human socio-cognitive niche and its evolutionary origins. Philosophical Transactions of the Royal Society of London. Series B. Biological Sciences. 2012;367(1599):2119–2129. doi: 10.1098/rstb.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Sackett J, Mieczkowski B, Richie A, Thoemke K, Rumbley J, et al. Fertilization in C. elegans requires an intact C-terminal RING finger in sperm protein SPE-42. BMC Developmental Biology. 2011;11(1):10. doi: 10.1186/1471-213X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson J. C. I love you with all my brain: Laying aside the intellectually dull sword of biological determinism. Socioaffective Neuroscience & Psychology. 2012;2:17334. doi: 10.3402/snp.v2i0.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav J. S, Joshi B. V, Gurjar M. K. An enantiospecific synthesis of (4R,5R)-5-hydroxy-4-decanolide from d-glucose. Carbohydrate Research. 1987;165(1):116–119. [Google Scholar]
- Yan J, Jiang X, West A. A, Perry C. A, Malysheva O. V, Devapatla S, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. The American Journal of Clinical Nutrition. 2012;95(5):1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- Zafon C. Evolutionary endocrinology: A pending matter. Endocrinologia y Nutricion (English Edition) 2012;59(1):62–68. doi: 10.1016/j.endonu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y, Warren S. T. Neurogenetics: Advancing the ‘next-generation’ of brain research. Neuron. 2010;68(2):165–173. doi: 10.1016/j.neuron.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]