Abstract
Infection-induced preterm birth is the largest cause of infant death and of neurological disabilities in survivors. Silibinin, from milk thistle, exerts potent anti-inflammatory activities in non-gestational tissues. The aims of this study were to determine the effect of silibinin on pro-inflammatory mediators in (i) human fetal membranes and myometrium treated with bacterial endotoxin lipopolysaccharide (LPS) or the pro-inflammatory cytokine IL-1β, and (ii) in preterm fetal membranes with active infection. The effect of silibinin on infection induced inflammation and brain injury in pregnant mice was also assessed. Fetal membranes and myometrium (tissue explants and primary cells) were treated with 200 μM silibinin in the presence or absence of 10 μg/ml LPS or 1 ng/ml IL-1β. C57BL/6 mice were injected with 70 mg/kg silibinin with or without 50 μg LPS on embryonic day 16. Fetal brains were collected after 6 h. In human fetal membranes, silibinin significantly decreased LPS-stimulated expression of IL-6 and IL-8, COX-2, and prostaglandins PGE2 and PGF2α. In primary amnion and myometrial cells, silibinin also decreased IL-1β-induced MMP-9 expression. Preterm fetal membranes with active infection treated with silibinin showed a decrease in IL-6, IL-8 and MMP-9 expression. Fetal brains from mice treated with silibinin showed a significant decrease in LPS-induced IL-8 and ninjurin, a marker of brain injury. Our study demonstrates that silibinin can reduce infection and inflammation-induced pro-labour mediators in human fetal membranes and myometrium. Excitingly, the in vivo results indicate a protective effect of silibinin on infection-induced brain injury in a mouse model of preterm birth.
Introduction
Preterm birth is the single leading cause of neonatal death worldwide, after exclusion of congenital defects, and can lead to numerous long-term health consequences for those surviving babies [1]. Spontaneous preterm birth, which accounts for almost 70% of all preterm births, may result from preterm labour with intact membranes or preterm pre-labour rupture of membranes (PPROM) [2]. Intrauterine infection, commonly presented as chorioamnionitis, is an acute inflammation of the membranes and chorion of the placenta, typically due to ascending bacterial infection. Chorioamnionitis complicates a third of patients with preterm labour [3] and is the most common complication associated with PPROM [4]. Chorioamnionitis predisposes the preterm infant to numerous organ disease, affecting cardiopulmonary, cerebral, and renal systems [5]; developmental delay and lifelong neurological impairments, such as mental retardation, cerebral palsy and learning and behavioural deficits, are caused by perinatal brain damage [6]–[8]. Besides the emotional burden on families, direct and indirect costs of preterm birth accounts for billions of health care dollars each year.
Labour, as an inflammatory event, is associated with increased expression of pro-inflammatory mediators in intrauterine tissues. These include pro-inflammatory cytokines IL-6, IL-8, TNF-α and IL-1β in the cervix, myometrium, fetal membranes and placenta [9]-[12]; prostaglandins PGE2 and PGF2α in the cervix, fetal membranes and myometrium [13]; and matrix metalloproteinase (MMP)-9 in the fetal membranes and placenta [14]. The regulation of these mediators leads to tissue remodelling of the cervix and fetal membranes, myometrial contractions, and membrane rupture, leading to successful delivery at term. It is untimely activation of these processes that can lead to spontaneous preterm birth [2].
There is no single drug that can treat all the underlying mechanisms that lead to spontaneous preterm birth. While there are currently measures to delay preterm labour, in an effort to provide enough time to administer antenatal corticosteroids or to transport the mother to a tertiary care facility, each have limitations and possible side effects. Tocolytics can delay preterm labour for a short time, including magnesium sulphate, beta-mimetics, calcium channel blockers, prostaglandin inhibitors and oxytocin receptor antagnonists [15]; however, the use of tocolytics is controversial due to the lack of evidence of significant benefits in neonatal outcomes [16]. Progesterone treatment has been associated with a reduction in rates of preterm birth and risk of infant birthweight of less than 2500 g [17]. Limitations include maternal side effects and a lack of information regarding optimal doses, mode of administration and gestation to commence therapy (prior to, or after 20 weeks gestation), as well as longer-term infant and childhood outcomes. Some clinical trials in progress include the tocolytic nifedipine [18], [19], nifedipine based on cervical length [20], and dietary supplementation of the omega-3 long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA), which resulted in longer gestation and larger infant size [21].
Silibinin, also known as silybin, is the main active compound of silymarin, the standardised extract of milk thistle. Silibinin has a long history of safe use by humans, exhibiting extremely low toxicity [22]. Silibinin has been utilised as an effective treatment for hepatic disease [23] and as an adjunctive cancer therapy [24], [25]. Silibinin has been shown to have potent anti-inflammatory and anti-cancer effects (reviewed in [26]); silibinin reduced pro-inflammatory mediators in vitro in canine hepatocytes [27], an HIV viral infection model [28], in vitro and in vivo models of mouse skin inflammation [29], [30], and skin and prostate cancer cells from animal models [24]. In mouse models of allergic inflammation [31] and infection-induced sepsis [32], silibinin decreased inflammation and increased survival rate. Chelation of iron may be a mode of action in the anti-cancer properties of silibinin [33]. While the effect of silibinin on reactive oxygen species and pro-inflammatory cytokines has been explored in peripheral blood monocytes from women with preeclampsia [34], [35], there are no other studies describing the effect of silibinin on labour mediators in human gestational tissues. Thus, the aims of this study were to determine the effect of silibinin on pro-inflammatory mediators in (i) human fetal membranes and myometrium treated with bacterial endotoxin lipopolysaccharide (LPS) or the pro-inflammatory cytokine IL-1β, and (ii) in preterm fetal membranes with chorioamnionitis. Further, the in vivo effect of silibinin was determined in a mouse model, where LPS was used to induce inflammation in C57BL/6 time mated mice. Maternal tissues and fetal brains were assessed for markers of inflammation and brain injury.
Materials and Methods
Ethics
Written informed consent was obtained from all participating patients. Ethics approval was obtained from the Mercy Hospital for Women's Research and Ethics Committee. Pregnant women were recruited to the study by a clinical research midwife.
Tissue collection
Human placentae with attached fetal membranes and myometrium were collected for two studies: from women who delivered at (i) term (>37 weeks gestation) at elective Caesarean section (indications for Caesarean section were breech presentation and/or previous Caesarean section) in the absence of labour; and (ii) preterm (<37 weeks gestation) with or without chorioamniontis. All tissues were obtained within 15 min of delivery.
For the term studies, fetal membranes, obtained 2 cm from the periplacental edge, and myometrial biopsies, obtained from the upper margin of the incision made in the lower uterine segment, were obtained from women who delivered healthy, singleton infants from elective Caesarean section in the absence of labour. Indications for Caesarean section included repeat Caesarean section or breech presentation. Women with any underlying medical conditions such as diabetes, asthma, polycystic ovarian syndrome, preeclampsia and macrovascular complications were excluded. Additionally, women with multiple pregnancies, obese women, fetuses with chromosomal abnormalities were excluded.
For the preterm studies, fetal membranes were obtained 2 cm from the periplacental edge (n = 7 patients). All the preterm placentas were swabbed for microbiological culture investigations and assessed for histopathological evidence of infection. Chorioamnionitis was diagnosed pathologically according to standard criteria which included histological evidence of macrophages and neutrophils permeating the chorionic cell layer and often infiltrating the amniotic cell. Four of the cases had histologically proven chorioamnionitis from mild to severe. The chorioamnionitis tissues were collected from women either after spontaneous labour onset at Caesarean section or after spontaneous vaginal delivery. The preterm women without infection all went into spontaneous labour and delivered vaginally or at Caesarean section (n = 3 patients). The mean gestation was 30±1.9 weeks; five of the women had PPROM with rupture occurring from 2 to 26 days before delivery (two cases with PPROM were negative for chorioamnionitis). None of the women had any underlying medical conditions such as diabetes, asthma, polycystic ovarian syndrome, preeclampsia and macrovascular complications. Additionally, women with multiple pregnancies, obese women, fetuses with chromosomal abnormalities were excluded.
