Abstract
This study investigated B16F10 melanoma cell death induced by melatonin combined with endoplasmic reticulum (ER) stress through the PI3K/Akt/mTOR pathway. Cell viability was significantly decreased after treatment with melatonin combined with ER stress from thapsigargin or tunicamycin compared to no treatment or treatment with melatonin only. Combined melatonin and ER stress also significantly reduced expression of p85β, p-Akt (Ser473, Thr308), and p-mTOR (Ser2448, Ser2481) compared to treatment with melatonin only. The ER stress protein p-PERK and p-eIF2α were significantly increased under combined melatonin and ER stress treatment compared to no treatment or treatment with melatonin only. Combined melatonin and ER stress significantly reduced Bcl-2 protein and augmented Bax protein compared to melatonin-only treatment. Also, the combined treatment significantly lowered expression of catalase, Cu/Zn-SOD, and Mn-SOD proteins compared to melatonin only. Expression of p85β was significantly more decreased under treatment with melatonin and thapsigargin or tunicamycin plus the PI3K inhibitors LY294002 or wortmannin than under treatment with only melatonin or a PI3K inhibitor. The PI3K downstream target p-Akt (Ser473, Thr308) showed significantly decreased expression under treatment with melatonin and thapsigargin or tunicamycin plus PI3K inhibitors than under treatment with melatonin or PI3K inhibitors only. These results indicate that survival of B16F10 melanoma cells after combined treatment with melatonin and ER stress inducers is suppressed through regulation of the PI3K/Akt/mTOR pathway. Melatonin combined with thapsigargin or tunicamycin appears to be a promising strategy for effective melanoma treatment.
Introduction
Melanomas are malignant tumors that arise from melanocytes, which produce black or brown melanin pigment in skin, but are also found in other parts of the body such as the bowel and the eye. Melanocytes synthesize melanin from tyrosine to protect the body from damaging ultraviolet radiation [1]. Melanocytes are found in various areas of the body including the skin, bowel, and eyes but are predominantly located in the epidermis; over 90% of all melanomas are cutaneous [1]–[3]. Melanoma is the fifth most common cancer in the United States, causing up to 75% of deaths related to skin cancer [4]. Melanoma is curable if detected early; however, metastatic melanoma requires continued therapy [5].
Melatonin has direct anticancer or anti-apoptotic effects on different types of human tumors [6]–[11] and functions as a broad-spectrum antioxidant [12]–[18]. Melatonin is also reported to induce apoptotic or autophagic cell death [19]–[21]. Numerous studies have demonstrated that melatonin has anti-proliferative effects in melanoma cells [22]–[29], and that these effects are related to the cell line-specific response of melatonin-binding receptors [25]–[29].
The phosphatidyl inositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway is an important target for therapies for numerous cancers such as lung carcinoma, thyroid carcinoma, breast cancer, gastrointestinal carcinoma, and bladder carcinoma [30]–[33]. PI3K and its downstream targets AKT/PKB and mTOR are central in physiological processes such as cell growth, survival, motility, differentiation, and proliferation, and in the development of malignant disease [30]. The PI3K family is divided into three classes [34]. Class IA contains the PI3K proteins that are important for regulating proliferation and tumorigenesis. Class IA PI3Ks are heterodimeric molecules composed of a p110 catalytic subunit and a p85 regulatory subunit. The PI3K/Akt/mTOR pathway is frequently activated in human melanoma and is a possible therapeutic target for melanoma treatment [35]–[38].
Zha et al. [39] demonstrate that melatonin sensitizes human hepatoma cells to endoplasmic reticulum (ER) stress-induced cell death. In this study, we found that combined melatonin and ER stress treatment induced cell death in B16F10 melanoma cells through the PI3K/Akt/mTOR pathway. This finding suggests that targeting PI3K/Akt/mTOR could be an effective strategy for the melanoma therapy.
Materials and Methods
Cell Culture
B16F10 cells were obtained from Korea Cell Line Bank (Seoul, Korea) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GibcoBRL, Gaithersburg, MD, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS, GibcoBRL) at 37°C with 5% CO2 in a humidified incubator.
ER Stress and Treatment of PI3k Inhibitors
B16F10 cells were cultured in DMEM medium plus 1% heat-inactivated FBS with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) (Calbiochem, San Diego, MO, USA) for 6 hr, or with tunicamycin (5 μg/mL) (Calbiochem) for 16 hr in a 37°C and 5% CO2 incubator. Melatonin (Sigma, St Louis, MO, USA) was dissolved in dimethyl sulfoxide, and cells were treated with melatonin for 24 hr. To determine the effects of 20 μM LY294002 and 2 μM wortmannin (Calbiochem), cells were treated for 1 hr and co-exposed with or without melatonin and/or thapsigargin or tunicamycin.
