Abstract
The fungal pathogen Penicillium marneffei produces melanin-like pigment in vitro. The synthetic pathway of melanin and its possible influence in the protective yeast cells surviving within macrophage cells are not known. In this work, P. marneffei produced brown black pigment in the presence of L-DOPA and black particles were extracted from yeast cells treated with proteolytic enzymes, denaturant and concentrated hot acid. Kojic acid inhibited the brown-black pigment production of P. marneffei yeast grown on brain heart infusion agar. Transmitting electron microscopy showed spherical granular electron-dense particles with an average diameter of 100 nm in a beaded arrangement in the innermost cell wall. Electron-paramagnetic resonance revealed that the black particles contain a stable free radical compound. The UV-visible and Fourier transform infrared spectra of particles extracted from P. marneffei and synthetic DOPA-melanin showed a high degree of similarity. Melanized yeast cells decreased phagocytosis by macrophage cells and increased resistance to intracellular digestion in vitro. These results indicate that P. marneffei can synthesize DOPA-melanin or melanin-like compounds in vitro and suggest that the DOPA-melanin pathway is associated with cell wall structure and enhances the resistance to phagocytosis by macrophages.
Introduction
Penicillium marneffei, recently renamed as Talaromyces marneffei [1], is a thermally dimorphic fungus that appears in mycelia form at 25°C and yeast form at 37°C. The fungus is endemic in Southeast Asia and causes systemic infection in humans, especially in immune suppressed individuals. P. marneffei infection is fatal if untreated in immunocompromised hosts. The pathogen has become one of the most common HIV-related infections in Southeast Asia [2], [3].
Once P. marneffei invades the host, it proliferates first in the reticuloendothelial system before disseminating throughout the host. The ability of P. marneffei yeast cells to survive within host cells is essential to enable the fungus to produce systemic infection, and this survival ability renders the fungus difficult to be completely cleared from the body. The mechanisms by which P. marneffei protects itself from the host immune defense remain unclear. Expression of acid phosphatase activity in P. marneffei yeast cells [4], and the upregulation of the cpeA and sodA genes in this yeast phase [5], [6], may be associated with the oxidative stress response during intracellular infection.
Melanin pigments are dark brown and black pigments formed by oxidative polymerization of phenolic compounds. These high molecular weight amorphous polymers are widely found in bacteria, fungi, plants and animals. Many fungi synthesize melanins, and several types of melanins are known to exist in the fungal kingdom. Two major types of melanin, 1,8-dihydroxynaphthalene (DHN) and L-3,4-dihydroxyphenylalanine (DOPA), are found in fungi. Fungal melanin contributes to virulence in an array of human pathogen fungi, including Cryptococcus neoformans [7], [8], Paracoccidioides brasiliensis [9], Aspergillus fumigatus [10], Histoplasma capsulatum [11], Blastomyces dermatitidis [12] and Malassezia furfur [13]. Fungal melanin can influence the immune response of the host by several pathways, including reduction of the oxidative burst capacity of macrophages [14], inhibition of apoptosis in macrophages [10], and inhibition of cytokine production in the host [15].
P. marneffei conidia and yeast cells can produce melanin or melanin-like compounds in vitro and in vivo [16]. The polyketide synthase gene of the melanin-biosynthesis gene cluster (alb1) in P. marneffei contributes to melanin pigment production and the pathogen virulence [17]. Whether P. marneffei synthesizes melanin via the DHN pathway, DOPA pathway, or both pathways has not been determined.
The distribution of melanin in P. marneffei yeast cells and the role of melanin during intracellular infection remain unclear. There are two possible starting molecules in the DOPA pathway of melanin synthesis, L-DOPA or tyrosine. In the present study, we examined the role of L-DOPA on the synthesis of melanin in P. marneffei and the physicochemical properties of melanin isolation from P. marneffei yeast cells. Given the potential role of melanin in the virulence of P. marneffei, we also investigated the effect of melanin in yeast cells on their phagocytosis by Ana-1 mouse macrophage cells.
Materials and Methods
Fungal strain and preparation of inocula
The P. marneffei FRR2161 strain was generously donated by Dr. Alex Andronopolous (Melbourne University, Australia). The GXMU110608 P. marneffei strain was isolated from an HIV positive patient through regular fungal culture for diagnosis at the first affiliated hospital of Guangxi Medical University in 2006. Stock cultures were maintained in our laboratory with 6-month transfers to potato dextrose agar (PDA). To obtain the yeast phase of P. marneffei, colonies grown on PDA at 25°C were transferred to culture on brain heart infusion agar (BHI) at 37°C and maintained by continuous weekly passages until 70% or more cells transformed into yeast-like cells. The cells were harvested with 0.1% Tween80 and the number of fungi was counted with a hemocytometer. A conidia suspension that contained approximately 1–5×106 cell per milliliter was used as the inoculum for shake culture.
