Abstract
In vivo confocal microscopy (IVCM) is an emerging technology that provides minimally invasive, high resolution, steady-state assessment of the ocular surface at the cellular level. Several challenges still remain but, at present, IVCM may be considered a promising technique for clinical diagnosis and management. This mini-review summarizes some key findings in IVCM of the ocular surface, focusing on recent and promising attempts to move “from bench to bedside”. IVCM allows prompt diagnosis, disease course follow-up, and management of potentially blinding atypical forms of infectious processes, such as acanthamoeba and fungal keratitis. This technology has improved our knowledge of corneal alterations and some of the processes that affect the visual outcome after lamellar keratoplasty and excimer keratorefractive surgery. In dry eye disease, IVCM has provided new information on the whole-ocular surface morphofunctional unit. It has also improved understanding of pathophysiologic mechanisms and helped in the assessment of prognosis and treatment. IVCM is particularly useful in the study of corneal nerves, enabling description of the morphology, density, and disease- or surgically induced alterations of nerves, particularly the subbasal nerve plexus. In glaucoma, IVCM constitutes an important aid to evaluate filtering blebs, to better understand the conjunctival wound healing process, and to assess corneal changes induced by topical antiglaucoma medications and their preservatives. IVCM has significantly enhanced our understanding of the ocular response to contact lens wear. It has provided new perspectives at a cellular level on a wide range of contact lens complications, revealing findings that were not previously possible to image in the living human eye. The final section of this mini-review provides a focus on advances in confocal microscopy imaging. These include 2D wide-field mapping, 3D reconstruction of the cornea and automated image analysis.
Keywords: Confocal microscopy, dry eye, glaucoma, keratitis, keratoplasty
INTRODUCTION
In vivo confocal microscopy (IVCM) is a rapidly evolving imaging and diagnostic tool, which offers an exciting bridge between clinical and laboratory observations, enabling clinicians and scientists to gain insight into alterations of the ocular surface microstructure, both in health and disease.1
In contrast to conventional microcopy in which the image can be observed directly (all points in the specimen are imaged parallel), a CM optimizes illumination and detection for a single spot only.2
At present, the most widely used IVCM reported in the published literature are the Confoscan Series (Nidek Co. Ltd., Gamagori, Japan) and the Heidelberg Retina Tomograph II (HRT-II)/Rostock Cornea Module (RCM) (Heidelberg Engineering GmbH, Heidelberg, Germany).1
The firsts are white light slit scanning confocal microscopes. With this technology many points along the axis of the slit can be scanned in parallel, greatly reducing scanning times and the required intensity of the light source. However, these instruments are only truly confocal in the axis perpendicular to the slit height and provide lower transverse and axial resolution.1
The HRT-II/RCM (Heidelberg Engineering GmbH, Heidelberg, Germany) is a laser scanning confocal microscope. This instrument uses a coherent high-intensity light source and the laser beam is scanned over the back of the microscope objective by a set of galvanometer scanning mirrors.1 Because of the high-depth resolution, optical sections of only a few micrometers can be imaged and precisely measured in combination with a high contrast.2
Studying the cornea, the Confoscan 4 (the last of the Confoscan series) produces high-quality images throughout the depth of the cornea with accurate depth information.3 In comparison, the HRT-III/RCM (Heidelberg Engineering GmbH, Heidelberg, Germany) produces very detailed images of the anterior cornea but is less accurate in determining depth or producing high-quality images of the posterior cornea. The ability of the laser IVCM to examine the different components of the ocular surface has opened new lanes for studying the physiology and pathology of this complex morphofunctional unit.
This mini-review does not provide an exhaustive review of the literature but rather summarizes some key findings in IVCM of the ocular surface, focusing on recent and promising attempts to move “from bench to bedside”. The role of IVCM in the management of infectious keratitis, corneal transplantation, refractive surgery, dry eye disease and imaging of corneal nerves, assessment of the ocular surface in glaucomatous patients and contact lens wearers are the main topics of this review. The final section provides a focus on advances in confocal microscopy imaging.
IVCM IN MANAGEMENT OF INFECTIOUS KERATITIS
Infectious keratitis is a common condition that can lead to sight-threatening complications and blindness.4 Early and accurate diagnosis, as well as subsequent initiation of appropriate therapy have been demonstrated to be the key in prevention of permanent vision loss.4 Although clinically, patient history and slit-lamp examination are essential in raising suspicion for the underlying etiology, microbiology remains the gold standard. However, the high false-negative rate and delay of corneal cultures due to slow-growing organisms such as fungi and Acanthamoeba are important limitations to their diagnostic ability.5 Given the importance of timing in diagnosis and initiation of therapy, there is an exciting emerging role for IVCM evolving not only for the diagnosis, but also potentially in the management of this disease.6
Acanthamoeba Keratitis
Acanthamoeba is a protozoan that is ubiquitously found in soil, water and air. While the infection rate was reported to be 1.2 per million adults and 0.2–1 per 10,000 contact lens wearers per year,7 rates have recently risen to more than seven-fold in contact lens wearers.8 Acanthamoeba keratitis is often misdiag-nosed due to initially non-specific presentation. The typical ring infiltrate and radial perineuritis may develop at later stages and raise suspicion, at which time the prognosis is guarded and cases require surgical intervention.9 Therefore, there is great value in more rapid identification of the organism. Unfortunately, cultures can take weeks to become positive and sensitivity ranges from 0 to 68%.9 Further, although polymerase chain reaction (PCR) diagnosis has a better sensitivity for more superficial cases,10 it is costly, needs technical expertise and is not yet available in all laboratories. The pharmaco-therapy for acanthamoeba keratitis is expensive, prolonged, and toxic and toxicity may become difficult to distinguish from persistent infection. IVCM may thus be helpful in determination of therapeutic efficacy and persistence or absence of Acanthamoeba non-invasively, potentially improving patient outcomes and decrease the need for surgical intervention.11
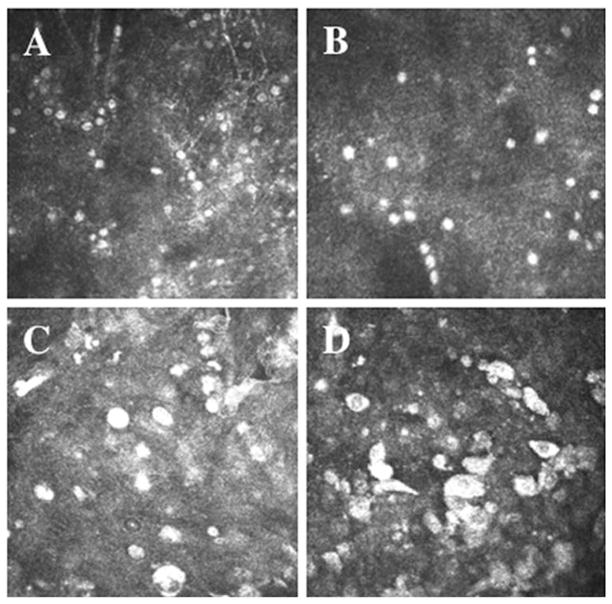
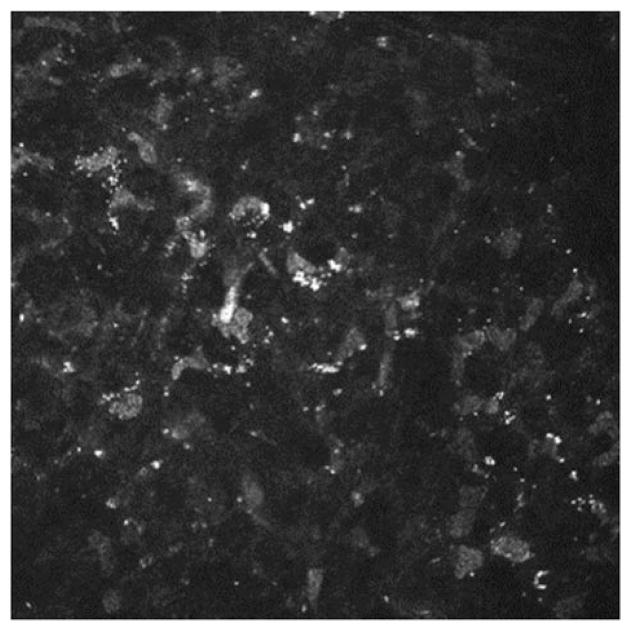
Acanthamoeba presents as active trophozoites and/or quiescent cysts, with both forms being identifiable on IVCM, allowing for rapid, same-day diagnosis. Detection of Acanthamoeba by IVCM was first reported in 199212 and was recently supported by The American Academy of Ophthalmology as an adjunctive diagnostic modality.13 Double-walled cysts appear as hyper-reflective, spherical, occasionally ovoid, structures, ranging 15–28 μm in diameter (Figure 1A and B). The double-wall may not always be apparent by IVCM, making it occasionally difficult to differentiate cysts from leukocyte or epithelial nuclei. Clustering (Figure 1A) and rows (Figure 1B) of cysts are typically suggestive of active proliferative disease. Trophozoites are 25–40 μm in diameter,7 and appear as hyper-reflective and ovoid structures on IVCM (Figure 1C and D) but are difficult to distinguish from leukocytes and keratocyte nuclei.
FIGURE 1.
Laser IVCM appearance of (A) Acanthamoeba cysts demonstrating clustering of cysts, (B) Acanthamoeba cysts demonstrating linear alignment of cysts and (C and D) Acanthamoeba trophozoites.
Several single-observer studies have demonstrated a sensitivity of 90.9–100%, and a specificity of 77.3–100% by IVCM.14–16 Another study, studying grading by different observers with variable or no experience, demonstrated a sensitivity of up to 55.8% and specificity up to 84.2%,17 underlining the importance of training and experience in evaluation of IVCM images.
Fungal Keratitis
The clinical presentation of fungal keratitis is non-specific and indolent, resulting in delayed therapy and potential development into endophthalmitis and loss of the eye. Alternatively, the permanent scarring associated with the delay in treatment can lead to significant vision loss. The gold standard for diagnosis are corneal smear and cultures, both of which have a limited sensitivity and cultures can take several days to weeks to obtain growth.
