Summary
Following initial platelet activation, arachidonic acid is metabolised by cyclooxygenase-1 and 12-lipoxygenase (12-LOX). While the role of 12-LOX in the platelet is not well defined, recent evidence suggests that it may be important for regulation of platelet activity and is agonist-specific in the manner in which it regulates platelet function. Using small molecule inhibitors selective for 12-LOX and 12-LOX-deficient mice, the role of 12-LOX in regulation of human platelet activation and thrombosis was investigated. Pharmacologically inhibiting 12-LOX resulted in attenuation of platelet aggregation, selective in hibition of dense versus alpha granule secretion, and inhibition of platelet adhesion under flow for PAR4 and collagen. Additionally, 12-LOX-deficient mice showed attenuated integrin activity to PAR4-AP and convulxin compared to wild-type mice. Finally, platelet activation by PARs was shown to be differentially dependent on COX-1 and 12-LOX with PAR1 relying on COX-1 oxidation of arachi donic acid while PAR4 being more dependent on 12-LOX for normal platelet function. These studies demonstrate an important role for 12-LOX in regulating platelet activation and thrombosis. Furthermore, the data presented here provide a basis for potentially targeting 12-LOX as a means to attenuate unwanted platelet activation and clot formation.
Keywords: Platelet activation, 12-lipoxygenase, eicosanoids, aggregation, thrombosis
Introduction
Platelet activation plays a crucial role in haemostasis and thrombosis (1, 2) and a central role in the pathophysiology of cardiovascular disorders such as acute coronary syndrome (3–5). Activation of human platelets is initiated through primary signalling by thrombin or collagen and reinforced by secondary signalling events initiated by arachidonic acid (AA) release from the phospholipids, formation of bioactive metabolites, and granule secretion. The clinical importance of this positive feedback to platelet activation is evident by the widespread use of aspirin, which specifically blocks AA metabolism to thromboxane A2(6). An equally important metabolic pathway which utilises AA as its substrate is platelet-type 12-Lipoxygenase (12-LOX), resulting in the formation of 12-(S)-hydroperoxyeicosatetraenoic acid (12-HpETE) (7, 8). 12-LOX is primarily expressed in the platelet and megakaryocyte and its metabolic products have been shown to play a role in regulating vascular and non-vascular processes such as integrin activation, vascular hypertension, and progression of certain types of cancer (9–15). While we have recently shown an important role for 12-LOX in platelet activation (16), the extent to which 12-LOX activation directly regulates platelet activity and its agonist-specific role in regulating thrombosis is unclear. We there fore sought to determine if pharmacological inhibition or genetic ablation of 12-LOX activity plays a role in PAR and collagen-mediated platelet activation and clot formation.
12-LOX utilizes AA as a substrate to form 12-(S)-hydroperoxy-eicosatetraenoic acid (12-HpETE) (7) which is further metabolised by glutathione peroxidase to form 12-HETE (17). Once formed, the majority of 12-HETE is released to the extracellular space and is postulated to signal through a number of potential mechanisms including GPCRs (18, 19), cytosolic signalling complexes, or alter natively incorporation into the plasma membrane (20–22). The biological function of 12-LOX activation in platelets has been complicated by the observation that its metabolites are reported to mediate both an increase as well as decrease in platelet reactivity (11, 16, 23–26). In support of 12-LOX as a mediator of platelet activation, 12-LOX activity has been linked α-granule secretion (12) and surface integrin expression and activation in both the platelet and tumour cells (16, 27–29). Additionally, although 12-LOX has been postulated to play a role in dense granule secretion in platelets (26), specific inhibitors which would enable direct investi gation of 12-LOX regulation of platelet reactivity, adhesion, and clot formation have been lacking (2, 30, 31). The recent development, however, of selective platelet 12-LOX inhibitors has allowed for a more detailed investigation of how 12-LOX selectively regu lates platelet reactivity, clot formation, and dense granule secretion in the human platelet (16, 31, 32). While these inhibitors selectively block 12-LOX activity in human platelets while not affecting activity of other enzymes in this pathway (5-LOX, 15-LOX, COX-1, thromboxane synthase, cPLA2, or PKC) (16, 33), their effectiveness in inhibiting the 12-LOX homolog expressed in murine platelets (12/15-LOX) is unclear limiting the use of these selective inhibitors in the murine system in vivo.