Tissue explants
For the term studies, fetal membranes (combined amnion and choriodecidua) and myometrium (collected as described above) were dissected and tissue fragments placed in DMEM at 37°C in a humidified atmosphere of 8% O2 and 5% CO2 for 1 h. Tissues were blotted dry on sterile filter paper and transferred to 24-well tissue culture plates (100 mg wet weight/well for fetal membranes and 50 mg wet weight/well for myometrium). The explants were incubated, in duplicate, in 1 ml DMEM containing penicillin G (100 U/ml) and streptomycin (100 μg/ml). Explants were pre-incubated with 200 μM silibinin (S0417, Sigma, St. Louis, USA) for 1 h, then incubated, for 24 h, in the presence of 10 μg/ml LPS (to facilitate the production of pro-inflammatory mediators) [36]–[38]. After 24 h incubation, tissue and media were collected separately and stored at −80°C for further analysis as detailed below. Experiments were performed in fetal membranes and myometrium from six patients. Based on previously published studies [39]–[41], a dose response was initially performed to determine the optimal concentration of silibinin. Fetal membranes were incubated in the absence or presence of 10 μg/ml LPS and silibinin at 50, 100 and 200 μM. While all concentrations of silibinin decreased LPS-stimulated IL-6 release, treatment with 200 μM silibinin was closer to basal readings, and was thus used in subsequent experiments (data not shown).
For the preterm study, the effect of silibinin was determined in fetal membranes that either already had an infection or had undergone preterm labour. Explants were incubated with or without 200 μM silibinin for 24 h. After 24 h incubation, tissue and media were collected separately and stored at −80°C for further analysis as detailed below. Experiments were performed from seven patients. For the preterm studies, due to the large variability in basal release or expression of the endpoints, all data were normalised to the untreated samples (basal), which was set at 1.
Primary amnion and myometrial cell culture
Primary amnion epithelial cultures and myometrium cells were used to investigate the effects of silibinin on pro-labour mediators. The amnion and myometrial cells were stimulated with IL-1β, as LPS does not induce an inflammatory response (observed in our laboratory, also by [42]). For these studies, reflected amnion (obtained approximately 2 cm from the peri-placental edge) was obtained from women who delivered healthy, singleton infants at term (>37 weeks gestation) undergoing elective Caesarean section in the absence of labour. Amnion cells were prepared as previously described [37], [38], [43]–[47]. Myometrial cells were isolated and cultured as previously described [37], [38], [48], [49]. Primary cells at 80–90% confluence were incubated in 1 ng/ml IL-1β (amnion) or 200 pg/ml IL-1β (myometrium) in the absence or presence of 200 μM silibinin. After 24 h incubation, cells and media were collected separately and stored at -80°C for further analysis as detailed below. The medium was assayed for cytokine and prostaglandin release, and cells were assayed for cytokine, COX-2 and MMP-9 gene expression. Experiments were performed on cells collected from six patients.
Model of infection-induced inflammation in the pregnant mouse
Animal studies were conducted with approval from the Austin Health's Animal Ethics Committee (A2012/04451). Time mated C57BL/6 mice were obtained from the University of Adelaide on day 12 of gestation and allowed to acclimatise for 4 days prior to experiments. They received food and water ad libitum and were on a 12-hour light/dark cycle. An initial dose response was performed to determine the optimal dose to induce inflammation in gestational tissues and fetal brain. Based on other studies [50]–[52], 10–75 μg of LPS per mouse was administered intraperitoneally, with the 50 μg/mouse dose being the final concentration used. The concentration of silibinin used was 70 mg/kg, based on other studies [53]–[55] as well as solubility of our reagent. For these studies, on day 16, the mice were intraperitoneally injected with 500 μl of either saline (n = 7), LPS (n = 6), or LPS with silibinin (n = 5). As silibinin was solubilised in DMSO, equal amounts of DMSO (10% v/v) were included in the injections for control and LPS groups. Six hours after injection, mice were euthanised with carbon dioxide and cervical dislocation. This time point was based on the LPS dose response where inflammation was induced, but before preterm delivery occured. Myometrium and placenta were harvested, as well as fetal brains from the pups. Tissues were flash frozen then stored at −80°C until further analysis by qRT-PCR as detailed below.
Cytokine and prostaglandin assays
Conditioned medium from cell and tissue culture experiments was assessed for IL-6 and IL-8 concentrations using commercial ELISA according to the manufacturer's instructions (Invitrogen, Carlsbad, USA). The concentration of PGE2 and PGF2α into the incubation medium were assayed using commercially available competitive enzyme immunoassay kits according to the manufacturer's specifications (Kookaburra Kits from Sapphire Bioscience, Redfern, Australia). The calculated interassay and intraassay coefficients of variation (CV) were all less than 10%. Data was corrected for total protein and expressed as either ng or pg per mg protein. The protein content of tissue homogenates was determined using BCA protein assay (Pierce, Rockford, USA), using BSA as a reference standard, as previously described [56]. For the preterm explant studies, due to patient variability, data were normalised to the untreated samples (basal), which was set at 1.
Gelatin zymography
Assessment of enzymes of ECM weakening and rupture (MMP-9) was performed by gelatin zymography as previously described [37], [38], [44], [46], [47] on conditioned media collected from primary amnion and myometrium cells, and myometrial explants. Proteolytic activity was visualised as clear zones of lysis on a blue background of undigested gelatin. For the term explant studies, data were corrected for background, and fold change was calculated relative to LPS or IL-1β-stimulated cells, which was set at 1. For the preterm explant studies, data were normalised to the untreated samples (basal), which was set at 1.
RNA extraction and qRT-PCR
Analysis of human gene expression by qRT-PCR was performed as we have previously described [37], [38], [46]-[49]. Total RNA from cells and tissues was extracted using TRIsure according to manufacturer's instructions (Bioline, London, UK). RNA concentrations were quantified using a spectrophotometer (NanoDrop, Thermo Fisher Scientific, Waltham, USA). RNA quality and integrity was determined via the A260/A280 ratio. One μg of RNA was converted to cDNA using the Tetro cDNA Synthesis Kit (Bioline) according to the manufacturer's instructions. The cDNA was diluted ten-fold and 4 μl of this was used to perform qRT-PCR using SensiFAST SYBR No-ROX kit (Bioline) and 100 nM of primers: GAPDH (QT01192646), IL-6 (QT00083720), IL-8 (QT00000322), COX-2 (QT00040586), MMP-9 (QT00040040) (Qiagen, Germantown, Maryland, USA). The specificity of the product was assessed from melting curve analysis. RNA without reverse transcriptase during cDNA synthesis as well as PCR reactions using water instead of template showed no amplification. Average gene CT values were normalised to the average GAPDH mRNA CT values of the same cDNA sample. For the term explant studies, fold differences in target gene expression were determined by the comparative CT method, relative to LPS or IL-1β treatment. For the myometrial cells, as we have previously published [49], due to variability of patient response to IL-1β, data was expressed as fold change relative to IL-1β-stimulated cells, which was set at 1. For the preterm explant studies, data were normalised to the untreated samples (basal), which was set at 1.
For the mouse studies, RNA was extracted as described above. Mouse primers used for qRT-PCR were GAPDH [57], IL-6 [58], IL-8 [59], IL-1β [60], COX-2 [61] and ninjurin [62]. For the mouse studies, data were normalised to the control mice, which was set at 1.
Statistical analysis
Statistical analyses were performed using a commercially available statistical software package (Statgraphics Plus version 3.1, Statistical Graphics Corp., Rockville, USA). Homogeneity of data was assessed by Bartlett's test, and when significant, data were logarithmically transformed before further analysis. Data were analysed by one-way ANOVA, and comparisons between groups (to LPS or IL-1β treatment for human studies, and LPS for mice studies) were performed using LSD multiple-range tests. Statistical difference was indicated by a P value of less than 0.05. Data are expressed as mean ± standard error of the mean (SEM).
