Abstract
The complete nucleotide sequence of plasmids pMCBF1 and pMCBF6 was determined and analyzed. pMCBF1 and pMCBF6 form a novel clade within the IncP-1 plasmid family designated IncP-1 ς. The plasmids were exogenously isolated earlier from a marine biofilm. pMCBF1 (62 689 base pairs; bp) and pMCBF6 (66 729 bp) have identical backbones, but differ in their mercury resistance transposons. pMCBF1 carries Tn5053 and pMCBF6 carries Tn5058. Both are flanked by 5 bp direct repeats, typical of replicative transposition. Both insertions are in the vicinity of a resolvase gene in the backbone, supporting the idea that both transposons are “res-site hunters” that preferably insert close to and use external resolvase functions. The similarity of the backbones indicates recent insertion of the two transposons and the ongoing dynamics of plasmid evolution in marine biofilms. Both plasmids also carry the insertion sequence ISPst1, albeit without flanking repeats. ISPs1is located in an unusual site within the control region of the plasmid. In contrast to most known IncP-1 plasmids the pMCBF1/pMCBF6 backbone has no insert between the replication initiation gene (trfA) and the vegetative replication origin (oriV). One pMCBF1/pMCBF6 block of about 2.5 kilo bases (kb) has no similarity with known sequences in the databases. Furthermore, insertion of three genes with similarity to the multidrug efflux pump operon mexEF and a gene from the NodT family of the tripartite multi-drug resistance-nodulation-division (RND) system in Pseudomonas aeruginosa was found. They do not seem to confer antibiotic resistance to the hosts of pMCBF1/pMCBF6, but the presence of RND on promiscuous plasmids may have serious implications for the spread of antibiotic multi-resistance.
Introduction
Plasmids are independent from the bacterial host chromosome and conjugative broad-host range (BHR) plasmids can transfer between many different species in a bacterial community. Thus, plasmids are a particularly fluid part of the bacterial genome and one that may provide the cell with new traits that allow adaptation to selection forces, especially to transient environmental changes and challenges. Plasmids are likely to evolve differently in different bacterial backgrounds [1] and might also be influenced by environmental conditions [2]. Thus analysis of plasmids from different environments is important for our understanding of these important mobile elements (MGE). There are a number of studies about plasmids in marine environments [3], [4], [5], [6], [7], [8], [9], [10], [11], as well as reports about the role of marine plasmids in antibiotic resistance [12], [13], [14], [15] and population dynamics [16], [17], for excellent reviews on this topic see [18], [19]. Still, compared with many other environments, plasmids from marine habitats are not well studied and fully sequenced plasmids with marine origins are underrepresented in the databases.
Using an exogenous isolation strategy, we have earlier isolated plasmids from marine bulk water, biofilms and the surface microlayer [20]. One of these plasmids, pMCBF1 isolated from a biofilm, was shown to have a BHR and transferred to many Gram-negative bacteria, including Planctomyces maris [21]. We also measured comparatively high transfer rates of pMCBF1 from a P. putida donor to indigenous bacteria, directly in seawater [4]. pMCBF1 was therefore an interesting BHR marine biofilm plasmid to characterize further. We determined the complete nucleotide sequences of pMCBF1 and the related pMCBF6, and a brief summary of the plasmids was presented earlier [1]. It was also shown that they form a novel clade within the promiscuous IncP-1 plasmid incompatibility group [1].
Since pMCBF1 and pMCBF6 are the only representatives of their IncP-1 clade, and except for pMLUA1, pMLU3 and pMUA4 [22], so far the only sequenced IncP-1 plasmids isolated from marine environments, it is interesting to analyze both their backbone genes and their accessory genes, and to compare these with other IncP-1 plasmids. Here we present a detailed analysis of pMCBF1 and pMCBF6.
Materials and Methods
Bacterial strains, plasmids and growth conditions
P. putida UWC1 [23] and P. putida KT2440 [24] with pMCBF1 and pMCBF6 were grown overnight at 26°C in Luria-Bertani media [25] with 10 g NaCl per liter and supplemented with HgCl2 at 17 mg per liter. Escherichia coli with pMCBF1 and pMCBF6 were grown overnight at 37°C in the same medium supplemented with ampicillin (50 mg/liter).
Mercury and antibiotic resistance tests
To test for phenyl mercury resistance, P. putida KT2440 with either pMCBF1 or pMCBF6 were grown overnight and spread as lawns on LB plates. Discs that were dipped in solutions of phenyl mercury chloride (saturated solution in ether) were dried and placed on the lawns. Plates were inoculated overnight at 30°C after which the diameters of clearing zones around the discs were recorded. P. putida KT2440 without plasmids was used as controls. For the antibiotic resistance tests susceptibility discs (OXOID, UK) were placed on bacterial lawns of E. coli HB101 [26] and CAG [27] with and without pMCBF1 and pMCBF6 and incubated in 37°C overnight and clearing zones recorded as above.