L-DOPA culture
P. marneffei FRR2161 and GXMU110608 yeast cultures were grown on a chemically defined liquid medium (0.22 M glucose, 2.0 mM MgSO4.7H2O, 1.8 mM KH2PO4, 15.2 mM asparagine, and 1.7 mM vitamin B1) with or without 1.0 mM L-DOPA (Sigma Chemical Co., U.S.) for 15 days at 37°C in a rotary shaker at 200 rpm. The chemically defined liquid medium with 1.0 mM DOPA but no P. marneffei was designated as control. All cultures were incubated in the dark to avoid photo polymerization of L-DOPA into melanin. Yeast cells were smeared and stained by cotton blue, and then samples were examined under optical microscope. Cells were collected by centrifugation at 2000 rpm for 30 min, washed with PBS, and stored at 4°C until use.
Culture with or without melanin inhibitors
Kojic acid and tricyclazole have been used as inhibitors to study the pathway of melanin synthesis in a few fungal species [13], [18], [19]. To investigate the melanin pathway in P. marneffei, FRR2161 and GXMU110608 strains were grown on BHI slant culture at 37°C. An inoculation loop was used to transfer yeast cells from slant culture to BHI plates, BHI plates with 100 μg/ml kojic acid, or BHI plates with 100µg/ml tricyclazole (Sigma Chemical Co., U.S.). A BHI plate with 1.0 mM DOPA but no P. marneffei was designated as control. After culture at 37°C for 7 days, the color of colonies was observed.
Electron microscopy
The culture suspensions of FRR2161 and GXMU110608 strains from chemically defined liquid medium were placed in 3% glutaraldehyde and fixed overnight at 4°C. Samples were then applied to a polylysine-coated coverslip, serially dehydrated in alcohol and replaced with isoamyl acetate for further dehydration. Samples were then dried by the critical-point drying method and further evaporated with carbon. The sample was coated with gold-palladium and the surface structures of yeast cells were observed by a scanning electron microscope (JSM-T300, Japan). For transmission electron microscopic observation, the culture suspension was allowed to stand for 1 h, and the precipitate was embedded in 2% agar and then fixed overnight in 3% glutaraldehyde at 4°C. The sample was incubated in 1% osmic acid for 2 h, dehydrated, and embedded in epoxy resin. Ultrathin sections were placed on nickel grids and examined using a Hitachi H-500 transmission electron microscope.
Isolation and purification of yeast melanin particles
Melanin particles were isolated from FRR2161 and GXMU110608 strains grown on chemically defined liquid medium supplemented with 1.0 mM L-DOPA. Yeast cells were collected by centrifugation at 5000 rpm for 10 min. The pellet was washed three times with PBS, and suspended in 1.0 M sorbitol/0.1 M sodium citrate (pH 5.5). Cells were collected by centrifugation, wall-lysing enzymes (from Trichoderma harzianum [Sigma-Aldrich]) dissolved in doubled distilled water were added at 15 mg/ml, and the suspension was incubated overnight at 30°C to generate protoplasts. The protoplasts were collected by centrifugation, washed with PBS, and incubated in 4.0 M guanidine thiocyanate overnight at room temperature. Cell debris were collected by centrifugation, washed three times with PBS, and digested with 1.0 mg/ml proteinase K overnight at 37°C. The resulting product was washed three times with PBS and then boiled in 6.0 M HCl for 1.5 h. The insoluble residue was collected by centrifugation, washed extensively with distilled water, and dried to obtain a black pigment crude product. Black pigment crude product was fully dissolved in 1 M NaOH; the supernatant was collected by centrifugation, adjusted with 6 M HCI to pH 2–3, allowed to stand overnight, and the precipitate was collected by centrifugation. The procedure was repeated three times. The precipitate was washed extensively with distilled water and dried to obtain a black pigment purified product.