Results
Effect of silibinin on pro-inflammatory cytokines in human gestational tissues
The first aim of this study was to determine the effect of silibinin on infection (LPS) and inflammation (IL-1β) induced pro-labour mediators in human fetal membranes and myometrium. Human fetal membranes (combined amnion and choriodecidua) and myometrium were incubated with 200 μM silibinin in the absence or presence of LPS for 24 h. Primary amnion cells and primary myometrial cells were incubated with 200 μM silibinin in the absence or presence of IL-1β for 24 h. The concentration of silibinin was determined from an initial dose response using 50, 100 and 200 μM of silibinin in the presence of LPS. All concentrations of silibinin decreased LPS-stimulated IL-6 concentration, however treatment with 200 μM give a similar reading to basal (data not shown) and was thus used in subsequent experiments. IL-6 and IL-8 gene expression was quantified by qRT-PCR and the concentration of IL-6 and IL-8 in conditioned media assessed by ELISA.
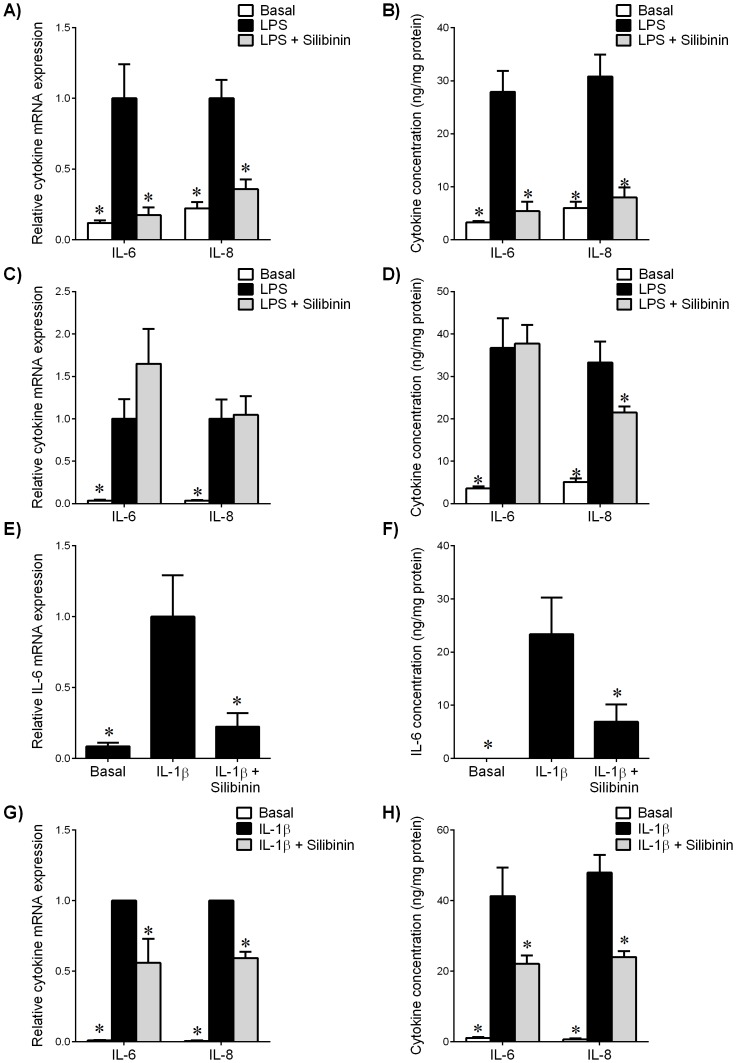
The effect of silibinin on cytokine expression and release is shown in Figure 1. In fetal membranes, LPS treatment increased IL-6 and IL-8 mRNA expression (Figure 1A) and release (Figure 1B). Treatment with silibinin significantly decreased LPS-induced IL-6 and IL-8 mRNA expression (Figure 1A) and release (Figure 1B). In myometrium, LPS treatment significantly increased IL-6 and IL-8 mRNA expression (Figure 1C) and release (Figure 1D). However, there was no effect of silibinin treatment on LPS-induced IL-6 and IL-8 mRNA expression (Figure 1C). Silibinin significantly decreased LPS-stimulated IL-8 release (Figure 1D) but there was no change in IL-6 expression.
Figure 1. Effect of silibinin on pro-inflammatory cytokines in human gestational tissues.
(A,B) Fetal membranes and (C,D) myometrium were incubated 24 h in the absence or presence of 10 μg/ml LPS with or without 200 μM silibinin (n = 6 patients). (E,F) Human amnion cells and (G,H) myometrial cells were incubated 24 h in the absence or presence of IL-1β with or without silibinin (n = 6 patients). (A,C,E,G) Gene expression for IL-6 and IL-8 was analysed by qRT-PCR. Cytokine mRNA expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to LPS or IL-1β-stimulated expression. Data is displayed as mean ± SEM (one-way ANOVA). *P<0.05 vs. LPS or IL-1β-stimulated expression. (B,D,F,H) The incubation medium was assayed for concentration of IL-6 and IL-8 release by ELISA. Each bar represents mean concentration ± SEM (one-way ANOVA). *P<0.05 vs. LPS or IL-1β-stimulated release.
In primary amnion cells, IL-1β-induced IL-6 mRNA expression (Figure 1E) and release (Figure 1F) was significantly attenuated by treatment with silibinin. In myometrial cells, silibinin significantly diminished IL-1β-induced IL-6 and IL-8 mRNA expression (Figure 1G) and release (Figure 1H).
Effect of silibinin on the COX-prostaglandin pathway in human gestational tissues
Human fetal membranes, primary amnion cells, myometrium, and primary myometrial cells were treated with silibinin as described above. To determine if the effect of silibinin on prostaglandin release occurs at the transcriptional level, the mRNA expression of COX-2, the time-limiting enzyme involved in prostaglandin formation, was measured using qRT-PCR.
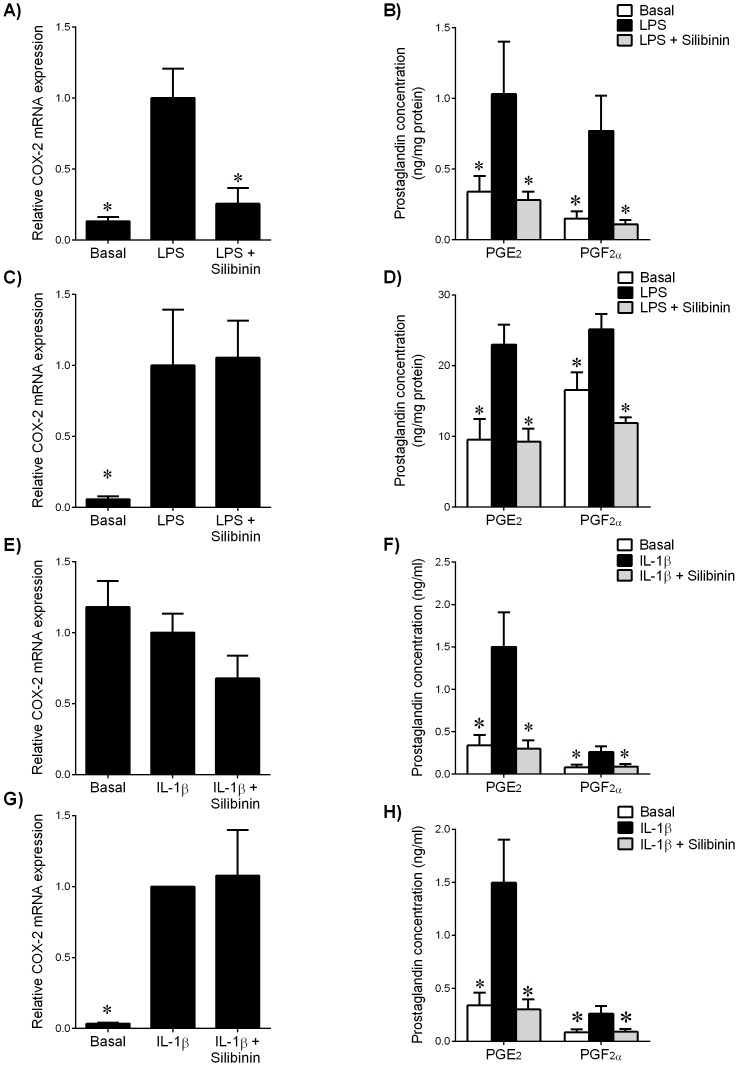
In fetal membranes, treatment with LPS increased COX-2 mRNA expression, while silibinin decreased LPS-stimulated COX-2 mRNA expression (Figure 2A). Release of PGE2 and PGF2α was increased in the presence of LPS, an effect abrogated with silibinin (Figure 2B). In myometrium, treatment with LPS significantly augmented COX-2 mRNA expression (Figure 2C) and prostaglandin release (Figure 2D). While there was no effect of silibinin on LPS-stimulated COX-2 mRNA expression, silibinin significantly decreased LPS-induced prostaglandin release (Figure 2D).