Sequencing and sequence analysis
Sequencing of pMCBF1 and pMCBF6 was performed earlier in our laboratory using standard techniques [1]. Sequence alignments presented here were created using the eBioX program, the phylogenetic network was created using the SplitsTree program [28], and the similarity analysis was carried out using the SimPlot program [29]. The content of the genomes of the IncP-1 plasmids vary and all genes are not present in all plasmids [1]. The network constructed here was based on the largest segment that we could identify in which all genes were present in all analyzed plasmids (TraC to TraM).
Nucleotide sequence accession numbers
The complete sequences from pMCBF1 and pMCBF6 are deposited with GeneBank CoreNucleotide (accession # AY950444 and EF107516, respectively).
Results and Discussion
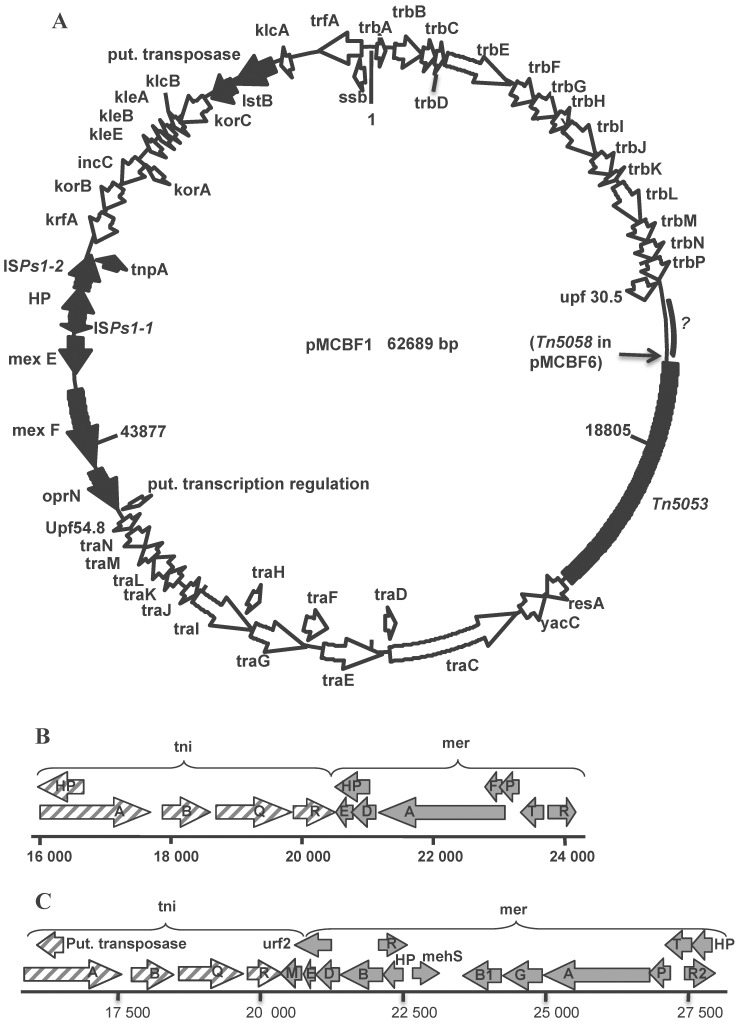
pMCBF1 and pMCBF6 represent the first fully sequenced IncP-1 plasmids from marine environments. Generally the difference between the IncP-1 plasmids sequenced so far lies in the various insertions of resistance and catabolic genes, often as part of MGEs, onto a more or less conserved backbone structure. pMCBF1 and pMCBF6 are obvious examples of this since they differ only in their mercury resistance (HgR) transposons that have inserted on otherwise identical backbones (Fig. 1A). Furthermore, the similarity of pMCBF1 and pMCBF6 strongly indicates that the insertions of Tn5053 in pMCBF1 and Tn5058 in pMCBF6 are recent events.
Figure 1. Genetic map of the IncP-1 ς plasmids pMCBF1 and pMCBF6.
The backbones of the two plasmids are identical and differ only with regard to inserted transposons. Panel (A) shows pMCBF1 with Tn5053. Coding regions are shown by arrows, indicating the direction of transcription. Unfilled arrows denote plasmid backbone genes, black arrows denote accessory genes. An arrow points to the insertion site of Tn5058 in the pMCBF6 backbone. The section of the pMCBF1/pMCBF6 backbone that has a low similarity to other sequences in the databases is marked with a question mark. Panels B and C shows transposon Tn5053 on pMCBF1 and Tn5058 on pMCBF6, respectively. Striped arrows denote genes in the transposition module (tni) and gray arrows denote mercury resistance (mer) genes. HP means hypothetical proteins.
