Abstract
Cervical cancer is the second most common malignancy among women worldwide, with over 490000 cases diagnosed and 274000 deaths each year. Although current screening methods have dramatically reduced cervical cancer incidence and mortality in developed countries, a “See and Treat” method would be preferred, especially in developing countries. Results from our previous work have suggested that Raman spectroscopy can be used to detect cervical precancers; however, with a classification accuracy of 88%, it was not clinically applicable. In this paper, we describe how incorporating a woman's hormonal status, particularly the point in menstrual cycle and menopausal state, into our previously developed classification algorithm improves the accuracy of our method to 94%. The results of this paper bring Raman spectroscopy one step closer to being utilized in a clinical setting to diagnose cervical dysplasia.
Posterior probabilities of class membership, as determined by MRDF-SMLR, for patients regardless of menopausal status, and for pre-menopausal patients only
Keywords: optical diagnosis, hormonal status, cervix, multi-class-statistical analysis
1. Introduction
Cervical cancer is the second most common malignancy among women worldwide, with over 490000 cases diagnosed and 274000 deaths each year [1]. In the US, it is estimated that 3,870 deaths will occur and 11070 new cases of invasive cervical cancer will be diagnosed in 2008 [2]. Due to the introduction of early detection methods, incidences of clinically invasive cervical cancer have been shown to decrease by 78% and mortality to decrease by 72% [3]. Early identification of cervical precancers is the key for successful treatment of the disease and for decreasing mortality from it.
The normal cervix is covered by two types of epithelia: squamous and columnar. Their interface is called the squamo-columnar junction. Over time, the columnar epithelium is replaced by squamous epithelium, causing the squamo-columnar junction to move towards the os or the opening of the ectocervix. This transitional epithelium is termed squamous metaplasia [4]. Virtually all squamous cervical neoplasms begin at the squamo-columnar junction. The extent and limit of their precursors coincide with the distribution of the transformation zone [5].
There are a variety of clinically normal abnormalities that can result in an atypical cervix. Cervicitis, or inflammation, which may be infectious or non-infectious, is usually the response of tissue to injury and is a byproduct of its natural repair mechanism [5]. As described above, the transitional epithelium or squamous metaplasia is a normal abnormality that occurs in the cervix. Pregnancy is another normal state that causes a variety of biochemical and functional changes to occur in cervical connective tissue [6]. This process of cervical ripening results in the softening, dilation, and effacement of the cervix, a normal process during pregnancy that drastically changes the makeup of the cervix [7]. A woman's menstrual cycle can also lead to normal changes in the cervix. During the cycle, a woman's hormonal level fluctuates as levels of estrogen and progesterone peak during and after ovulation, respectively. Although these changes are small compared to those found during pregnancy, they do impact the biochemical makeup of the cervix [8]. Menopause, the permanent cessation of the menstrual cycle, may also affect the cervix due to hormonal changes. Peri-menopause is defined as the transitional period from normal menstrual periods to no periods at all, often taking up to 10 years.
Clinically speaking, cervical lesions can be divided into low grade squamous intraepithelial lesions (LGSIL) and high grade squamous intraepithelial lesions (HGSIL). This distinction is important, as patients with low grade lesions are usually followed but not treated, while patients with high grade lesions are usually treated immediately with extended follow-up. Patients with specific strains of human papillomavirus (HPV) are typically placed in the same category as patients with low grade lesions and are treated as such. Certain strains of HPV may be involved in the early stages of cervical cancer, while other strains may aid in the progression of the disease [9]. These viruses infect skin and mucosal membranes to produce characteristic epithelial proliferation, which can have the capacity to undergo malignant transformations [5].
Currently, the primary screening tool for cervical precancer is the Papanicolaou (Pap) smear, where cells scraped from the walls of the ecto- and endocervix are examined and diagnosed [10]. The widespread application of the Pap smear as a screening tool has greatly decreased the incidence of cervical cancer, especially in the US [11]. While the specificity of the Pap smear is generally very high (95%) [11], its sensitivity can be as low as 20% to 50% depending on the prevalence of the disease within the population [12]. Colposcopy usually follows an abnormal Pap smear in the US and is used to direct the taking of biopsies [13]. After applying 4–6% acetic acid to the cervix to turn abnormal areas white, a colposcope is used to visualize these abnormal areas on the surface of the cervix. Multiple biopsies are then taken using standard punch biopsy forceps, and these tissue samples are fixed in formalin and sent for histological examination. Colposcopy has a sensitivity of 62% to 98% in pin-pointing the presence and grade of lesions [14–16]. The result of the biopsy decides treatment of the disease. This procedure requires extensive training, is time-consuming, and its sensitivity is variable and limited, even in the hands of expert practitioners [17]. A “see and treat” tool that can provide high sensitivity in diagnosing cervical cancer would be optimal in addressing the limitations of colposcopy-guided biopsy.