Characterization of melanin pigment
The solubility of purified P. marneffei pigment from FRR2161 and GXMU110608 strains and synthetic DOPA-melanin (2 mg) were tested with distilled water, hot 1.0 M NaOH solution and common organic solvents (methanol, absolute ethanol, propanol, acetone, acetonitrile, iso-amyl alcohol, and chloroform). Reactions with oxidizing agents such as 30% hydrogen peroxide (H2O2) were determined. Purified P. marneffei melanin was subjected to various analyses such as UV-visible absorption (Varian Cary50), Fourier transform infrared (FT-IR) spectra (Perkin Elmer Spectrum100) and electron-paramagnetic resonance (EPR) (Bruker EMX-10/12). The UV absorption was performed using 40 μg/ml P. marneffei melanin dissolved in sodium hydroxide scanning at approximately 200–800 nm wavelength. The dried 2 mg P. marneffei melanin was mixed with 200 mg potassium bromide by grinding in an agate mortar for 5 min, and the sample was examined by infrared spectral scanning at approximately 4000–400 cm-1. The various instrumental parameters of EPR were set at 100 kHZ modulation frequency, 1.0 G modulation amplitude, 5.0 mW microwave power, 9.75 GHZ microwave frequency, 3475.0 G center magnetic field strength, 100.0 G scan width and 83.89 s scan time.
In vitro phagocytosis
The FRR2161 strain was inoculated in BHI slants at 37°C, and generations were transferred every three days until pure yeast cells were acquired. The P. marneffei colonies were flooded with PBS and the number of fungi was counted with a hemocytometer after washing. The concentration of yeast suspension was adjusted to 1×107/ml for experimental use. Ana-1 mouse macrophage cells (1×106/bottle) were cultured in RPMI-1640 medium containing 10% fetal bovine serum. Yeast cells in a 200 μl suspension were cultured with the Ana-1 mouse macrophage cell line pretreated with 1 μg/ml recombinant murine IFN-γ. Culture groups were divided into the experimental group cultured with 1 mM L-DOPA and the control group cultured without L-DOPA. Ana-1 mouse macrophage cells cultured with 1 mM L-DOPA without yeast cells were designated as blank controls. The macrophage cells and yeast cells were observed under an inverted microscope during the 7 days of culture, after which cells were collected by centrifugation at 3000 rpm for 5 min. The method of sample preparation for transmission electron microscopy was described as above. The tests were repeated three times on different days.
Statistical analysis
The average number of yeast cells per macrophage in P. marneffei cultured with L-DOPA was compared to control. The data was analyzed by t-test for two independent samples, with significance defined as P<0.05.
Results
Colors of medium and isolation from culture
The liquid medium colors of the cultured FRR2161 and GXMU110608 strains supplemented with L-DOPA at 37°C by shake culture were brown-black. No pigmentations were observed in P. marneffei strains cultured without L-DOPA and in control medium (Fig. 1A). Pigment production was not observed in control flasks, indicating that L-DOPA autopolymerization does not occur. The yeast cells of P. marneffei isolated from the chemically defined medium with L-DOPA appeared as dark brown (Fig. 1B) and darkly pigmented particles was isolated from the yeast cells (Fig. 1C). When cultured on BHI at 37°C, the yeast colony surface was smooth, its color was black/tan, and brown-black pigment was produced around the colony (Fig. 1D). However, when cultured on BHI at 37°C with kojic acid (Fig. 1E) or tricyclazole (Fig. 1F), the color of the yeast colony became lighter, and no brown-black pigment was produced around the colony cultured with kojic acid. Pigment production was also not observed in control plate (Fig.1G).
Figure 1. Black pigment production of P. marneffei in media under various culture conditions.
FRR2161 strain grown on chemically defined liquid medium with or without L-DOPA, control flask (A) and the yeast cells isolated from the medium (B). Black particles isolated from the dark brown yeast cells(C). FRR2161 strain grown on BHI (D), BHI plates with 100 μg/ml kojic acid (E) or BHI plates with 100 μg/ml tricyclazole (F) and a BHI plate with DOPA but no P. marneffei(G).