Plasmid backbone
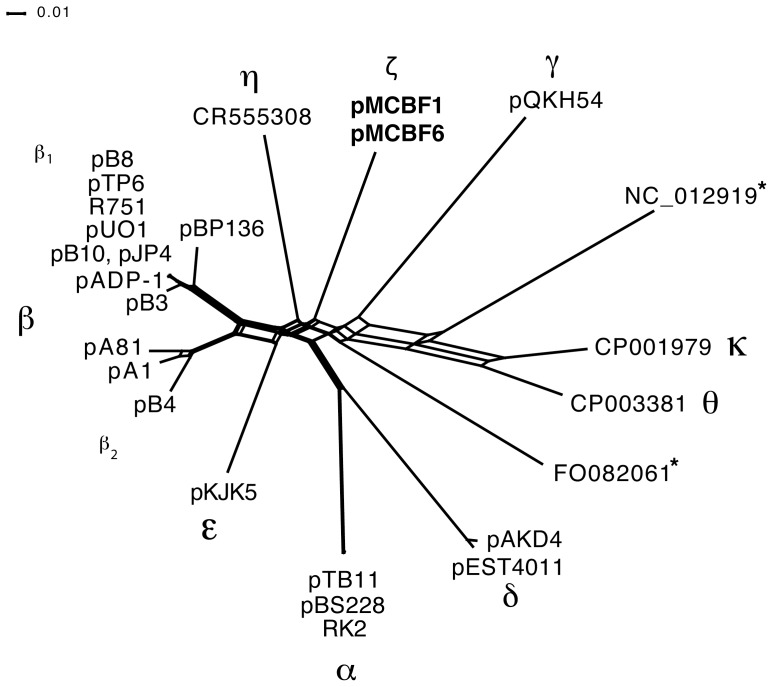
Although the focus of most previous studies has been on accessory genes and MGEs, it has been shown that also the IncP-1 plasmid backbones vary and can be divided into different evolutionary clades. Hitherto, twelve phylogenetic clades have been described for the IncP-1 plasmid group, which are designated α, β-1, β-2, γ, δ, ε, ς, κ, η, θ [1], [30], [31], [32], [33], [34], [35] and two unnamed groups (Fig. 2). The pMCBF1/pMCBF6 plasmids described here are so far the only described plasmids from the IncP-1 ς-clade.
Figure 2. Phylogenetic network of clades of the IncP-1 plasmid family.
The network is based on representative plasmids from each of the twelve previously described phylogenetic clades of the IncP-1 plasmid family. The network is based on the genetic segment harboring the traC - traM genes for all analyzed plasmids. The two plasmids pMCBF1 and pMCBF6 described in this study are highlighted in bold. Clades without designated names are marked with *. Previously described intra-clade recombinants [1] were not included in the analysis. The figure is updated from [1].
As shown in Fig 2, the members of the IncP-1 plasmid backbone family form a star-phylogeny. Such topology is typical when there has been extensive recombination [36], [37], exponential population growth [38], [39], or if members of each clade have evolved in different distinct separated populations (i.e., long time of evolution since a common ancestor result in long external branches). It has previously been demonstrated that homologous recombination has contributed to the evolution of the IncP-1 backbones [1]. IncP-1 plasmids have also been found in different environments, such as soil, wastewater treatment plants, and in human pathogens and fish pathogens [35], and as described here in a marine biofilm. Furthermore, it was recently demonstrated that the IncP-1 plasmid backbone has evolved in, and genetically adapted to, vastly different host bacterial species [1]. It is thus likely that the IncP-1 backbone at least periodically has evolved in different separated populations, which may explain the diversity and the star-shaped phylogeny of this BHR plasmid family.
We have identified 44 open reading frames (ORFs) in the pMCBF1/pMCBF6 backbone. pMCBF1 has 24 ORFs and pMCBF6 28 ORFs that are related to accessory genes or functions such as transposons, various resistance functions, IS elements and multi-drug efflux systems. There are various genetic distances between the pMCBF1/pMCBF6 backbone genes and the corresponding genes in plasmids from the other clades, which is probably explained by the history of recombination [1]. As an example, most of the backbone genes such as replication, conjugation and stable maintenance genes share 58–95% similarity with genes from plasmids pADP-1 [40], pB4 [41], R751 [31] and pTSA [42] from the β clade, which is the most studied IncP-1 clade.
The origin of replication (oriV) of pMCBF1/pMCBF6 resembles other IncP-1 plasmids. The nine TrfA DNA binding sites (iterons) in the vicinity of the pMCBF1/pMCBF6 oriV have the 17 bp consensus sequence N(T/C/G)GCCCCTCA(A/T)(A/G/C)T(A/G)T(C/T)A, and have been conserved to some degree compared to both RK2 and R751. The R751 iterons by comparison have the sequence (A/C/G)NGCCCC(T/C)C A(A/T)(G/C)(T/G)GTCA. The pMCBF1/pMCBF6 iterons are arranged as eight direct repeats and one inverted repeat, similar to the iterons close to oriV in R751.
