Abstract
The objective of this study was to elucidate the role of the proteasome pathway or multicatalytic proteinase complex in the induction of immunologic nitric oxide (NO) synthase (iNOS) in rat alveolar macrophages activated by lipopolysaccharide. Macrophages were incubated in the presence of lipopolysaccharide plus test agent for up to 24 hr. Culture media were analyzed for accumulation of stable oxidation products of NO (NO2- + N03-, designated as NOX-), cellular RNA was extracted for determination of iNOS mRNA levels by Northern blot analysis, and nuclear extracts were prepared for determination of NF-kappa B by electrophoretic mobility-shift assay. Inhibitors of calpain (alpha-N-acetyl-Leu-Leu-norleucinal; N-benzyloxycarbonyl-Leu-leucinal) and the proteasome (N-benzyloxycarbonyl-Ile-Glu-(O-t-Bu)-Ala-leucinal) markedly inhibited or abolished the induction of iNOS in macrophages. The proteinase inhibitors interfered with lipopolysaccharide-induced NOX- production by macrophages, and this effect was accompanied by comparable interference with the appearance of both iNOS mRNA and NF-kappa B. Calpain inhibitors elicited effects at concentrations of 1-100 microM, whereas the proteasome inhibitor was 1000-fold more potent, producing significant inhibitory effects at 1 nM. The present findings indicate that the proteasome pathway is essential for lipopolysaccharide-induced expression of the iNOS gene in rat alveolar macrophages. Furthermore, the data support the view that the proteasome pathway is directly involved in promoting the activation of NF-kappa B and that the induction of iNOS by lipopolysaccharide involves the transcriptional action of NF-kappaB.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard E. E., Henderson S. A., Arabolos N. S., Griscavage J. M., Castro F. E., Barrett C. T., Ignarro L. J. Nonsteroidal anti-inflammatory drugs inhibit expression of the inducible nitric oxide synthase gene. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1053–1059. doi: 10.1006/bbrc.1995.1441. [DOI] [PubMed] [Google Scholar]
- Beg A. A., Baldwin A. S., Jr The I kappa B proteins: multifunctional regulators of Rel/NF-kappa B transcription factors. Genes Dev. 1993 Nov;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995 Mar 10;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Bush P. A., Gonzalez N. E., Griscavage J. M., Ignarro L. J. Nitric oxide synthase from cerebellum catalyzes the formation of equimolar quantities of nitric oxide and citrulline from L-arginine. Biochem Biophys Res Commun. 1992 Jun 30;185(3):960–966. doi: 10.1016/0006-291x(92)91720-b. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira M. E., Berg K. A., Wilk S. A new inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces accumulation of ubiquitin-protein conjugates in a neuronal cell. J Neurochem. 1994 Oct;63(4):1578–1581. doi: 10.1046/j.1471-4159.1994.63041578.x. [DOI] [PubMed] [Google Scholar]
- Fukuto J. M., Wood K. S., Byrns R. E., Ignarro L. J. NG-amino-L-arginine: a new potent antagonist of L-arginine-mediated endothelium-dependent relaxation. Biochem Biophys Res Commun. 1990 Apr 30;168(2):458–465. doi: 10.1016/0006-291x(90)92343-x. [DOI] [PubMed] [Google Scholar]
- Grilli M., Chiu J. J., Lenardo M. J. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- Griscavage J. M., Rogers N. E., Sherman M. P., Ignarro L. J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993 Dec 1;151(11):6329–6337. [PubMed] [Google Scholar]
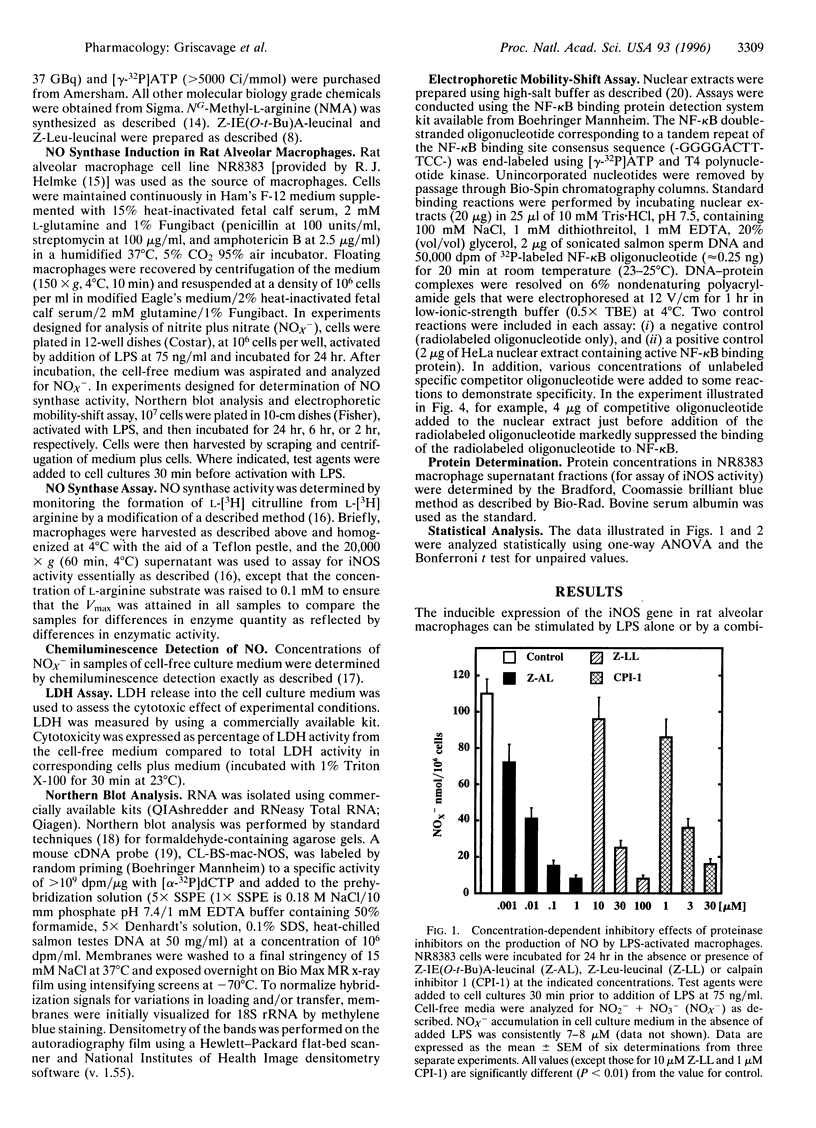