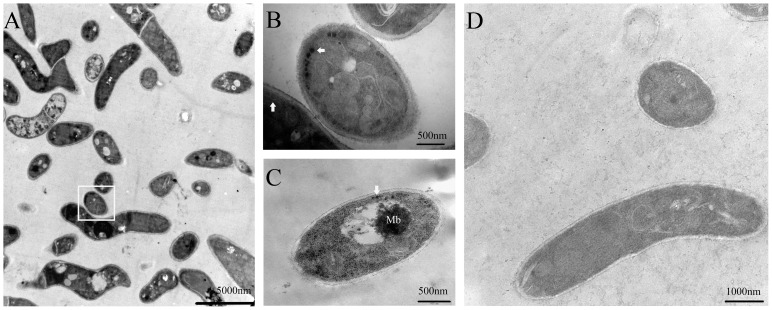
Morphological analysis by electron microscopy
Yeast cells grown with or without L-DOPA showed no difference as observed by scanning electron microscopy. Transmission electron microscopy analysis of yeast cells supplemented with L-DOPA showed spherical granular electron-dense particles with an average diameter of 100 nm in a beaded arrangement in the innermost cell wall (Fig. 2A, B, C). Enlargement of microbodies size with electron-dense material increased significantly was observed (Fig. 2C). The other cell organelles, such as mitochondrion and liposome body, had no significant changes. Spherical granular electron-dense particles and electron-dense material deposited in microbodies were not seen in the yeast cells cultured without L-DOPA (Fig. 2D).
Figure 2. TEM examination of P. marneffei FRR2161 cultured in chemically defined liquid medium with or without L-DOPA.
Spherical granular electron-dense particles (white arrowheads) were in a beaded arrangement in the innermost cell wall in the presence of L-DOPA (A, B, C).Magnification of area indicated by rectangle in panel A (B). Enlargement of microbody (Mb) size with electron-dense material increased was observed(C). No spherical granular electron-dense particles were observed in yeast cells without L-DOPA (D).
Physicochemical analysis of P. marneffei melanin
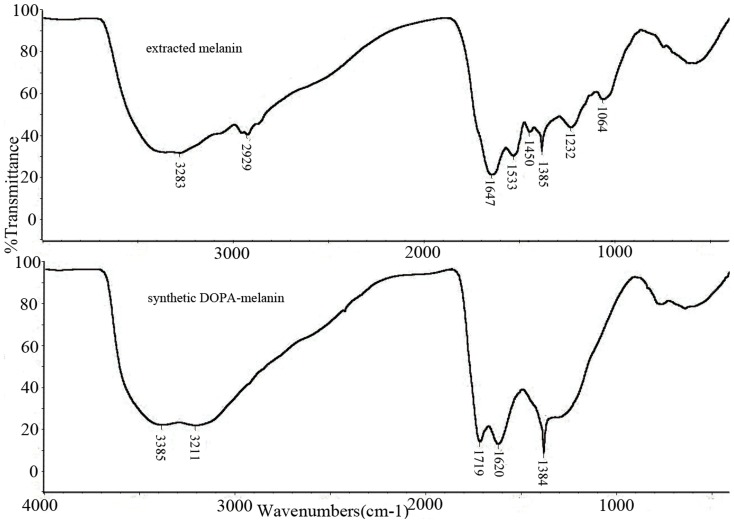
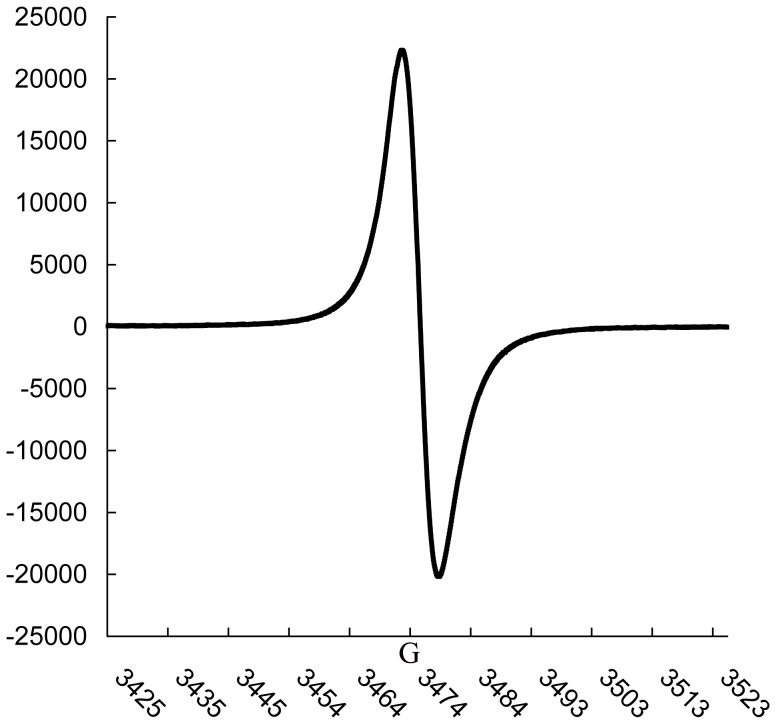
The physicochemical properties of the melanin from P. marneffei were very similar to synthetic DOPA-melanin. Both substances were insoluble in water and common organic solvents, but were soluble in hot NaOH; they precipitated in concentrated HCl and decolorized in the presence of H2O2. These characteristics are common to various melanin described in literature [20], [21], [22]. UV-vis analysis indicated that melanin extracted from P. marneffei had a typical absorption peak at 205 nm and absorbed progressively less as the wavelength increased. In the FT-IR, the sample showed a broad absorption at 3283 cm-1, indicating the presence of an OH group and NH2 group. Absorption at 1647 cm-1 was attributed to aromatic ring C = C stretching spectra. The extracted melanin and synthetic DOPA-melanin showed similar spectrum (Fig. 3). However, the absorption peaks of extracted melanin had several different peaks compared with synthetic DOPA-melanin: (i) the peak occurred at 2929 and 1450 cm-1, which indicated a