The TrfA protein is necessary for the replication of iteron containing theta replication plasmids (e.g. [43]) and trfA has been used as a base for plasmid classification [44]. We suggested earlier that pMCBF1 and pMCBF6 had replication functions that were different from known plasmid incompatibility groups based on lack of hybridization with the inc/rep Couturier probes [20]. However, the general lack of similarity of the Couturier probes with many environmentally isolated plasmids has been noted in several investigations [9], [18], [45], [46], [47]. We now know that the IncP-1 Couturier probe only detects the IncP-1 α clade [48]. In fact, the nucleotide similarity between pMCBF1/pMCBF6 and the Couturier IncP-1 probe is only about 60% and these plasmids could therefore not be targeted by the probe.
The pMCBF1/pMCBF6 backbone has no insert between the replication initiation gene trfA and the oriV. Except for the IncP-1 β2 plasmids pBP136 and pA1, most other IncP-1 plasmids have an insert, usually several kilobases (kb), that separates trfA and oriV.
Just as for the IncP-1 β plasmids, pMCBF1/pMCBF6 lack the parABCDE genes that play a key role in stability of IncP-1 α plasmids. Although the parABCDE genes may be absent from pMCBF1/pMCBF6, they apparently possess the korA-incC-korB locus that mediates accurate plasmid segregation. Surface exclusion systems also protect the resident plasmid from competition, by preventing new plasmids to enter the cell. pMCBF1/pMCBF6 carry the trbK homologue, which confers surface exclusion in plasmid RK2. We know from recombination analyses that pMCBF1/pMCBF6 have been involved in recombination events [1], which strongly suggest that these plasmids have been in the same host cell as other IncP-1 plasmids [1]. Thus, it seems that the surface exclusion systems of pMCBF1/pMCBF6 have a “leakiness” that allow recombination to occur.
One block of about 2.5 kb, between the inserted HgR transposons and the trb section (marked with a question mark in Fig.1) has a low similarity (73% identity) to Methylophaga sp. JAM7 [49].
Accessory genes, transposons and insertion sequences
The DNA segment bearing the mercury resistance determinant on pMCBF1 (Fig. 1B) shows 99% nucleotide similarity to Tn5053 [50]. There are five base pair direct repeats in the flanking plasmid DNA, which are typically formed after Tn5053 transposition [50]. Interestingly, Tn5053 in pMCBF1 is inserted close to the resolvase gene (resA) in the backbone (Fig. 1A) which supports the suggestion that Tn5053 is a “resolvase-site-hunter” that insert in “hot-spots” associated with res sites and resolvase genes [51]. Minakhina et al. suggested that the plasmid resolvase may participate in the transposition event, but the exact mechanism is not clear [51]. If so, this is an interesting case in which the transposon is dependent on an external site-specific recombination system. The two inverted repeats (IR) that form the ends of Tn5053 in pMCBF1 have 2 and 4 mismatches compared with other published sequences [52]. There are also 4 mismatches between the two IR of Tn5053 in pMCBF1. However, this apparently did not stop transposition of Tn5053 into pMCBF1. Tn5053 belongs to a superfamily of diverse elements including retroviruses, phage Mu, insertion sequences of the IS3 family, and transposons Tn552, Tn7 and Tn5058 [53]. They are found on plasmids within the IncP-1 group (pPUO1, pB11 and pMCBF1/pMCBF6) as well as on other plasmid groups, such as the Enterobacter cloacae plasmid pELC_A [54].
The DNA segment bearing the mercury resistance determinant on pMCBF6 (Fig. 1C) is highly similar to Tn5058 in Pseudomonas sp. ED23-33 [55]. The length of both sequences are 12, 373 bp and the nucleotide similarity varies from 96 to 99% except for the first 257 bp where similarity is 84%, but the IR at the start of the element again has a perfect match with Tn5058. The element has 25 bp inverted repeats with 2 mismatches. The complex Tn5058 is part of the Tn5053 family (see above) [53], [56]. Judging from comparisons with the closest relatives of Tn5058, such as Tn21, Tn501, Tn5053, Tn5041D, Tn5718 and others, it was suggested that Tn5058 was formed by a complicated series of recombination events involving these elements [56]. Tn5058 is also carried by other IncP-1 plasmids such as pIJB1, pWEC911 and pTP6. Interestingly, Tn5058 has been detected in bacteria preserved for 15.000–40.000 years in permafrost grounds [56]. When compared to several Tn5058 collected in modern days, there were high sequence similarities suggesting that these transposons are genetically stable over time. We compared the Tn5058 in plasmid pMCBF6 with the Tn5058 isolated from permafrost, using the sliding window protocol implemented in the SimPlot program. Our results support previous suggestions that the Tn5058 is indeed well conserved and there is a high nucleotide identity between the transposon Tn5058 isolated here and the transposon Tn5058 isolated from permafrost in most regions. Least conserved was the gene tniA with a 200 bp region of only than 80% identity and merB and merR with regions of less than 95% identity between the Tn5058 in pMCBF6 and the Tn5058 isolated from permafrost. Tn5058 has two copies of merR, merB and merD. Interestingly, it was suggested that the duplications in some of the mer genes in Tn5041D were the result of an integration, via homologous recombination, of a mer containing circular DNA structure [55]. The circular cassette was speculated to originate from an ancestral donor in which the mer genes were flanked by IS elements. The duplication of mer genes in Tn5058 might also have been the result of a similar event [55]. Just as for Tn5053 in pMCBF1, Tn5058 is flanked by 5 base pair direct repeats in pMCBF6, which strongly indicate a transposition event [50], [52]. These flanking direct repeats are also seen when Tn5058 is inserted in IncP-1 plasmid pIJB1. The insertion of Tn5058 in pMCBF6 is only 63 bp from the site where Tn5053 inserted in pMCBF1, which is close to the resolvase gene (resA) in the plasmid backbone. The insertion site further confirms that Tn5058 is a “res-site hunter” [51].