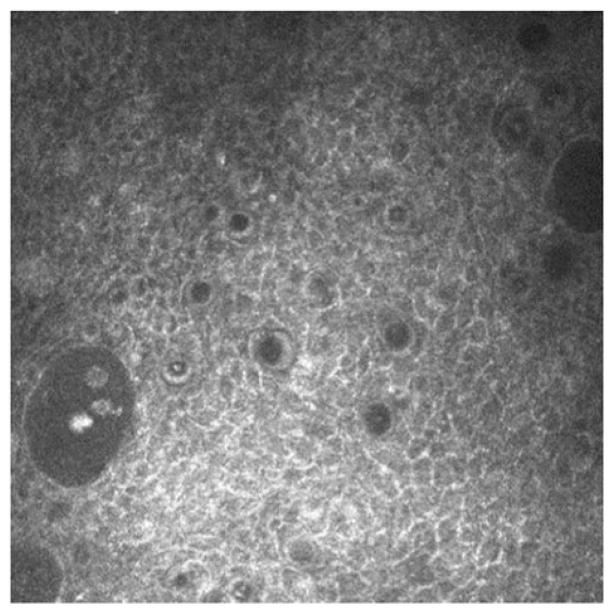
IVCM is a tool for identification of both filamentous fungi and yeast. Aspergillus hyphae are hyper-reflective 5–10 μm in diameter and have septate hyphae with dichotomous branches at 45° (Figure 2A).6 In contrast, Fusarium typically branches at 90°.8 Paecilomyces have variable branching and demonstrate loops on IVCM as well (Figure 2B).18 The hyper-reflective elements seen on IVCM must be differentiated from subbasal corneal nerves, which have a more regular branching pattern, as well as from stromal nerves that are much larger in diameter (25–50 μm). Filamentous fungi are 200–400 μm long. In addition, yeast such as Candida albicans, have round, budding bodies that may develop pseudohyphae, and are 10–40 μm in length and 5–10 μm in width.8 Candida parapsilosis, in contrast, present as small hyper-reflective round 3–5 μm structures (Figure 2C).18
FIGURE 2.
Laser ICVM appearance of (A) Fusarium solani hyphae, (B) Paecilomyces lilacinus hyphae and (C) Candida parapsilosis.
Two recent studies14,16 in patients with microbial keratitis demonstrated that IVCM had sensitivity from 89.2 to 94% and a specificity from 78 to 92.7% in patients with fungal keratitis. As with acanthamoeba keratitis, IVCM can be applied not only for diagnosis of fungal keratitis, but for monitoring and guidance of treatment as well. In fungal keratitis, the depth of invasion is an important prognostic factor, and IVCM is currently the only method that allows determination of the depth of infection. This information helps the clinician to decide the timing for surgical intervention when the disease is progressing despite medical treatment.6
Conclusion
IVCM is a non-invasive tool that may allow for rapid diagnoses of potentially blinding atypical infections, such as acanthamoeba and fungal keratitis, allowing prompt initiation of treatment. IVCM may also provide the unique advantage of following the disease course and guiding treatment, as well as shed light on the pathogenesis in these potentially devastating diseases.19,20 Currently, smaller organisms, including bacteria and viruses are not visible on examination, and the use of IVCM in these cases is not helpful. It is important to note that IVCM testing requires skilled operators, and interpretation requires an experienced and well-trained observer. Furthermore, in some cases, the evidence of its high sensitivity and specificity is still poor and therefore IVCM cannot at present replace microbiologic testing.6
IVCM IN CORNEAL TRANSPLANTATION AND REFRACTIVE SURGERY
IVCM is a valuable imaging modality for examining corneal cells and ultrastructure after transplantation or other surgical procedures. Confocal studies of the donor cornea after penetrating keratoplasty (PK) have revealed decreased innervation,21,22 increased backscatter22 and decreased cellularity.23 With current interest in lamellar keratoplasty techniques, this review will concentrate more on recent findings after lamellar keratoplasty and other lamellar procedures.
Corneal Transplantation
Lamellar keratoplasty has resurged over the last decade for the treatment of anterior and posterior corneal disease.24 While donor changes would be similar to those after PK, corneas after lamellar keratoplasty also contain residual host tissue and a new surgical lamellar interface. Currently, the most common lamellar keratoplasty procedure is endothelial keratoplasty (EK), specifically, Descemet’s stripping endothelial keratoplasty (DSEK), in which nearly all the host cornea is retained with the exception of host Descemet’s membrane and endothelium. IVCM has improved our knowledge of the residual changes in the host cornea after EK, and has enabled understanding of some of the processes that affect the visual outcome after EK, as discussed below.
Corneal Backscatter after Corneal Transplantation
Corneal backscatter refers to light scattered toward an observer from the cornea and is of interest after EK because backscatter from the surgical interface has been assumed to degrade postoperative visual acuity.25 With appropriate calibration of the incident light source and the optical detection system, changes in corneal backscatter can be determined prospectively by using IVCM.26 After DSEK for Fuchs’ dystrophy, significant corneal backscatter is noted from the surgical interface in the early period, but this decreases progressively over the first 2 years after surgery.27 Contrary to popular belief, backscatter from this region has not been associated with changes in visual acuity or disability glare after EK.27 In contrast, more backscatter can be measured from the subepithelial and anterior stromal region of the cornea.27,28 High backscatter in this region is present preoperatively (in Fuchs’ dystrophy) and is caused by subtle basal epithelial edema, increased scatter from the anterior stromal extracellular matrix, and the presence of subepithelial cells (presumed fibroblasts), despite the cornea appearing clear by slit-lamp examination.29 After DSEK, scatter from this anterior region declines over the first 6 months, mainly because of resolution of epithelial edema, but thereafter persists and remains elevated compared to normal through 2 years after surgery. Scatter from the subepithelial region has not been associated with visual acuity after DSEK, but has been associated with disability glare.25,27,30
Corneal Cellularity after Corneal Transplantation
Corneal nerves are not affected by EK except those traversing the site of the incision, whereas all the corneal nerves are severed during PK.23,31 Nevertheless, IVCM has revealed significant abnormalities of sub-basal and stromal nerves before and after EK for Fuchs’ dystrophy. Stromal nerves are frequently tortuous and closely associated with keratocytes, suggesting interactions between these cell types.31 In addition, an abnormal and depleted subbasal nerve plexus (SNP) has been associated with decreased corneal sensitivity.31,32
Abnormal subepithelial cells (presumed fibroblasts) are brightly reflective cells visible before and after DSEK for Fuchs’ dystrophy.29 These cells contribute to corneal backscatter and possibly to increased anterior surface aberrations.33 Abnormalities of keratocytes, which repair and maintain the cormealstroma, have been shown by IVCM. Keratocyte density is reduced in the most anterior cornea in Fuchs’ dystrophy, and remains reduced with no evidence of repopulation through 3 years after DSEK.29,34 The consequence of this loss of cells is unknown, but possibly results in impaired repair of the anterior stromal extracellular matrix after EK. Posterior host keratocytedensity has been found to decrease at 2 and 3 years after EK,29 and might be the consequence of loss of proximity, and therefore disrupted communication, between these cells and the endothelial cells.
The donor corneal endothelium is easily examined by IVCM after EK, and it is an ideal imaging technique for examining these thickened and potentially hazy corneas when compared to non-contact specular microscopy.35 Microfolds of the posterior donor cornea after EK can be visualized by confocal microscopy, but these folds have not been associated with postoperative visual acuity.36
Excimer Keratorefractive Surgery
Laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) are the most common corneal surgical procedures. Both involve tissue removal by excimer photoablation, and LASIK involves anterior corneal flap creation resulting in a lamellar interface.37 Long-term changes in corneal backscatter and cellularity in both procedures have been studied by IVCM.
Corneal Backscatter after Excimer Keratorefractive Surgery
Aside from transient increases in corneal backscatter in the early period after LASIK and PRK, corneal backscatter is decreased over the longer term, as measured by IVCM.26,38 The decrease can only be partly explained by decreased keratocyte density (see below). Other possible factors are also thought to contribute to decreased reflectivity in the LASIK flap (unpublished data). Of interest is that the anterior keratocyte depletion after LASIK and in Fuchs’ dystrophy is associated with opposite effects on anterior corneal reflectivity.
Corneal Cellularity after Excimer Keratorefractive Surgery
Keratocyte density decreases after LASIK in the stromal flap, and after PRK, because of removal of the highest density of anterior cells.39 Loss of anterior keratocytes has not been associated with a detrimental visual outcome, with increased backscatter or with unstable anterior surface aberrations.26,40 Corneal subbasal nerves become depleted after LASIK and PRK, with a slow recovery over several years, although decreased corneal sensitivity recovers within months.41,42 No long-term detrimental effects have been observed in the corneal endothelium by IVCM.43,44
IVCM OF OCULAR SURFACE IN DRY EYE
The International Dry Eye Workshop45 (2007) and the International Workshop on Meibomian Gland Dysfunction46 (2010) recently defined dry eye and meibomian gland dysfunction (MGD). Although these definitions provide clear descriptions of the disease components, in-depth investigation of the pathophysiologic mechanisms are still required.47
IVCM is a minimally invasive and powerful tool which allows detection of changes in the ocular surface epithelium, immune and inflammatory cells, corneal nerves, keratocytes, and meibomian gland (MG) structures at cellular level.44,45,48
IVCM studies show dry eye-related changes in the corneal epithelium,49–52 including decreased superficial cell density and increased basal density. Changes in the stroma are also evident by IVCM including the presence of abnormal hyper-reflective keratocytes.50,53,54 Some authors referred to these as “activated” and interpreted them to be in a particular state of metabolic activation, induced by pro-inflammatory mediators.50,54,55
Confocal studies confirmed a key role of the SNP in dry eye disease, reporting increased tortuosity of the nerves.50,51,55 The nerves also show an increased density of bead-like formations50,51 that could reflect nerve damage or, alternatively, reflect increased metabolic activity of nerve fibers in response to abnormal changes in the epithelium and to neuro-inflammation. The meaning of changes in subbasal nerve tortuosity and bead-like formations is currently subject to interpretation as there is conflicting data about those changes and how they correlate with sensitivity.49,50,55–59 A more extensive discussion on this issue is included in the next section.
IVCM demonstrated that the density of epithelial DCs, which are interpreted as antigen presenting cells, and other inflammatory cells increases in the central and peripheral cornea in patients with dry eye.20,60 Dynamic in vivo assessment of the central corneal inflammatory cell density may not only be of help in evaluating dry eye severity, but can likely guide clinical treatment and aid in the evaluation of the efficacy of anti-inflammatory drugs in clinical trials.47,61
IVCM has recently been used to examine the MGs. With this application, it provides a new tool to assess morphologic changes and allows the quantitative study of MG acinar unit diameter and density, orifice diameter and periglandular inflammatory cells density.62–65 The method also allows semi-quantitative assessment of meibum secretion reflectivity and the inhomogeneity of the glandular interstices and acinar wall.64,65 Confocal data showed the potential to diagnose simple MGD (Figure 3) with high sensitivity and specificity and to assess MGD response to treatment.63–66 Finally, confocal examination provided new information about particular patterns of MG changes associated with Sjogren’s syndrome (SS),64 graft-versus-host disease,67 contact lens wear65 and aging.68
FIGURE 3.
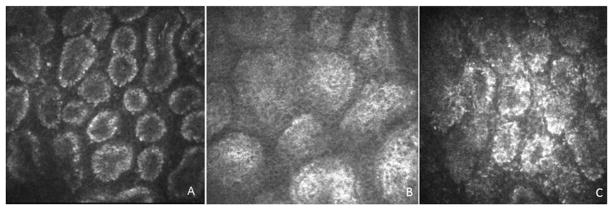
Representative confocal microscopy of MGs scans of (A) normal control subject, (B) MGD patients and (C) SS patient. Please note the decreased density and the enlargement of acinar unit and the increased secretion reflectivity in MGD patient (B). SS patient shows small acinar units, with increased density of inflammatory cells and increased inhomogeneity of the periglandular interstice (C).