Figure 2. Effect of silibinin on COX-prostaglandin pathway in human gestational tissues.
(A,B) Fetal membranes and (C,D) myometrium were incubated 24 h in the absence or presence of 10 μg/ml LPS with or without 200 μM silibinin (n = 6 patients). (E,F) Human amnion cells and (G,H) myometrial cells were incubated 24 h in the absence or presence of IL-1β with or without silibinin (n = 6 patients). (A,C,E,G) Gene expression for COX-2 was analysed by qRT-PCR. COX-2 mRNA expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to LPS or IL-1β-stimulated expression. Data is displayed as mean ± SEM (one-way ANOVA). *P<0.05 vs. LPS or IL-1β-stimulated expression. (B,D,F,H) The incubation medium was assayed for concentration of PGE2 and PGF2α release by ELISA. Each bar represents mean concentration ± SEM (one-way ANOVA). *P<0.05 vs. LPS or IL-1β-stimulated release.
In primary amnion cells, IL-1β did not induce COX-2 gene expression (Figure 2E); however, IL-1β induced prostaglandin release (Figure 2F), an effect abrogated by silibinin. In primary myometrial cells, there was no change in IL-1β-induced COX-2 mRNA expression (Figure 2G), however silibinin significantly decreased IL-1β-induced PGE2 and PGF2α secretion (Figure 2H).
Effect of silibinin on MMP-9 expression and activity in human gestational tissues
Human myometrium, primary amnion cells, and primary myometrial cells were used to determine the effect of silibinin on MMP-9 expression and activity. Fetal membranes are not responsive to LPS treatment with respect to MMP-9 expression and activity (data not shown). For these studies, MMP-9 gene expression was assessed by qRT-PCR and secreted MMP-9 activity by gelatin zymography.
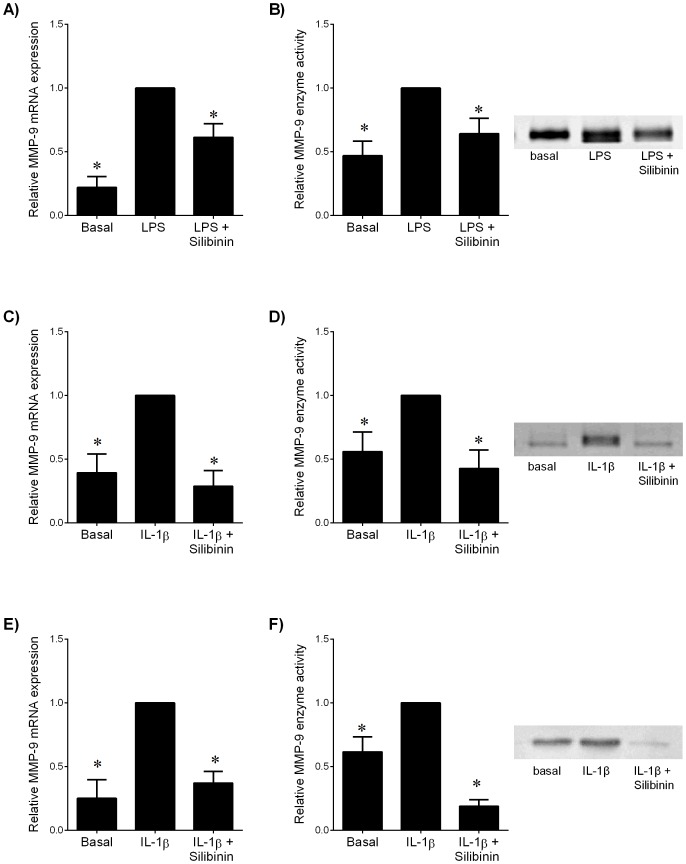
In myometrial tissue, LPS treatment significantly increased MMP-9 mRNA expression (Figure 3A) and activity (Figure 3B). Silibinin significantly decreased LPS-induced MMP-9 mRNA expression (Figure 3A) and activity (Figure 3B).
Figure 3. Effect of silibinin on MMP-9 expression in human gestational tissues.
(A,B) Myometrium was incubated 24 h in the absence or presence of 10 μg/ml LPS with and without 200 μM silibinin (n = 6 patients). (C,D) Human amnion cells and (E,F) myometrial cells were incubated 24 h in the absence or presence of IL-1β with and without silibinin (n = 6 patients). (A,C,E) Gene expression for MMP-9 was analysed by qRT-PCR. MMP-9 mRNA expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to LPS or IL-1β-stimulated expression. Data is displayed as mean ± SEM (one-way ANOVA). *P<0.05 vs. LPS or IL-1β-stimulated expression. (B,D,F) The incubation medium was assayed for MMP-9 activity by gelatin zymography. Zymography from one patient per tissue type is shown depicting MMP-9 activity.
In amnion cells, treatment with IL-1β significantly increased MMP-9 mRNA expression (Figure 3C). Treatment with silibinin significantly decreased IL-1β-induced MMP-9 mRNA expression. Silibinin also decreased IL-1β-induced MMP-9 activity (Figure 3D). Myometrial cells treated with IL-1β showed an increase in MMP-9 mRNA expression (Figure 3E) and activity (Figure 3F). Treatment with silibinin decreased IL-1β-induced MMP-9 mRNA expression (Figure 3E) and activity (Figure 3F).
Effect of silibinin on fetal membranes from preterm deliveries
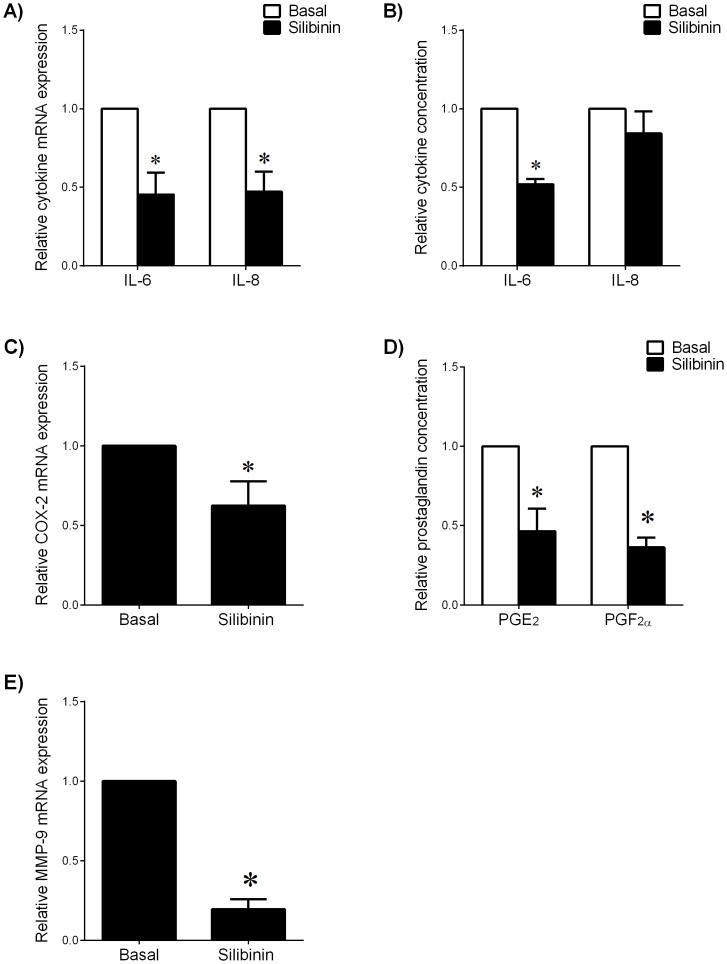
The above studies demonstrate that silibinin can decrease LPS- and IL-1β-induced pro-inflammatory mediators in vitro. The next aim was to determine if silibinin could decrease pro-labour mediators in an ex vivo model using fetal membranes from spontaneous preterm deliveries with and without chorioamnionitis. Cases of chorioamnionitis ranged from mild to severe. Fetal membranes were treated with or without silibinin or vehicle control. The effect of silibinin was found to be equally effective in both non-infected and infected membranes, and thus all subsequent data are combined. The effect of silibinin on cytokine expression and release in preterm fetal membranes is shown in Figures 4A-B. Treatment with silibinin significantly decreased IL-6 and IL-8 mRNA expression (Figure 4A) compared to untreated membranes. Silibinin decreased release of IL-6 (Figure 4B); however the decrease in IL-8 release did not reach significance. Silibinin treatment decreased COX-2 mRNA expression (Figure 4C) and release of prostaglandins (Figure 4D). Silibinin decreased MMP-9 mRNA expression (Figure 4E); however there was no effect on MMP-9 activity (data not shown).