Here we show that inhibition of 12-LOX activity inhibits platelet aggregation, which is coupled to a significant shift in the ability of the platelet to induce normal integrin activation, dense granule secretion, and platelet adhesion under venous shear. Additionally, αIIbβ3 activation in the 12-LOX−/− mouse is attenuated pro viding strong evidence for the involvement of 12-LOX in formation and long-term stability of the platelet clot. Finally, we show that while COX-1, but not 12-LOX activation, is essential for normal platelet activation through PAR1 (34), 12-LOX activation plays an important role in normal platelet function through PAR4 (16). Thus, these studies provide the first reported evidence supporting an important physiological role for 12-LOX in adhesion of platelets under shear which is coupled to platelet aggregation, integrin activation, and secretion and support further investigation of 12-LOX as a potential target for regulation of platelet activation and uncontrolled thrombus formation.
Materials and methods
Materials
12-LOX compounds (NCTT-956 and -694) were synthesised at the NIH Chemical Genomics Center (Rockville, MD, USA). For a list of all materials, see Suppl. Methods (available online at www.thrombosis-online.com).
Human platelets
Human platelets were obtained from healthy volunteers from within the Thomas Jefferson University community and the Phil adelphia area. These studies were approved by the Thomas Jefferson University Institutional Review Board and informed consent was obtained from all donors before blood draw. Experiments were performed on both whole blood and washed platelets as previously described (35, 36). Unless otherwise noted, all experiments were conducted with a platelet concentration of 3 × 108 platelets/ml.
Mice
12-LOX−/− mice and strain-matched controls (B6129SF2/J) were obtained from Jackson Labs. Experimental procedures were approved by the Animal Care and Use Committee of Thomas Jefferson University.
Platelet Aggregation and dense granule secretion
Washed platelets were pretreated with inhibitors for 10 minutes (min). The aggregation response to agonist was measured using alumi-aggregometer (Model 700D, Chronolog Corp) with stirringat 1,100 rounds per minute (rpm) at 37°C. ATP luminescence was used to detect dense granule secretion. Aggregation in whole-blood was measured with a lumi-aggregometer following manufacturers recommendations.
Platelet adhesion under flow
In vitro platelet adhesion under venous and arterial shear rates was performed in a microfluidic apparatus (37). Whole blood was in cubated with NCTT-956 or NCTT-694 for 15 min followed by in cubation with calcein green fluorophore and infused at venous (400-S) or arterial (2,000-S) wall shear rates for 5 min. Adhesion of platelets was monitored continuously with a Nikon Ti-U inverted microscope equipped with a Retiga EXL monochrome camera. Images were analysed using Nikon NIS Elements software.
Flow cytometry
P-selectin expression and integrin αIIbβ3 activation on the surface of the platelet were measured by flow cytometry (Accuri C6) as a marker α-granule secretion and integrin activation, respectively (16, 38).
Tail-bleeding assay
Tail bleeding of ALOX12−/− and wild-type mice was performed on an anesthetised mouse as previously described (39). Time in seconds required for cessation of blood flow was recorded.
Statistical analysis
Comparison between experimental groups was made using appro priate statistical analyses (paired t-test program or ANOVA with post-test analysis) using Prism software. Differences in mean values (measured as standard error of the mean) were considered significant at p < 0.05.