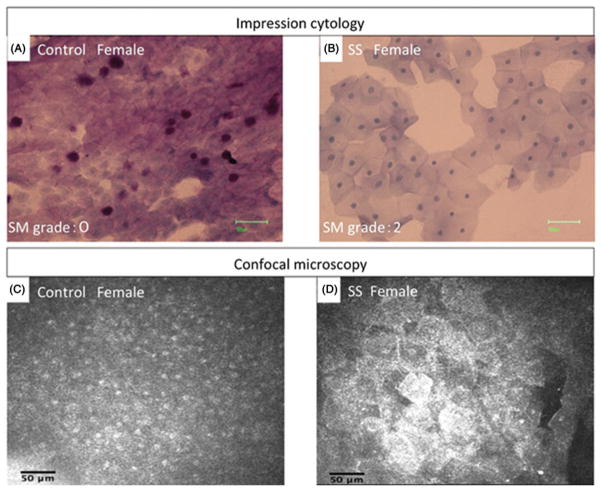
Recent IVCM reports studied the conjunctiva in dry eye patients, focusing on confocal correlations with impression cytology, inflammatory glands and goblet cell evaluation. In SS, cellular changes resembling squamous metaplasia were present, and the mean cell area and nucleus–cytoplasm ratio in impression cytology showed a significant correlation with the corresponding confocal microscopy parameters (Figure 4).69 IVCM was effective in the study of inflammatory conjunctival cell density and response to treatment in Sjogren’s patients,70,71 while data are conflicting about the evaluation of goblet cells (repeatability, inter-observer agreement, images interpretation and chances to discriminate these cells.).70,72 Finally, IVCM was successfully applied to lacrimal gland examination in some Sjogren’s patients, revealing inflammatory cell infiltration and perilobular fibrosis.73
FIGURE 4.
Representative impression cytology imprint and confocal microscopy scans of bulbar conjunctiva in (A and C) normal control subject and (B and D) SS patient. The squamous metaplasia was observed both in impression cytology and confocal microscopy examination.
In conclusion, IVCM is providing new opportunities to better understand the complex pathophysiologic mechanisms of dry eye. Recent confocal studies have shown several potential clinical applications of this technology in the assessment of dry eye disease grading and prognosis, in the improvement of differential diagnosis, in the optimization of a tailored approach to treatment and in the evaluation of the responses to treatments.48,74
IVCM OF CORNEAL NERVES
The cornea is the most densely innervated tissue in the body with a density of nerve endings 200–300 times greater than the skin.75 The majority of nerves in the cornea have a sensory function. Sensory nerve endings termed “nociceptors” function in maintaining homeostasis, in wound healing and in sensing the environment to regulate tear secretion and distribution.
IVCM allows the description of the morphology, density and disease-induced or surgically induced alterations of corneal nerves, particularly the SNP.76,77 It is particularly useful for imaging corneal nerves near the corneal apex because the central cornea is more easily applanated to obtain high-quality tangential images. Although IVCM is capable of providing excellent images, it lacks sufficient resolution to image nerve terminals in the epithelium and very small diameter nerves in the subbasal plexus. Furthermore, the HRT laser scanning confocal microscope (Heidelberg Engineering GmbH, Heidelberg, Germany) that has been used in many of the published studies is only capable if imaging a 400 ×400 μm area, making it difficult to locate and serially re-image the same region of the cornea.
IVCM Imaging of Nerves in Ocular Surface and Corneal Diseases
IVCM has been used to image corneal nerves in a variety of corneal and ocular surface diseases/ conditions, including non-Sjögren’s syndrome (SS) or SS-associated aqueous tear deficiency (ATD),49,50,56,78 following LASIK surgery41,79–84 (including post-LASIK dysesthesia) and neurotrophic epitheliopathy before and after treatment with autologous serum.85
Benıtez del Castillo et al.78 reported decreased density of nerves in the subbasal plexus in both non-SS and SS ATD compared to normal subjects <60 years of age. Furthermore, both ATD groups had a greater number of beaded nerves in the subbasal plexus than control subjects less than and greater than age 60 years. Similar findings were also observed by Villani et al.50 who reported a decreased number and increased tortuosity of subbasal nerves compared to the control group. Tuominen et al.49 observed nerve sprouting in the SNP in 40% of eyes with SS. The authors hypothesized that this finding may represent sensory nerve regeneration. Nerve sprouting was noted in neonatal rat corneas after treatment with capsaicin which induces release of neuropeptides from sensory nerve endings,86 and overexpression of nerve growth factor (NGF) was found to induce hypertrophy of the peripheral nervous system.87
In another study by Tuisku et al.,56 corneal subbasal nerve density was similar in patients with SS and control subjects; however, thickening and cone-like structures were seen in nerves and this was accompanied by hyperalgesia to an air jet esthesiometer and increased density of dendritic cells (DCs) surrounding corneal nerves in the SS corneas. The density of DCs correlated with the severity of irritation symptoms.
Post-LASIK Hypoesthesia or Dysesthesia
Following LASIK, patients may rarely develop neurotrophic epitheliopathy or chronic pain, termed “keratoneuralgia”.88–90 This may be due to abnormal regeneration or increased sodium ion channels that lower the threshold to excitation, resulting in greater sensitivity to normal environmental stimuli (e.g. air drafts, temperature change).90 IVCM can confirm loss of corneal innervation or the presence of abnormal nerve regeneration. It may identify patients who would benefit from protective and/or regenerative therapy. Confocal microscopy of the cornea after LASIK has shown decreased corneal nerve density in the early postoperative period that increases with time, albeit at a slower rate than return of corneal sensitivity.41 The subbasal nerve fiber density is barely detectable for up to 6 months post-LASIK,79 and 1 year after LASIK, the number of subbasal and stromal nerves in the corneal flap are less than half the preoperative density.80 Immediately after LASIK, most patients develop a relatively hypoesthetic cornea. Studies have found that corneal sensitivity progressively improves and approaches the preoperative or “normal” range by 6–12 months when measured with Cochet–Bonnet esthesiometry91,92 and by up to 2 years when measured by gas esthesiometry.93
Nerve morphology has been found to correlate with corneal sensitivity after LASIK. One study found that low sensitivity in specific corneal areas was associated not only with the absence of nerves in confocal images, but also with short nerves lacking connections between nerve bundles.81 Another study found a significant association in LASIK patients between long nerve fiber bundles and greater sensitivity by non-contact esthesiometry.82 Finally, Stachs et al.83 concluded that “normal” sensation returns to eyes by 6 months post-LASIK, despite a markedly abnormal SNP with abnormally curved bundles or with thin nerves without the connections between nerve bundles as seen in healthy, non-surgical eyes.
Tuisku et al.84 found that 2–5 years after LASIK for high myopia, most patients experienced ocular surface discomfort consistent with dry eye syndrome, but in the absence of clinical signs of ocular surface disease and with normal sensitivity measured with non-contact esthesiometry. IVCM of these corneas showed abnormal nerve morphology with excessive nerve sprouting. Interestingly, patients with diabetes mellitus and severe peripheral neuropathy have also been found to have tortuous subbasal nerves which may represent nerve regeneration.81 Many of the patients with post-LASIK dysesthesia, evaluated at the Ocular Surface Center of the Cullen Eye Institute (Houston, TX), have an abnormal subbasal plexus with small, branched nerve bundles that lack interconnections.
Neurotrophic Keratopathy
IVCM was found to be useful in monitoring corneas for evidence of nerve regeneration in patients with neurotrophic keratopathy who were treated with autologous serum drops.85 In a study performed at the Ocular Surface Center of the Cullen Eye Institute (Houston, TX), 11 eyes of 6 patients with neurotrophic keratopathy from a variety of conditions, including diabetes mellitus, herpes zoster ophthalmicus and trigeminal nerve ablation were evaluated. Four images of the SNP in the central cornea were randomly selected for analysis of the corneal nerves. Autologous plasma drops were instilled 6–8 times per day. Autologous serum therapy resulted in improved best-corrected visual acuity, decreased mean corneal fluorescein staining scores and significantly increased corneal sensitivity measured by the Cochet–Bonnet and modified Belmonte esthesiometers. The mean number, length, width and density of subbasal nerves increased significantly after serum treatment.
These studies indicate that IVCM is a useful technology to image the SNP in normal and diseased corneas. Additional studies are necessary to standardize examination methods and confirm the findings of these preliminary studies.
Peripheral Neuropathy
A promising future clinical application of IVCM is the assessment and quantification of peripheral neuropathies, related to diabetes mellitus or to other conditions known to cause small nerve fiber damage, including hereditary sensory and autonomic neuropathy,94 autoimmune neuropathy95 and chemotherapy-associated neuropathy.96
Diabetic neuropathy, specifically, is a common and significant clinical problem, as highlighted by The American Diabetes Association, which reports that 8.3% of US population is affected by diabetes and that 60–70% of people with diabetes have mild-to-severe forms of nervous system damage.97 The accurate detection, characterization and quantification of this condition are important in order to define at-risk patients, anticipate deterioration, monitor progression, assess new therapies and potentially reduce the incidence of foot ulcerations and amputations.98
Several confocal studies reported corneal nerves changes in diabetic patients, including reduced nerves density and branching and increased tortuosity.81,99–101 The most recent researches showed early detection,101,102 severity stratification100,103 and moderate-to-high sensitivity and specificity of IVCM for diagnosis of diabetic neuropathy,99,104 suggesting the prospect to use in vivo confocal features of corneal nerves as biomarkers of diabetic neuropathy.105,106 Larger longitudinal studies and a larger normative database, together with ongoing IVCM technical progress, promise to allow the optimization and diffusion of the clinical use of this approach.
IVCM FOR ASSESSING THE OCULAR SURFACE IN GLAUCOMA
In glaucoma, IVCM constitutes an interesting aid to evaluate filtering blebs and to better understand the conjunctival wound healing process. IVCM has also been used to assess corneal changes induced by topical antiglaucoma medications and their preservatives.
The long-term success of glaucoma filtering surgery is mainly dependent on the development of a functioning filtering bleb. The formation and the maintenance of this functioning bleb, with regard to wound healing and conjunctival scarring, are therefore of primary importance. Because in some cases the appearance of the bleb is not correlated to intraocular pressure (IOP) control and because the reason of failure is often unclear, some authors have looked for new in vivo evaluation techniques such as IVCM. This imaging technique allows the visualization of epithelial microcysts, subepithelial connective tissue, blood vessels and inflammatory cells within the conjunctival bleb tissues (Figure 5).107–110 Functioning blebs show a normal conjunctival epithelium with numerous microcysts and a loose hypo-reflective sub-epithelial tissue with a high number of optically clear spaces. In contrast, non-functioning blebs show no or very few microcysts with hyper-reflective subepithelial tissue, dense connective tissue and numerous blood vessels.107,108 In functioning blebs with mitomycin C, numerous clear spaces corresponding to large confluent microcysts and a loosely arranged connective tissue with numerous large clear spaces may also be observed.107,108,110 The infiltration of bleb tissues by inflammatory cells can be also observed and monitored during the postoperative period.109 Clinicians, with images at a cellular level, would be able to better predict the outcome of these blebs and eventually provide specific treatments in order to enhance the success rates of their surgical procedures.107
FIGURE 5.