Cell Viability Assay
Cell survival was determined using a Cell Counting Kit-8 (Dojindo, Tokyo, Japan). Briefly, B16F10 cells were cultured in 96-well plates (Corning Inc., Corning, NY, USA) at 5×103 cells per well with or without dissolved melatonin. After 24 hr, cells were washed and treated with the Cell Counting Kit-8, and the plate was incubated in the dark for 4 hr. Absorbance at 450 nm was read using a microplate reader (Epoch System, BioTek Instruments, Winooski, VT, USA). Percent viability was calculated as (absorbance of melatonin-treated cells/absorbance of control cells)×100.
Western Blotting
Cells were harvested, washed twice with ice-cold PBS, and resuspended in 20 mM Tris-HCl buffer (pH 7.4) containing protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin, 5 μg/mL pepstatin A, and 1 μg/mL chymostatin) and phosphatase inhibitors (5 mM Na3VO4 and 5 mM NaF). Whole cell lysates were prepared using 20 strokes of a Dounce homogenizer, followed by centrifugation at 13,000×g for 20 min at 4°C. Protein concentration was determined using the Bicinchoninic acid assay (BCA) (Sigma, St Louis, MO, USA). Proteins (40 μg) or media (20 μL) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes. Membranes were incubated with antibodies against p-mTOR (Ser2448, Ser2481) and mTOR (1∶1000) (Cell Signaling Technology, Beverly, MA, USA); p-Akt (Thr308, Ser473) and Akt (1∶1000) (Cell Signaling Technology); p85α and p85β (1∶500) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); p-PERK and PERK (1∶1000) (Cell Signaling Technology); p-eIF2α and eIF2α (1∶1000 dilution) (Cell Signaling Technology); Bax and Bcl-2 (1∶500) (Santa Cruz Biotechnology); catalase (1∶500) (Santa Cruz Biotechnology); Cu/Zn-SOD (1∶500) (Santa Cruz Biotechnology); Mn-SOD (1∶500) (Santa Cruz Biotechnology) and actin (1∶1000) (Santa Cruz Biotechnology). Membranes were incubated with anti-rabbit or anti-mouse IgG-conjugated horseradish peroxidase secondary antibodies (Santa Cruz Biotechnology) and ECL Western blotting reagents (Pierce Biotechnology, Rockford, IL, USA). Immunoreactive proteins were visualized by exposure to X-ray film. Protein bands were visualized by image scanning, and optical density was measured using ImageJ analysis software (version 1.37; Wayne Rasband, NIH, Bethesda, MD, USA) after data were corrected by background subtraction and normalized to actin as an internal control.
Statistical Analysis
Significant differences were detected by ANOVA, followed by Tukey’s test for multiple comparisons. Analysis was performed using Prism Graph Pad v4.0 (Graph Pad Software Inc., San Diego, CA, USA). Values are expressed as mean ± standard deviation (SD) of at least three separate experiments, with representative experiments shown in the figures. P<0.05 was considered statistically significant.
Results
Cell Survival in Thapsigargin or Tunicamycin Combined with Melatonin Treatment
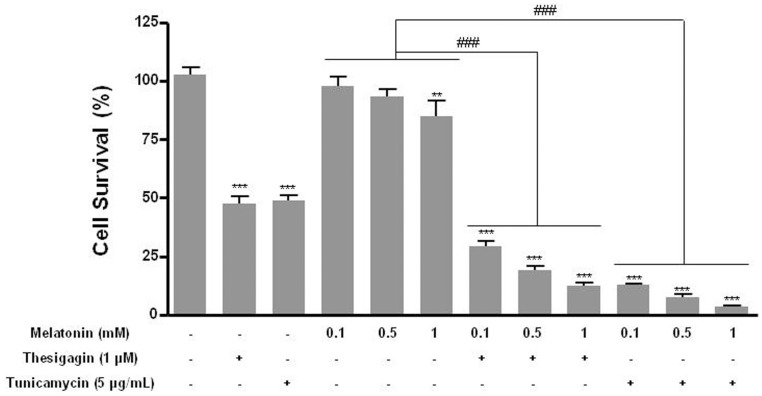
We investigated whether melatonin combined with ER stress induced death in B16F10 melanoma cells. Cell survival was significantly reduced by about 48% in ER stress conditions of thapsigargin or tunicamycin treatment and by about 30–37% when ER stress induced by thapsigargin or tunicamycin was combined with melatonin treatment. Comparisons were to treat with or without melatonin only (Fig. 1).
Figure 1. Cell viability of B16F10 cells after treatment with melatonin with or without thapsigargin or tunicamycin.