Optical methods are prime candidates for “see and treat” procedures for the cervix because they can provide automated, fast, and non-intrusive characterization of normal and abnormal tissues in vivo. Choosing the right optical method depends on the particular requirements of a given problem. For example, optical coherence tomography (OCT) can produce high-resolution, cross-sectional images, but since its ability to detect disease depends on visualizing changes in the tissue microstructure, it is difficult for OCT to detect early cases of cervical precancer [18]. Fluorescence spectroscopy has been studied extensively for screening and diagnosing cervical pre-cancers [19–23], but the results have had unacceptably high false positive rates for detecting benign abnormalities such as inflammation, hyperplasia, and metaplasia [24, 25]. Previous studies using infrared microspectroscopy have shown differences in spectra due to the normal, cyclical changes of cervical epithelium [26]. However, this application is limited because tissue must be removed before spectra are acquired. For a tissue like the cervix, where many normal changes occur, the optical technique applied must be able to discern among the various conditions such as cervicitis, LGSIL, and HGSIL. Raman spectroscopy is a molecular specific technique providing information about the biochemical composition of a tissue by probing vibrational and rotational bond transitions in various biomolecules [27]. Our previous work suggests that Raman spectroscopy combined with automated multivariate statistical analysis can be used to detect changes in tissue associated with the progression of cervical cancer [28]. With a sensitivity of 88%, our previous application cannot be applied within the clinic since it does not provide sufficient improvement over traditional methods. While this may be due to Raman spectroscopy's inability to be utilized in this capacity, we believe that including small, normal variations like menopausal or hormonal status in our classification algorithms will improve the sensitivity to over 90%.
The goal of this paper is to examine the effect of incorporating normal variations into our classification algorithm prior to data analysis. Raman spectra were acquired from 145 patients undergoing either a routine Pap smear or biopsy guided by colposcopy. The effects that hormonal status, particularly the point in the menstrual cycle and menopausal state, had on Raman spectra gathered from normal cervix areas were examined. These effects were then accounted for to improve the classification ability of a previously developed statistical algorithm for detecting cervical precancer, highlighting the need to account for such variation in any further analysis.
2. Materials and Methods
2.1. Clinical Study Design-Pap Smear Patients
A total of 102 patients undergoing a routine Pap smear were recruited to participate in the study as approved by the Copernicus and Vanderbilt Institutional Review Boards (IRBs). To be eligible for enrollment, the patient had to be undergoing a routine Pap smear, be between the ages of 18–75, and still have a cervix. Informed consent was obtained from each patient prior to the procedure. The cervix was exposed and visually examined by the attending physician, and the Pap procedure was done according to standard clinical protocol. The cervix was wiped clean with a dry cotton swab, after which Raman measurements were taken from three locations on the ectocervix. The spectra were considered normal if the Pap smear was negative. The patient's age, date of last menstrual period, use of artificial hormones, menopausal status, and any previous abnormal Pap smears were all noted upon chart review.
2.2. Clinical Study Design-Dysplasia Patients
A total of 43 patients undergoing colposcopy-guided biopsy were recruited to participate in the study as approved by the Copernicus and Vanderbilt IRBs. To be eligible for enrollment, the patient had to be undergoing a colposcopy-guided biopsy, be between the ages of 18–75, and still have a cervix. After informed consent was obtained from each patient, the cervix was exposed and visually examined by the attending physician. Acetic acid was applied to the cervix to turn abnormal areas white for visualization, and spectra were acquired from multiple areas of abnormal tissue and 1–2 visually normal areas. Aceto-white tissue was then removed and placed in fixative solution for pathology examination. Based on pathology, spectra were placed into four categories for analysis: normal, metaplasia, LGSIL, and HGSIL.
2.3. Instrumentation
Raman spectra were collected from multiple sites in vivo using a portable Raman spectroscopy system consisting of a 785 nm diode laser (Process Instruments, Inc., Salt Lake City, UT), a beam-steered fiber optic probe (Visionex Inc., Atlanta, GA), an imaging spectrograph (Kaiser Optical Systems, Inc., Ann Arbor, MI), and a back-illuminated, deep-depletion, thermo-electrically cooled charge coupled device (CCD) camera (Roper Scientific, Inc., Princeton, NJ), all controlled with a laptop computer. Details of the system have been previously reported [30]. For each measurement, the fiber optic probe delivered 80mW of incident light onto the tissue for 3 seconds, and the overhead fluorescent lights were turned off. Spectra were obtained from the surface of the cervix. As the penetration depth is about 300 μm, information from both the epithelium and the stroma are present in the spectra.
Spectral calibration of the system was performed each day using a neon-argon lamp and naphthalene and acetaminophen standards to correct for system wavenumber, laser excitation, and throughput variations. The spectra were processed for fluorescence subtraction and noise smoothing using the modified polynomial fit method described previously [29]. Following data processing, each spectrum was normalized to its mean spectral intensity across all Raman bands to account for overall intensity variability. These normalized spectra were categorized according to menopausal status and histopathological classification as determined by the pathologist.
2.4. Statistical Analysis
The two-step multivariate analysis technique used in this paper has been previously described [30]. In the first step, using maximum representation and discrimination feature (MRDF), the processed data set undergoes a two-part, non-linear transform to extract relevant features that provide the best class separation. The second step uses sparse multinomial logistic regression (SMLR) for classifying the MRDF output features into corresponding tissue categories. SMLR is a probabilistic multi-class model based on a Bayesian machine-learning framework of statistical pattern recognition, which separates a set of labeled input data into their classes by predicting the posterior probabilities of class membership. An unbiased classification algorithm was developed using these two methods and was tested using leave-one-patient-out cross-validation. The classification accuracy, defined as the total number of spectra correctly classified divided by the total number of spectra, is used to determine the validity of the method. The use of acetic acid should not affect the analysis because statistical comparisons were done with either the presence or absence of acetic acid unless otherwise noted.