IVCM appearance of microcysts of aqueous humor in the conjunctival epithelium over a filtering bleb after trabeculectomy.
Moreover, the chronic use of topical IOP-lowering drugs and their preservatives is known to cause significant changes in the ocular surface. Several studies have evaluated the subbasal corneal nerves with IVCM in patients treated for glaucoma or ocular hypertension. IVCM analysis showed ocular surface alterations in glaucomatous patients treated chronically with benzalkonium chloride (BAK)-containing eye drops (Figure 6). These changes included a reduction of the density of superficial epithelial cells,111 activation of stromal keratocytes, decrease in subbasal nerves and increase in subbasal nerve tortuosity. Baratz et al.112 showed a decrease in the number and density of subbasal corneal nerves in the medication group of the Ocular Hypertension Treatment Study (OHTS). Martone et al.111 observed similar nerve modifications but in association with an increased number of nerve beadings and tortuosity. These authors also demonstrated a direct relationship between nerve tortuosity and corneal sensation in glaucoma patients. Labbe et al.113 recently evaluated the relationship between the IVCM morphology of subbasal corneal nerves and corneal sensitivity in patients with dry eye and patients treated for glaucoma and ocular hypertension. The density and number of subbasal corneal nerves were significantly decreased in dry eye and glaucoma patients compared to controls. In the dry eye group, corneal sensitivity correlated with the density and the number of nerves. However, in the glaucoma group, corneal sensitivity correlated only with the tortuosity of subbasal nerves. Although patients with dry eyes and those medically treated for glaucoma experience similar ocular surface symptoms and signs, the different correlations found between corneal sensation and nerve morphology in these two groups suggest that nerve alterations and/or dysfunction could also be different. Despite the decreased number of subbasal nerves observed in those patients, an additional anesthetic effect of IOP-lowering medications and preservatives could explain these results.113
FIGURE 6.
Corneal epithelial changes in glaucoma patient, showing anisocytosis and inflammatory cells.
In relation to drug-induced toxic effects, IVCM has also been widely used by researchers to explore the ocular surface changes induced by topical medications and their preservatives. Clinical observations of toxic keratopathy are important findings in glaucoma patients.114,115 Additionally IVCM may be used indifferent animal models of ocular toxicity, in order to compare several formulations of topical antiglaucoma eye drops and preservatives. Liang et al.116 also investigated with IVCM the effects of antiglaucoma prostaglandin analogs with or without BAK preservative on the conjunctiva-associated lymphoid tissue (CALT). The CALT reaction after instillation of BAK-containing eye drops was characterized by inflammatory cell infiltration in the dome and intrafollicular layers and by cell circulation inside the lymph vessels confirming the concentration-dependent toxic effects of BAK. IVCM analyses of the ocular surface in animal models as well as in humans could thus become pertinent tools in the future for evaluating and understanding immunotoxicologic challenges on the ocular surface and would provide useful criteria for investigating newly developed eye drops.
CONFOCAL MICROSCOPY IN CONTACT LENS WEAR
IVCM has significantly enhanced our understanding of the ocular response to contact lens wear in two ways. First, it has provided new perspectives, at a cellular level, on a wide range of contact lens complications that have already been well documented in the literature, such as stromal edema and changes in endothelial cell morphology. Second, it has revealed phenomena that were not previously possible to image in the living human eye, such as the appearance of stromal microdots and changes in keratocyte cell density. At present, IVCM is a precious complementary tool in contact lens wearers’ evaluation and management.53 This technology, in addition to its previously discussed essential role in contact lens-related infectious keratitis, allows early detection of ocular surface changes, finer identification of the pathogenic mechanisms and improved tailored clinical approach. As for several other areas, clinical utility of IVCM in contact lens wearers is suggested by a strong background and supported by preliminary clinical experiences, but a satisfactory level of evidence is still an unmet need. The following is a brief review of contact lens-associated ocular changes observed using IVCM in relation to the various substructures of the anterior eye.
Tear Film
Mucin balls form in the tear layer in patients wearing silicone hydrogel lenses. They have been demonstrated using IVCM to vary in size from 40 to 80 μm,117 and to display a highly reflective core with a more poorly reflective, apparently translucent, outer layer.118 These inclusions are spherical and can apparently penetrate the full thickness of the epithelium,117,119 leading to activation of keratocytes in the underlying anterior stroma.117 Such observations indicate a potential for mucin balls to render the cornea to be more susceptible to infection.
Corneal Epithelium
Epithelial cell size has been observed with the IVCM to increase in response to all forms of lens wear, with the greatest effect seen in rigid lens wearers. Lenses of higher oxygen transmissibility (Dk/t) have been shown to interfere least with the normal process of epithelial desquamation.120 The degree of epithelial disturbance in response to various concentrations of preservatives used in contact lens disinfecting solutions depends on the duration of lens wear.121 IVCM studies have confirmed that extended wear of high Dk/t rigid lenses induces more epithelial thinning than hydrogel lenses, which in turn induces more thinning than silicone hydrogel lenses.122 Basal cell morphology is unaffected by short123 or long124 term wear of high Dk/t soft lenses, but visible alterations to basal cells (less regular in appearance) is associated with long-term wear of low Dk/t soft lenses.124
Corneal Nerves
The current consensus is that contact lens wear does not alter corneal nerves;125,126 however, a qualitative difference in appearance, such as slight blurring of nerves and less contrast with the background, have been noted among contact lens wearers, which have been attributed to an artifact due to lens-induced edema.127
Corneal DCs
Among contact lens wearers, a higher density of DCs, which are presumed to be Langerhans’ cells, occurs in the layer of the SNP in both the central and peripheral cornea. This suggests that contact lens wear can alter the immune status of the cornea.128 Increased numbers of DCs have also been observed in association with various combinations of soft contact lenses and lens care solutions.129
Corneal Stroma
Dark lines and folds are observed with the IVCM in the edematous cornea in response to contact lens wear.130 The lack of keratocyte clarity at high levels of edema probably corresponds with the corneal haze observed with the slit-lamp biomicroscope.130 Apparent keratocyte loss following overnight lens wear131 is most likely an artifact due to uni-dimensional volumetric stromal expansion, causing keratocytes to be more spread out within the tissue. Consequently, when viewed by IVCM, fewer keratocytes are observed within the fixed depth of focus (this phenomenon can be explained by the binomial expansion theory130). As well, a degradation of image quality at higher levels of edema renders keratocytes more difficult to detect.130 These artifacts need to be taken into account, or properly controlled for, in studies of keratocyte loss during contact lens wear.
Only a few of the studies that have investigated keratocyte loss associated with contact lens wear have attempted to account for these artifacts. Nevertheless, most of these studies have concluded that contact lens wear results in keratocyte apoptosis, although there is some disagreement as to the magnitude of this effect.125,130–137 Mechanical stimulation of the corneal surface, due to the physical presence of a contact lens, and the consequent release of inflammatory mediators, is likely to be the main cause of the observed reduction in the number of keratocytes.133,138 Keratocyte loss may play a role in contact lens-induced stromal thinning in view of the role of keratocytes in maintaining the structural integrity of the cornea.
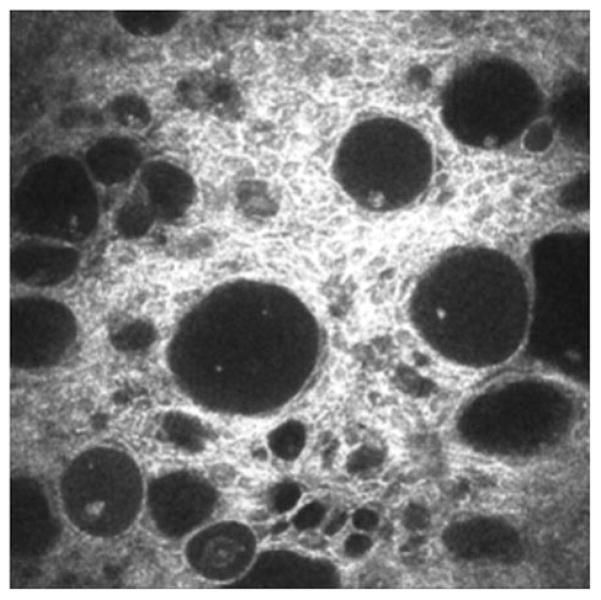
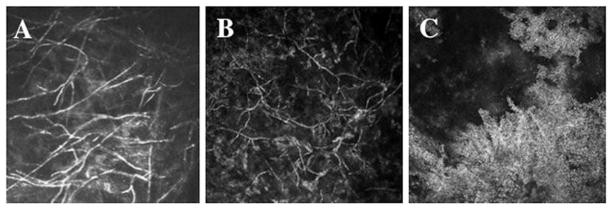
Highly reflective stromal “microdot deposits” can be observed with the IVCM throughout the entire stroma of lens wearers and non-lens wearers (Figure 7);139–142 however, microdots appear in greater numbers in lens wearers and in patients with some disorders such as chloroquine-induced keratopathy,143 exfoliation syndrome144 and mucopolysaccharidoses.145 These findings’ interpretation is still unclear in many cases and we may hypothesize that, in different conditions, similar images are due to different mechanisms. Contact lens-related microdot deposits may represent granules of lipofuscin-like material within the corneal stroma of long-term contact lens wearers, formed as a result of chronic oxygen deprivation and chronic microtrauma to the cornea.
FIGURE 7.
Extensive formation of microdots in the corneal stroma of a rigid lens wearer.
Corneal Endothelium
Endothelial blebs are an acute response to lens wear which take on a different appearance with the IVCM compared with the slit lamp biomicroscope due to the different optical configuration of these two instruments. With the IVCM, endothelial blebs have a bright center and dark annular surround, set against a bright background field of the remainder of the “non-blebbing” endothelium.142 Optical models have been constructed to explain these differences.146 Chronic morphologic changes to the endothelium in response to lens wear observed with the IVCM,147 such as increased polymegethism and increased light scatter, confirm previous observations made using specular microscopy.
Limbus
Long-term soft contact lens wear can induce many changes in corneal limbus, such as microcystic formations in epithelial cells, microdot deposits in corneal stroma, increased Langerhans cell density and decreased of keratocyte density.148 There are more rolling leukocytes in limbal vessels in patients wearing low Dk/t lenses (versus non-lens wearers),149 highlighting the potential of this measure as an indication of sub-clinical inflammation.
Contact Lens-Associated Keratitis
Because bacteria involved in contact lens-related infections are beyond the resolution of the IVCM, this instrument cannot be used to diagnose contact lens-associated bacterial keratitis. However, the IVCM may be used to view damage to the various corneal layers caused by bacterial infection, such as needle-like deposits in crystalline keratitis150 and to indirectly assess the infectious process by monitoring the activity of Langerhans’ cells.151 Contact lens wear is a significant risk factor for acanthamoeba and fungal keratitis. IVCM role in these severe affections is discussed in a previous section of this review.