The sequence data suggest that Tn5053 confers mercury resistance towards inorganic mercury while Tn5058 also carries resistance towards organo-mercury compounds. When the two plasmids were tested for phenyl mercury chloride (PhHg) resistance the diameter of the clearing zones around PhHg discs was only 6 mm for P. putida 2440(pMCBF6) but 13 mm for P. putida 2440(pMCBF1) and the plasmid-free P. putida 2440, confirming the PhHg resistance of pMCBF6 but not of pMCBF1.
A 2982 bp sequence between kfrA and mexE in both pMCBF1 and pMCBF6 shows 99% (nucleotide) similarity with insertion sequence ISPs1 (Fig. 1A). The 24 bp IR regions with 4 mismatches characteristic of ISPs1 [57] are found in pMCBF6/pMCBF1. ISPs1 is related to the ISL3 family, but is larger than the usual ISL3 elements which are 1300–1550 bp. ISPs1 has been found in several copies in Pseudomonas stutzeri, where it has inserted into, and inactivated, catabolic genes [57], and in Yersinia ruckeri plasmid pYR1 [58]. Interestingly, ISPs1 was also recently found on plasmid pAMEC615 from the marine Alteromonas macleodii isolated from the English Channel [59]. In Pseudomonas stutzeri, ISPs1 was flanked by eight bp direct repeats (DR), indicating a replicative transposition event [57], but no such flanking DR was found in any of pMCBF1/pMCBF6, pAMEC615 or pYR1. If DR were formed during a replicative transposition in these plasmids, they may have degenerated with time, perhaps because these sequences are not under selective pressure. Alternatively, the ISPs1was inserted by some other recombination mechanism than replicative transposition. The insertion point of ISPs1 in pMCBF1/6, within the plasmid control region between kfrA and upf54.8, seems to be unique among IncP-1 plasmids, indicating that insertion points other than the ones between tra and trb regions and between the replication region and oriV, are possible.
A region containing a putative remnant of an insertion event is located between klcA and klcB. This region contains an ORF with closest similarity (74%) to a putative transposase, as well as an IstB homolog. IstB is associated with IS21 family insertion sequences. The function of IstB is unknown, but it may assist in transposition [60]. We find no DR or IR as signs of a recent insertion. Insertions of transposons in the klcA/klcB region are seen also in IncP-1 plasmid RP1/RK2 [61].
Adjacent to ISPs1 is an inserted region that shows a high similarity to part of the recently sequenced plasmid pALIDE201 from Alicycliphilus denitrificans isolated from anaerobic sewer sludge [62]. Furthermore, three genes within this region show similarity (73–67%) to mexEF and a gene from the NodT family, which are part of tripartite multi-drug resistance-nodulation-division (RND) families. Such efflux systems are common on chromosomes of many Gram-negative bacteria [63], e.g. the mexEF-oprN in Pseudomonas aeruginosa. The IncP-1 β plasmid pB4 has a region with about 80% similarity to a RND efflux system in P. denitrificans, this was the first plasmid that was shown to carry a RND efflux system [41]. Adjacent to the RND region in pB4 a putative transposon terminus was found close to a Tn3-like transposase, and the authors speculated that the RND efflux system might have been transferred to pB4 from the chromosome of an unknown gram-negative bacterium by transpositional cointegrate formation [41]. Although ISPs1 is found adjacent to the mex-region in pMCBF6/pMCBF1, we do not know if this IS element was involved in the mex insertion. The MexEF-OprN system in P. aeruginosa confers resistance to aromatic hydrocarbons, fluoroquinolones, chloramphenicol, triclosan and trimethoprim [63]. Our experimental data shows that when tested in disk diffusion tests for antibiotic sensitivity on agar plates, E. coli with pMCBF1 or pMCBF6 did not confer a higher resistance to chloramphenicol, nalidixic acid or trimethoprim than plasmid free cells. Further studies are needed to define the possible function of MexEF-OprN in pMCBF1/pMCBF6. Our finding that plasmids from a marine biofilm carry a multi-drug efflux system shows that pB4 is not unique, and that many different efflux systems may potentially be borne by promiscuous plasmids. The possibly serious implications of multi-drug efflux systems on such plasmids and the effects this may have on the spread of multi-resistance to human pathogens have been discussed [41].