Cell survival of B16F10 cells was determined after 24± SD of three experiments. *P<0.05; **P<0.01; ***P<0.001 vs. untreated control; ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
p85β, p-Akt, and p-mTOR in Thapsigargin or Tunicamycin Combined with Melatonin Treatment
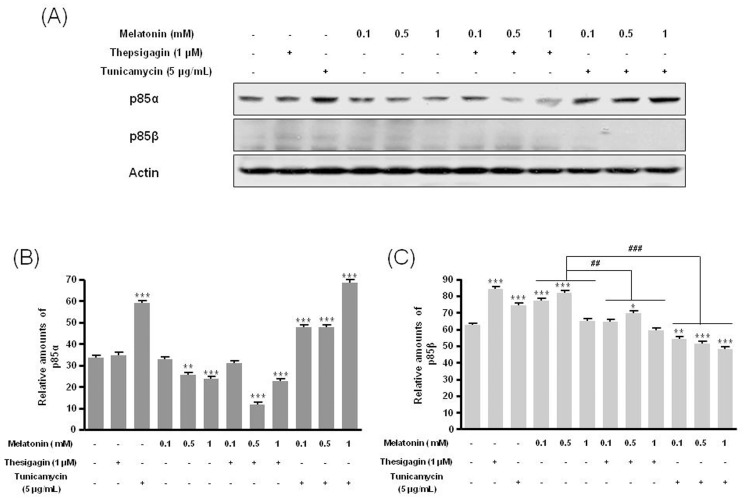
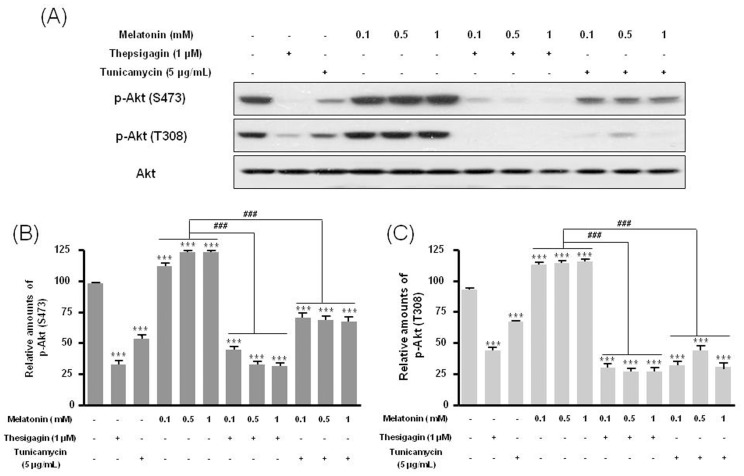
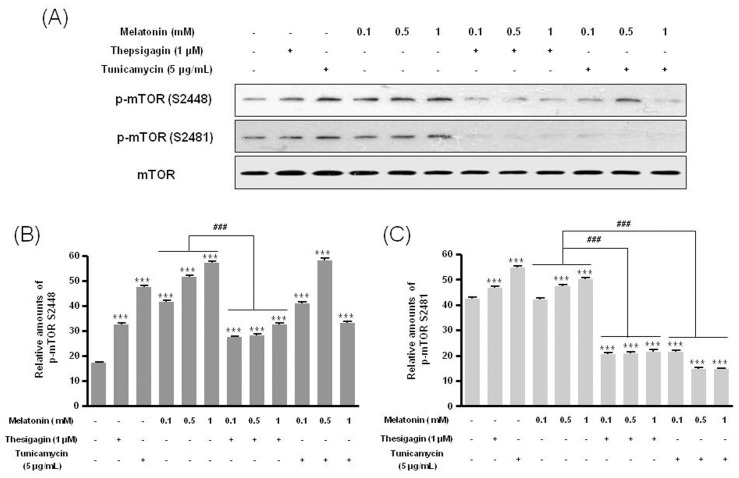
Combined melatonin and ER stress significantly decreased p85β protein expression compared to melatonin-only treatment (Fig. 2). Under combined treatment, expression of the PI3K downstream proteins p-Akt (Ser473, Thr308) and p-mTOR (Ser2448, Ser2481) was significantly reduced compared to melatonin-only treatment (Figs. 3, 4).