Figure 4. Effect of silibinin on pro-labour mediators in preterm fetal membranes.
Preterm fetal membranes with histological chorioamnionitis and following spontaneous preterm labour were incubated for 24 μM silibinin (n = 7 patients). (A,C,E) Gene expression for IL-6, IL-8, COX-2 and MMP-9 were analysed by qRT-PCR. mRNA expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to basal expression. Data is displayed as mean ± SEM (one-way ANOVA). *P<0.05 vs. basal expression. (B,D) The incubation medium was assayed for concentration of IL-6, IL-8, PGE2 and PGF2α release by ELISA. Data was normalised to untreated (basal) levels, which was set at 1. Each bar represents mean ± SEM (one-way ANOVA). *P<0.05 vs. basal release.
Effect of silibinin on infection-induced inflammation in vivo
The final aim of this study was to determine the efficacy of silibinin in a pregnant mouse model of infection-induced inflammation. Mice were injected with either saline (control), LPS, or LPS with silibinin. After 6 h, placenta and myometrium were collected and pro-inflammatory markers assessed by qRT-PCR. Fetal brains were collected and also assessed for markers of inflammation and fetal brain injury.
In mouse placenta, LPS treatment significantly increased IL-6, IL-8 and IL-1β mRNA expression compared to control (Figure 5A). Treatment with silibinin did not change LPS-stimulated response to these genes. In mouse myometrium, LPS treatment significantly increased IL-6, IL-8, IL-1β and COX-2 mRNA expression (Figure 5B). However, there was no effect of silibinin on LPS-stimulated response in myometrium.
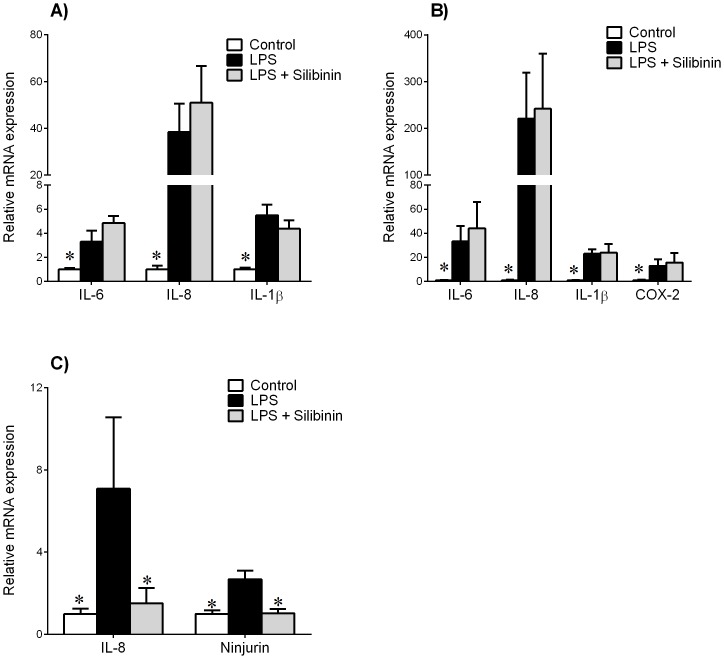
Figure 5. Effect of silibinin in a mouse model of infection-induced preterm birth.
Time mated C57BL/6 mice were intraperitoneally injected with either saline (control, n = 7), 50 μg LPS (n = 6), or LPS with 70 mg/kg silibinin (n = 5). Tissues were collected after 6 h. Gene expression for IL-6, IL-8, IL-1β and COX-2 were analysed by qRT-PCR in (A) placenta and (B) myometrium. Gene expression of IL-8 and ninjurin were analysed in (C) fetal brain. Gene expression was normalized to GAPDH mRNA expression and the fold change was calculated relative to control expression. Data is displayed as mean ± SEM (one-way ANOVA). *P<0.05 vs. control gene expression.
In fetal brain, LPS treatment significantly increased IL-8 and ninjurin mRNA expression compared to control (Figure 5C). Treatment with silibinin significantly decreased LPS-induced IL-8 and ninjurin mRNA expression. We had also assessed other markers of inflammation (IL-6 and IL-1β) and injury (microtubule-associated protein 2 (MAP2), glial fibrillary acidic protein (GFAP) and myelin basic protein (MBP)). However, for these five markers, there was no effect of LPS treatment in the fetal brain compared to controls (data not shown), thus the effect of silibinin was only assessed for IL-8 and ninjurin.
Discussion
A diet consisting of many serves of fruits and vegetables has been attributed to many health benefits, such as decreased risk of coronary heart disease [63] and decreased incidence of preeclampsia [64], [65]. It is unclear as to why they are beneficial, and it may be due to the presence of dietary phytophenols. In preeclamptic women, the polyphenolic plant flavanoid silibinin has been shown to reduce oxidative metabolism and cytokine production in peripheral blood mononuclear cells [34], [35]. However, the effect of silibinin on mediators of labour in human gestational tissues has not previously been explored. The data presented in this study demonstrate that in human gestational tissues, silibinin decreases LPS and IL-1β-induced mRNA expression and secretion of pro-inflammatory cytokines, COX-2 mRNA expression and resultant prostaglandin release (fetal membranes, but not myometrium), and MMP-9 mRNA expression and activity. In fetal membranes from spontaneous preterm labour and with active infection (confirmed by pathology), silibinin treatment decreased pro-inflammatory cytokine expression and release, COX-2 gene expression and prostaglandin release, and MMP-9 gene expression. We also investigated the ability of silibinin to decrease inflammation and brain injury using a mouse model of infection-induced preterm birth. Fetal brains from C57BL/6 time mated mice treated with silibinin showed a decrease in LPS-induced IL-8 and ninjurin expression. However, silibinin did not change LPS-induced inflammation in placenta or myometrium.
As an inflammatory event, human labour is associated with an induction of pro-inflammatory cytokines such as IL-6, IL-8 and TNF-α, but more so in preterm deliveries [66]. In cases of infection-induced preterm labour, bacterial invasion of the choriodecidual space activates the fetal membranes and decidua to produce pro-inflammatory cytokines; these cytokines stimulate prostaglandin synthesis and release, which also leads to the synthesis and release of MMPs (reviewed in [67]). Prostaglandins are critical in the onset of preterm and term labour, promoting myometrial contractility [13] and cervical ripening [68]. One of the main enzymes responsible for prostaglandin synthesis is COX-2, which is present in human gestational tissues before the onset of labour [13]. COX-2 is induced in tissues after exposure to bacteria as well as inflammatory cytokines, with IL-1β increasing COX-2 expression in myometrium and subsequent prostaglandin production [69]. The activation of pro-inflammatory cytokines, prostaglandins and MMPs that attack the fetal membranes lead to uterine contractions and rupture of membranes. Our study demonstrates that in fetal membranes and primary amnion cells, treatment with silibinin decreased LPS- and IL-1β-induced IL-6 and IL-8 expression, COX-2 expression and subsequent prostaglandin release, and MMP-9 gene expression and activity. In myometrium, silibinin did not affect LPS-induced cytokine mRNA expression, but decreased IL-1β-stimulated cytokine mRNA and release, perhaps suggesting that in myometrium, silibinin may exert anti-inflammatory effects based on the inflammatory stimulus. Silibinin decreased inflammation-induced prostaglandin release but did not affect COX-2 mRNA expression in myometrium; this could be due to silibinin exerting its effects on COX-2 protein expression or activity, which was not assessed in this study. It is also possible that in myometrium silibinin acts by blocking enzymes downstream of COX-2, such as PGH2, synthase or PGE and PGF synthases.
