Meningitis is associated with high mortality and long-term complications among survivors in the neonatal period.1–3 Adequate treatment relies on proper diagnosis, and only cerebrospinal fluid (CSF) culture can definitively diagnose meningitis.1,4–8 When evaluating an infant for sepsis, many clinicians choose to obtain a blood culture and await a positive result before performing a lumbar puncture. This strategy underestimates the incidence of meningitis; furthermore, up to 50% of very low birth weight (<1500 g) infants with meningitis have a negative blood culture.8
The concordance between blood, urine, and CSF cultures may affect management strategies for sepsis and meningitis. Meningitis in the neonate is classified as early- or late-onset depending on the time after birth when it occurs. The majority (61.4%) of late-onset sepsis cases are caused by Gram-positive organisms.9 Prior studies have found 39% of blood and CSF cultures to be concordant with Gram-positive organisms compared with 16% of blood and urine cultures.10,11 However, the relationship between individual organisms present in blood and CSF cultures, as well as between concordance and the timing of blood and CSF cultures, have not been fully described. We sought to determine the concordance of organisms between blood and CSF cultures and to evaluate predictors of concordance adjusting for markers of severity of illness.
METHODS
We identified all infants discharged from 333 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from 1997–2010 with at least one positive blood culture paired with a CSF culture in the first 120 postnatal days of life. The Pediatrix Medical Group maintains an administrative database that prospectively captures information from notes generated by clinicians on all infants cared for by the group. Data on multiple aspects of care are entered into the system to generate admission notes, daily progress notes, procedure notes, and discharge summaries. Information is collected regarding maternal history and demographics, medications, laboratory results, culture results, and diagnoses.
We defined a blood-CSF culture pair obtained in the same infant by the following rules: (1) We excluded blood cultures positive for organisms considered contaminants, including non-speciated streptococci, Bacillus sp., Corynebacterium sp., diphtheroids sp., Gram-positive rods (not including Listeria sp.), Lactobacillus sp., Micrococcus sp., Stomatococcus sp., and Bacteroides sp. (2) When multiple positive blood cultures with the same organism were obtained within a 21-day period, they were considered to be a common infectious episode, and only the first positive culture was retained for analysis. (3) Multiple CSF cultures within the same 21-day period were also considered to be a common infectious episode, and only the first positive culture was retained for analysis. (4) Blood and CSF cultures positive with >1 organism were treated as separate positive cultures. If one of the organisms was considered a contaminant, we counted only the true organism as a positive culture and ignored the contaminant.
We included positive blood cultures in the analysis if they could be paired with a CSF culture obtained in the same infant on the same day as the blood culture or up to three days before or three days after the positive blood culture. If multiple blood cultures could be paired with the same CSF culture, they were each counted as separate blood-CSF culture pair. If multiple CSF cultures could be paired with the same blood culture, we kept, in order of preference, a positive CSF culture over a negative CSF culture, a CSF culture obtained on the same day over a CSF culture obtained before or after the blood culture, and then a CSF culture obtained closest in time before or after the blood culture. All blood cultures that could not be paired with a CSF culture and all CSF cultures that could not be paired with a blood culture were excluded from the analysis.
We defined blood-CSF culture concordance as the growth of the same organism in the blood and CSF of the blood-CSF culture pair. We defined blood-CSF culture discordance as either the growth of an organism in the blood culture but not the CSF culture, or the growth of two different organisms in each one of the two cultures of each pair. We also examined group B streptococcal (GBS) concordance in early- and late-onset GBS sepsis. We determined concordance for early-onset (<3 days of life) as well as late-onset (≥3 days of life) GBS sepsis in infants with a GBS-positive blood culture.