Figure 2. Expression of p85α and p85β in B16F10 cells after melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. p85α and p85β expression was analyzed by Western blot (A). Relative amounts of p85α (B) and p85β (C) quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ## P<0.01, ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
Figure 3. Phosphorylation of Akt (Ser473) and Akt (Thr308) in B16F10 cells after melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. p-Akt (Ser473) and p-Akt (Thr308) were analyzed by Western blot (A). Relative amounts of p-Akt (Ser473) (B) and p-Akt (Thr308) (C) quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
Figure 4. Phosphorylation of mTOR (Ser2448) and mTOR (Ser2481) in B16F10 cells after melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. p-mTOR (Ser2448) and p-mTOR (Ser2481) were analyzed by Western blot (A). Relative amounts of p-mTOR (Ser2448) (B) and p-mTOR (Ser2481) (C) were quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
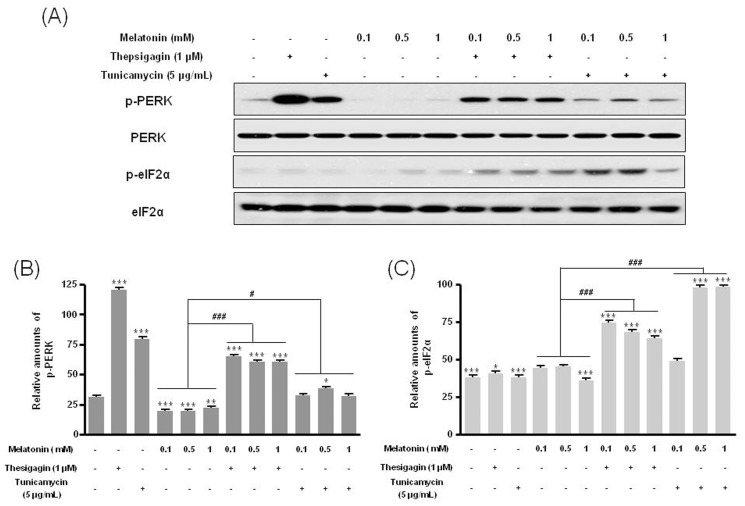
p-PERK and p-eIF2α in Thapsigargin or Tunicamycin Combined with Melatonin Treatment
The ER stress protein p-PERK was significantly increased under treatment with melatonin and ER stress compared to treatment with or without melatonin (Figs. 5A, B). The protein p-eIF2α also significantly increased under combination treatment (Figs. 5A, C).
Figure 5. Phosphorylation of PERK and eIF2α proteins in B16F10 cells after melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. p-PERK and p-eIF2α proteins were analyzed by Western blot (A). Relative amounts of p-PERK (B) and p-eIF2α (C) were quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; # P<0.05, ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
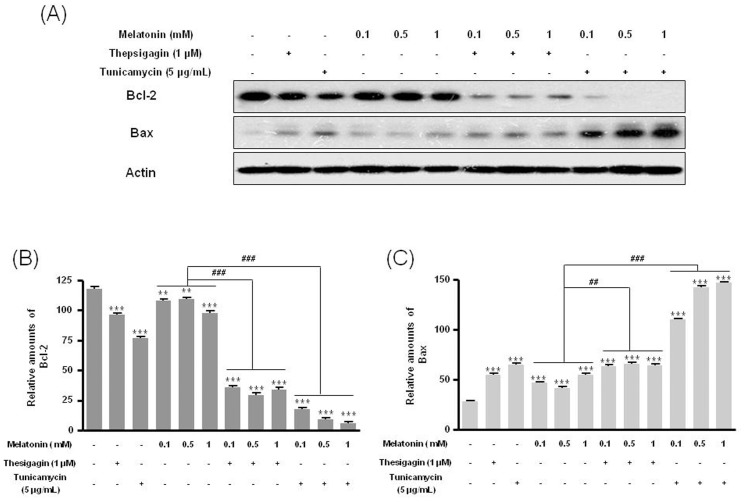
Bcl-2/Bax, Catalase, Cu/Zn-SOD, and Mn-SOD in Thapsigargin or Tunicamycin Combined with Melatonin Treatment
Combined melatonin and ER stress significantly reduced Bcl-2 protein expression and augmented Bax expression compared to treatment with or without melatonin (Fig. 6). Also, the combined condition significantly decreased expression of catalase, Cu/Zn-SOD, and Mn-SOD proteins compared to melatonin only (Fig. 7).
Figure 6. Expression of Bcl-2 and Bax in B16F10 cells after melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16. Bcl-2 and Bax were analyzed by Western blot (A). Relative amounts of Bcl-2 (B) and Bax (C) were quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ## P<0.01, ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
Figure 7. Expression of catalase, Cu/Zn-SOD, and Mn-SOD in B16F10 cells under melatonin with or without thapsigargin or tunicamycin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. Catalase, Cu/Zn-SOD, and Mn-SOD were analyzed by Western blot (A). Relative amounts of catalase (B), Cu/Zn-SOD (B), and Mn-SOD (D) quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ### P<0.001, melatonin vs. melatonin with thapsigargin or tunicamycin.