Investigation of the chronic morbidity of corneal infiltrative events associated with contact lens wear by IVCM revealed no significant impact upon basal epithelial cell density, anterior or posterior keratocyte density, or endothelial cell density, polymegethism or pleomorphism.152 However, markedly reduced pan-corneal cell counts and increased endothelial polymegethism were observed in the affected eye of a patient who had suffered from a severe corneal infiltrative event.152
Conjunctiva
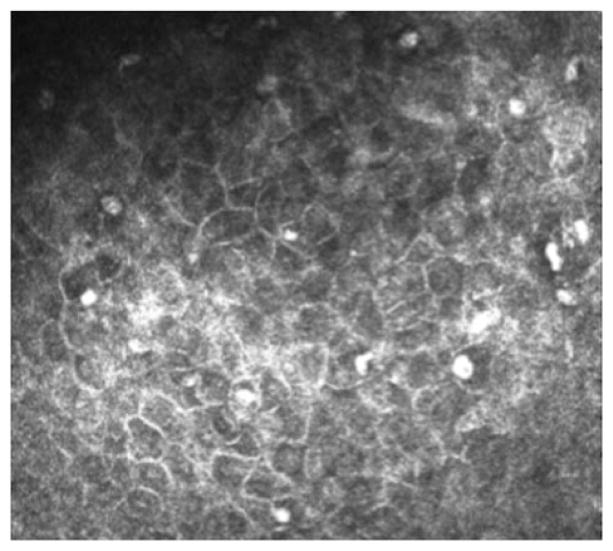
Contact lens wear can induce changes in the bulbar conjunctiva as observed by IVCM. For instance, epithelial thinning, accelerated formation and enlargement of microcysts (Figure 8), and increased epithelial cell density occur and can be documented by IVCM.153 A preliminary study found no impact of soft contact lens wear on bulbar conjunctival goblet or Langerhans’ cell density. Examination of MGs of contact lens wearers showed decreased basal epithelial cell density, lower acinar unit diameters, higher glandular orifice diameters, greater secretion reflectivity and greater inhomogeneity of the periglandular interstices.65 These morphologic changes are interpreted as signs of MGs dropout, duct obstruction and glandular inflammation.154
FIGURE 8.
IVCM appearance of microcysts in the bulbar conjunctiva of an asymptomatic soft contact lens wearer.
ADVANCES IN IVCM IMAGING
Current developments of IVCM include (i) two-dimensional wide-field mapping, (ii) 3D reconstruction of the cornea and (iii) automated image analysis with the resulting quantification of cornea parameters, including the SNP.
2D Wide-Field Mapping
One of the crucial limitations of the confocal microscopy is the small field of imaging. Images generated by confocal microscopes are relatively small (HRT-II/RCM 400 ×400 μm; Confoscan 4/Nidek 460 ×345 μm)155 and contain only a very small portion of the SNP. The analysis of the depicted nerve fibers may result in misleading findings after quantification. Therefore, several study groups are working on solutions with off- and on-line mapping procedures for corneal structures.
Real-time mapping of the corneal emphasizing structures of the SNP was presented by Zhivov et al.156 Source data (384 ×384 pixels, 400 ×400 μm) were used to create large-scale maps of the scanned area by selecting the automatic real-time (ART) composite mode. The algorithm is based on an affine transformation. The maximal ART composite image size using source images is 3072 ×3072 pixels (3.2 ×3.2 mm) and corresponds to 8 ×8 non-overlapping images. The acquisition time for a single composite image was up to 3 min. Given that the total CLSM time for a single composite image is <3 min with no post-processing, the advantages of the online system are apparent.
Another wide-field mapping technique was shown by Edwards et al.157 The method uses the same source data (384 ×384 pixels, 400 ×400 μm, maximum number of 100 images, duration 20 s) and images could be mapped with a post-processing time of ~10 min.
3D Reconstruction
The idea for 3D imaging of the living cornea was originally suggested by Petroll et al.158 in 1993 when the first micrographs of rabbit cornea were published. A further image processing algorithm based on phase correlation was used by Allgeier et al.159 to analyze and reduce motion distortions in volume scan image sequences (30 images, volume depth 60 μm, constant interslice distance 2 μm, duration 4 s). 3D tracing of the SNP was performed in order to reconstruct images containing only the SNP layer in humans.
Petroll et al.160 used the real-time “streaming mode” of the HRT-II/RCM (Heidelberg Engineering GmbH, Heidelberg, Germany). A device modification allowed z-scans through the whole thickness of the rabbit cornea with a 2-μm interslice distance. The external software (ImageJ, http://rsbweb.nih.gov/ij/; Imaris, Bitplane Inc., Zurich, Switzerland) allowed the determination of the epithelial and total corneal thickness as well as keratocyte density data.
Imaging and Quantification of the SNP
Presently, an automatic analysis of the SNP structures is the promising standard in SNP image analysis. Zhivov et al.161 performed the evaluation of SNP on the basis of the best artifact-free single image using in-house developed software tool. The analysis was based on morphologic (length, diameter and density) and topologic (continuity and connectivity) parameters derived from segmented nerve fibers. It also used the skeletonized medial lines that were transformed into networks of undirected graphs consisting of nerve paths and various types of connecting nodes. A more recent approach (Winter, unpublished) employs different local adaptive image filters for image contrast homogenization during image preprocessing and an adapted Gabor filter model for final image segmentation.
Dabbah et al.162,163 presented an analysis and classification system for detecting nerve fibers in confocal images. It was based on a multi-scale adaptive dual-model detection algorithm that exploited the curvilinear structure of the nerve fibers and adapted itself to the local image information. Holmes et al.164 proposed a segmentation and skeletonization algorithm for the detection of nerve fibers based on ridge map calculation. During the process nerve fibers are identified, gaps are reconnected and finally quantified.
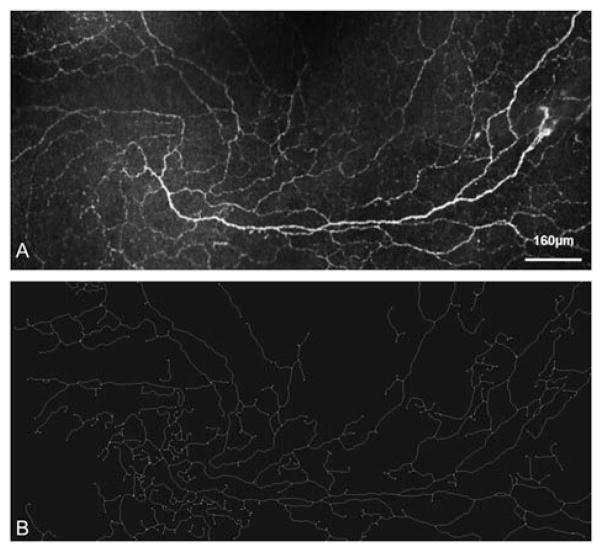
In summary, automated analysis of corneal nerve fibers employed and generated many sophisticated methods and strategies in the field of medical image processing. Currently, a number of different approaches in relation to the principal problem of small field of imaging of the SNP is in progress which hopefully will provide promising solutions. Imaging of large areas of the SNP will soon enable a considerably more accurate and thus more reliable quantification of nerve fiber networks in the context of research and clinical diagnosis (Figure 9).
FIGURE 9.
Real-time mapping of (A) SNP structures and (B) results of automatic quantification of SNP structures using in house developed software.
CLOSING REMARKS
The application of IVCM to the ocular surface opened a new era in ocular science.
The slit lamp biomicroscopy has been the mainstay of ophthalmic examination of the anterior eye for more than half a century, and this is unlikely to change. With its full color, wide-field view, low-to-medium magnification, binocular objective, highly versatile illumination system and relatively low cost, the slit lamp will probably remain the instrument of first choice for examining any ocular disease.53
At present, IVCM provides new opportunities to view ocular surface structures at a cellular level, through a quick, minimally invasive (maybe non-invasive at all, in the next future) and steady-state respectful examination.
The impact of IVCM on cornea and ocular surface research has been immediate and rapidly growing, with 907 articles published from 1985 (when Lemp et al.165 first reported confocal examination of the cornea) to July 2013, 455 of them published in the last 5 years (data by PubMed; research performed with the key words “in vivo confocal microscopy cornea” or “in vivo confocal microscopy ocular surface”). This quantitative progression has been associated to a qualitative evolution, moving from corneal apex to the whole-ocular surface, from qualitative assessments to attempts to standardize and, in some cases, automatize quantitative evaluations, from small case series or pilot studies to well-constructed clinical trials.
Several challenges still remain. One of the main limitations of IVCM is the small field of view. IVCMs currently used in ophthalmology can image only a very small area. For instance, the HRT-II/RCM (Heidelberg Engineering GmbH, Heidelberg, Germany) images a 400 ×400 μm field (0.160 mm2) and the Confoscan 4 [(Nidek Co. Ltd., Gamagori, Japan) images a 460 ×345 μm field (0.159 mm2]. As a consequence of small fields of view and limitations of applicability in eyes with small palpebral apertures, the reproducible investigation and quantification of the same areas over time is virtually impossible. Additional concerns exist about the standardization of image acquisition, interpretation and quantification.1
At present, these difficulties, together with the high cost of this technology, are factors preventing widespread deployment and use of IVCM in clinical practice. However, despite the open challenges, IVCM is ready to be used as a precious complementary in vivo technique for clinical diagnosis and management and its clinical applications seem to be extremely promising and still largely to be explored.
Acknowledgments
This work was supported in part by: Research to Prevent Blindness Grant Program, New York, NY (Unrestricted Departmental Grant; to S.V.P. as Olga Keith Wiess Scholar and to S.C.P.), the Mayo Foundation, Rochester, MN [National Institutes of Health Grant EY11915 (to S.C.P.), the Oshman Foundation, Houston, TX (to S.C.P.), the William Stamps Farish Fund, Houston, TX (to S.C.P.) and the Hamill Foundation, Houston, TX (to S.C.P.)