Statistical Analysis
The unit of observation for this study was a blood-CSF culture pair. We used standard summary statistics to describe the study variables. We compared infant-level and culture-level characteristics, including organism genus and group (Gram-positive, Gram-negative, or fungal organisms) between the categories of concordant versus discordant blood-CSF culture pairs using chi-square tests of association and Wilcoxon rank sum tests where appropriate. We used univariable and multivariable logistic regression analysis to evaluate the odds of a concordant versus discordant blood-CSF culture pair, controlling for the organism grown in the blood culture, gestational age (GA) in weeks, postnatal age in days, fraction of inspired oxygen (FiO2) <30% or ≥30% on the day of the blood culture, mechanical ventilation on the day of the blood culture, use of inotropes (dopamine, dobutamine, epinephrine, norepinephrine, phenylephrine, or vasopressin) on the day of the blood culture, the day of CSF culture with respect to the blood culture, the presence of a ventriculo-peritoneal (VP) shunt, and exposure to CSF-penetrating antibiotics (cefepime, cefotaxime, ceftriaxone, ceftazidime, meropenem, imipenem/cilastatin) before the day of the CSF culture. We did not include ampicillin as a CSF-penetrating antibiotic in our cohort due to absence of dosing information and the required high dose for CSF penetration. We used the method of generalizing estimating equations with an exchangeable (compound symmetry) covariance structure to account for correlation between multiple blood-CSF culture pairs obtained in the same infant. We repeated these analyses separately in infants who grew only Gram-negative and Gram-positive organisms from their blood culture.
We used Stata 12 (College Station, TX), and statistical significance was defined as a p<0.05. The study was approved by the Duke University Institutional Review Board without the need for written informed consent because the data were collected without identifiers.
RESULTS
Infant characteristics
We identified 8839 infants with at least one positive blood culture that could be paired with a CSF culture (Table 1); 220 infants (3%) had a VP shunt. The median GA was 30 weeks (5th and 95th percentile; 24, 40), and the median birth weight was 1407 g (600, 3762). The median day of life of first positive blood culture for each infant was 12 (0, 66).
TABLE 1.
Demographics
| Infants with blood-CSF culture concordance N=437 n (%) |
Infants without blood-CSF culture concordance N=8402 n (%) |
|
|---|---|---|
| Gestational age, weeks | ||
| ≤25 | 81 (19) | 1359 (16) |
| 26–32 | 182 (42) | 3675 (44) |
| 33–36 | 49 (11) | 1099 (13) |
| ≥37 | 122 (28) | 2235 (27) |
| Birth weight, g | ||
| <1000 | 134 (31) | 2695 (32) |
| 1000–1499 | 88 (20) | 1765 (21) |
| 1500–2499 | 79 (18) | 1414 (17) |
| 2500–3499 | 98 (22) | 1656 (20) |
| ≥3500 | 34 (8) | 836 (10) |
| Sex | ||
| Male | 236 (54) | 4631 (55) |
| Race/ethnicity | ||
| White | 205 (47) | 3887 (46) |
| Black | 100 (23) | 1962 (24) |
| Hispanic | 101 (23) | 1836 (22) |
| Other | 16 (4) | 387 (5) |
| VP shunt | ||
| Yes | 44 (10) | 176 (2) |
Blood-CSF culture pairs
There were 9408 positive blood cultures paired with a CSF culture, and 440 (5%) were concordant (Figure 1). Of the 8968 discordant cultures, 73 (<1%) were due to growth of two different organisms in the blood and CSF, and the remaining 8895 (99%) discordant pairs were a result of a positive blood culture and a negative CSF culture. Of the 440 concordant pairs, 232 (53%) were Gram-positive, 168 (38%) were Gram-negative, and 40 (< 1%) were attributable to fungi or other organisms. Of the 5540 Gram-positive blood-CSF culture pairs, the most commonly cultured organisms in the blood were GBS (1950, 35%), coagulase-negative staphylococci (CoNS) (1265, 23%), and S. aureus (1169, 21%). Of the 3151 Gram-negative blood-CSF culture pairs, the most commonly cultured organisms from the blood were E. coli (1297, 41%) and Klebsiella (634, 20%). The most commonly concordant organisms of the 440 concordant pairs were GBS (145, 33%), E. coli (84, 19%), S. aureus (49, 11%), and C. albicans (28, 6%).
Figure 1.