3. Results
3.1.Variations in Menopausal Status
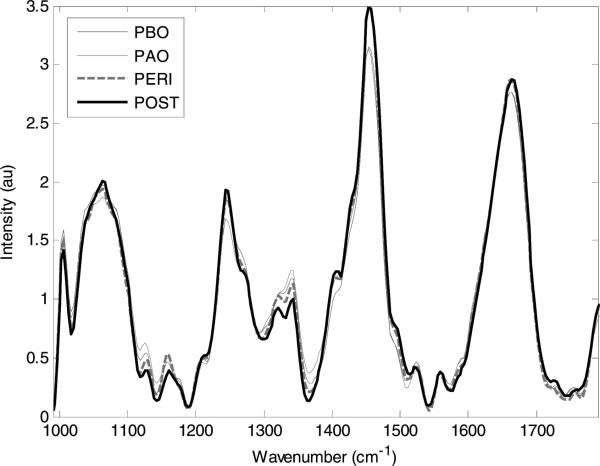
The Raman spectra in Figure 1 are shown stratified by menopausal status into four groups: pre-menopausal proliferative phase (days 1–14 of the menstrual cycle) or pre-menopausal before ovulation (PBO); pre-menopausal secretory phase (days 15–28+ of the menstrual cycle) or pre-menopausal after ovulation (PAO); peri-menopausal (PERI); and post-menopausal (POST). These terms are defined in Table 1. Some spectra were thrown out due to slipping of the probe or if mucus was present on the cervix. From the spectra that remained, there were subtle but consistent differences according to location within the menstrual cycle and menopausal status, especially at the 1250 cm–1 peak, typically associated with collagen, and the 1300–1320 cm–1 region, typically associated with cellular features [31]. As seen in the confusion matrix in Table 2, the spectra were classified into their respective hormonal and menopausal groups by the MRDF-SMLR algorithm with an overall accuracy of 98%. The number along the diagonal of the confusion matrix represents the number of spectra that were classified correctly. In this case, only three spectra were misclassified.
Figure 1.
Average Raman spectra for post menopausal normal cervix (POST-29), peri menopausal normal cervix (PERI-34), pre-menopausal after ovulation normal cervix (PAO-54) and pre-menopausal before ovulation normal cervix (PBO-47).
Table 1.
Description of abbreviations for menopausal and hormonal status.
| Category | Abbreviation | Age | Time since last period |
|---|---|---|---|
| Pre-menopausal before ovulation | PBO | 18–45 | 5–14 days |
| Pre-menopausal after ovulation | PAO | 18–45 | 15–28 days |
| Peri-menopausal | PERI | 45–55 | Variable periods |
| Post-menopausal | POST | 50+ | 2+ years |
Table 2.
Classification of different hormonal variations using MRDF and SMLR leave-one- patient-out cross-validation.
| Histological Classification |
|||||
|---|---|---|---|---|---|
| PBO | PAO | PERI | POST | ||
| Raman Classification | PBO | 47 | 0 | 0 | 0 |
| PAO | 0 | 53 | 0 | 0 | |
| PERI | 0 | 0 | 33 | 1 | |
| POST | 0 | 1 | 1 | 28 | |
3.2. Dysplasia Study
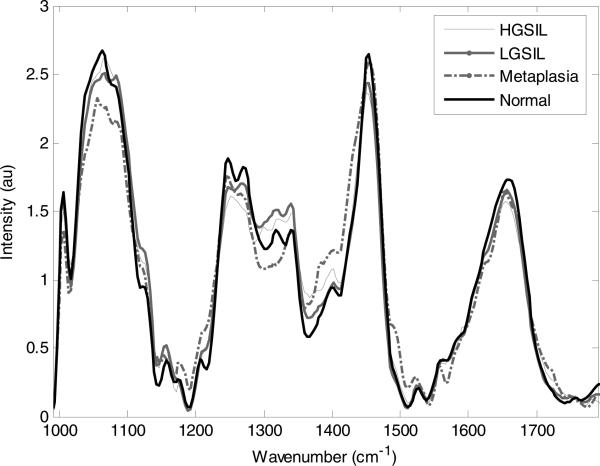
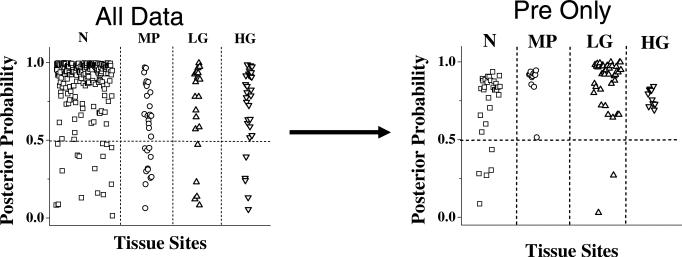
Average spectra from cervical tissues categorized as LGSIL, HGSIL, normal, and metaplasia are displayed in Figure 2. These spectra were obtained before colposcopy-guided biopsy. The most prominent spectral differences among the classes are seen in the region between 1230 cm–1 and 1300 cm–1, where a number of biological molecules contribute to the spectral features. Using the MRDF-SMLR algorithm, the overall classification accuracy was 88%, with a sensitivity of 86% and specificity of 97% for separating diseased from non-diseased tissues [32]. These results are somewhat undesirable for use in the clinic, especially in the LGSIL category, where the misclassification rate was 19%. Based on the results of the above study, the spectra were further stratified by menopausal status with only pre-menopausal spectra used. Table 3 shows the confusion matrix comparing Raman diagnosis to histopathology diagnosis using the following four classes: normal, metaplasia, LGSIL, and HGSIL. In this analysis, only six out of 95 spectra were misclassified – two LGSIL and four normal. This stratification of spectra based on menopausal state results in an improvement of overall classification accuracy from 88% to 94%. This result is shown in Figure 3, which displays the posterior probabilities of class membership, as determined by MRDF-SMLR, for all patients regardless of menopausal status (as published by Kanter et al. [32]), and for pre-menopausal patients only. Besides the improvement in classification rate, Figure 3 also shows an increase in the algorithm's confidence in its classification, as more spectra receive probabilities of class membership closer to one.