The majority of the well-known IncP-1 plasmids, and plasmids in general, originate from soil environments or from a clinical environment and other man-made systems, such as wastewater treatment plants [35]. pMCBF1/pMCBF6 are among the first IncP-1 plasmids that are sequenced from marine environments. Three IncP-1ε plasmids (pMLUA1, pMLUA3, pMLUA4) were recently isolated from the air-water interface from an estuary in Portugal [22], [64]. Analysis of these, and other plasmids from marine bacteria, has often revealed new features [7], [10], [11], [22]. Analysis of the pMCBF1/pMCBF6 plasmids also showed, among other things, that they form a new clade within the IncP-1 plasmid group.
Acknowledgments
We would like to thank Robert Almstrand for skillful technical assistance.
Funding Statement
Financial support for this study was provided by The Swedish Research Council (contract no. 2002-214), to M.H. and by the University of Gothenburg. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Norberg P, Bergström M, Jethava V, Dubhashi D, Hermansson M (2011) The IncP-1 plasmid backbone adapts to different host bacterial species and evolves through homologous recombination. Nat Comm 2: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirzhner V, Paz A, Volkovich Z, Nevo E, Korol A (2007) Different clustering of genomes across life using the A-T-C-G and degenerate R-Y alphabets: early and late signaling on genome evolution? J Mol Evol 64: 448–456. [DOI] [PubMed] [Google Scholar]
- 3. Cook M, Osborn A, Bettandorff J, Sobecky P (2001) Endogenous isolation of replicon probes for assessing plasmid ecology of marine sediment microbial communities. Microbiology 147: 2089. [DOI] [PubMed] [Google Scholar]
- 4. Dahlberg C, Bergström M, Hermansson M (1998) In situ detection of high levels of horizontal plasmid transfer in marine bacterial communities. Appl Environ Microbiol 64: 2670–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hermansson M, Jones GW, Kjelleberg S (1987) Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria in the marine air-water interface. Appl Environ Microbiol 53: 2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Paulsen IT, Palenik B (2011) Analysis of two marine metagenomes reveals the diversity of plasmids in oceanic environments. Environ Microbiol 14: 453–466. [DOI] [PubMed] [Google Scholar]
- 7. Petersen J, Frank O, Göker M, Pradella S (2013) Extrachromosomal, extraordinary and essential—the plasmids of the Roseobacter clade. Appl Microbiol Biotech 97: 2805–2815. [DOI] [PubMed] [Google Scholar]
- 8. Sobecky P, Mincer T, Chang M, Toukdarian A, Helinski D (1998) Isolation of broad-host-range replicons from marine sediment bacteria. Appl Environ Microbiol 64: 2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sobecky PA, Mincer TJ, Chang MC, Helinski DR (1997) Plasmids isolated from marine sediment microbial communities contain replication and incompatibility regions unrelated to those of known plasmid groups. Appl Environ Microbiol 63: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhong Z, Caspi R, Helinski D, Knauf V, Sykes S, et al. (2003) Nucleotide sequence based characterizations of two cryptic plasmids from the marine bacterium Ruegeria isolate PR1b. Plasmid 49: 233–252. [DOI] [PubMed] [Google Scholar]
- 11. Zhong Z, Toukdarian A, Helinski D, Knauf V, Sykes S, et al. (2001) Sequence analysis of a 101-Kilobase plasmid required for agar degradation by a Microscilla isolate. Appl Environ Microbiol 67: 5771–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang J, Wang C, Shu C, Liu L, Geng J, et al. (2013) Marine sediment bacteria harbor antibiotic resistance genes highly similar to those found in human pathogens. Microb Ecol 65: 975–981. [DOI] [PubMed] [Google Scholar]
- 13. Phelan RW, Clarke C, Morrissey JP, Dobson ADW, O'Gara F, et al. (2011) Tetracycline resistance-encoding plasmid from Bacillus sp. strain #24, isolated from the marine sponge haliclona simulans. Appl Environ Microbiol 77: 327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baya AM, Brayton PR, Brown VL, Grimses DJ, Russek-Cohen E, et al. (1986) Coincident plasmids and antimicrobial resistance in marine bacteria isolated from polluted and unpolluted Atlantic Ocean samples. Appl Environ Microbiol 51: 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sizemore RK, Colwell RR (1977) Plasmids carried by antibiotic-resistant marine bacteria. Antimicrob Ag Chemoth 12: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palenik B, Ren Q, Tai V, Paulsen IT (2009) Coastal Synechococcus metagenome reveals major roles for horizontal gene transfer and plasmids in population diversity. Environ Microbiol 11: 349–359. [DOI] [PubMed] [Google Scholar]