Results
12-LOX inhibition attenuates platelet aggregation
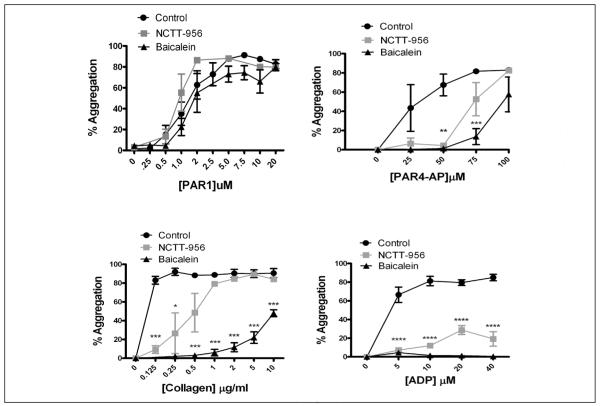
Platelet aggregation is essential for agonist-mediated clot formation. To confirm the specificity of the 12-LOX inhibitor, levels of TxB2 and 12-HETE were measured in the presence of NCTT-956 following platelet stimulation with 200 μM PAR4-AP (Suppl. Figure 1, available online at www.thrombosis-online.com). The role of 12-LOX in agonist-induced platelet aggregation was examined by measuring the dose-response for PAR1-AP, PAR4-AP, collagen, and ADP-mediated aggregation in the absence or presence of NCTT-956 or baicalein (12-LOX inhibitors [16, 31, 32]) (▶ Figure 1). In the absence of 12-LOX inhibitors, all agonists were able to induce full and stable aggregation. Interestingly, in contrast to PAR1 sensitivity to COX-1 for normal aggregation (34), PAR1-mediated platelet aggregation was insensitive to 12-LOX inhibition. A significant shift in the dose-response for aggregation was observed with all other agonists tested. ADP, which normally induces full aggregation at 5 μM, was not able to overcome the inhibition of 12-LOX and full aggregation was not reached even at an ADP concentration of 40 μM. These data support an important role for 12-LOX in regulating agonist-induced platelet aggregation through a number of receptor pathways.
Figure 1. 12-LOX is an important determinant of platelet aggregation.
Washed platelets were treated with or without 50 μM baicalein or 25 μM NCTT-956, and platelet aggregation was measured following stimulation with increasing concentrations of PAR1-AP, PAR4-AP, collagen, or ADP. All agonists tested other than PAR1-AP showed a shift to the right in the EC50 for platelet aggregation (N=3). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
12-LOX regulation of dense granule secretion
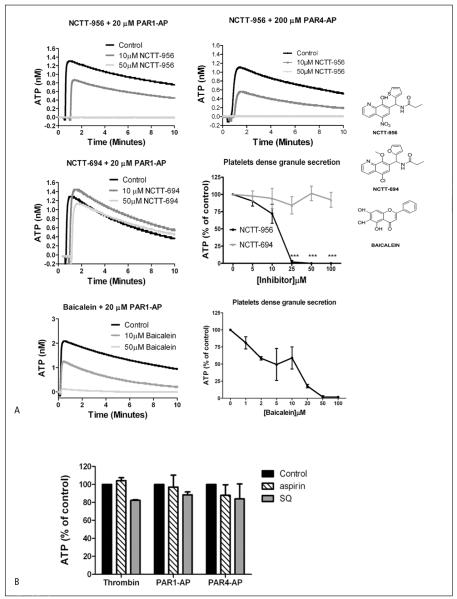
As granule secretion plays an important role in the regulation of platelet clot growth and stability, secretion of dense granules was measured in platelets treated with or without 12-LOX inhibitors (▶ Figure 2). Treatment with the active 12-LOX inhibitor (NCTT-956) resulted in complete inhibition of PAR1-AP and PAR4-AP-induced ATP secretion, while treatment with the inactive analog (NCTT-694) had no effect on dense granule secretion at concentrations as high as 100 μM NCTT-694 (▶ Figure 2A). The effects of NCTT-956 were also compared to the 12-LOX inhibitor baicalein. Similar to NCTT-956, baicalein induced a dose-dependent inhibition of PAR1-AP-mediated dense granule secretion. To ascertain that the analogs did not interfere directly with the luciferase-based assay of ATP release, control reactions were performed where a known concentration of ATP was measured in presence of analogs or vehicle. The results showed that neither NCTT-956 nor NCTT-694 inhibit the luciferase assay (Suppl. Figure 2, available online at www.thrombosis-online.com). This was a surprising result based on the observation in ▶ Figure 1 that inhibition of 12-LOX attenuated PAR4, but not PAR1-mediated platelet aggregation. However, this is in line with the observation that PAR4 is more sensitive to positive feedback through secretion and ADP compared to PAR1 (36).