CH2 group and CH3 group, respectively, and (ii) the peaks of 1533, 1385, 1232 and 1064 cm-1 indicated N-H, C-N, C-OH and C-O stretching spectra, respectively. EPR spectroscopy is a particularly effective method for studying melanin pigments, since these pigments uniquely contain a stable population of organic free radicals [23]. In this study, EPR revealed that particles from the yeast cells of P. marneffei contained a stable free radical compound (Fig.4). The presence of stable paramagnetic species is a characteristic of the melanin pigment [23].
Figure 3. FT-IR analysis of the melanin extracted from P. marneffei and the synthetic DOPA-melanin.
Figure 4. EPR spectral analysis of the black particles extracted from P. marneffei.
Effect of DOPA-melanin on phagocytosis
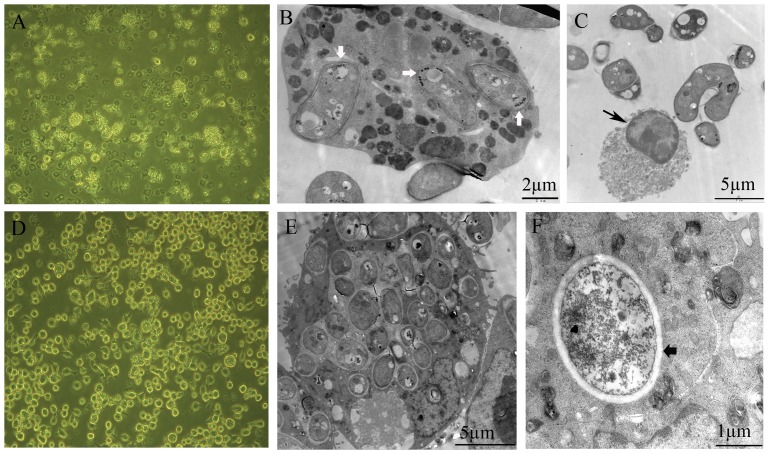
The number of macrophage cells infected with P. marneffei yeast cells in the presence of L-DOPA decreased compared to the control culture infected with P. marneffei yeast cells without L-DOPA after 7 days of culture as observed by an inverted microscope (Fig.5A, D). The number of macrophage cells in the blank control (culture without yeast cells in the presence of L-DOPA) increased with normal cell morphology. Transmission electron microscopy was used to analyze cells, and yeast cells with the beaded arrangement of spherical granular electron-dense particles were defined as melanized cells. Under transmission electron microscopy, phagocytosis of 1–18 melanized yeast cells by a single macrophage cell was observed, with an average 6.5±4.3 (mean±SD) yeast cells per macrophage in an examination of 100 macrophages (Fig. 5B). The yeast cells with the beaded arrangement of spherical granular electron-dense particles in the inner cell wall maintained cell structural integrity within macrophage cells. Disintegration of macrophage cells was observed in the group infected with melanized yeast cells (Fig. 5C). Phagocytosis of 5–25 non-melanized yeast cells by a single macrophage cell was observed, with an average 11.6±4.4 (mean±SD) yeast cells per macrophage in an examination of 100 macrophages (Fig. 5E). Intracellular digestion of non-melanized yeast cell was observed (Fig. 5F), but the frequency was low. The average number of yeast cells per macrophage in the group in the presence of L-DOPA was significantly less than that of the control group (P < 0.01).
Figure 5. Microscopic examination of macrophages cultured with P. marneffei.