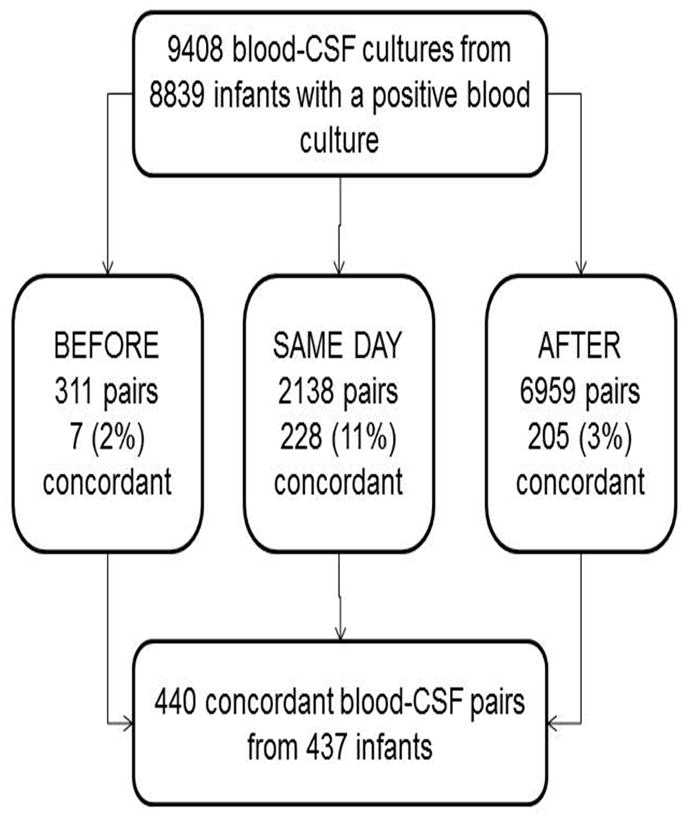
Study population. CSF=cerebrospinal fluid.
We found that the most frequently cultured organisms in the blood were not the most commonly concordant organisms (Table 2). CoNS represented 1265/9408 (13%) of the total blood-CSF culture pair population, but only 4/1265 (<1%) of the paired CSF cultures were positive for CoNS. Serratia represented 227/9408 (2%) of the total blood-CSF culture pair population, and 24/227 (11%) of the paired blood-CSF cultures were positive for Serratia. Streptococcus pnemoniae grew in 64/9408 (< 1%) of blood cultures, and 7/64 (11%) of the paired blood and CSF cultures were positive for S. pneumoniae.
TABLE 2.
Blood-CSF Culture Concordance
| No VP shunt, N = 8619 Concordant blood-CSF pairs (%) |
VP shunt, N = 220 Concordant blood-CSF pairs (%) |
Total, N = 8839 Concordant blood-CSF pairs (%) |
|
|---|---|---|---|
| Gram-negative organisms | 142/3055 (5) | 26/96 (27) | 168/3151 (5) |
| Acinetobacter | 0/43 (0) | 0/1 (0) | 0/44 (0) |
| Citrobacter | 2/56 (4) | 0/3 (0) | 2/59 (3) |
| Escherichia coli | 72/1273 (6) | 12/24 (50) | 84/1297 (6) |
| Enterobacter | 13/376 (3) | 3/14 (21) | 16/390 (4) |
| Hemophilus influenza | 2/125 (2) | 0/2 (0) | 2/127 (2) |
| Klebsiella | 17/612 (3) | 1/22 (5) | 18/634 (3) |
| Proteus | 1/22 (5) | 0/0 (0) | 1/22 (5) |
| Pseudomonas | 4/130 (3) | 4/6 (67) | 8/136 (6) |
| Salmonella | 5/23 (22) | 0/0 (0) | 5/23 (22) |
| Serratia marcescens | 20/209 (10) | 4/18 (22) | 24/227 (11) |
| Other | 6/186 (3) | 2/6 (33) | 8/192 (4) |
| Gram-positive organisms | 216/5412 (4) | 16/128 (13) | 232/5540 (4) |
| CoNS | 2/1217 (<1) | 2/48 (4) | 4/1265 (<1) |
| Enterococcus | 10/521 (2) | 3/23 (13) | 13/544 (2) |
| Group B Streptococcus | 140/1940 (7) | 5/10 (50) | 145/1950 (7) |
| Listeria | 4/49 (8) | 0/1 (0) | 4/50 (8) |
| Staphylococcus aureus | 43/1133 (4) | 6/36 (17) | 49/1169 (4) |
| Streptococcus pneumoniae | 7/64 (11) | 0/0 (0) | 7/64 (11) |
| Other | 10/488 (2) | 0/10 (0) | 10/498 (2) |
| Fungus | 26/472 (6) | 2/23 (9) | 28/495 (6) |
| Candida | 26/469 (6) | 2/23 (9) | 28/492 (6) |
| Malassezia | 0/3 (0) | 0/0 (0) | 0/3 (0) |