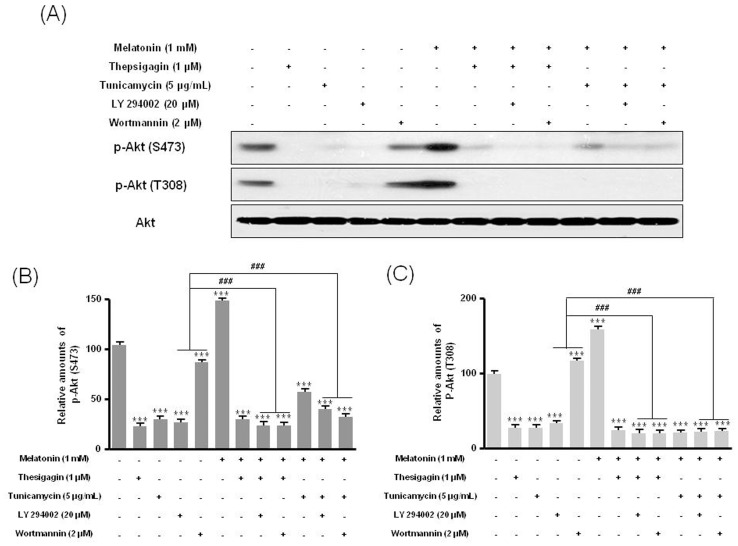
The PI3K Inhibitors LY294002 or Wortmannin Confirms the Expression of p85β Protein
Last, we found that the PI3K inhibitors LY294002 or wortmannin suppressed the expression of PI3K subunit p85β protein seen after combined melatonin and ER stress treatment of B16F10 melanoma cells. Expression of p85β decreased significantly after treatment with melatonin and thapsigargin or tunicamycin plus PI3K inhibitors than after treatment with only melatonin or PI3K inhibitors (Fig. 8). In addition, the PI3K downstream protein p-Akt (Ser473, Thr308) showed significantly lower expression after treatment with melatonin and thapsigargin or tunicamycin and PI3K inhibitors than after treatment with only melatonin or PI3K inhibitors (Fig. 9). These results indicate that survival of B16F10 melanoma cells after combined treatment with melatonin and ER stress inducers was suppressed through regulation of the PI3K/Akt/PKB-mTOR (p85β) pathway.
Figure 8. Expression of p85α, p85β, and p110 proteins in B16F10 cells after melatonin with or without thapsigargin/tunicamycin or LY 294002 or wortmannin treatment.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. Cells were treated with 20 μM LY 294002 or 2 μM wortmannin for 1 hr. Expression of p85α, p85β, and p110 was analyzed by Western blot (A). Relative amounts of p85α (B) and p85β (C) quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ### P<0.001, LY294002 or wortmannin vs. melatonin with thapsigargin or tunicamycin or LY294002 or wortmannin.
Figure 9. Phosphorylation of Akt (Ser473) and Akt (Thr308) in B16F10 cells under melatonin with or without thapsigargin or tunicamycin or LY294002 or wortmannin.
B16F10 cells were cultured with or without melatonin (0.1, 0.5, 1 mM) and/or thapsigargin (1 μM) for 6 hr or tunicamycin (5 μg/mL) for 16 hr. Cells were treated with 20 μM LY294002 or 2 μM wortmannin for 1 hr. p-Akt (Ser473) and p-Akt (Thr308) were analyzed by Western blot (A). Relative amounts of p-Akt (Ser473) (B) and p-Akt (Thr308) (C) quantified as described in Materials and methods. Data are mean ± SD of three experiments. ***P<0.001 vs. untreated control; ### P<0.001, LY294002 or wortmannin vs. melatonin with thapsigargin or tunicamycin or LY294002 or wortmannin.
Discussion
This study found that melatonin combined with thapsigargin or tunicamycin directly influenced cell death of B16F10 melanoma cells via the PI3K/Akt/mTOR pathway, suggesting that melatonin combined with ER stress could be a new therapeutic strategy for effective melanoma treatment.
Melatonin has an anti-proliferative function in melanoma cells with either a direct antitumor effect [22]–[25] or through melatonin receptors [26]–[29]. For direct anti-proliferation effects, the effective melatonin dose is 1 μM to 1 mM, and the effective time is 24 to 72 hr. For potential interactions with melatonin receptors, the dose is 10 pM to 0.1 μM for 72 hr in melanoma cells [22]–[29]. However, this pharmacological action of melatonin does not offer a promising strategy for preventing proliferation of melanoma cells. Instead, new strategies and potential alternative approaches using melatonin are needed for melanoma treatment. In this study, the PI3K/Akt/mTOR pathway in melanoma cells was demonstrated to be a new therapeutic target for melanoma treatment [30]–[38].