Culture of Ana-1 mouse macrophage cells with P. marneffei in the presence of L-DOPA (A:×200). Phagocytosis of the melanized yeast cells (short white arrowheads) by Ana-1 mouse macrophage cells (B), disintegration of macrophage cells (black arrowhead) and the extracellular melanized yeast cells (C). Culture of Ana-1 mouse macrophage cells with P. marneffei without L-DOPA (D:×200). Phagocytosis of the non-melanized yeast cells by Ana-1 mouse macrophage cells (E), and intracellular digestion of non-melanized yeast cells (short black arrowhead) (F).
Discussion
Several fungi, such as C. neoformans [24] and P. brasiliensis [25], synthesize melanin via the DOPA pathway in a process resembling mammalian melanin biosynthesis. There are two possible starting molecules in this pathway, either L-DOPA or tyrosine. In this study, we found that P. marneffei can synthesize DOPA-melanin or melanin-like compounds in vitro. Our evidence supporting the DOPA-melanin production of P. marneffei yeast is as follows: (i) darkly pigmented liquid medium of P. marneffei culture in the presence of L-DOPA, (ii) spherical granular electron-dense particle beaded arrangement at the inner cell wall observed by transmission electron microscopic in yeast cells cultured both in defined liquid medium and in RPMI-1640 medium supplemented with L-DOPA; (iii) isolation of black particles from yeast cells of P. marneffei cultured in the presence of L-DOPA; (iv) EPR demonstration of particles from the yeast cells of P. marneffei containing a stable free radical compound; (v) particles extracted from P. marneffei and synthetic DOPA-melanin showed similar physicochemical characterization and spectra analysis by UV-vis and FT-IR; and (vi) kojic acid inhibition of the brown-black pigment production of P. marneffei grown on BHI. Kojic acid effectively inhibits the rate of formation of pigmented product due to the direct effect of kojic acid on tyrosinase [26], [27]. Kojic acid significantly reduced the production of DOPA-melanin or melanin-like compounds in Malassezia furfur [13] and Klebsiella sp. GSK [22]. Kojic acid effectively inhibited the black pigment production of P. marneffei grown on BHI, indicating the yeast cells produce DOPA-melanin or melanin-like compounds. In the present study, we also observed that tricyclazole inhibited the brown-black pigment production of P. marneffei grown on BHI at 37°C, and a previous study confirmed that the PKS gene cluster regulates the melanin pigment production [17]. These results indicate that P. marneffei can synthesize DHN-melanin in vitro. All evidence supports P. marneffei synthesis of melanin by both the DOPA pathway and DHN pathway in vitro. It is noteworthy that both melanin pathways are found in another dimorphic fungus Sporothrix schenckii [28]. The observation that fungi have both DOPA and DHN pathways for melanin production may be associated with a concomitant increase in protection against unfavorable conditions in the environment and during infection.
Fungal melanin is associated with cell wall structure. Solid-state nuclear magnetic resonance techniques were used to track the process of melanin synthesis in C. neoformans, and results suggested that melanin may act as a cell wall component attached to polysaccharide [29]. The arrangement of melanin in the cell wall is dependent on the fungal species. Melanin in C. neoformans was found deposited in the innermost layer of the cell wall and composed of granular particles approximately 40–130 nm in diameter, and the melanin was synthesized at sites of vesicles associated with lipid [30], [31], [32]. In contrast, in Histoplasma capsulatum, melanin was found on the outer layer, and yeast cells grown with L-DOPA formed tufts on the cell surface [11]. Similarly, Fonsecaea pedrosoi showed patched granules with an average diameter of 47 nm on the cell surface [19]. However, melanin in P. brasiliensis was found at the cell surface and cytoplasm [9]. In this study, DOPA-melanin of P. marneffei composed of spherical granular particles approximately 100 nm in diameter were found in the innermost layer of the cell wall, and the melanin particles were in a beaded arrangement. The beaded arrangement of melanin particles has not been previously found in other fungi, and thus may be a characteristic feature for DOPA-melanin of P. marneffei. Recent evidence supports the hypothesis that fungal melanosomes exist. In Fonsecaea pedrosoi, melanin is stored in intracellular vesicles, named melanosomes. The melanosome fuses with the fungal cell membrane, where the melanin is released and reaches the cell wall [33]. The ultrastructure of P. marneffei cells grown on PDA or BHI palates without L-DOPA, such as mitochondria, endoplastic reticulum, liposome bodies and microbodies were observed by TEM in our previous study [34], no beaded electron-dense particles were observed. The beaded structure of P. marneffei cells in the presence of L-DOPA may be a melanosome of this yeast pathogen. Aliphatic groups (CH2 group and CH3 group) in the melanin from P. marneffei suggest that melanin synthesis may have an association with yeast cell wall components, such as polysaccharide attachment to melanin. The enlarged microbodies and electron-dense material increased significantly when the yeast cells of P. marneffei were cultured with L-DOPA. However, other cell organelles showed no significant changes. This suggests that the microbodies may be the synthesis site of melanin.