Of all preterm births, spontaneous preterm labour, with intact membranes, accounts for 40–45% of cases [2] and pre-labour rupture of membranes (PROM) occurs in 30–40% of preterm deliveries [70]. While the precise causes are unknown, both are strongly associated with infection and/or inflammation. Women with PPROM commonly begin labour spontaneously; however a proportion remain undelivered for weeks or months, increasing the risk of infection. Abnormal degradation of the fetal membranes has also been proposed as the final pathway of PPROM, with increased MMP-9 activity linked to the increase of inflammation-related cytokines [71]. We determined the effect of silibinin in fetal membranes from preterm deliveries with and without histological infection, with most cases in our study group having PPROM. The one case that did undergo spontaneous preterm labour had acute chorioamnionitis. Silibinin treatment in the preterm fetal membranes decreased pro-inflammatory cytokine expression, COX-2 expression and subsequent prostaglandin release, and MMP-9 gene expression. These results indicate that silibinin can reduce the inflammation already present in the preterm fetal membranes and has some potential as a therapy for threatened cases. However, it may also signify a need for treatment, or a dietary intake that contains silibinin, before PPROM and preterm labour occurs. Myhre et al report that intake of garlic is associated with decreased risk of preterm delivery, and dried fruits, particularly raisins, is associated with reduced risk of PPROM [72]. The effects of garlic were attributable to the component allicin, which has strong antimicrobial properties and may have lowered the overall inflammation level.
In survivors of preterm birth, perinatal brain damage is a primary cause of developmental delay and lifelong neurological impairments such as mental retardation and cerebral palsy [6], [7]. Antenatal infection has been linked to white matter damage and cerebral palsy; in babies born within one hour of membrane rupture (with low risk of infection subsequent to the rupture) whose placentas had histologic chorioamnionitis, the presence of leukocytes in the umbilical cord and chorionic plate were associated with an eleven-fold increase in the risk of white matter damage [73]. Studies involving mice show that inflammation-induced preterm birth caused neuropathology in the fetal brains, but not in preterm mice induced by a non-inflammatory method, indicating that it is not the process of preterm birth that affects neuronal morphology [74]. In this study, gestational day 15.5 mice were given an intraperitoneal injection of LPS with or without silibinin and compared to a basal group. In the fetal brain, a marker of inflammation (IL-8) and a marker of neuronal damage (ninjurin) were assessed. Silibinin significantly decreased LPS-induced mRNA expression of ninjurin and IL-8 in the fetal brain. Ninjurin was used as an indicator of neuropathy and inflammation in the fetal brain. It is associated with the increased activity of microglial cells, the immune cells of the brain, during inflammation and tissue remodelling [75]. It also has been found to regulate the migration of macrophages to sites of inflammation in the central nervous system [76]. The phytonutrient supplement study to prevent preeclampsia [77] demonstrated a non-significant reduction in neonatal death, NICU admission and respiratory distress syndrome with the phytonutrient group, promising improved neonatal outcome if a large cohort could be achieved. The ability to reduce the expression of IL-8 and ninjurin in the fetal brain demonstrates that silibinin has great potential as a treatment for infection-induced preterm birth.
Our study demonstrated that in vitro, silibinin decreases LPS and IL-1β-induced inflammation in human gestational tissues, and in vivo, decreased inflammation and injury in the fetal brain; however, in the mouse, there was no effect of silibinin on LPS-stimulated inflammation in placenta and myometrium. All tissues were collected 6 h after injection of treatments; it is known that a low dose of LPS in a mouse model causes fetal brain injury while not inducing preterm birth [78] (intrauterine infusion of 50 μg LPS rather than high dose of 250 μg). A number of issues could be addressed in future studies: can silibinin delay infection-induced preterm birth, and decrease inflammation at the later time point; would a higher dose of silibinin achieve the reduction of inflammation in maternal tissues; what is the effect of silibinin in the pregnant mouse without LPS; and should silibinin be given as a treatment at the start of pregnancy, regularly, before onset of infection. It is this last point that would be of most interest in human trials to determine whether silibinin, if taken regularly as a supplement or part of a diet, could be a treatment for the prevention of infection-induced preterm birth.
While we must be appropriately cautious to suggest administering silibinin to pregnant women during critical periods of fetal development, pilot studies have previously been performed in humans as a colorectal cancer chemopreventative agent. Initially, studies were performed in mice where dietary feeding of silibinin at 75 or 150 mg/kg for 60 days after receiving a tumour xenograph implant, or fed 3 weeks before tumour implantation and 63 days after, showed that silibinin decreased tumour volume, and that animals did not show weight loss or reduced food consumption [79]. This led to the pilot study by Hoh et al, confirming that oral administration of silibinin (silibinin formulated with phosphatidylcholine, as silipide capsules) in humans at daily doses (extrapolated by the mice studies) of up to 1.44 mg for a week, is safe [80], and achieved levels in the colorectal tract similar to those known to exert pharmacologic activity. Of note, our study involved a single dose of silibinin at 70 mg/kg at the same time as LPS injection; repeat doses of silibinin, perhaps orally, to the pregnant mouse before infection-induced preterm birth could possibly give the desired effect of reducing the inflammation associated with preterm birth. While evidence from the previous studies bodes well for safe consumption, further studies are required to determine any side effects and the ability of silibinin to cross the placenta and act on the fetus. For example, silibinin has known anti-tumoral effects mediated, among others, by inhibiting cell cycle progression [81]. Therefore, although the data in our study is promising, we must be cautious before recommending a therapeutic regime using silibinin or other flavonoids in pregnancy, due to the potential existence of serious side-effects on the fetus.
The transcription factor nuclear factor-kappa B (NF-κB) is a common, central pathway involved in promoting the formation of pro-inflammatory and pro-labour mediators in human gestational tissues [82], [83]. For example, we have previously shown that inhibition of NF-κB activity in ex situ human gestational tissues, suppresses the formation of labour-mediating effectors, including pro-inflammatory cytokines, COX-prostaglandins and ECM enzymes. Thus, it is possible that silibinin is exerting its anti-inflammatory actions by inhibiting the activity of NF-κB. Indeed, in non-gestational tissues, silibinin has been shown to exert its effects by inhibiting the activity of NF-κB [27], [40], [55].
In conclusion, we demonstrate that silibinin can reduce infection and inflammation-induced pro-labour mediators in human fetal membranes and myometrium, and can reduce pro-inflammatory cytokines, prostaglandins and gene expression of MMP-9 in preterm fetal membranes both with active infection and from spontaneous preterm labour. Of most promise is our in vivo data, where silibinin decreased inflammation and injury in fetal brains in an infection-induced model of inflammation in the pregnant mouse. While the in vivo data is preliminary, the ramifications of our findings is that silibinin could be a novel treatment that not only reduces the inflammation associated with preterm labour, but also improves the adverse outcomes that are commonly linked with this global predicament. However, further studies are required to fully understanding the mechanisms of action of silibinin and importantly its effects on the fetus.
Acknowledgments
The authors gratefully acknowledge the assistance of the Clinical Research Midwives Genevieve Christophers, Renee Grant, Gabrielle Fleming, Debra Jinks and Rachel Murdoch, and the Obstetrics and Midwifery staff of the Mercy Hospital for Women for their co-operation. We also thank A/Prof Laura Parry (Department of Zoology, University of Melbourne) for her generous assistance and advice with the collection of mouse tissues, and Marin Poljak (Department of Obstetrics & Gynaecology, University of Melbourne) for his contribution to the mice studies.