- 17. Pradella S, Päuker O, Petersen J (2010) Genome organisation of the marine Roseobacter clade member Marinovum algicola. Arch Microbiol 192: 115–126. [DOI] [PubMed] [Google Scholar]
- 18. Smalla K, Sobecky PA (2002) The prevalence and diversity of mobile genetic elements in bacterial communities of different environmental habitats: insights gained from different methodological approaches. FEMS Microbiol Ecol 42: 165–175. [DOI] [PubMed] [Google Scholar]
- 19. Sobecky PA, Hazen TH (2009) Horizontal gene transfer and mobile genetic elements in marine systems. Methods in molecular biology (Clifton, NJ) 532: 435–453. [DOI] [PubMed] [Google Scholar]
- 20. Dahlberg C, Linberg C, Torsvik VL, Hermansson M (1997) Conjugative plasmids isolated from bacteria in marine environments show various degrees of homology to each other and are not closely realted to well characterized plasmids. Appl Environ Microbiol 63: 4692–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dahlberg C, Bergström M, Andreason M, Christensen BB, Molin S, et al. (1998) Interspecies bacterial conjugation by plasmids from marine environmnents visualized by gfp expression. Mol Biol Evol 15: 385–390. [Google Scholar]
- 22. Oliveira CS, Moura A, Henriques I, Brown CJ, Rogers LM, et al. (2013) Comparative genomics of IncP-1ε plasmids from water environments reveals diverse and unique accessory genetic elements. Plasmid 70: 412–419. [DOI] [PubMed] [Google Scholar]
- 23. McClure NC, Weightman AJ, Fry JC (1989) Survival of Pseudomonas putida UWC1 containing cloned catabolic genes in a model activated-sludge unit. Appl Environ Microbiol 55: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, et al. (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4: 799–808. [DOI] [PubMed] [Google Scholar]
- 25.Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbour Laboratory.
- 26. Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41: 459–472. [DOI] [PubMed] [Google Scholar]
- 27. Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, et al. (1989) A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli . Microbiol Rev 53: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huson DH (2005) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 29. Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, et al. (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pansegrau W, Lanka E, Barth PT, Figurski DH, Guiney DG, et al. (1994) Complete nucleotide sequence of Birmingham IncP alpha plasmids. Compilation and comparative analysis. J Mol Biol 239: 623–663. [DOI] [PubMed] [Google Scholar]
- 31. Thorsted PB, Macartney DP, Akhtar P, Haines AS, Ali N, et al. (1998) Complete sequence of the IncP plasmid R751: Implications for evolution and organisation of the IncP backbone. J Mol Biol 282: 969–990. [DOI] [PubMed] [Google Scholar]
- 32. Vedler E, Vahter M, Heinaru A (2004) The completely sequenced plasmid pEST4011 contains a novel IncP1 backbone and a catabolic transposon harboring tfd genes for 2,4-dichlorophenoxyacetic acid degradation. J Bacteriol 186: 7161–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haines AS, Akhtar P, Stephens ER, Jones K, Thomas CM, et al. (2006) Plasmids from freshwater environments capable of IncQ retrotransfer are diverse and include pQKH54, a new IncP-1 subgroup archetype. Microbiology (Reading, England) 152: 2689–2701. [DOI] [PubMed] [Google Scholar]
- 34. Bahl MI, Hansen LH, Goesmann A, Sørensen SJ (2007) The multiple antibiotic resistance IncP-1 plasmid pKJK5 isolated from a soil environment is phylogenetically divergent from members of the previously established alpha, beta and delta sub-groups. Plasmid 58: 31–43. [DOI] [PubMed] [Google Scholar]
- 35. del Castillo CS, Bin Jang H, Hikima J-i, Jung TS, Morii H, et al. (2013) Comparative analysis and distribution of pP9014, a novel drug resistance IncP-1 plasmid from Photobacterium damselae subsp. piscicida. Int J Antimicrob Agents 42: 10–18. [DOI] [PubMed] [Google Scholar]
- 36. Schierup MH, Hein J (2000) Recombination and the molecuar clock. Mol Biol Evol 17: 1578–1579. [DOI] [PubMed] [Google Scholar]
- 37. Schierup MH, Hein J (2000) Consequences of Recombination on Traditional Phylogenetic Analysis. Genetics 156: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes EC, Pybus OG, Harvey PH (1999) The molecular population dynamics of HIV-1. In: Crandall KA, editor. The Evolution of HIV. Baltimore: The Johns Hopkins University Press. pp. 177–207.