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2010;29:30–58. doi: 10.1016/j.preteyeres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea – a major review. Clin Experiment Ophthalmol. 2009;37:100–117. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 3.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with the ConfoScan 4 and Z-ring adapter. Eye Contact Lens. 2007;33:185–190. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PA, Geraldine P. Infectious keratitis. CurrOpin Infect Dis. 2007;20:129–141. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- 5.Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–116. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Kumar RL, Cruzat A, Hamrah P. Current state of in vivo confocal microscopy in management of microbial keratitis. Semin Ophthalmol. 2010;25:166–170. doi: 10.3109/08820538.2010.518516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto Y, Dogru M, Sato EA, Katono Y, Uchino Y, Shimmura S, et al. The application of in vivo confocal scanning laser microscopy in the management of acanth-amoeba keratitis. Mol Vis. 2007;13:1319–1326. [PubMed] [Google Scholar]
- 8.Labbé A, Khammari C, Dupas B, Gabison E, Brasnu E, Labetoulle M, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf. 2009;7:41–52. doi: 10.1016/s1542-0124(12)70291-4. [DOI] [PubMed] [Google Scholar]
- 9.Claerhout I, Goegebuer A, Van Den Broecke C, Kestelyn P. Delay in diagnosis and outcome of acanthamoeba keratitis. Graefes Arch Clin Exp Ophthalmol. 2004;242:648–653. doi: 10.1007/s00417-003-0805-7. [DOI] [PubMed] [Google Scholar]
- 10.Mathers WD, Nelson SE, Lane JL, Wilson ME, Allen RC, Folberg R. Confirmation of confocal microscopy diagnosis of acanthamoeba keratitis using polymerase chain reaction analysis. Arch Ophthalmol. 2000;118:178–183. doi: 10.1001/archopht.118.2.178. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Alipour F, Cruzat A, Zheng L, Hamrah P. In vivo confocal microscopy in diagnosis and management of acanthamoeba keratitis improves patient outcome. Invest Ophthalmol Vis Sci. 2010;51:ARVO E-Abstract No. 2891. [Google Scholar]
- 12.Chew SJ, Feuerman RW, Assouline M, Kaufman HE, Barron BA, Hill JM. Early diagnosis of infectious keratitis with in vivo real time confocal microscopy. CLAO J. 1992;18:197–201. [PubMed] [Google Scholar]
- 13.Kaufman SC, Musch DC, Belin MW, Cohen EJ, Meisler DM, Reinhart WJ, et al. Confocal microscopy: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:396–406. doi: 10.1016/j.ophtha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Kanavi MR, Javadi M, Yazdani S, Mirdehghanm S. Sensitivity and specificity of confocal scan in the diagnosis of infectious keratitis. Cornea. 2007;26:782–786. doi: 10.1097/ICO.0b013e318064582d. [DOI] [PubMed] [Google Scholar]
- 15.Tu EY, Joslin CE, Sugar J, Booton GC, Shoff ME, Fuerst PA. The relative value of confocal microscopy and superficial corneal scrapings in the diagnosis of acanthamoeba keratitis. Cornea. 2008;27:764–772. doi: 10.1097/ICO.0b013e31816f27bf. [DOI] [PubMed] [Google Scholar]
- 16.Vaddavalli PK, Garg P, Sharma S, Sangwan VS, Rao GN, Thomas R. Role of confocal microscopy in the diagnosis of fungal and acanthamoeba keratitis. Ophthalmology. 2011;118:29–35. doi: 10.1016/j.ophtha.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Hau SC, Dart JK, Vesaluoma M, Parmar DN, Claerhout I, Bibi K, et al. Diagnostic accuracy of microbial keratitis with in vivo scanning laser confocal microscopy. Br J Ophthalmol. 2010;94:982–987. doi: 10.1136/bjo.2009.175083. [DOI] [PubMed] [Google Scholar]
- 18.Baniasadi N, Cruzat A, Witkin D, Stacey R, Jakobiec FA, Chodosh J, et al. In vivo confocal microscopy for Paecilomyces lilacinus and Candida parapsilosis fungal keratitis. Invest Ophthalmol Vis Sci. 2011;52:ARVO E-Abstract No. 5856. [Google Scholar]
- 19.Kurbanyan K, Hoesl LM, Schrems WA, Hamrah P. Corneal nerve alterations in acute acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye. 2012;26:126–132. doi: 10.1038/eye.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, et al. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollingsworth JG, Efron N, Tullo AB. A longitudinal case series investigating cellular changes to the transplanted cornea using confocal microscopy. Cont Lens Anterior Eye. 2006;29:135–141. doi: 10.1016/j.clae.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Patel SV, McLaren JW, Hodge DO, Bourne WM. The effect of corneal light scatter on vision after penetrating keratoplasty. Am J Ophthalmol. 2008;146:913–919. doi: 10.1016/j.ajo.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel SV, Erie JC, McLaren JW, Bourne WM. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and in late endothelial failure. Arch Ophthalmol. 2007;125:1693–1698. doi: 10.1001/archopht.125.12.1693. [DOI] [PubMed] [Google Scholar]
- 24.Tan DTH, Mehta JS. Future directions in lamellar corneal transplantation. Cornea. 2007;26:S21–S28. doi: 10.1097/ICO.0b013e31812f685c. [DOI] [PubMed] [Google Scholar]
- 25.McLaren JW, Patel SV. Modeling the effect of forward scatter and aberrations on visual acuity after endothelial keratoplasty. Invest Ophthalmol Vis Sci. 2012;53:5545–5551. doi: 10.1167/iovs.12-10011. [DOI] [PubMed] [Google Scholar]
- 26.McLaren JW, Bourne WM, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010;51:5610–5616. doi: 10.1167/iovs.10-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baratz KH, McLaren JW, Maguire LJ, Patel SV. Corneal haze determined by confocal microscopy 2 years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol. 2012;130:868–874. doi: 10.1001/archophthalmol.2012.73. [DOI] [PubMed] [Google Scholar]
- 28.Patel SV, Baratz KH, Hodge DO, Maguire LJ, McLaren JW. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–160. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- 29.Patel SV, McLaren JW. In vivo confocal microscopy of Fuchs endothelial dystrophy before and after endothelial keratoplasty. JAMA Ophthalmol. 2013;131:611–618. doi: 10.1001/jamaophthalmol.2013.799. [DOI] [PubMed] [Google Scholar]
- 30.van der Meulen IJ, Patel SV, Lapid-Gortzak R, Nieuwendaal CP, McLaren JW, van den Berg TJ. Quality of vision in patients with Fuchs endothelial dystrophy and after Descemet stripping endothelial keratoplasty. Arch Ophthalmol. 2011;129:1537–1542. doi: 10.1001/archophthalmol.2011.247. [DOI] [PubMed] [Google Scholar]
- 31.Ahuja Y, Baratz KH, McLaren JW, Bourne WM, Patel SV. Decreased corneal sensitivity and abnormal corneal nerves in Fuchs endothelial dystrophy. Cornea. 2012;31:1257–1263. doi: 10.1097/ICO.0b013e31823f7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Aqaba M, Alomar T, Lowe J, Dua HS. Corneal nerve aberrations in bullous keratopathy. Am J Ophthalmol. 2011;151:840–849. doi: 10.1016/j.ajo.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Patel SV, Baratz KH, Maguire LJ, Hodge DO, McLaren JW. Anterior corneal aberrations after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Ophthalmology. 2012;119:1522–1529. doi: 10.1016/j.ophtha.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 34.Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in Fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129:555–561. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raecker ME, McLaren JW, Kittleson KM, Patel SV. Endothelial image quality after Descemet stripping with endothelial keratoplasty: a comparison of three microscopy techniques. Eye Contact Lens. 2011;37:6–10. doi: 10.1097/ICL.0b013e318203dc19. [DOI] [PubMed] [Google Scholar]
- 36.Seery LS, Nau CB, McLaren JW, Baratz KH, Patel SV. Graft thickness, graft folds, and aberrations after Descemet stripping endothelial keratoplasty for Fuchs dystrophy. Am J Ophthalmol. 2011;152:910–916. doi: 10.1016/j.ajo.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shortt AJ, Allan BD, Evans JR. Laser-assisted in-situ keratomileusis (LASIK) versus photorefractive keratectomy (PRK) for myopia. Cochrane Database Syst Rev. 2013;1:CD005135. doi: 10.1002/14651858.CD005135.pub3. [DOI] [PubMed] [Google Scholar]
- 38.McLaren JW, Bourne WM, Patel SV. Stromal reflectance after photorefractive keratectomy: a paired comparison between epithelial removal by rotary brush and excimer laser-scrape. Invest Ophthalmol Vis Sci. 2012;53:AVRO E-Abstract No. 1469. [Google Scholar]
- 39.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Corneal keratocyte deficits after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol. 2006;141:799–809. doi: 10.1016/j.ajo.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Calvo R, McLaren JW, Hodge DO, Bourne WM, Patel SV. Corneal aberrations and visual acuity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Am J Ophthalmol. 2010;149:785–793. doi: 10.1016/j.ajo.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–1064. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. Arch Ophthalmol. 2010;128:1413–1419. doi: 10.1001/archophthalmol.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klingler KN, McLaren JW, Bourne WM, Patel SV. Corneal endothelial cell changes 5 years after laser in situ keratomileusis: femtosecond laser versus mechanical microkeratome. J Cataract Refract Surg. 2012;38:2125–2130. doi: 10.1016/j.jcrs.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel SV, Bourne WM. Corneal endothelial cell loss 9 years after excimer laser keratorefractive surgery. Arch Ophthalmol. 2009;127:1423–1427. doi: 10.1001/archophthalmol.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.International Dry Eye WorkShop (DEWS) The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 46.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27:138–148. doi: 10.3109/08820538.2012.711416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villani E, Magnani F, Viola F, Santaniello A, Scorza R, Nucci P, et al. In vivo confocal evaluation of the ocular surface morphofunctional unit in dry eye. Optom Vis Sci. 2013;90:576–586. doi: 10.1097/OPX.0b013e318294c184. [DOI] [PubMed] [Google Scholar]
- 49.Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helintö M, Tervo TM. Corneal innervation and morphology in primary Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2003;44:2545–2549. doi: 10.1167/iovs.02-1260. [DOI] [PubMed] [Google Scholar]
- 50.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48:2017–2022. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 51.Villani E, Galimberti D, Viola F, Mapelli C, Del Papa N, Ratiglia R. Corneal involvement in rheumatoid arthritis: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2008;49:560–564. doi: 10.1167/iovs.07-0893. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Chen Q, Chen W, Cui L, Ma H, Lu F. Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology. 2011;118:902–907. doi: 10.1016/j.ophtha.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 53.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Villani E, Viola F, Sala R, Salvi M, Mapelli C, Currò N, et al. Corneal involvement in Graves’ orbitopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2010;51:4574–4578. doi: 10.1167/iovs.10-5380. [DOI] [PubMed] [Google Scholar]