Figure 2.
Average Raman spectra for normal ectocervix, metaplasia, LGSIL and HGSIL.
Table 3.
Classification of pre-menopausal samples- normal, metaplasia, LGSIL and HGSIL using MRDF and SMLR leave-one-patient-out cross-validation.
| Histological Classification |
|||||
|---|---|---|---|---|---|
| Normal | Metaplasia | LGSIL | HGSIL | ||
| Raman Classification | Normal | 27 | 0 | 2 | 0 |
| Metaplasia | 0 | 12 | 0 | 0 | |
| LGSIL | 4 | 0 | 39 | 0 | |
| HGSIL | 0 | 0 | 0 | 11 | |
Figure 3.
Posterior probabilities of classification as normal ectocervix (N), metaplasia (MP), LGSIL (LG) and HGSIL (HG) from the entire data set (classification accuracy 88%) and the pre-menopausal data only (classification accuracy 94%) [33].
3.3. Independent Validation
Based on the results of the previous study, a small independent validation was performed. Spectra obtained from patients undergoing routine Pap smear that had resulted in an abnormal diagnosis were classified using the above algorithm (from the dysplasia study). When the spectra were taken, the location of the measurement on the cervix was noted. The spectra were then run through the MRDF-SMLR algorithm developed for the dysplasia classification using only the pre-menopausal data. A colposcopy was done as normal, and those results were compared to the algorithm results, as shown in Table 4. Of the six spectra that were analyzed in this manner, three were classified as LGSIL and three as normal. All of the spectra came from a cervix that the Pap smear suggested may contain areas of LGSIL. In all areas that were classified by the algorithm as normal, no biopsies were taken, and the colposcopic examination suggested that these areas were indeed normal. In the areas that classified as LGSIL, two of these regions were biopsied and confirmed to be LGSIL. The other region was not biopsied, but was noted in the examination as having a mosaic appearance, meaning it may have had some disease, although there is no way to confirm this.
Table 4.
Results of the independent validation.
| Raman Classification | Pathology |
|---|---|
| LGSIL | LGSIL confirmed by biopsy |
| LGSIL | LGSIL confirmed by biopsy |
| LGSIL | Unknown |
| Normal | Suspected Normal |
| Normal | Suspected Normal |
| Normal | Suspected Normal |
4. Discussion
Raman spectroscopy can be used in the cervix, despite variations in both patient history and physiology. This study brings Raman spectroscopy one step closer to clinical use by improving its specificity in diagnosing cervical dysplasia. This improvement was accomplished by incorporating variations in the normal cervix to differentiate LGSIL, HGSIL, and metaplasia from normal tissues. Our results suggest that Raman spectroscopy is sensitive enough to detect subtle changes in the cervix such as those that occur due to hormonal or menopausal status as well as small changes that are associated with dysplasia.
Previous fluorescence studies have shown that there is a statistical difference between pre and post-menopausal fluorescence data and that the post-menopausal women have a higher average fluorescence signal [33] which may need to be considered when using fluorescence spectroscopy for disease detection. Another study suggests that fluorescence spectroscopy is predominantly affected by hemoglobin absorption and these effects can be avoided if measurements are not taken during the first eight days of the cycle [34], indicating that fluorescence spectroscopy is not sensitive to subtle, hormonal changes in the cervix. Fluorescence spectroscopy is capable of distinguishing HGSIL from normal, however in one study, the sensitivity and specificity of detecting squamous normal cervix tissue and LGSIL of the cervix in 161 patients are only 55% and 63%, respectively [35] and thus fluorescence spectroscopy cannot be used to find early indicators of disease. Raman spectroscopy at first glance seems to have similar results to fluorescence spectroscopy. But since Raman is very sensitive to biochemical changes, it should be able to discriminate small changes associated with disease and small changes associated with other factors. Therefore, it is necessary to account for such changes, such as menopausal status, when using Raman to detect early disease where only a few disease-related biochemical changes have occurred.
This new approach was necessary because hormonal changes, such as menopausal status and location in the menstrual cycle, have the potential to change the composition of the ectocervix [36]. The ectocervix consists primarily of collagenous connective tissue, which is approximately 15% smooth muscle, a small amount of elastic tissue, and a ground substance of mucopolysaccharides. During the menstrual cycle, the cervix becomes softer and more elastic as the level of estrogen increases. After ovulation, this process is reversed and the cervix loses some of its elasticity. During peri-menopause, the layer of epithelial cells thins and the vascularity and cellular content of the cervix is erratic, but the spectra remain consistent. The most variable and therefore the hardest group to classify is the post-menopausal group. The absence of ovarian estrogen and progesterone causes the cervix to change, leading to both dryness and atrophy, although these conditions are considered normal in a woman who has gone through menopause. The cervical Raman signatures vary significantly depending on location within the menstrual cycle and with the onset and completion of menopause. These spectral differences are shown in Figure 1; the most notable differences occur around 1250 cm–1, 1300 cm–1, and 1320 cm–1, most likely due to changes in protein levels, especially elastin and collagen, for reasons noted above. The 98% classification accuracy provided by the MRDF-SMLR algorithm for discriminating spectra into PBO, PAO, PERI, and POST categories indicates the validity and necessity of including hormonal variations when analyzing cervical Raman spectra.