Sánchez-Hernández et al. [40] reported that the RAF inhibitor sorafenib induces apoptosis by dual targeting of BRAF and PI3K/AKT/mTOR signaling to effectively control melanoma disease. Marone et al. [41] used newly identified ATP-competitive PI3K/mTOR inhibitors to demonstrate that the PI3K and mTOR pathways inhibit tumor growth in a syngeneic B16 mouse melanoma tumor model. Furthermore, a combination of PI3K inhibitors and rapamycin has cooperative effects in other tumors in vitro [42]. Sinnberg et al. [43] studied combinations of the PI3K inhibitor LY294002 or the mTOR inhibitor rapamycin with the chemotherapeutics cisplatin or temozolomide, finding that these combinations significantly induced apoptosis of melanoma cells and completely suppressed invasive tumor growth of melanoma cells. These results indicate that the PI3K/mTOR signaling pathway effectively regulates melanoma disease and prevents tumor progression. In this study, we demonstrated that a combination of melatonin and thapsigargin or tunicamycin induced cell death through the PI3K/Akt/mTOR pathway in B16F10 melanoma cells. This treatment could be a new strategy for effective suppression of B16F10 melanoma cell proliferation.
In melanomas, the anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 appear to increase while the pro-apoptotic protein Bax decreases with the progression of primary melanoma tumors and melanoma cells and might be involved in resistance to conventional therapies [44]–[46]. In our study, the combination of melatonin and thapsigargin or tunicamycin significantly downregulated Bcl-2 and upregulated Bax in melanoma cells. These and other studies suggest that the PI3K/AKT/mTOR signaling pathway modulates the expression of Bcl-2 and Bax family proteins [47]–[50]. Inhibition of mTOR with rapamycin- or mTOR-specific small interfering RNA downregulated Bcl-2 and Mcl-1 in anaplastic large-cell lymphoma cells [51]. Likewise, the RAF inhibitor sorafenib downregulates Bcl-2 and Mcl-1 through independent inhibition of mitogen-activated protein kinase (MAPK) [52]–[54]. Also, sorafenib together with rapamycin downmodulates Bcl-2 and Mcl-1 through mechanisms that are dependent on RAF and MAPK inhibition [46]. Therefore, Bcl-2 and Bax family proteins modulate melanoma proliferation through PI3K/mTOR or MAPK signaling pathways and could effectively regulate melanoma disease and prevent tumor progression.
The PI3K inhibitors LY294002 and wortmannin and the mTOR inhibitor rapamycin significantly augment growth inhibition by cisplatin and temozolomide. This results indicate a possible synergistic effect on melanoma cells treated with combinations of rapamycin or wortmannin and temozolomide [43]. Combinations of the PI3K inhibitors LY294002 and wortmannin or the mTOR inhibitor rapamycin with the chemotherapeutics cisplatin or temozolomide lead to a significant 2- to 3-fold increase in melanoma cell apoptosis, but MAPK pathway inhibitors do not significantly increase chemosensitivity [43]. Similarly, in our study, the PI3K inhibitors LY294002 or wortmannin in combination with melatonin and thapsigargin or tunicamycin induced cell death. We demonstrated that the PI3K inhibitors LY294002 and wortmannin in combination with melatonin and thapsigargin or tunicamycin influenced the expression of a PI3K subunit and p-Akt in B16F10 melanoma cells. PI3K inhibitors in combination with melatonin and thapsigargin or tunicamycin decreased expression of the PI3K subunit p85β and p110 proteins and the PI3K downstream molecule p-Akt (Ser473, Thr308) significantly more than treatment with melatonin or PI3K inhibitors only. These results indicate that the PI3K inhibitors LY294002 and wortmannin in combination with melatonin and thapsigargin or tunicamycin enhance the PI3K/AKT/mTOR signaling pathway.
Recent studies imply that intrinsic pathways for induction of cell death in cancer therapy are initiated by ER stress. Abnormal protein folding or calcium imbalance in the ER triggers ER stress and subsequently induces destruction responses in cells [55]–[58]. Cell death of human melanoma cells by ER stress induced to upregulate p-PERK and p-eIF2α could benefit the pharmaceutical development of anti-melanoma drugs [57], [58]. In our study, a combination treatment with melatonin and ER stress-inducing agents significantly increased p-PERK and p-eIF2α compared to treatment with or without melatonin only, downregulating translation and resulting in cell death.
In conclusion, our data suggest that inhibition of the PI3K/AKT/mTOR signaling pathway by melatonin combined with thapsigargin or tunicamycin efficiently attenuates growth and proliferation of B16F10 melanoma cells. The PI3K inhibitors LY294002 or wortmannin in combination with melatonin and thapsigargin/tunicamycin may enhance cell death.