Figure 2. 12-LOX activation is required for dense granule secretion in platelets.
Washed platelets were treated with active or inactive 12-LOX inhibitors and the level of dense granule secretion was monitored by measuring ATP release following stimulation with different agonists. A) ATP release induced by 20 μM PAR1-AP (N=5) or 200 μM PAR4-AP (N=4) with increasing concentration of NCTT-956, or NCTT-694 (N=3). Composite analysis of dose-response for dense granule secretion with PAR1-AP in the presence of in creasing concentrations of NCTT-956 (N=5) and NCTT-694 (N=3). ***P<0.001. Representative and composite for dense granule secretion following PAR1-AP in the presence of increasing concentrations of baicalein (N=3). Structure of NCTT-956, NCTT-694, and baicalein are shown for comparison. B) Composite analysis of ATP secretion in the presence of the 100 μM aspirin or 100 nM SQ29548 followed by stimulation with 10 nM thrombin, 20 μM PAR1-AP, or 200 μM PAR4-AP for 10 min. C) Dense granule secretion was measured in absence or presence of 12-LOX metabolites to determine their role in the regulation of agonist-mediated secretion. Washed platelets were treated with either 250 nM 12(S)-HETE (N=3) or 12(S)-HpETE (N=3). Dense granule secretion was measured following activation with PAR1-AP. ***P<0.001; *P<0.05. Metabolite alone did not induce dense granule secretion in washed platelets (N=3).
To determine if 12-LOX regulation of dense granule secretion was agonist specific, the same experiments were repeated with a number of agonists known to induce dense granule secretion (Suppl. Figure 3, available online at www.thrombosis-online.com). Inhibition of 12-LOX completely eliminated dense granule secretion induced by thrombin, PAR4-AP, collagen, U46619 (an agonist for the TPα receptor), and ADP, giving strong evidence that 12-LOX activation is important to platelet dense granule secretion. To determine if inhibition of dense granule secretion was due to an inhibition of 12-LOX signalling or a potential indirect effect on thromboxane signalling, platelets were treated without or with 100 μM aspirin (COX-1 inhibitor) or 100 nM SQ29548 (TPα inhibitor) followed by stimulation with 10 nM thrombin, 20 μM PAR1-AP, or 200 μM PAR4-AP. Neither inhibition of COX-1 nor TPα resulted in a significant change in agonist-mediated dense granule secretion (▶ Figure 2B).
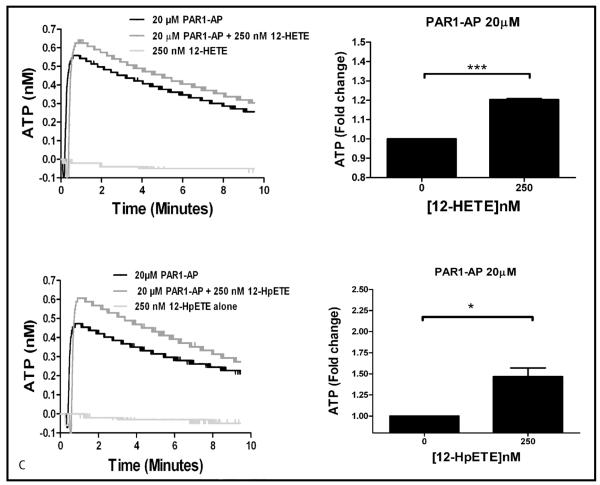
Role of 12-HETE in dense granule release
To determine if 12-HETE, or its precursor, 12-HpETE, plays a role in regulation of agonist-induced secretion, dense granule secretion was measured following PAR1-AP stimulation either in the presence or absence of exogenous metabolite (250 nM 12-HETE or 12-HpETE). Exogenous 12-HETE and 12-HpETE significantly increased PAR1-AP-mediated dense granule secretion by 20% compared to agonist alone (▶ Figure 2C) while metabolite alone did not induce dense granule secretion. These data are in agreement with other published work implicating 12-HETE as a potential modulator of platelet function (26) and our current observation that absence of 12-LOX activity and 12-HETE results in inhibition of agonist-mediated dense granule secretion. Exogenously added 12-HpETE resulted in an even larger increase in PAR1-AP-induced dense granule secretion of 60% compared to control suggesting a potential role for other 12-LOX metabolites in modulating agonist-mediated platelet activation.