In certain fungal species, melanized cells of fungi inhibit phagocytosis by macrophages and decrease susceptibility to killing by free radicals or hydrolytic enzymes [9], [14], [35]. The average number of yeast cells per Ana-1 mouse macrophage in the presence of L-DOPA was significantly less than that of cultures without L-DOPA, which indicated that melanized P. marneffei inhibit phagocytosis by macrophages. Yeast cells of P. marneffei cultured with L-DOPA were not observed undergoing intracellular digestion by Ana-1 mouse macrophage cells, indicating that DOPA-melanin increased the resistance of yeast cells to hydrolytic enzymes. Furthermore, the reduction of the number of Ana-1 mouse macrophage cells and disintegration of macrophages in the presence of P. marneffei cultured with L-DOPA suggest that melanized yeast cells increase virulence to macrophages.
Taken together, the results obtained in this study indicate that P. marneffei can synthesize DOPA-melanin or melanin-like compounds when grown in vitro. The melanin particles may be associated with the cell wall structure in yeast form of P. marneffei. Melanized P. marneffei enhance the resistance to phagocytosis by macrophages and it even enhances the virulence to macrophage cells. Further studies should be performed on the genetic regulation of the DOPA pathway in the P. marneffei yeast form and the role of melanin as a potential target for therapeutic intervention in penicilliosis.
Acknowledgments
The authors would like to thank Dr. Alex Andronopolous (Melbourne University, Australia) for providing the P. marneffei cells.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 81160191). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, et al. (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol 70: 159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ustianowski AP, Sieu TP, Day JN (2008) Penicillium marneffei infection in HIV. Curr Opin Infect Dis 21: 31–36. [DOI] [PubMed] [Google Scholar]
- 3. Cao C, Liang L, Wang W, Luo H, Huang S, et al. (2011) Common Reservoirs for Penicillium marneffei Infection in Humans and Rodents, China. Emerg Infect Dis 17: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youngchim S, Vanittanakom N, Hamilton AJ (1999) Analysis of the enzymatic activity of mycelial and yeast phases of Penicillium marneffei . Med Mycol 37: 445–450. [DOI] [PubMed] [Google Scholar]
- 5. Pongpom P, Cooper CR Jr, Vanittanakom N (2005) Isolation and characterization of a catalase-peroxidase gene from the pathogenic fungus, Penicillium marneffei . Med Mycol 43: 403–411. [DOI] [PubMed] [Google Scholar]
- 6. Thirach S, Cooper CR Jr, Vanittanakom P, Vanittanakom N (2007) The copper, zinc superoxide dismutase gene of Penicillium marneffei: cloning, characterization, and differential expression during phase transition and macrophage infection. Med Mycol 45: 409–417. [DOI] [PubMed] [Google Scholar]
- 7. Nosanchuk JD, Rosas AL, Lee SC, Casadevall A (2000) Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 355: 2049–2050. [DOI] [PubMed] [Google Scholar]
- 8. Rosas AL, Nosanchuk JD, Feldmesser M, Cox GM, McDade HC, et al. (2000) Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun 68: 2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, et al. (2006) Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect 8: 197–205. [DOI] [PubMed] [Google Scholar]
- 10. Volling K, Thywissen A, Brakhage AA, Saluz HP (2011) Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell Microbiol 13: 1130–1148. [DOI] [PubMed] [Google Scholar]
- 11. Nosanchuk JD, Gomez BL, Youngchim S, Diez S, Aisen P, et al. (2002) Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect Immun 70: 5124–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nosanchuk JD, van Duin D, Mandal P, Aisen P, Legendre AM, et al. (2004) Blastomyces dermatitidis produces melanin in vitro and during infection. FEMS Microbiol Lett 239: 187–193. [DOI] [PubMed] [Google Scholar]
- 13.Youngchim S, Nosanchuk JD, Pornsuwan S, Kajiwara S, Vanittanakom N (2013)The role of L-DOPA on melanization and mycelial production in Malassezia furfur. PLoS One 8: e63764. Available:. Accessed 31 August 2013. [DOI] [PMC free article] [PubMed]