Funding Statement
Associate Professor Martha Lappas was in recipient of a National Health and Medical Research Council (NHMRC) Career Development Award (grant no. 1047025). Dr. Ratana Lim was, in part, supported by an early career researcher grant from the University of Melbourne. This work was supported by the Austin Hospital Medical Research Foundation (AMRF), Medical Research Foundation for Women and Babies, and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) Research Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, et al. (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoon BH, Romero R, Moon JB, Shim SS, Kim M, et al. (2001) Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 185: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 4. Menon R, Fortunato SJ (2007) Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol 21: 467–478. [DOI] [PubMed] [Google Scholar]
- 5. Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ (2013) The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy 2013: 412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, et al. (2006) Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res 59: 478–483. [DOI] [PubMed] [Google Scholar]
- 7. Redline RW, Minich N, Taylor HG, Hack M (2007) Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr Dev Pathol 10: 282–292. [DOI] [PubMed] [Google Scholar]
- 8. Suppiej A, Franzoi M, Vedovato S, Marucco A, Chiarelli S, et al. (2009) Neurodevelopmental outcome in preterm histological chorioamnionitis. Early Hum Dev 85: 187–189. [DOI] [PubMed] [Google Scholar]
- 9. Denison FC, Kelly RW, Calder AA, Riley SC (1998) Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod 13: 3560–3565. [DOI] [PubMed] [Google Scholar]
- 10. Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, et al. (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 11. Sennstrom MB, Ekman G, Westergren-Thorsson G, Malmstrom A, Bystrom B, et al. (2000) Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod 6: 375–381. [DOI] [PubMed] [Google Scholar]
- 12. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, et al. (2002) Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 66: 445–449. [DOI] [PubMed] [Google Scholar]
- 13. Olson DM (2003) The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol 17: 717–730. [DOI] [PubMed] [Google Scholar]
- 14. Xu P, Alfaidy N, Challis JR (2002) Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab 87: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 15. Abramovici A, Cantu J, Jenkins SM (2012) Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am 39: 77–87. [DOI] [PubMed] [Google Scholar]
- 16. Alfirevic Z (2012) Tocolytics: do they actually work? BMJ 345: e6531. [DOI] [PubMed] [Google Scholar]
- 17. Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA (2013) Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev 7: CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roos C, Spaanderman ME, Schuit E, Bloemenkamp KW, Bolte AC, et al. (2013) Effect of maintenance tocolysis with nifedipine in threatened preterm labor on perinatal outcomes: a randomized controlled trial. JAMA 309: 41–47. [DOI] [PubMed] [Google Scholar]
- 19. Salim R, Garmi G, Nachum Z, Zafran N, Baram S, et al. (2012) Nifedipine compared with atosiban for treating preterm labor: a randomized controlled trial. Obstet Gynecol 120: 1323–1331. [DOI] [PubMed] [Google Scholar]
- 20. Chawanpaiboon S, Sutantawibul A (2012) Effect of cervical length to the efficacy of nifedipine and bed rest for inhibiting threatened preterm labor. J Med Assoc Thai 95: 636–643. [PubMed] [Google Scholar]
- 21. Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, et al. (2013) DHA supplementation and pregnancy outcomes. Am J Clin Nutr 97: 808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hackett ES, Twedt DC, Gustafson DL (2013) Milk thistle and its derivative compounds: a review of opportunities for treatment of liver disease. J Vet Intern Med 27: 10–16. [DOI] [PubMed] [Google Scholar]
- 23. Tamayo C, Diamond S (2007) Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther 6: 146–157. [DOI] [PubMed] [Google Scholar]
- 24. Deep G, Agarwal R (2007) Chemopreventive efficacy of silymarin in skin and prostate cancer. Integr Cancer Ther 6: 130–145. [DOI] [PubMed] [Google Scholar]
- 25. Ladas EJ, Kelly KM (2003) Milk thistle: is there a role for its use as an adjunct therapy in patients with cancer? J Altern Complement Med 9: 411–416. [DOI] [PubMed] [Google Scholar]
- 26. Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB (2006) Anticancer potential of silymarin: from bench to bed side. Anticancer Res 26: 4457–4498. [PubMed] [Google Scholar]
- 27. Au AY, Hasenwinkel JM, Frondoza CG (2011) Silybin inhibits interleukin-1beta-induced production of pro-inflammatory mediators in canine hepatocyte cultures. J Vet Pharmacol Ther 34: 120–129. [DOI] [PubMed] [Google Scholar]
- 28. McClure J, Lovelace ES, Elahi S, Maurice NJ, Wagoner J, et al. (2012) Silibinin inhibits HIV-1 infection by reducing cellular activation and proliferation. PLoS One 7: e41832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W, Otkur W, Zhang Y, Li Q, Ye Y, et al. (2013) Silibinin protects murine fibroblast L929 cells from UVB-induced apoptosis through the simultaneous inhibition of ATM-p53 pathway and autophagy. FEBS J 280: 4572–4584. [DOI] [PubMed] [Google Scholar]
- 30. Wang Q, Ye Y, Liu W, Jiang S, Tashiro S, et al. (2012) Dual effects of silibinin treatment on autophagy-regulated dermal apoptosis retardation and epidermal apoptosis up-regulation in UVB-induced skin inflammation. J Asian Nat Prod Res 14: 688–699. [DOI] [PubMed] [Google Scholar]
- 31. Choi YH, Jin GY, Guo HS, Piao HM, Li L, et al. (2012) Silibinin attenuates allergic airway inflammation in mice. Biochem Biophys Res Commun 427: 450–455. [DOI] [PubMed] [Google Scholar]
- 32. Kang JS, Jeon YJ, Park SK, Yang KH, Kim HM (2004) Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1beta and prostaglandin E2 synthesis by silymarin. Biochem Pharmacol 67: 175–181. [DOI] [PubMed] [Google Scholar]
- 33. Kovacevic Z, Kalinowski DS, Lovejoy DB, Yu Y, Suryo Rahmanto Y, et al. (2011) The medicinal chemistry of novel iron chelators for the treatment of cancer. Curr Top Med Chem 11: 483–499. [DOI] [PubMed] [Google Scholar]
- 34. Cristofalo R, Bannwart-Castro CF, Magalhaes CG, Borges VT, Peracoli JC, et al. (2013) Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic Res 47: 268–275. [DOI] [PubMed] [Google Scholar]
- 35. Giorgi VS, Peracoli MT, Peracoli JC, Witkin SS, Bannwart-Castro CF (2012) Silibinin modulates the NF-kappab pathway and pro-inflammatory cytokine production by mononuclear cells from preeclamptic women. J Reprod Immunol 95: 67–72. [DOI] [PubMed] [Google Scholar]
- 36. Lappas M (2013) NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod. 89: 14. [DOI] [PubMed] [Google Scholar]
- 37. Lim R, Barker G, Wall CA, Lappas M (2013) Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. Mol Hum Reprod 19: 451–462. [DOI] [PubMed] [Google Scholar]
- 38. Wall C, Lim R, Poljak M, Lappas M (2013) Dietary flavonoids as therapeutics for preterm birth: luteolin and kaempferol suppress inflammation in human gestational tissues in vitro. Oxid Med Cell Longev 2013: 485201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, et al. (2009) Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther 8: 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raina K, Agarwal C, Agarwal R (2013) Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-kappaB signaling. Mol Carcinog 52: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim BR, Seo HS, Ku JM, Kim GJ, Jeon CY, et al.. (2013) Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-kappaB signaling pathway in HMC-1 human mast cells. Inflamm Res. [DOI] [PMC free article] [PubMed]
- 42. Helmer H, Tretzmuller U, Brunbauer M, Kaider A, Husslein P, et al. (2002) Production of oxytocin receptor and cytokines in primary uterine smooth muscle cells cultivated under inflammatory conditions. J Soc Gynecol Investig 9: 15–21. [DOI] [PubMed] [Google Scholar]
- 43. Bennett PR, Rose MP, Myatt L, Elder MG (1987) Preterm labor: stimulation of arachidonic acid metabolism in human amnion cells by bacterial products. Am J Obstet Gynecol 156: 649–655. [DOI] [PubMed] [Google Scholar]