As shown in Figure 2, changes in Raman spectra due to cervical dysplasia are different than those associated with hormonal variations. The largest difference when comparing LGSIL Raman spectra to normal Raman spectra is in the 1230–1300 cm–1 region. This peak range is usually associated with proteins (amide III), DNA (guanine), and lipids (CH transformations) [31]. It is expected that there will be variations in the protein and lipid content when dysplasia occurs, as well as an increase in the ratio of nucleus to cytoplasm due to increased DNA content in dysplastic cells. Another expected change is a reduction in glycogen peaks that occur around 1300 cm–1 [37]. This difference is expected to be minimal in LGSIL because the disease affects only a small portion of the epithelium. As the disease progresses towards HGSIL, this drop in the glycogen peak is expected to become more drastic.
As indicated in Figure 3, restricting the dysplasia diagnostic algorithm to only pre-menopausal spectra resulted in improving the overall accuracy for discriminating among normal, metaplasia, LGSIL, and HGSIL from 88% to 94%. Although there were insufficient numbers of PERI and POST spectra to achieve statistical significance, it is expected that such analyses would achieve similar results. Due to the large effect of hormonal and menopausal status on the spectra, the optimal strategy for future clinical implementation would first stratify by hormonal/menopausal status before applying a separate diagnostic algorithm for each hormonal/menopausal category.
The classification technique used in this paper, a non-linear multi-class algorithm, yields a posterior probability of how likely the spectra are to fit into a particular tissue category. This powerful tool quantifies the confidence with which a sample is classified correctly with Raman spectroscopy. If the value is high, a biopsy may not be needed; if the probability is low and the area is suspicious based on the doctor's observation, a biopsy could be taken as is the current clinical protocol. Results from the small independent validation set provide good examples of these ideas although this study has several limitations (biopsies cannot be taken from normal subjects and the spot the measurement is taken from is approximate). In patients with abnormal Pap smears, Raman measurements were taken from several areas of the cervix, and the previously developed diagnostic algorithm successfully classified the spectra in accordance with colposcopy and/or biopsy results. Since these patients had LGSIL, the biopsies were ultimately unnecessary and could have been avoided based on their Raman diagnoses.
This application of Raman spectroscopy would be particularly useful in developing countries, where “see and treat” methods are optimal. One major problem with screening alone is poor follow-up testing among women with abnormal Pap smears. Usually, an abnormal Pap smear requires a follow-up biopsy and a return visit 3–6 months later depending on the result, but an estimated 10–61% of women with abnormal Pap smears do not show up for follow-up testing [38]. Additionally, only an estimated 19% of women in developing countries have been screened for cervical dysplasia in the past five years, compared with around 60% of women in developed countries [39]. In developing countries, standard practice is for a nurse to photograph the cervix to send to a doctor for diagnosis, but this time-consuming process also has a high error rate and level of ambiguity. Using Raman spectroscopy would allow a nurse to find suspicious areas, take Raman measurements, report an accurate diagnosis, and decide on appropriate treatment all on the same day. This process could reduce the number of patients who are treated unnecessarily and ensure that all patients receive the appropriate treatment. Besides developing countries, this technique for cervical dysplasia diagnosis would greatly benefit rural communities and has to the potential to reduce the number of cervical biopsies taken.
Acknowledgements
The authors would like to acknowledge the financial support of the NCI/NIH (R01-CA95405). We would also like to thank the doctors and staff at Vanderbilt University Medical Center and Tri-state Women's Health for all their help.
Biography
 Elizabeth Kanter received her B.E. in biomedical engineering from Vanderbilt University in 2003, her M.S. from the University of Arizona in 2005 and her Ph.D. from Vanderbilt University in 2008. Her Ph.D. research focused on detection of cervical pre-cancers using Raman Spectroscopy. She currently holds a post-doctoral position at the University of Texas-Dallas.
Elizabeth Kanter received her B.E. in biomedical engineering from Vanderbilt University in 2003, her M.S. from the University of Arizona in 2005 and her Ph.D. from Vanderbilt University in 2008. Her Ph.D. research focused on detection of cervical pre-cancers using Raman Spectroscopy. She currently holds a post-doctoral position at the University of Texas-Dallas.
 Elizabeth Vargis received her B.S. in bioengineering from the University of California at Berkeley in 2004 and her M.S. in biomedical engineering from Vanderbilt University in 2007. She also worked at the Lawrence Berkeley National Lab from 2003–2005 where she focused on understanding the relationship between levels of hormone receptors and the occurrence of breast cancer. Elizabeth is currently pursuing a Ph.D. in biomedical engineering from Vanderbilt looking at how normal changes in the cervix affect the ability of Raman spectroscopy to detect cervical abnormalities and precancers.