- 55.Benítez del Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 56.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjögren’s syndrome. Exp Eye Res. 2008;86:879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Hoşal BM, Ornek N, Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye. 2005;19:1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 58.Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea. 2005;24:818–824. doi: 10.1097/01.ico.0000154402.01710.95. [DOI] [PubMed] [Google Scholar]
- 59.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves—analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin H, Li W, Dong N, Chen W, Liu J, Chen L, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51:122–128. doi: 10.1167/iovs.09-3629. [DOI] [PubMed] [Google Scholar]
- 61.Villani E, Galimberti D, Del Papa N, Nucci P, Ratiglia R. Inflammation in dry eye associated with rheumatoid arthritis: cytokine and in vivo confocal microscopy study. Innate Immun. 2013;19:420–427. doi: 10.1177/1753425912471692. [DOI] [PubMed] [Google Scholar]
- 62.Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K. The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis. 2008;14:1263–1271. [PMC free article] [PubMed] [Google Scholar]
- 63.Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665–672. doi: 10.1016/j.ophtha.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 64.Villani E, Beretta S, De Capitani M, Galimberti D, Viola F, Ratiglia R. In vivo confocal microscopy of meibomian glands in Sjögren’s syndrome. Invest Ophthalmol Vis Sci. 2011;52:933–939. doi: 10.1167/iovs.10-5995. [DOI] [PubMed] [Google Scholar]
- 65.Villani E, Ceresara G, Beretta S, Magnani F, Viola F, Ratiglia R. In vivo confocal microscopy of meibomian glands in contact lens wearers. Invest Ophthalmol Vis Sci. 2011;52:5215–5219. doi: 10.1167/iovs.11-7427. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto Y, Shigeno Y, Sato EA, Ibrahim OM, Saiki M, Negishi K, et al. The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol. 2009;247:821–829. doi: 10.1007/s00417-008-1017-y. [DOI] [PubMed] [Google Scholar]
- 67.Ban Y, Ogawa Y, Ibrahim OM, Tatematsu Y, Kamoi M, Uchino M, et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. 2011;17:2533–2543. [PMC free article] [PubMed] [Google Scholar]
- 68.Villani E, Canton V, Magnani F, Viola F, Nucci P, Ratiglia R. The aging meibomian gland: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2013;54:4735–4740. doi: 10.1167/iovs.13-11914. [DOI] [PubMed] [Google Scholar]
- 69.Kojima T, Matsumoto Y, Dogru M, Tsubota K. The application of in vivo laser scanning confocal microscopy as a tool of conjunctival in vivo cytology in the diagnosis of dry eye ocular surface disease. Mol Vis. 2010;16:2457–2464. [PMC free article] [PubMed] [Google Scholar]
- 70.Villani E, Beretta S, Galimberti D, Viola F, Ratiglia R. In vivo confocal microscopy of conjunctival roundish bright objects: young, older, and Sjögren subjects. Invest Ophthalmol Vis Sci. 2011;52:4829–4832. doi: 10.1167/iovs.10-6215. [DOI] [PubMed] [Google Scholar]
- 71.Wakamatsu TH, Sato EA, Matsumoto Y, Ibrahim OM, Dogru M, Kaido M, et al. Conjunctival in vivo confocal scanning laser microscopy in patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2010;51:144–150. doi: 10.1167/iovs.08-2722. [DOI] [PubMed] [Google Scholar]
- 72.Hong J, Zhu W, Zhuang H, Xu J, Sun X, Le Q, et al. In vivo confocal microscopy of conjunctival goblet cells in patients with Sjögren’s syndrome dry eye. Br J Ophthalmol. 2010;94:1454–1458. doi: 10.1136/bjo.2009.161059. [DOI] [PubMed] [Google Scholar]
- 73.Sato EA, Matsumoto Y, Dogru M, Kaido M, Wakamatsu T, Ibrahim OM, et al. Lacrimal gland in Sjögren’s syndrome. Ophthalmology. 2010;117:1055–1055. doi: 10.1016/j.ophtha.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 74.Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13:569–576. doi: 10.1097/ACI.0b013e328364ec92. [DOI] [PubMed] [Google Scholar]
- 75.Kenchegowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2010;51:879–891. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20:374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Patel DV, McGhee CN. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. Br J Ophthalmol. 2009;93:853–860. doi: 10.1136/bjo.2008.150615. [DOI] [PubMed] [Google Scholar]
- 78.Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45:3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 79.Lee SJ, Jin KK, Kyung YS, Kim EK, Lee HK. Comparison of corneal nerve regeneration and sensitivity between LASIK and laser epithelial keratomileusis (LASEK) Am J Ophthalmol. 2006;141:1009–1015. doi: 10.1016/j.ajo.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 80.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–3664. [PubMed] [Google Scholar]
- 81.Kallinikos P, Berhanu M, O’Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–422. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 82.Stapleton F, Hayward KB, Bachand N, Trong PH, Teh DW, Deng KM, et al. Evaluation of corneal sensitivity to mechanical and chemical stimuli after LASIK: a pilot study. Eye Contact Lens. 2006;32:88–93. doi: 10.1097/01.icl.0000174757.49938.82. [DOI] [PubMed] [Google Scholar]
- 83.Stachs O, Zhivov A, Kraak R, Hovakimyan M, Wree A, Guthoff R. Structural-functional correlations of corneal innervation after LASIK and penetrating keratoplasty. J Refrac Surg. 2010;26:159–167. doi: 10.3928/1081597X-20100224-01. [DOI] [PubMed] [Google Scholar]
- 84.Tuisku IS, Lindbohm N, Wilson SE. Dry eye and corneal sensitivity after high myopic LASIK. J Refract Surg. 2007;23:338–342. doi: 10.3928/1081-597X-20070401-05. [DOI] [PubMed] [Google Scholar]
- 85.Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–591. doi: 10.1136/bjo.2009.164780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marfurt CF, Ellis LC, Jones MA. Sensory and sympathetic nerve sprouting in the rat cornea following neonatal administration of capsaicin. Somatosens Mot Res. 1993;10:377–398. doi: 10.3109/08990229309028845. [DOI] [PubMed] [Google Scholar]
- 87.Albers KM, Wright DE, Davis BM. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J Neurosci. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ambrósio R, Jr, Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. 2008;24:396–407. doi: 10.3928/1081597X-20080401-14. [DOI] [PubMed] [Google Scholar]
- 89.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain. Is it real? Ocul Surf. 2009;7:28–40. doi: 10.1016/s1542-0124(12)70290-2. [DOI] [PubMed] [Google Scholar]
- 90.Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocular Surf. 2010;8:135–145. doi: 10.1016/s1542-0124(12)70224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benitez-del-Castillo JM, del Rio T, Iradier T, Hernández JL, Castillo A, Garcia-Sanchez J. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20:30–32. doi: 10.1097/00003226-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 92.Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108:1230–1235. doi: 10.1016/s0161-6420(01)00623-6. [DOI] [PubMed] [Google Scholar]
- 93.Gallar J, Acosta MC, Moilanen JA, Holopainen JM, Belmonte C, Tervo TM. Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. J Refract Surg. 2004;20:229–235. doi: 10.3928/1081-597X-20040501-06. [DOI] [PubMed] [Google Scholar]
- 94.Mimura T, Amano S, Fukuoka S, Honda N, Arita R, Ochiai M, et al. In vivo confocal microscopy of hereditary sensory and autonomic neuropathy. Curr Eye Res. 2008;33:940–945. doi: 10.1080/02713680802450992. [DOI] [PubMed] [Google Scholar]
- 95.Lalive PH, Truffert A, Magistris MR, Landis T, Dosso A. Peripheral autoimmune neuropathy assessed using corneal in vivo confocal microscopy. Arch Neurol. 2009;66:403–405. doi: 10.1001/archneurol.2008.587. [DOI] [PubMed] [Google Scholar]
- 96.Ferrari G, Gemignani F, Macaluso C. Chemotherapy-associated peripheral sensory neuropathy assessed using in vivo corneal confocal microscopy. Arch Neurol. 2010;67:364–365. doi: 10.1001/archneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 97.American Diabetes Association. [last accessed 15 July 2013];Data from the 2011 National Diabetes Fact Sheet (released 26 January 2011) Available at http://www.diabetes.org/diabetes-basics/diabetes-statistics/?loc=DropDownDB-stats.
- 98.Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21:1071–1075. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 99.Tavakoli M, Quattrini C, Abbott CA, Kallinikos P, Marshall A, Finnigan J, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 101.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769–773. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 102.Ziegler D, Zhivov A, Allgeier S, Winter K, Papanas N, Ziegler I, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetic subjects. Diabetologia. 2012;55:A44. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 103.Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013 doi: 10.2337/dc13-0193. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, et al. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35:821–828. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, Malik RA, et al. Corneal markers of diabetic neuropathy. Ocul Surf. 2011;9:17–28. doi: 10.1016/s1542-0124(11)70006-4. [DOI] [PubMed] [Google Scholar]
- 106.Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13:488–499. doi: 10.1007/s11892-013-0390-z. [DOI] [PubMed] [Google Scholar]
- 107.Labbe A, Dupas B, Hamard P, Baudouin C. In vivo confocal microscopy study of blebs after filtering surgery. Ophthalmology. 2005;112:1979. doi: 10.1016/j.ophtha.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 108.Guthoff R, Klink T, Schlunck G, Grehn F. In vivo confocal microscopy of failing and functioning filtering blebs: results and clinical correlations. J Glaucoma. 2006;15:552–558. doi: 10.1097/01.ijg.0000212295.39034.10. [DOI] [PubMed] [Google Scholar]
- 109.Messmer EM, Zapp DM, Mackert MJ, Thiel M, Kampik A. In vivo confocal microscopy of filtering blebs after trabeculectomy. Arch Ophthalmol. 2006;124:1095–1103. doi: 10.1001/archopht.124.8.1095. [DOI] [PubMed] [Google Scholar]
- 110.Amar N, Labbe A, Hamard P, Dupas B, Baudouin C. Filtering blebs and aqueous pathway an immunocytological and in vivo confocal microscopy study. Ophthalmology. 2008;115:1154–1161. e4. doi: 10.1016/j.ophtha.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 111.Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–735. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 112.Baratz KH, Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25:1046–1052. doi: 10.1097/01.ico.0000230499.07273.c5. [DOI] [PubMed] [Google Scholar]
- 113.Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 114.Liang H, Brignole-Baudouin F, Riancho L, Baudouin C. Reduced in vivo ocular surface toxicity with polyquad-preserved travoprost versus benzalkonium-preserved travoprost or latanoprost ophthalmic solutions. Ophthalmic Res. 2012;48:89–101. doi: 10.1159/000335984. [DOI] [PubMed] [Google Scholar]
- 115.Labbe A, Pauly A, Liang H, Brignole-Baudouin F, Martin C, Warnet JM, et al. Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J Ocul Pharmacol Ther. 2006;22:267–278. doi: 10.1089/jop.2006.22.267. [DOI] [PubMed] [Google Scholar]