| Other | 12/211 (6) | 0/11 (0) | 12/222 (5) |
| Total | 396/9150 (4) | 44/258 (17) | 440/9408 (5) |
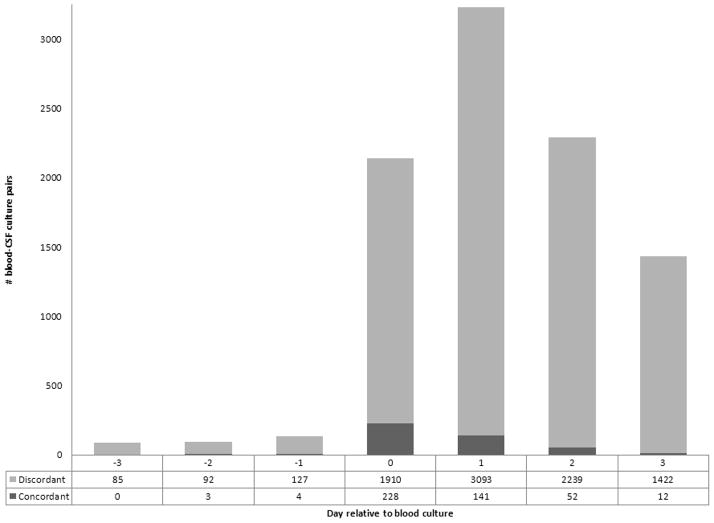
The majority of blood-CSF culture pairs (3234/9408, 34%) were paired on the first day following the blood culture, with 141 (4%) concordant (Figure 2). When blood and CSF cultures were obtained on the same day, the percentage of concordance was higher (228/2138, 11%) than when the CSF was obtained before (7/311, 2%) or after (205/6959, 3%) the blood culture (Figure 2). For GBS-positive blood culture obtained in the first three days of life (1403), 52 (4%) of the blood and CSF cultures were concordant. For GBS-positive blood culture obtained after the first three days of life (547), 93 (17%) of the blood and CSF cultures were concordant (p <0.001).
Figure 2.
Concordance of blood-CSF culture pairs. CSF=cerebrospinal fluid.
Comparison of culture pairs in infants with and without a VP shunt
Of the 9150 blood-CSF culture pairs from infants without a VP shunt, only 396 (4%) were concordant (Table 2). The most frequently cultured organisms from the blood were GBS (1940, 21%), E. coli (1273, 14%), CoNS (1217, 13%), and S. aureus (1133, 12%). The most commonly concordant organisms between the blood and CSF in which there were at least 10 positive blood cultures were Salmonella (5/23, 22%), S. pneumo (7/64, 11%), Serratia (20/209, 10%), and Listeria (4/49, 8%).
Of the 258 blood-CSF culture pairs from infants with a VP shunt, 44 (17%) were concordant. The most frequently cultured organisms from the blood in the infants with a VP shunt were CoNS (48/258, 19%) and S. aureus (36/258, 14%). Organisms with at least 10 positive blood cultures with the highest concordance included E. coli (12/24, 50%), Serratia (4/18, 22%), Enterobacter (3/14, 21%), and S. aureus (6/36, 17%) (Table 2).
Logistic regression
On multivariable analysis, infants with a VP shunt had an increased odds of concordance compared to infants without a VP shunt (Table 3). When CSF cultures were obtained on the same day as the blood culture, the odds of concordance was higher than when CSF cultures were obtained before or after the blood cultures.
TABLE 3.