- 39. Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial-DNA sequences in stable and exponentially growing populations. Genetics 129: 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martinez B, Tomkins J, Wackett LP, Wing R, Sadowsky MJ (2001) Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J Bacteriol 183: 5684–5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tauch A, Schlüter A, Bischo N, Goesmann A, Meyer F, et al. (2003) The 79,370-bp conjugative plasmid pB4 consists of an IncP-1b backbone loaded with a chromate resistance transposon, the strA- strBstreptomycin resistance gene pair, the oxacillinase gene blaNPS-1, and a tripartite antibiotic efflux system of the resistance-nodulation-division family. Mol Gen Genomics 268: 570–584. [DOI] [PubMed] [Google Scholar]
- 42. Tralau T, Cook AM, Ruff J (2001) Map of the IncP1{beta} plasmid pTSA encoding the widespread genes (tsa) for p-toluenesulfonate degradation in Comamonas testosteroni T-2. Appl Environ Microbiol 67: 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas CM, editor (2000) The Horizontal gene pool. Amsterdam, The Netherlands: Harwood Academic Publ. 419 p. [Google Scholar]
- 44. Couturier MF, Bex P, Bergquist L, Maas WK (1988) Identification and classification of bacterial plasmids. Microbiol Rev 52: 375–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kobayashi N, Bailey M (1994) Plasmids isolated from the sugar beet phyllosphere show little or no homology to molecular probes currently available for plasmid typing. Microbiology 140: 289–296. [DOI] [PubMed] [Google Scholar]
- 46. Osborn AM, Pickup RW, Saunders JR (2000) Development and application of molecular tools in the study of IncN-related plasmids from lakewater sediments. FEMS Microbiol Lett 186: 203–208. [DOI] [PubMed] [Google Scholar]
- 47. van Elsas JD, McSpadden Gardener BB, Wolters AC, Smit E (1998) Isolation, characterization, and transfer of cryptic gene-mobilizing plasmids in the wheat rhizosphere. Appl Environ Microbiol 64: 880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heuer H, Smalla K (2012) Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol Rev 36: 1083–1104. [DOI] [PubMed] [Google Scholar]
- 49. Villeneuve C, Martineau C, Mauffrey F, Villemur R (2012) Complete genome sequences of Methylophaga sp. strain JAM1 and Methylophaga sp. strain JAM7. J Bacteriol 194: 4126–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kholodii GY, Yurieva OV, Lomovskaya OL, Gorlenko ZM, Mindlin SZ, et al. (1993) Tn5053, a mercury resistance transposon with integron's ends. J Mol Biol 230: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 51. Minakhina S, Kholodii G, Mindlin S, Yurieva O, Nikiforov V (1999) Tn5053 family transposons are res unters sensing plasmidal res sites occupied by cognate resolvases. Mol Microbiol 33: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 52. Partridge SR (2011) Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev 35: 820–855. [DOI] [PubMed] [Google Scholar]
- 53. Kholodii GY, Mindlin SZ, Bass IA, Yurieva OV, Minakhina SV, et al. (1995) Four genes, two ends, and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol Microbiol 17: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 54. Ren Y, Ren Y, Zhou Z, Guo X, Li Y, et al. (2010) Complete Genome Sequence of Enterobacter cloacae subsp. cloacae Type Strain ATCC 13047. J Bacteriol 192: 2463–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kholodii G, Gorlenko Z, Mindlin S, Hobman J, Nikiforov V (2002) Tn5041-like transposons: molecular diversity, evolutionary relationships and distribution of distinct variants in environmental bacteria. Microbiology 148: 3569–3582. [DOI] [PubMed] [Google Scholar]
- 56. Mindlin S, Minakhin L, Petrova M, Kholodii G, Minakhina S, et al. (2005) Present-day mercury resistance transposons are common in bacteria preserved in permafrost grounds since the Upper Pleistocene. Res Microbiol 156: 994–1004. [DOI] [PubMed] [Google Scholar]
- 57. Bolognese F, di Lecce C, Galli E, Barbieri P (1999) Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl Environ Microbiol 65: 1876–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welch TJ, Fricke WF, McDermott PF, White DG, Rosso M-L, et al. (2007) Multiple antimicrobial resistance in plague: An emerging public health risk. PLoS ONE 2: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lopez-Perez M, Gonzaga A, Rodriguez-Valera F (2013) Genomic diversity of deep ecotype Alteromonas macleodii isolates: evidence for pan-Mediterranean clonal frames. Gen Biol Evol 5: 1220–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith CA, Pinkney M, Guiney DG, Thomas CM (1993) The ansestral IncP replication system consisted of contigous oriV and trfA segments as deduced from a comparison of the nucleotide sequences of diverse IncP plasmids. J Gen Microbiol 139: 1761–1766. [DOI] [PubMed] [Google Scholar]
- 61. Pinyon JL, Hall RM (2011) Evolution of IncP-1α Plasmids by Acquisition of Antibiotic and Mercuric Ion Resistance Transposons. Microb Drug Resist 17: 339–343. [DOI] [PubMed] [Google Scholar]
- 62. Oosterkamp MJ, Veuskens T, Plugge CM, Langenhoff AAM, Gerritse J, et al. (2011) Genome sequences of Alicycliphilus denitrificans strains BC and K601T. J Bacteriol 193: 5028–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Poole K (2001) Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol 4: 500–508. [DOI] [PubMed] [Google Scholar]
- 64. Oliveira CS, Lázaro B, Azevedo JSN, Henriques I, Almeida A, et al. (2012) New molecular variants of epsilon and beta IncP-1 plasmids are present in estuarine waters. Plasmid 67: 252–258. [DOI] [PubMed] [Google Scholar]