12-Lipoxygenase does not regulate α-granule secretion
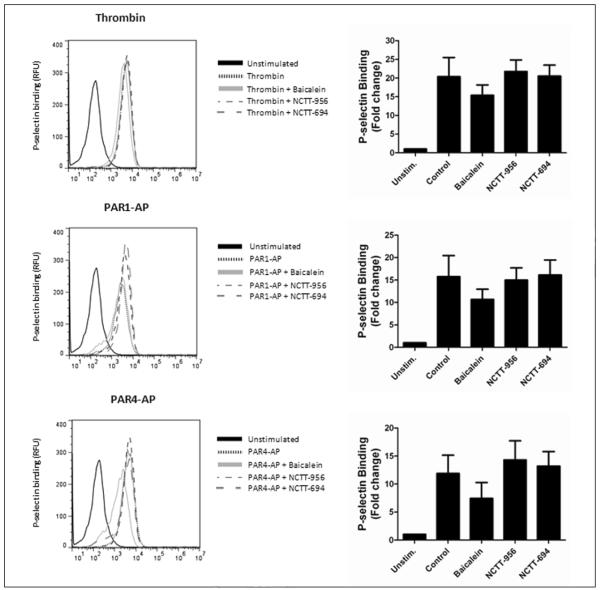
Secretion of α-granules from human platelets is induced by both PAR1 and PAR4 (35, 40). To assess if 12-LOX specifically regulates dense granule secretion or both dense and α-granule secretion, platelets were treated with or without NCTT-956, NCTT-694, or baicalein for 10 min followed by stimulation with thrombin, PAR1-AP, or PAR4-AP. α-granule secretion was determined by measuring P-selectin levels on the platelet surface by flow cytometry (▶ Figure 3). α-granule secretion was not significantly attenuated following 12-LOX inhibition in the presence of all three agonists. These data suggest that α-granule secretion is not regulated via the 12-LOX pathway.
Figure 3. 12-LOX is not critical for agonist-mediated α-granule secretion.
α-granule secretion was determined following stimulation of platelets with 10 nM thrombin, 20 μM PAR1-AP, or 200 μM PAR4-AP in the presence or absence of active and inactive 12-LOX inhibitors (N=4). P-selectin expression following treatment with 25 μM NCTT-956, 50 μM baicalein, or 25 μM NCTT-694 was not significantly different from control.
Regulation of platelet function in vivo by 12-LOX
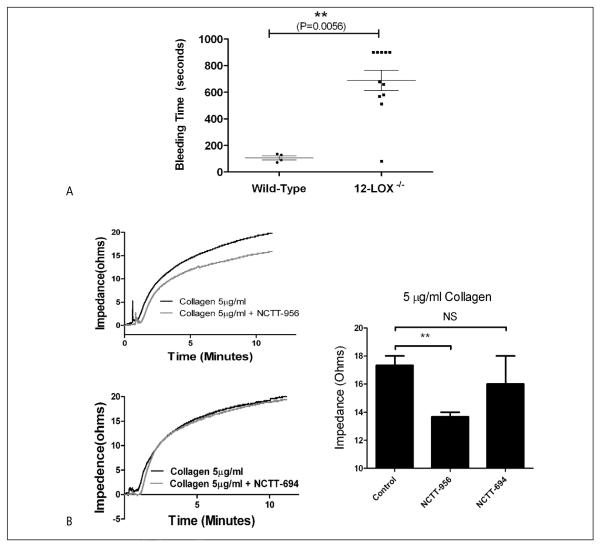
To determine if 12-LOX plays a role in platelet function in vivo, bleeding time was assessed in 12-LOX−/− and strain-matched wild-type mice using the tail-bleeding assay (39) (▶ Figure 4A). While wild-type mice showed a normal cessation time for bleeding following clipping of the tail vein (N=10), the time to cessation of bleeding in 12-LOX−/− mice was significantly prolonged (N=11).