Adjusted Odds Ratios for Concordance of CSF Cultures with Blood Cultures
| Multivariable Regression
|
||
|---|---|---|
| Odds Ratio | P | |
| Organism type in blood-CSF pair | ||
| Gram-positive cocci | 1.00 (reference) | |
| Gram-negative rods | 1.27 (1.02–1.61) | 0.04 |
| Fungus | 1.27 (0.82–1.96) | 0.29 |
| Other | 1.34 (0.66–2.72) | 0.41 |
| Gestational age (weeks) | ||
| <25 | 1.00 (reference) | |
| 26–28 | 0.94 (0.68–1.32) | 0.75 |
| 29–32 | 1.23 (0.86–1.78) | 0.26 |
| 33–36 | 1.24 (0.79–1.94) | 0.34 |
| ≥37 | 1.71 (1.12–2.62) | 0.01 |
| Day of life of blood culture | ||
| 0–3 days | 1.00 (reference) | |
| 4–28 days | 1.23 (0.90–1.68) | 0.19 |
| ≥28 days | 0.94 (0.64–1.37) | 0.73 |
| Exposure to CSF penetrating antibiotic before blood-CSF culture pairing | ||
| Yes | 2.18 (1.65–2.89) | <0.01 |
| CSF culture day with respect to blood culture | ||
| Before | 1.00 (reference) | |
| Same day | 6.11 (2.81–13.24) | <0.01 |
| After | 1.48 (0.68–3.22) | 0.32 |
| Mechanical ventilation on day of blood culture | ||
| Yes | 1.51 (1.15–1.97) | <0.01 |
| FiO2 on day of blood culture | ||
| ≥30% | 1.11 (0.88–1.41) | 0.39 |
| Inotropes on day of blood culture | ||
| Yes | 1.84 (1.26–2.67) | <0.01 |
| VP shunt | ||
| Yes | 3.87 (2.59–5.78) | <0.01 |
We also observed higher concordance in infants exposed to CSF-penetrating antibiotics the day before drawing the CSF culture (OR=2.18 [95% confidence interval; 1.65–2.89]). Of the 1240 CSF cultures obtained following infant exposure to these antibiotics, 85 (7%) were concordant in the blood and CSF compared to only 355/8168 (4%) concordant blood-CSF cultures from infants who were not exposed to CSF-penetrating antibiotics prior to obtaining the CSF culture. The most common organisms found concordant from blood-CSF culture pairs in which the CSF culture was obtained after administration of a CSF-penetrating antibiotic were GBS (20/109, 18%), Citrobacter (2/16, 13%), E. coli (26/250, 10%), C. albicans (9/94, 10%), and Pseudomonas (3/29, 10%). Among infants with Gram-negative organisms isolated in the blood, infants with a VP shunt were more likely to have concordant CSF cultures (OR=7.60 [4.26 – 13.58]) compared to infants without a VP shunt. Among infants with Gram-positive organisms isolated in the blood, infants with a VP shunt were more likely to have concordant CSF cultures (OR=3.17 [1.65 – 6.11]) compared to infants without a VP shunt.
DISCUSSION
In this large cohort of infants, we observed that blood-CSF culture concordance was associated with the presence of Gram-negative and fungal organisms, presence of a VP shunt, and timing of the CSF culture on the same day as the blood culture. We also observed increased concordance in cultures from infants exposed to CSF-penetrating antibiotics prior to blood-CSF culture pairing. Gram-positive organisms were cultured more frequently in our cohort than any other organism, which is consistent with previous studies.12,13 The overall incidence of culture-positive meningitis, as well as the distribution of organisms in this cohort, was similar to previously reported cohorts of hospitalized infants.13–15
Infants with blood-CSF concordance were more likely to have indicators related to increased severity of illness, such as mechanical ventilation, inotropic drug use, and presence of a VP shunt. Overall, there was a four-fold increase in blood-CSF culture concordance in infants with a VP shunt, regardless of the organism type. The increase in all-organism concordance and Gram-negative concordance in infants with VP shunts is likely due to placement of the VP shunt in the peritoneal cavity.16,17 Because meningitis in the infant is often due to the spread of pathogens from the blood into the CSF, and organisms frequently associated with peritoneal infection are Gram-negative rods including E. coli, it is possible that these pathogens can cause meningitis in several ways: They may spread from a local peritoneal infection to the blood and continue to spread to the ventricles, or they may spread from the peritoneum into the ventricles of the brain through the shunt, leading to meningitis.16,18–20 Alternatively, it is possible that infection starts within the shunt itself and spreads to the blood stream, causing sepsis due to structural abnormalities in the shunts.17
Serratia had one of the highest incidences of concordance between the blood and CSF in our data, both in infants with (22%) and without (10%) a VP shunt. Serratia bloodstream infections have been associated with morbidities including brain abscesses, which are difficult to treat even with appropriate CSF-penetrating antibiotics, and development of ventriculitis and parenchymal necrosis.21
The clinical presentation for meningitis in infants is non-specific. It may present as a multitude of symptoms, including feeding difficulties, temperature instability, respiratory distress, as well as vomiting, diarrhea, and seizures, making it difficult to separate from the clinical presentation of other etiologies (e.g., apnea of prematurity, urinary tract infection, necrotizing enterocolitis). Although CSF culture is the gold standard for diagnosis of meningitis, the optimal time for a lumbar puncture in infants with clinical signs and symptoms of sepsis and meningitis is uncertain.6,8,22,23 We found that approximately one out of 20 positive blood cultures grew the same organism in the CSF culture.