Funding Statement
This research was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-00757). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84: 1155–1228. [DOI] [PubMed] [Google Scholar]
- 2. Liang KV, Sanderson SO, Nowakowski GS, Arora AS (2006) Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc 81: 511–516. [DOI] [PubMed] [Google Scholar]
- 3. Kivelä T, Kujala E. (20130 Prognostication in eye cancer: the latest tumor, node, metastasis classification and beyond. Eye (Lond) 27: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerant AF, Johnson JT, Sheridan CD, Caffrey TJ. (2000) Early detection and treatment of skin cancer. Am Fam Physician 62: 357–368, 375–376, 381–382. [PubMed]
- 5. Weinstock MA (2000) Early detection of melanoma. JAMA 284: 886–889. [DOI] [PubMed] [Google Scholar]
- 6. Jung-Hynes B, Reiter RJ, Ahmad N (2010) Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res 48: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, et al. (2011) Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res 51: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui P, Yu M, Peng X, Dong L, Yang Z (2012) Melatonin prevents human pancreatic carcinoma cell PANC-1-induced human umbilical vein endothelial cell proliferation and migration by inhibiting vascular endothelial growth factor expression. J Pineal Res 52: 236–243. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Xiao X, Zhang Y, Shi D, Chen W, et al. (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res 53: 77–90. [DOI] [PubMed] [Google Scholar]
- 10. Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, et al. (2012) Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res 53: 91–98. [DOI] [PubMed] [Google Scholar]
- 11. Min KJ, Kim HS, Park EJ, Kwon TK (2012) Melatonin enhances thapsigargin-induced apoptosis through reactive oxygen species-mediated upregulation of CCAAT-enhancer-binding protein homologous protein in human renal cancer cells. J Pineal Res 53: 99–106. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, et al. (2004) Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 36: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1: 57–60. [Google Scholar]
- 14. Bonnefont-Rousselot D, Collin F, Jore D, Gardès-Albert M (2011) Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res 50: 328–235. [DOI] [PubMed] [Google Scholar]
- 15. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51: 1–16. [DOI] [PubMed] [Google Scholar]
- 16. Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pineal Res 47: 109–126. [DOI] [PubMed] [Google Scholar]
- 17. Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM (2010) Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res 48: 297–310. [DOI] [PubMed] [Google Scholar]
- 18. Kilic U, Yilmaz B, Ugur M, Yüksel A, Reiter RJ, et al. (2012) Evidence that membrane-bound G protein-coupled melatonin receptors MT1 and MT2 are not involved in the neuroprotective effects of melatonin in focal cerebral ischemia. J Pineal Res 52: 228–235. [DOI] [PubMed] [Google Scholar]
- 19. Joo SS, Yoo YM (2009) Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res 47: 8–14. [DOI] [PubMed] [Google Scholar]
- 20. Kim CH, Yoo YM (2010) Melatonin induces apoptotic cell death via p53 in LNCaP cells. Korean J Physiol Pharmacol 14: 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim CH, Kim KH, Yoo YM (2012) Melatonin-induced autophagy is associated with degradation of MyoD protein in C2C12 myoblast cells. J Pineal Res 53: 289–297. [DOI] [PubMed] [Google Scholar]
- 22. Narita T, Kudo H (1985) Effect of melatonin on B16 melanoma growth in athymic mice. Cancer Res 45: 4175–4177. [PubMed] [Google Scholar]
- 23. Cos S, Garcia-Bolado A, Sánchez-Barceló EJ (2001) Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16BL6 and PG19). Melanoma Res 11: 197–201. [DOI] [PubMed] [Google Scholar]
- 24. Yerneni LK, Jayaraman S (2003) Pharmacological action of high doses of melatonin on B16 murine melanoma cells depends on cell number at time of exposure. Melanoma Res 13: 113–117. [DOI] [PubMed] [Google Scholar]
- 25. Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, et al. (2010) Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res 49: 45–54. [DOI] [PubMed] [Google Scholar]
- 26. Souza AV, Visconti MA, Castrucci AM (2003) Melatonin biological activity and binding sites in human melanoma cells. J Pineal Res 34: 242–248. [DOI] [PubMed] [Google Scholar]
- 27. Kadekaro AL, Andrade LN, Floeter-Winter LM, Rollag MD, Virador V, et al. (2004) MT-1 melatonin receptor expression increases the antiproliferative effect of melatonin on S-91 murine melanoma cells. J Pineal Res 36: 204–211. [DOI] [PubMed] [Google Scholar]
- 28. Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, et al. (2006) Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol 29: 665–672. [DOI] [PubMed] [Google Scholar]
- 29. Helton RA, Harrison WA, Kelley K, Kane MA (1993) Melatonin interactions with cultured murine B16 melanoma cells. Melanoma Res 3: 403–413. [DOI] [PubMed] [Google Scholar]
- 30. Bjornsti MA, Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348. [DOI] [PubMed] [Google Scholar]
- 31. Morgensztern D, McLeod HL (2005) PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 16: 797–803. [DOI] [PubMed] [Google Scholar]