- 116.Liang H, Baudouin C, Labbe A, Riancho L, Brignole-Baudouin F. Conjunctiva-associated lymphoid tissue (CALT) reactions to antiglaucoma prostaglandins with or without BAK-preservative in rabbit acute toxicity study. PLoS One. 2012;7:e33913. doi: 10.1371/journal.pone.0033913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ladage PM, Petroll WM, Jester JV, Fisher S, Bergmanson JP, Cavanagh HD. Spherical indentations of human and rabbit corneal epithelium following extended contact lens wear. CLAO J. 2002;28:177–180. doi: 10.1097/01.ICL.0000033621.46837.21. [DOI] [PubMed] [Google Scholar]
- 118.Craig JP, Sherwin T, Grupcheva CN, McGhee CN. An evaluation of mucin balls associated with high-DK silicone-hydrogel contact lens wear. Adv Exp Med Biol. 2002;506:917–923. doi: 10.1007/978-1-4615-0717-8_128. [DOI] [PubMed] [Google Scholar]
- 119.Millar TJ, Papas EB, Ozkan J, Jalbert I, Ball M. Clinical appearance and microscopic analysis of mucin balls associated with contact lens wear. Cornea. 2003;22:740–745. doi: 10.1097/00003226-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 120.Ladage PM, Yamamoto K, Ren DH, Li L, Jester JV, Petroll WM. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 121.Imayasu M, Moriyama T, Ichijima H, Ohashi J, Petroll WM, Jester JV, et al. The effects of daily wear of rigid gas permeable contact lenses treated with contact lens care solutions containing preservatives on the rabbit cornea. CLAO J. 1994;20:183–188. doi: 10.1097/00140068-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 122.Ren DH, Petroll WM, Jester JV, Ho-Fan J, Cavanagh HD. The relationship between contact lens oxygen permeability and binding of Pseudomonas aeruginosa to human corneal epithelial cells after overnight and extended wear. CLAO J. 1999;25:80–100. [PubMed] [Google Scholar]
- 123.Kalogeropoulos G, Chang S, Bolton T, Jalbert I. The effects of short-term lens wear and eye rubbing on the corneal epithelium. Eye Contact Lens. 2009;35:255–259. doi: 10.1097/ICL.0b013e3181b4ec39. [DOI] [PubMed] [Google Scholar]
- 124.Jalbert I, Sweeney DF, Stapleton F. The effect of long-term wear of soft lenses of low and high oxygen transmissibility on the corneal epithelium. Eye. 2009;23:1282–1287. doi: 10.1038/eye.2008.307. [DOI] [PubMed] [Google Scholar]
- 125.Bansal AK, Mustonen RK, McDonald MB. High resolution in vivo scanning confocal microscopy of the cornea in long term contact lens wear. Invest Ophthalmol Vis Sci. 1997;38 [Google Scholar]
- 126.Wu T, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, et al. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med. 2012;29:e297–e303. doi: 10.1111/j.1464-5491.2012.03678.x. [DOI] [PubMed] [Google Scholar]
- 127.Oliveira-Soto L, Efron N. Morphology of corneal nerves in soft contact lens wear. A comparative study using confocal microscopy. Ophthal Physiol Opt. 2003;23:163–174. doi: 10.1046/j.1475-1313.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 128.Zhivov A, Stave J, Vollmar B, Guthoff R. In vivo confocal microscopic evaluation of langerhans cell density and distribution in the corneal epithelium of healthy volunteers and contact lens wearers. Cornea. 2007;26:47–54. doi: 10.1097/ICO.0b013e31802e3b55. [DOI] [PubMed] [Google Scholar]
- 129.Sindt CW, Grout TK, Critser DB, Kern JR, Meadows DL. Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study. Clin Ophthalmol. 2012;6:511–519. doi: 10.2147/OPTH.S28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Efron N, Mutalib HA, Perez-Gomez I, Koh HH. Confocal microscopic observations of the human cornea following overnight contact lens wear. Clin Exp Optom. 2002;85:149–155. doi: 10.1111/j.1444-0938.2002.tb03027.x. [DOI] [PubMed] [Google Scholar]
- 131.Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. 2002;43:995–1003. [PubMed] [Google Scholar]
- 132.Jalbert I, Stapleton F. Effect of lens wear on corneal stroma: preliminary findings. Aust N Z J Ophthalmol. 1999;27:211–213. doi: 10.1046/j.1440-1606.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 133.Kallinikos P, Morgan PB, Efron N. Assessment of stromal keratocytes and tear film inflammatory mediators during extended wear of contact lenses. Cornea. 2006;25:1–10. doi: 10.1097/01.ico.0000167877.11687.7e. [DOI] [PubMed] [Google Scholar]
- 134.Weed KH, MacEwen CJ, Cox A, McGhee CN. Quantitative analysis of corneal microstructure in keratoconus utilising in vivo confocal microscopy. Eye. 2007;21:614–623. doi: 10.1038/sj.eye.6702286. [DOI] [PubMed] [Google Scholar]
- 135.Zhong X, Chen X, Xie RZ, Yang J, Li S, Yang X, et al. Differences between overnight and long-term wear of orthokeratology contact lenses in corneal contour, thickness, and cell density. Cornea. 2009;28:271–279. doi: 10.1097/ICO.0b013e318186e620. [DOI] [PubMed] [Google Scholar]
- 136.Yagmur M, Okay O, Sizmaz S, Unal I, Yar K. In vivo confocal microscopy: corneal changes of hydrogel contact lens wearers. Int Ophthalmol. 2011;31:377–383. doi: 10.1007/s10792-011-9466-4. [DOI] [PubMed] [Google Scholar]
- 137.Ohta K, Shimamura I, Shiraishi A, Ohashi Y. Confocal microscopic observations of stromal keratocytes in soft and rigid contact lens wearers. Cornea. 2012;31:66–73. doi: 10.1097/ICO.0b013e31821b71ff. [DOI] [PubMed] [Google Scholar]
- 138.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45:3011–3020. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 139.Böhnke M, Masters BR. Long term contact lens wear induces a corneal degeneration with microdot deposits in the corneal stroma. Ophthalmology. 1997;104:1887–1896. doi: 10.1016/s0161-6420(97)30011-6. [DOI] [PubMed] [Google Scholar]
- 140.Trittibach P, Cadez R, Eschmann R, Sarra GM, Boehnke M, Frueh BE. Determination of microdot stromal degenerations within corneas of long-term contact lens wearers by confocal microscopy. Eye Contact Lens. 2004;30:127–131. doi: 10.1097/01.icl.0000131297.62881.ae. [DOI] [PubMed] [Google Scholar]
- 141.Bastion ML, Mohamad MH. Study of the factors associated with the presence of white dots in the corneas of regular soft contact lens users from an Asian country. Eye Contact Lens. 2006;32:223–227. doi: 10.1097/01.icl.0000199891.35160.68. [DOI] [PubMed] [Google Scholar]
- 142.Efron N, Mutalib HA. Confocal microscopy observations of the cornea in response to contact lens wear. Die Kontaktlinse. 2001;35:4–16. [Google Scholar]
- 143.Ma X, He L, He D, Xu J. Chloroquine keratopathy of rheumatoid arthritis patients detected by in vivo confocal microscopy. Curr Eye Res. 2012;37:293–299. doi: 10.3109/02713683.2011.631719. [DOI] [PubMed] [Google Scholar]
- 144.Sbeity Z, Palmiero PM, Tello C, Liebmann JM, Ritch R. Non-contact in vivo confocal scanning laser microscopy in exfoliation syndrome, exfoliation syndrome suspect and normal eyes. Acta Ophthalmol. 2011;89:241–247. doi: 10.1111/j.1755-3768.2009.01678.x. [DOI] [PubMed] [Google Scholar]
- 145.Mocan MC, Eldem B, Irkec M. In vivo confocal microscopic findings of two siblings with Maroteaux-Lamy syndrome. Cornea. 2007;26:90–93. doi: 10.1097/ICO.0b013e31802be383. [DOI] [PubMed] [Google Scholar]
- 146.Efron N. Endothelial blebs. Contact lens complications. 3. Edinburgh: Elsevier-Saunders; 2012. [Google Scholar]
- 147.Nagel S, Wiegand W, Thaer AA, Geyer OC. Light scattering study of the cornea in contact lens patients. In vivo studies using confocal slit scanning microscopy. Ophthalmologe. 1996;93:252–256. [PubMed] [Google Scholar]
- 148.Rong B, Yan XM. Changes of corneal limbus in long-term soft contact lens wearers by using laser confocal microscope. Zhonghua Yan Ke Za Zhi. 2007;43:514–518. [PubMed] [Google Scholar]
- 149.Nguyen TH, Dudek LT, Krisciunas TC, Matiaco P, Planck SR, Mathers WD, et al. In vivo confocal microscopy: increased conjunctival or episcleral leukocyte adhesion in patients who wear contact lenses with lower oxygen permeability (Dk) values. Cornea. 2004;23:695–700. doi: 10.1097/01.ico.0000127482.00843.c8. [DOI] [PubMed] [Google Scholar]
- 150.Sutphin JE, Kantor AL, Mathers WD, Mehaffey MG. Evaluation of infectious crystalline keratitis with confocal microscopy in a case series. Cornea. 1997;16:21–26. [PubMed] [Google Scholar]
- 151.Su PY, Hu FR, Chen YM, Han JH, Chen WL. Dendritiform cells found in central cornea by in-vivo confocal microscopy in a patient with mixed bacterial keratitis. Ocul Immunol Inflamm. 2006;14:241–244. doi: 10.1080/09273940600732398. [DOI] [PubMed] [Google Scholar]
- 152.Efron N, Morgan PB, Makrynioti D. Chronic morbidity of corneal infiltrative events associated with contact lens wear. Cornea. 2007;26:793–799. doi: 10.1097/ICO.0b013e31806c79dc. [DOI] [PubMed] [Google Scholar]
- 153.Efron N, Al-Dossari M, Pritchard N. Confocal microscopy of the bulbar conjunctiva in contact lens wear. Cornea. 2010;29:43–52. doi: 10.1097/ICO.0b013e3181acf82a. [DOI] [PubMed] [Google Scholar]
- 154.Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116:379–384. doi: 10.1016/j.ophtha.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 155.Zhivov A, Stachs O, Stave J, Guthoff RF. In vivo three-dimensional confocal laser scanning microscopy of corneal surface and epithelium. Br J Ophthalmol. 2009;93:667–672. doi: 10.1136/bjo.2008.137430. [DOI] [PubMed] [Google Scholar]
- 156.Zhivov A, Blum M, Guthoff R, Stachs O. Real-time mapping of the subepithelial nerve plexus by in vivo confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:1133–1135. doi: 10.1136/bjo.2009.175489. [DOI] [PubMed] [Google Scholar]
- 157.Edwards K, Pritchard N, Gosschalk K, Sampson GP, Russell A, Malik RA, et al. Wide-field assessment of the human corneal subbasal nerve plexus in diabetic neuropathy using a novel mapping technique. Cornea. 2012;31:1078–1082. doi: 10.1097/ico.0b013e318245c012. [DOI] [PubMed] [Google Scholar]
- 158.Petroll WM, Cavanagh HD, Jester JV. Three-dimensional imaging of corneal cells using in vivo confocal microscopy. J Microsc. 1993;170:213–219. doi: 10.1111/j.1365-2818.1993.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 159.Allgeier S, Zhivov A, Eberle F, Koehler B, Maier S, Bretthauer G, et al. Image reconstruction of the subbasal nerve plexus with in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:5022–5028. doi: 10.1167/iovs.10-6065. [DOI] [PubMed] [Google Scholar]
- 160.Petroll WM, Weaver M, Vaidya S, McCulley JP, Cavanagh HD. Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope. Cornea. 2013;32:e36–e43. doi: 10.1097/ICO.0b013e31825ec44e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhivov A, Winter K, Peschel S, Guthoff RF, Stachs O, Harder V, et al. Quantitative analysis of corneal subbasal nerve plexus with in vivo confocal laser scanning microscopy. Klin Monbl Augenheilkd. 2011;228:1067–1072. doi: 10.1055/s-0031-1281663. [DOI] [PubMed] [Google Scholar]
- 162.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15:738–747. doi: 10.1016/j.media.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 163.Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv. 2010;13:300–307. doi: 10.1007/978-3-642-15705-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holmes TJ, Pellegrini M, Miller C, Epplin-Zapf T, Larkin S, Luccarelli S, et al. Automated software analysis of corneal micrographs for peripheral neuropathy. Invest Ophthalmol Vis Sci. 2010;51:4480–4491. doi: 10.1167/iovs.09-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lemp MA, Dilly PN, Boyde A. Tandem-scanning (confocal) microscopy of the full-thickness cornea. Cornea. 1985–1986;4:205–209. [PubMed] [Google Scholar]