In our study, the cases of meningitis may be underestimated because nearly 75% of the blood-CSF culture pairs were matched in the three days following the positive blood culture. The most likely explanation is that a positive blood culture prompted a lumbar puncture to rule out meningitis. Given that most clinicians choose to administer antibiotics while waiting for blood culture results, this delay in matching may have resulted in sterilization of the CSF and thus negative CSF cultures. We found that the proportion of concordant CSF and blood cultures was four times higher when the blood and CSF cultures were obtained on the same day compared with when the CSF was obtained before or after the blood culture. This demonstrates the importance of obtaining the CSF at the time of the sepsis evaluation to most accurately diagnose and treat potential meningitis in an infant. CSF should continue to be collected at the time of sepsis evaluation for diagnosis of meningitis confirmation and guidance of correct treatment, if the infant is able to withstand the procedure.
When we examined whether the infants were exposed to CSF-penetrating antibiotics (cefepime, cefotaxime, ceftriaxone, ceftazidime, meropenem, imipenem/cilistatin) the day before a CSF culture was drawn, the odds of concordance increased in the exposed infants. Potential reasons for this phenomenon include lower CSF penetration of these drugs than expected or a delay in reaching steady-state levels in the CSF (potentially longer than one day). Physicians may administer these antibiotics because of a high degree of suspicion that infants with a positive blood culture could have meningitis based on clinical signs and symptoms (e.g., seizure, vital sign instability, or other central nervous system symptoms). Therefore, it is possible that a clinician’s choice to initiate empirical antibiotics was meant to treat meningitis. However, if the antibiotics take a longer time to reach a steady state in the CSF, the meningitis would not be treated within the first day, and the culture would be positive once obtained. The symptoms an infant exhibited may also have been consistent with a diagnosis of meningitis, thus encouraging a physician to administer antibiotics earlier, before a lumbar puncture and CSF culture were obtained.
There were a few limitations to this study. Given the multicenter, retrospective design, there was not a systematic approach to evaluation of sepsis. The study was also limited by the lack of antibiotic sensitivities for each organism and lack of information regarding medication dosing. As an example, ampicillin was not classified as a CSF-penetrating antibiotic although concentrations were likely high enough to treat GBS but not Gram-negative rods. Study strengths included the large sample size and ability to examine organism-level characteristics across blood-CSF culture pairs. This cohort includes data on infants from 333 NICUs across North America, including both academic and community sites, allowing these results to be generalizable to many institutions as well as a wide range of NICU populations.
Conclusions
In this study, we found that the presence of a VP shunt, increased severity of illness, and obtaining blood and CSF cultures on the same day were all related to an increase in the possibility of a concordant blood-CSF culture pair. This concordance also varied depending on the specific organism. Overall, obtaining a lumbar puncture in a timely manner in these infants is essential. If more lumbar punctures are obtained on the same day and even at the same time as the blood culture, it is possible that more infants might be diagnosed with meningitis and treated accordingly. Due to wide variability in NICU site performance of lumbar punctures in infants with suspected meningitis, determining a clear relationship between blood culture and CSF culture concordance will potentially aid in correct diagnosis and treatment of neonatal meningitis, particularly in infants with VP shunts.