- 32. Samuels Y, Ericson K (2006) Oncogenic PI3K and its role in cancer. Curr Opin Oncol 18: 77–82. [DOI] [PubMed] [Google Scholar]
- 33. Slomovitz BM, Coleman RL (2012) The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res 18: 5856–5864. [DOI] [PubMed] [Google Scholar]
- 34. Leevers SJ, Vanhaesebroeck B, Waterfield MD (1999) Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol 11: 219–225. [DOI] [PubMed] [Google Scholar]
- 35. Xie X, White EP, Mehnert JM (2013) Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PLoS One 8: e55096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Werzowa J, Koehrer S, Strommer S, Cejka D, Fuereder T, et al. (2011) Vertical inhibition of the mTORC1/mTORC2/PI3K pathway shows synergistic effects against melanoma in vitro and in vivo. J Invest Dermatol 131: 495–503. [DOI] [PubMed] [Google Scholar]
- 37. Karbowniczek M, Spittle CS, Morrison T, Wu H, Henske EP (2008) mTOR is activated in the majority of malignant melanomas. J Invest Dermatol 128: 980–987. [DOI] [PubMed] [Google Scholar]
- 38. Meier F, Schittek B, Busch S, Garbe C, Smalley K, et al. (2005) The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci 10: 2986–3001. [DOI] [PubMed] [Google Scholar]
- 39. Zha L, Fan L, Sun G, Wang H, Ma T, et al. (2012) Melatonin sensitizes human hepatoma cells to endoplasmic reticulum stress-induced apoptosis. J Pineal Res 52: 322–331. [DOI] [PubMed] [Google Scholar]
- 40. Sánchez-Hernández I, Baquero P, Calleros L, Chiloeches A (2012) Dual inhibition of (V600E) BRAF and the PI3K/AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEK-independent mechanism. Cancer Lett 314: 244–255. [DOI] [PubMed] [Google Scholar]
- 41. Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, et al. (2009) Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res 7: 601–613. [DOI] [PubMed] [Google Scholar]
- 42. Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, et al. (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65: 3336–3346. [DOI] [PubMed] [Google Scholar]
- 43. Sinnberg T, Lasithiotakis K, Niessner H, Schittek B, Flaherty KT, et al. (2009) Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol 129: 1500–1515. [DOI] [PubMed] [Google Scholar]
- 44. Bush JA, Li G (2003) The role of Bcl-2 family members in the progression of cutaneous melanoma. Clin Exp Metastasis 20: 531–539. [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Rosdahl I (2006) Bcl-xL and bcl-2 proteins in melanoma progression and UVB-induced apoptosis. Int J Oncol 28: 661–666. [PubMed] [Google Scholar]
- 46. Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, et al. (2008) Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J Invest Dermatol 128: 2013–2023. [DOI] [PubMed] [Google Scholar]
- 47. Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, et al. (1999) The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol 19: 6195–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mori M, Uchida M, Watanabe T, Kirito K, Hatake K, et al. (2003) Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J Cell Physiol 195: 290–297. [DOI] [PubMed] [Google Scholar]
- 49. Asnaghi L, Calastretti A, Bevilacqua A, D’Agnano I, Gatti G, et al. (2004) Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene 23: 5781–5791. [DOI] [PubMed] [Google Scholar]
- 50. Tirado OM, Mateo-Lozano S, Notario V (2005) Rapamycin induces apoptosis of JN-DSRCT-1 cells by increasing the Bax:Bcl-xL ratio through concurrent mechanisms dependent and independent of its mTOR inhibitory activity. Oncogene 24: 3348–3357. [DOI] [PubMed] [Google Scholar]
- 51. Vega F, Medeiros LJ, Leventaki V, Atwell C, Cho-Vega JH, et al. (2006) Activation of mammalian target of rapamycin signaling pathway contributes to tumor cell survival in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Cancer Res 66: 6589–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rahmani M, Davis EM, Bauer C, Dent P, Grant S (2005) Apoptosis induced by the kinase inhibitor BAY 43–9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem 280: 35217–35227. [DOI] [PubMed] [Google Scholar]
- 53. Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, et al. (2005) The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43–9006. Oncogene 24: 6861–6869. [DOI] [PubMed] [Google Scholar]
- 54. Panka DJ, Wang W, Atkins MB, Mier JW (2006) The Raf inhibitor BAY 43–9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res 66: 1611–1619. [DOI] [PubMed] [Google Scholar]
- 55. Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 56. Boyce M, Bryant KF, Jousse C, Long K, Harding HP, et al. (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939. [DOI] [PubMed] [Google Scholar]
- 57. Mhaidat NM, Thorne R, Zhang XD, Hersey P (2008) Involvement of endoplasmic reticulum stress in Docetaxel-induced JNK-dependent apoptosis of human melanoma. Apoptosis 13: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 58. Su TR, Tsai FJ, Lin JJ, Huang HH, Chiu CC, et al. (2012) Induction of apoptosis by 11-dehydrosinulariolide via mitochondrial dysregulation and ER stress pathways in human melanoma cells. Mar Drugs 10: 1883–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]