Elizabeth Vargis received her B.S. in bioengineering from the University of California at Berkeley in 2004 and her M.S. in biomedical engineering from Vanderbilt University in 2007. She also worked at the Lawrence Berkeley National Lab from 2003–2005 where she focused on understanding the relationship between levels of hormone receptors and the occurrence of breast cancer. Elizabeth is currently pursuing a Ph.D. in biomedical engineering from Vanderbilt looking at how normal changes in the cervix affect the ability of Raman spectroscopy to detect cervical abnormalities and precancers.
 Shovan K. Majumder received his B.Sc (with Honors) and M.Sc degrees in Physics from Jadavpur University, Kolkata, India. After graduating (post M.Sc) from Bhabha Atomic Research Centre (BARC) Training School, Mumbai, India in 1992, he joined Raja Ra manna Centre for Advanced Technology, Indore, a Dept. of Atomic Energy Laboratory of Govt. of India. While working there in the Laser Biomedical Applications and Instrumentation Division as Scientist since then, he received his Ph.D. degree from Devi Ahilya University, Indore, India for his work on “Laser Induced Fluorescence Spectroscopy for Cancer Diagnosis” and held a postdoctoral position for two years at the Biomedical Optics Laboratory of Vanderbilt University. His research interest includes applications of fluorescence, diffuse reflectance and Raman spectroscopy for biomedical optical diagnosis and imaging. Other interests include applications of pattern recognition based methods for evaluation and development of discrimination algorithms for classification of tissue pathologies.
Shovan K. Majumder received his B.Sc (with Honors) and M.Sc degrees in Physics from Jadavpur University, Kolkata, India. After graduating (post M.Sc) from Bhabha Atomic Research Centre (BARC) Training School, Mumbai, India in 1992, he joined Raja Ra manna Centre for Advanced Technology, Indore, a Dept. of Atomic Energy Laboratory of Govt. of India. While working there in the Laser Biomedical Applications and Instrumentation Division as Scientist since then, he received his Ph.D. degree from Devi Ahilya University, Indore, India for his work on “Laser Induced Fluorescence Spectroscopy for Cancer Diagnosis” and held a postdoctoral position for two years at the Biomedical Optics Laboratory of Vanderbilt University. His research interest includes applications of fluorescence, diffuse reflectance and Raman spectroscopy for biomedical optical diagnosis and imaging. Other interests include applications of pattern recognition based methods for evaluation and development of discrimination algorithms for classification of tissue pathologies.
 Matt Keller received his B.E. and M.S. degrees in biomedical engineering from Vanderbilt in 2003 and 2006, respectively. He is currently a Ph.D. candidate in the same field at Vanderbilt. Matt has been supported in his graduate work by a Howard Hughes Medical Institute Pre-doctoral Fellowship and Department of Defense Breast Cancer Research Program Pre-doctoral Fellowship. His current research interest is the use of Raman spectroscopy for evaluating margin status during breast conserving surgery.
Matt Keller received his B.E. and M.S. degrees in biomedical engineering from Vanderbilt in 2003 and 2006, respectively. He is currently a Ph.D. candidate in the same field at Vanderbilt. Matt has been supported in his graduate work by a Howard Hughes Medical Institute Pre-doctoral Fellowship and Department of Defense Breast Cancer Research Program Pre-doctoral Fellowship. His current research interest is the use of Raman spectroscopy for evaluating margin status during breast conserving surgery.
 Gautam G. Rao received his B.S. and M.D. degrees from the University of Miami. He completed his residency in Obstetrics and Gynecology at Wayne State University in Detroit, Michigan and fellowship in Gynecologic Oncology at the University of Texas Southwestern Medical Center in Dallas, Texas. He served on the faculty in the Department of Obstetrics and Gynecology at Vanderbilt University Medical Center from 2005–2007. He is now in practice at The Sarah Cannon Cancer Center at Centennial Medical Center in Nashville, TN.
Gautam G. Rao received his B.S. and M.D. degrees from the University of Miami. He completed his residency in Obstetrics and Gynecology at Wayne State University in Detroit, Michigan and fellowship in Gynecologic Oncology at the University of Texas Southwestern Medical Center in Dallas, Texas. He served on the faculty in the Department of Obstetrics and Gynecology at Vanderbilt University Medical Center from 2005–2007. He is now in practice at The Sarah Cannon Cancer Center at Centennial Medical Center in Nashville, TN.
 Emily Woeste is a Board Certified Obstetrician and Gynecologist in a private practice in the Northern Kentucky community. She has been with her practice for two years. She completed residency at The Ohio State University in 2006. She attended medical school at the University of Kentucky College of Medicine and graduated in 2002. She graduated with a Bachelor in Science from the University of Kentucky in 1998. In her spare time, she enjoys spending time with her family, reading and running.
Emily Woeste is a Board Certified Obstetrician and Gynecologist in a private practice in the Northern Kentucky community. She has been with her practice for two years. She completed residency at The Ohio State University in 2006. She attended medical school at the University of Kentucky College of Medicine and graduated in 2002. She graduated with a Bachelor in Science from the University of Kentucky in 1998. In her spare time, she enjoys spending time with her family, reading and running.
 Anita Mahadevan-Jansen received her B.S. and M.S. degrees in physics from the University of Bombay, Bombay, India, in 1988 and 1990, respectively. She received her M.S. and Ph.D. degrees in biomedical engineering from the University of Texas at Austin in 1993 and 1996, respectively. She joined the faculty of the Department of Biomedical Engineering at Vanderbilt University, in the fall of 1998. Her expertise is in the area of optical spectroscopy and imaging, specifically on the application of fluorescence and Raman spectroscopy for the detection of tissue physiology, as well as pathologies such as cancers.