- 14.Cunha MM, Franzen AJ, Seabra SH, Herbst MH, Vugman NV, et al. (2010) Melanin in Fonsecaea pedrosoi: a trap for oxidative radicals. BMC Microbiol 10: 80. Available:. biomedcentral. com/1471-2180/10/80.Accessed 15 July 2013. [DOI] [PMC free article] [PubMed]
- 15. Chai LY, Netea MG, Sugui J, Vonk AG, van de Sande WW, et al. (2010) Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 215: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Youngchim S, Hay RJ, Hamilton AJ (2005) Melanization of Penicillium marneffei in vitro and in vivo. Microbiology 151: 291–299. [DOI] [PubMed] [Google Scholar]
- 17. Woo PC, Tam EW, Chong KT, Cai JJ, Tung ET, et al. (2010) High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei . Febs J 277: 3750–3758. [DOI] [PubMed] [Google Scholar]
- 18.Pal AK, Gajjar DU, Vasavada AR (2013) DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med Mycol: In press [DOI] [PubMed]
- 19. Franzen AJ, Cunha MM, Batista EJ, Seabra SH, De Souza W, et al. (2006) Effects of tricyclazole 5-methyl-1,2,4-triazol [3,4] benzothiazole), a specific DHN-melanin inhibitor, on the morphology of Fonsecaea pedrosoi conidia and sclerotic cells. Microsc Res Tech 69: 729–737. [DOI] [PubMed] [Google Scholar]
- 20. Nosanchuk JD, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50: 3519–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goncalves RC, Lisboa HC, Pombeiro-Sponchiado SR (2012) Characterization of melanin pigment produced by Aspergillus nidulans . World J Microbiol Biotechnol 28: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 22. Sajjan S, Kulkarni G, Yaligara V, Kyoung L, Karegoudar TB (2010) Purification and physiochemical characterization of melanin pigment from Klebsiella sp. GSK. J Microbiol Biotechnol 20: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 23. Enochs WS, Nilges MJ, Swartz HM (1993) A standardized test for the identification and characterization of melanins using electron paramagnetic resonance (EPR) spectroscopy. Pigment Cell Res 6: 91–99. [DOI] [PubMed] [Google Scholar]
- 24. Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, et al. (2007) Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153: 3954–3962. [DOI] [PubMed] [Google Scholar]
- 25. Gomez BL, Nosanchuk JD, Diez S, Youngchim S, Aisen P, et al. (2001) Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect Immun 69: 5760–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahn V (1995) Effect of kojic acid on the oxidation of DL-DOPA, norepinephrine, and dopamine by mushroom tyrosinase. Pigment Cell Res 8: 234–240. [DOI] [PubMed] [Google Scholar]
- 27. Kim YJ, Uyama H (2005) Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci 62: 1707–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almeida-Paes R, Frases S, Fialho Monteiro PC, Gutierrez-Galhardo MC, Zancope-Oliveira RM, et al. (2009) Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes Infect 11: 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhong J, Frases S, Wang H, Casadevall A, Stark RE (2008) Following fungal melanin biosynthesis with solid-state NMR: biopolymer molecular structures and possible connections to cell-wall polysaccharides. Biochemistry 47: 4701–4710. [DOI] [PubMed] [Google Scholar]
- 30. Eisenman HC, Nosanchuk JD, Webber JB, Emerson RJ, Camesano TA, et al. (2005) Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans . Biochemistry 44: 3683–3693. [DOI] [PubMed] [Google Scholar]
- 31. Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, et al. (2008) Extracellularvesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A (2009) Vesicle-associated melanization in Cryptococcus neoformans . Microbiology 155: 3860–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franzen AJ, Cunha MM, Miranda K, Hentschel J, Plattner H, et al. (2008) Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi . J Struct Biol 162: 75–84. [DOI] [PubMed] [Google Scholar]
- 34. Liu D, Liang L, Luo Q, Cao C (2011) Morphology of Penicillium marneffei under oxidative stress in vitro. Mycoses 54: 113–118. [DOI] [PubMed] [Google Scholar]
- 35. Rosas AL, Casadevall A (2001) Melanization decreases the susceptibility of Cryptococcus neoformans to enzymatic degradation. Mycopathologia 151: 53–56. [DOI] [PubMed] [Google Scholar]