- 44. Lappas M, Riley C, Lim R, Barker G, Rice GE, et al. (2011) MAPK and AP-1 proteins are increased in term pre-labour fetal membranes overlying the cervix: regulation of enzymes involved in the degradation of fetal membranes. Placenta 32: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 45. Lee Y, Allport V, Sykes A, Lindstrom T, Slater D, et al. (2003) The effects of labour and of interleukin 1 beta upon the expression of nuclear factor kappa B related proteins in human amnion. Mol Hum Reprod 9: 213–218. [DOI] [PubMed] [Google Scholar]
- 46. Lim R, Barker G, Lappas M (2013) SIRT6 is decreased with preterm labor and regulates key terminal effector pathways of human labor in fetal membranes. Biol Reprod 88: 17. [DOI] [PubMed] [Google Scholar]
- 47. Lim R, Barker G, Riley C, Lappas M (2013) Apelin is decreased with human preterm and term labor and regulates prolabor mediators in human primary amnion cells. Reprod Sci 20: 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lappas M (2013) Forkhead box O1 (FOXO1) in pregnant human myometrial cells: A role as a pro-inflammatory mediator in human parturition. J Reprod Immunol 99: 24–32. [DOI] [PubMed] [Google Scholar]
- 49. Lim R, Barker G, Lappas M (2013) A novel role for FOXO3 in human labor: increased expression in laboring myometrium, and regulation of proinflammatory and prolabor mediators in pregnant human myometrial cells. Biol Reprod 88: 156. [DOI] [PubMed] [Google Scholar]
- 50. Gross G, Imamura T, Vogt SK, Wozniak DF, Nelson DM, et al. (2000) Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol 278: R1415–1423. [DOI] [PubMed] [Google Scholar]
- 51. Kaga N, Katsuki Y, Obata M, Shibutani Y (1996) Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol 174: 754–759. [DOI] [PubMed] [Google Scholar]
- 52. Lee PR, Kim SR, Jung BK, Kim KR, Chung JY, et al. (2003) Therapeutic effect of cyclo-oxygenase inhibitors with different isoform selectivity in lipopolysaccharide-induced preterm birth in mice. Am J Obstet Gynecol 189: 261–266. [DOI] [PubMed] [Google Scholar]
- 53. Lu P, Mamiya T, Lu LL, Mouri A, Zou L, et al. (2009) Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol 157: 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meiss R, Heinrich U, Robenek H, Themann H (1982) Effect of silybin on hepatic cell membranes after damage by polycyclic aromatic hydrocarbons (PAH). Agents Actions 12: 254–257. [DOI] [PubMed] [Google Scholar]
- 55. Wang Q, Zou L, Liu W, Hao W, Tashiro S, et al. (2011) Inhibiting NF-kappaB activation and ROS production are involved in the mechanism of silibinin's protection against D-galactose-induced senescence. Pharmacol Biochem Behav 98: 140–149. [DOI] [PubMed] [Google Scholar]
- 56. Lappas M, Permezel M, Georgiou HM, Rice GE (2004) Regulation of phospholipase isozymes by nuclear factor-kappaB in human gestational tissues in vitro. J Clin Endocrinol Metab 89: 2365–2372. [DOI] [PubMed] [Google Scholar]
- 57. Park SW, Chen SW, Kim M, Brown KM, D'Agati VD, et al. (2010) Protection against acute kidney injury via A(1) adenosine receptor-mediated Akt activation reduces liver injury after liver ischemia and reperfusion in mice. J Pharmacol Exp Ther 333: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sebastian BM, Roychowdhury S, Tang H, Hillian AD, Feldstein AE, et al. (2011) Identification of a cytochrome P4502E1/Bid/C1q-dependent axis mediating inflammation in adipose tissue after chronic ethanol feeding to mice. J Biol Chem 286: 35989–35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pedersen L, Pilegaard H, Hansen J, Brandt C, Adser H, et al. (2011) Exercise-induced liver chemokine CXCL-1 expression is linked to muscle-derived interleukin-6 expression. J Physiol 589: 1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li J, Zhao H, Luo P, Gu Y (2010) Functional cooperation of of IL-1beta and RGS4 in the brachial plexus avulsion mediated brain reorganization. J Brachial Plex Peripher Nerve Inj 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Endoh Y, Chung YM, Clark IA, Geczy CL, Hsu K (2009) IL-10-dependent S100A8 gene induction in monocytes/macrophages by double-stranded RNA. J Immunol 182: 2258–2268. [DOI] [PubMed] [Google Scholar]
- 62. Cho SJ, Rossi A, Jung YS, Yan W, Liu G, et al. (2013) Ninjurin1, a target of p53, regulates p53 expression and p53-dependent cell survival, senescence, and radiation-induced mortality. Proc Natl Acad Sci U S A 110: 9362–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, et al.. (2013) Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr. [DOI] [PMC free article] [PubMed]
- 64. Brantsaeter AL, Haugen M, Samuelsen SO, Torjusen H, Trogstad L, et al. (2009) A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr 139: 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. North RA, McCowan LM, Dekker GA, Poston L, Chan EH, et al. (2011) Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ 342: d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, et al.. (2003) Cytokines, prostaglandins and parturition—a review. Placenta 24 Suppl A: S33–46. [DOI] [PubMed]
- 67. Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and preterm delivery. N Engl J Med 342: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 68. Sparey C, Robson SC, Bailey J, Lyall F, Europe-Finner GN (1999) The differential expression of myometrial connexin-43, cyclooxygenase-1 and -2, and Gs alpha proteins in the upper and lower segments of the human uterus during pregnancy and labor. J Clin Endocrinol Metab 84: 1705–1710. [DOI] [PubMed] [Google Scholar]
- 69. Rauk PN, Chiao JP (2000) Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 43: 152–159. [DOI] [PubMed] [Google Scholar]
- 70. Parry S, Strauss JF 3rd (1998) Premature rupture of the fetal membranes. N Engl J Med 338: 663–670. [DOI] [PubMed] [Google Scholar]
- 71. Vadillo-Ortega F, Estrada-Gutierrez G (2005) Role of matrix metalloproteinases in preterm labour. BJOG 112 Suppl 119–22. [DOI] [PubMed] [Google Scholar]
- 72. Myhre R, Brantsaeter AL, Myking S, Eggesbo M, Meltzer HM, et al. (2013) Intakes of garlic and dried fruits are associated with lower risk of spontaneous preterm delivery. J Nutr 143: 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dammann O, Kuban KC, Leviton A (2002) Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev 8: 46–50. [DOI] [PubMed] [Google Scholar]
- 74. Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, et al. (2010) Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res 88: 1872–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ahn BJ, Lee HJ, Shin MW, Choi JH, Jeong JW, et al. (2009) Ninjurin1 is expressed in myeloid cells and mediates endothelium adhesion in the brains of EAE rats. Biochem Biophys Res Commun 387: 321–325. [DOI] [PubMed] [Google Scholar]
- 76. Ifergan I, Kebir H, Terouz S, Alvarez JI, Lecuyer MA, et al. (2011) Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol 70: 751–763. [DOI] [PubMed] [Google Scholar]
- 77. Parrish MR, Martin JN Jr, Lamarca BB, Ellis B, Parrish SA, et al. (2013) Randomized, placebo controlled, double blind trial evaluating early pregnancy phytonutrient supplementation in the prevention of preeclampsia. J Perinatol 33: 593–599. [DOI] [PubMed] [Google Scholar]
- 78. Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, et al. (2011) Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci 29: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, et al. (2002) Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res 62: 3063–3069. [PubMed] [Google Scholar]
- 80. Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, et al. (2006) Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res 12: 2944–2950. [DOI] [PubMed] [Google Scholar]
- 81. Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R (2010) Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog 49: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lappas M, Permezel M, Georgiou HM, Rice GE (2002) Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod 67: 668–673. [DOI] [PubMed] [Google Scholar]
- 83. Lappas M, Rice GE (2007) The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta 28: 543–556. [DOI] [PubMed] [Google Scholar]