Anita Mahadevan-Jansen received her B.S. and M.S. degrees in physics from the University of Bombay, Bombay, India, in 1988 and 1990, respectively. She received her M.S. and Ph.D. degrees in biomedical engineering from the University of Texas at Austin in 1993 and 1996, respectively. She joined the faculty of the Department of Biomedical Engineering at Vanderbilt University, in the fall of 1998. Her expertise is in the area of optical spectroscopy and imaging, specifically on the application of fluorescence and Raman spectroscopy for the detection of tissue physiology, as well as pathologies such as cancers.
References
- 1.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, Vermund SH. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecologic Oncology. 2006;103:1017–1022. doi: 10.1016/j.ygyno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervical Cancer Resource Center. 2007 [Google Scholar]
- 3.Anderson GH, Boyes DA, Benedet JL, Le Riche JC, Matisic JP, Suen KC, Worth AJ, Mill-ner A, Bennett OM. Organisation and results of the cervical cytology screening programme in British Columbia, 1955–1985. Br. Med. J. (Clin. Res. Ed.) 1988;296:975–978. doi: 10.1136/bmj.296.6627.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krantz K. The Anatomy of the Human Cervix, Gross and Microscopic. University of Chicago Press; Chicago: 1973. [Google Scholar]
- 5.Wright T, Kurman R, Ferenczy A. Cervical Intraepithelial Neoplasia. Springer-Verlag; New York: 1994. [Google Scholar]
- 6.Leppert PC. Anatomy and physiology of cervical ripening. Clin. Obstet. Gynecol. 1995;38:267–279. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Maul H, Saade G, Garfield RE. Prediction of term and preterm parturition and treatment monitoring by measurement of cervical cross-linked collagen using light-induced fluorescence. Acta Obstet. Gynecol. Scand. 2005;84:534–536. doi: 10.1111/j.0001-6349.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 8.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Winter C, Meier A, Stamm WE. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 2000;30:901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 9.Coleman DV, Wickenden C, Malcolm AD. Association of human papillomavirus with squamous carcinoma of the uterine cervix. Ciba Found. Symp. 1986;120:175–189. doi: 10.1002/9780470513309.ch12. [DOI] [PubMed] [Google Scholar]
- 10.Bates B. A Guide to Physical Examination. J. B. Lippincott Co.; Philadelphia: 1974. [Google Scholar]
- 11.Myers ER, McCrory DC, Subramanian S, McCall N, Nanda K, Datta S, Matchar DB. Setting the target for a better cervical screening test: characteristics of a cost-effective test for cervical neoplasia screening. Obstet. Gynecol. 2000;96:645–652. doi: 10.1016/s0029-7844(00)00979-0. [DOI] [PubMed] [Google Scholar]
- 12.Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J. Epidemiol. 1995;141:680–689. doi: 10.1093/oxfordjournals.aje.a117485. [DOI] [PubMed] [Google Scholar]
- 13.Burke L, Antonioli DA, Ducatman BS. Colposcopy, text and atlas. Appleton and Large; Norwalk: 1991. [Google Scholar]
- 14.Minoru U. Cervical Adenocarcinoma: A Colposcopic Atlas. Ishiyaku – EuroAmerica Inc.; St. Louis: 1985. [Google Scholar]
- 15.Kierkegaard O, Byrjalsen C, Frandsen KH, Hansen KC, Frydenberg M. Diagnostic accuracy of cytology and colposcopy in cervical squamous intraepithelial lesions. Acta Obstetricia et Gynecologica Scandinavica. 1994;73:648–651. doi: 10.3109/00016349409013460. [DOI] [PubMed] [Google Scholar]
- 16.Monsonego J, Pintos J, Semaille C, Beumont M, Dachez R, Zerat L, Bianchi A, Franco E. Human papillomavirus testing improves the accuracy of colposcopy in detection of cervical intraepithelial neoplasia. International Journal of Gynecological Cancer. 2006;16:591–598. doi: 10.1111/j.1525-1438.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell M. Accuracy of Colposcopy. Consultations in Obstetrics and Gynecology. 1994;6 [Google Scholar]
- 18.Escobar PF, Rojas-Espaillat L, Tisci S, Enerson C, Brainard J, Smith J, Tresser NJ, Feldchtein FI, Rojas LB, Belinson JL. Optical coherence tomography as a diagnostic aid to visual inspection and colposcopy for preinvasive and invasive cancer of the uterine cervix. International Journal of Gynecological Cancer. 2006;16:1815–1822. doi: 10.1111/j.1525-1438.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 19.Chang SK, Mirabal YN, Atkinson EN, Cox D, Malpica A, Follen M, Richards-Kortum R. Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J. Biomed Opt. 2005;10:024031. doi: 10.1117/1.1899686. [DOI] [PubMed] [Google Scholar]