- Griscavage J. M., Wilk S., Ignarro L. J. Serine and cysteine proteinase inhibitors prevent nitric oxide production by activated macrophages by interfering with transcription of the inducible NO synthase gene. Biochem Biophys Res Commun. 1995 Oct 13;215(2):721–729. doi: 10.1006/bbrc.1995.2523. [DOI] [PubMed] [Google Scholar]
- Guesdon F., Ikebe T., Stylianou E., Warwick-Davies J., Haskill S., Saklatvala J. Interleukin 1-induced phosphorylation of MAD3, the major inhibitor of nuclear factor kappa B of HeLa cells. Interference in signalling by the proteinase inhibitors 3,4-dichloroisocoumarin and tosylphenylalanyl chloromethylketone. Biochem J. 1995 Apr 1;307(Pt 1):287–295. doi: 10.1042/bj3070287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmke R. J., German V. F., Mangos J. A. A continuous alveolar macrophage cell line: comparisons with freshly derived alveolar macrophages. In Vitro Cell Dev Biol. 1989 Jan;25(1):44–48. doi: 10.1007/BF02624409. [DOI] [PubMed] [Google Scholar]
- Henkel T., Machleidt T., Alkalay I., Krönke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993 Sep 9;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee H. S., Chang K. T., Ko T. H., Baek K. J., Kwon N. S. Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-kappa B. J Immunol. 1995 May 1;154(9):4741–4748. [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Brown K., Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Schray-Utz B., Mordvintcev P. I., Hauschildt S., Busse R. Diethyldithiocarbamate inhibits induction of macrophage NO synthase. FEBS Lett. 1993 Apr 26;321(2-3):215–218. doi: 10.1016/0014-5793(93)80111-7. [DOI] [PubMed] [Google Scholar]
- Nussler A. K., Billiar T. R. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leukoc Biol. 1993 Aug;54(2):171–178. [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Palombella V. J., Rando O. J., Goldberg A. L., Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994 Sep 9;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Peters J. M. Proteasomes: protein degradation machines of the cell. Trends Biochem Sci. 1994 Sep;19(9):377–382. doi: 10.1016/0968-0004(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Read M. A., Neish A. S., Luscinskas F. W., Palombella V. J., Maniatis T., Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995 May;2(5):493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A. L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994 Sep 9;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Sorimachi H., Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994 Aug;8(11):814–822. [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. P., Aeberhard E. E., Wong V. Z., Griscavage J. M., Ignarro L. J. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1301–1308. doi: 10.1006/bbrc.1993.1359. [DOI] [PubMed] [Google Scholar]
- Stefanovic-Racic M., Meyers K., Meschter C., Coffey J. W., Hoffman R. A., Evans C. H. N-monomethyl arginine, an inhibitor of nitric oxide synthase, suppresses the development of adjuvant arthritis in rats. Arthritis Rheum. 1994 Jul;37(7):1062–1069. doi: 10.1002/art.1780370712. [DOI] [PubMed] [Google Scholar]
- Thanos D., Maniatis T. NF-kappa B: a lesson in family values. Cell. 1995 Feb 24;80(4):529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- Traenckner E. B., Pahl H. L., Henkel T., Schmidt K. N., Wilk S., Baeuerle P. A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995 Jun 15;14(12):2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980 Nov;35(5):1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994 Feb 18;269(7):4705–4708. [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994 Nov;56(5):576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]