Acknowledgments
This work was supported by the American Recovery and Reinvestment Act, DHHS- 1R18AE000028-01 (P.B.S). This work was also supported by a grant from the Doris Duke Charitable Foundation to UNC-Chapel Hill School of Medicine to fund clinical research fellow Kristyn S. Beam. Dr. Laughon receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology and from NICHD (1K23HL092225-01). Dr. Cohen-Wolkowiez receives support for research from the National Institutes of Health (1K23HD064814); he also receives support from the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org) and from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Benjamin receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1K24HD058735-05, and NICHD contract HHSN2752010000031) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives salary support for research from the National Institutes of Health and the U.S. Department of Health and Human Services (NICHD 1K23HD060040-01, DHHS-1R18AE000028-01, and HHSN267200700051C); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Christoph P. Hornik and Reese H. Clark have no relevant conflicts to disclose.
The funding organizations played no role in the study design; collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. 1999;23:218–225. doi: 10.1016/s0146-0005(99)80066-4. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin DK, DeLong E, Cotten CM, Garges HP, Steinbach WJ, Clark RH. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004;24:175–180. doi: 10.1038/sj.jp.7211068. [DOI] [PubMed] [Google Scholar]
- 4.Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117:1094–1100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 5.de Louvois J. Acute bacterial meningitis in the newborn. J Antimicrob Chemother. 1994;34 (Suppl A):61–73. doi: 10.1093/jac/34.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CE, Whitwell JK, Pethe K, Saxena K, Super DM. Term newborns who are at risk for sepsis: are lumbar punctures necessary? Pediatrics. 1997;99:E10. doi: 10.1542/peds.99.4.e10. [DOI] [PubMed] [Google Scholar]
- 7.Wiswell TE, Baumgart S, Gannon CM, Spitzer AR. No lumbar puncture in the evaluation for early neonatal sepsis: will meningitis be missed? Pediatrics. 1995;95:803–806. [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen N, Fanaroff AA, et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics. 2004;113:1181–1186. doi: 10.1542/peds.113.5.1181. [DOI] [PubMed] [Google Scholar]
- 9.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88 (Suppl 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downey LC, Benjamin DK, Jr, Clark RH, et al. Urinary tract infection concordance with positive blood and cerebrospinal fluid cultures in the neonatal intensive care unit. J Perinatol. 2013;33:302–306. doi: 10.1038/jp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garges HP, Moody MA, Cotten CM, et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117:1094–1100. doi: 10.1542/peds.2005-1132. [DOI] [PubMed] [Google Scholar]
- 12.Jean-Baptiste N, Benjamin DK, Jr, Cohen-Wolkowiez M, et al. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2011;32:679–686. doi: 10.1086/660361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 14.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 16.Sacar S, Turgut H, Toprak S, et al. A retrospective study of central nervous system shunt infections diagnosed in a university hospital during a 4-year period. BMC Infect Dis. 2006;6:43. doi: 10.1186/1471-2334-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odio C, McCracken GH, Jr, Nelson JD. CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child. 1984;138:1103–1108. doi: 10.1001/archpedi.1984.02140500009004. [DOI] [PubMed] [Google Scholar]
- 18.Simon TD, Hall M, Riva-Cambrin J, et al. Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr. 2009;4:156–165. doi: 10.3171/2009.3.PEDS08215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuan TJ, Thorell EA, Hamblett NM, Kestle JR, Rosenfeld M, Simon TD. Treatment and microbiology of repeated cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J. 2011;30:731–735. doi: 10.1097/INF.0b013e318218ac0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP. Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg. 1984;60:1014–1021. doi: 10.3171/jns.1984.60.5.1014. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JR, Diacovo T, Baker CJ. Serratia marcescens meningitis in neonates. Pediatric Infectious Disease Journal. 1992;11:881–6. doi: 10.1097/00006454-199210000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Ajayi OA, Mokuolu OA. Evaluation of neonates with risk for infection/suspected sepsis: is routine lumbar puncture necessary in the first 72 hours of life? Trop Med Int Health. 1997;2:284–288. doi: 10.1046/j.1365-3156.1997.d01-270.x. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg RG, Benjamin DK, Jr, Cohen-Wolkowiez M, et al. Repeat lumbar punctures in infants with meningitis in the neonatal intensive care unit. J Perinatol. 2011;31:425–429. doi: 10.1038/jp.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]