- 20.Chang SK, Pavlova I, Marin NM, Follen M, Richards-Kortum R. Fluorescence spectroscopy as a diagnostic tool for detecting cervical pre-cancer. Gynecol. Oncol. 2005;99:S61–63. doi: 10.1016/j.ygyno.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Utzinger U, Trujillo EV, Atkinson EN, Mitchell MF, Cantor SB, Richards-Kortum R. Performance estimation of diagnostic tests for cervical pre-cancer based on fluorescence spectroscopy: effects of tissue type, sample size, population, and signal-to-noise ratio. IEEE Trans. Biomed. Eng. 1999;46:1293–1303. doi: 10.1109/10.797989. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Shuhatovich O, Price R, Pikkula B, Follen M, McKinnon N, Macaulay C, Knight B, Richards-Kortum R, Cox DD. Design and preliminary analysis of a study to assess intra-device and inter-device variability of fluorescence spectroscopy instruments for detecting cervical neoplasia. Gynecol. Oncol. 2005;99:S98–111. doi: 10.1016/j.ygyno.2005.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell MF, Cantor SB, Ramanujam N, Tortolero-Luna G, Richards-Kortum R. Fluorescence spectro scopy for diagnosis of squamous intraepithelial lesions of the cervix. Obstet. Gynecol. 1999;93:462–470. doi: 10.1016/s0029-7844(98)00385-8. [DOI] [PubMed] [Google Scholar]
- 24.Ramanujam N, Mitchell MF, Mahadevan-Jansen A, Thomsen SL, Staerkel G, Malpica A, Wright T, Atkinson N, Richards-Kortum R. Cervical pre-cancer detection using a multivariate statistical algorithm based on laser-induced fluorescence spectra at multiple excitation wavelengths. Photochem. Photo-biol. 1996;64:720–735. doi: 10.1111/j.1751-1097.1996.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 25.Schomacker KT, Frisoli JK, Compton CC, Flotte TJ, Richter JM, Nishioka NS, Deutsch TF. Ultraviolet laser-induced fluorescence of colonic tissue: basic biology and diagnostic potential. Lasers. Surg. Med. 1992;12:63–78. doi: 10.1002/lsm.1900120111. [DOI] [PubMed] [Google Scholar]
- 26.Romeo MJ, Wood BR, McNaughton D. Observing the cyclical changes in cervical epithelium using infrared microspectroscopy. Vibrational Spectroscopy. 2002;28:167. [Google Scholar]
- 27.Colthrup RJ. Infrared and Raman spectroscopy. 1991 [Google Scholar]
- 28.Keller M, Kanter E, Mahadevan-Jansen A. Raman spectroscopy for cancer diagnosis. Spectroscopy. 2006;21(9):33. [Google Scholar]
- 29.Lieber CA, Mahadevan-Jansen A. Automated method for subtraction of fluorescence from biological Raman spectra. Applied Spectroscopy. 2003;57:1363–1367. doi: 10.1366/000370203322554518. [DOI] [PubMed] [Google Scholar]
- 30.Majumder SK, Gebhart S, Johnson MD, Thompson R, Lin WC, Mahadevan-Jansen A. A probability-based spectroscopic diagnostic algorithm for simultaneous discrimination of brain tumor and tumor margins from normal brain tissue. Appl. Spectrosc. 2007;61:548–557. doi: 10.1366/000370207780807704. [DOI] [PubMed] [Google Scholar]
- 31.Bitar RA, Had Martinho S, Tierra-Criollo CJ, Zambelli Ramalho LN, Netto MM, Martin AA. Biochemical analysis of human breast tissues using Fourier-transform Raman spectroscopy. J. Biomed. Opt. 2006;11:054001. doi: 10.1117/1.2363362. [DOI] [PubMed] [Google Scholar]
- 32.Kanter EM, Majumder S, Vargis E, Robichaux-Viehoever A, Kanter GJ, Shappell HHWJ., III Mahadevan-Jansen A. Multi-class discrimination of cervical precancers using Raman spectroscopy. Journal of Raman Spectroscopy. 2008 doi: 10.1002/jrs.2108. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill EM, Malpica A, Alford RE, Nath AR, Follen M, Richards-Kortum RR, Ramanujam N. Relationship between collagen autofluorescence of the human cervix and menopausal status. Photo-chemistry and Photobiology. 2003;77:653–658. doi: 10.1562/0031-8655(2003)077<0653:rbcaot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Cox DD, Chang SK, Dawood MY, Staerkel G, Utzinger U, Richards-Kortum RR, Follen M. Detecting the signal of the menstrual cycle in fluorescence spectroscopy of the cervix. Applied Spectroscopy. 2003;57:67–72. doi: 10.1366/000370203321165223. [DOI] [PubMed] [Google Scholar]
- 35.Chang SK, Pavlova I, Marin NM, Follen M, Richards-Kortum R. Fluorescence spectroscopy as a diagnostic tool for detecting cervical pre-cancer. Gynecologic Oncology. 2005;99:S61–S63. doi: 10.1016/j.ygyno.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 36.Katz VL, Lobo RA, Lentz G, Gershenson D. Comprehensive Gynecology. 5th ed. Elsevier Inc.; Katz: 2008. [Google Scholar]
- 37.Sellors J, Sankaranarayanan R. Colpsocopy and Treatment of Cervical Intreaepithelial Neoplasia: A Beginners’ Manual. International Agency for Research on Cancer; Lyon: 2003. [PubMed] [Google Scholar]
- 38.Shinn E, Basen-Engquist K, Le T, Hansis-Diarte A, Bostic D, Martinez-Cross J, Santos A, Follen M. Distress after an abnormal Pap smear result: scale de velopment and psychometric validation. Prev. Med. 2004;39:404–412. doi: 10.1016/j.ypmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of Cervical Cancer Screening in 57 Countries: Low Average Levels and Large Inequalities. PLoS Medicine. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]