Figure 4. Inhibition of 12-LOX in whole blood attenuates platelet aggregation and adhesion.
In vivo haemostasis, as well as platelet aggregation and clot formation ex vivo in whole blood were measured to deter mine the role of 12-LOX in regulating platelet activation in the presence of confounding factors present in the blood. A) Tail-bleeding time of 12-LOX−/− (N=11) and strain-matched wild-type mice (N=10). Mean bleeding time is denoted by a horizontal line (****P<0.0001). B) Whole blood aggregometry was performed in the absence or presence of the 12-LOX inhibitor, NCTT-956 or the negative analog, NCTT-694. Blood was treated with or without 100 μM NCTT-956 or -694, stimulated with 5 μg/ml collagen, and impedance aggregometry was measured for 10 min post-stimulation (N=3). A significant decrease in impedance was observed in the presence of NCTT-956 but not NCTT-694 (**P<0.01; NS, not significant).
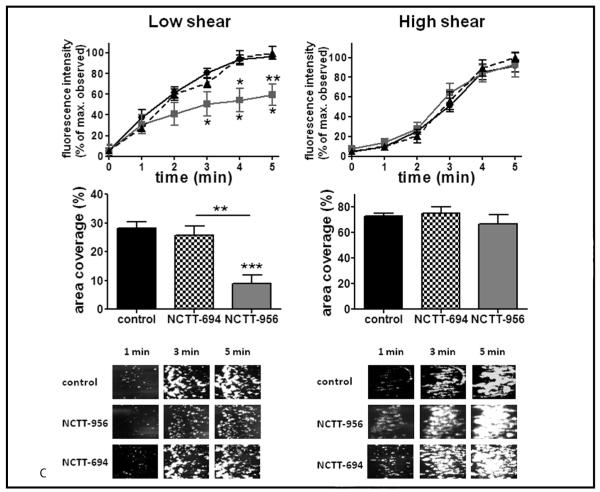
C) Platelet clot formation over a collagen strip under low or high shear conditions (400 s−1 or 2,000 s−1, respectively) was measured either with (□) or without (•) 100 μM NCTT-956 or NCTT-694 (△) (N=4). The rate of clot formation was significantly delayed and at tenuated following inhibition of 12-LOX compared to control.
Regulation of whole blood aggregation and adhesion by 12-LOX
Although aggregation in washed platelets shows sensitivity to 12-LOX activation (▶ Figure 1), its role in whole blood has not been well defined. To determine if platelet aggregation is altered in whole blood, freshly drawn human blood was treated with or without NCTT-956 or NCTT-694 for 10 min followed by stimulation with collagen (the first step in platelet activation following vessel injury). Using impedance aggregometry a significant decrease in platelet aggregation was observed in the presence of the 12-LOX inhibitor compared to untreated platelets or platelets treated with the negative analog (▶ Figure 4B). To determine if 12-LOX plays a role in platelet adhesion and clot formation under flow, whole blood treated with or without NCTT-956 or NCTT-694 was flowed over a collagen strip at low and high shear rates (400 and 2000 sec−1) (▶ Figure 4C). Although untreated blood showed significant clot growth within 1–3 min, inhibition of 12-LOX at low shear resulted in a significant delay in platelet adhesion as well as attenuation of the overall area and intensity of adhesion to collagen. Blood treated with the inactive analog NCTT-694, however, exhibited no delay in adhesion or clot growth. At high shear, 12-LOX activation did not appear to be essential as there was no observable difference in area coverage or clot formation at any time point measured. These data provide strong evidence that platelet reactivity and haemostasis rely in part on the enzymatic activity of platelet 12-LOX and supports a potential role for 12-LOX in the recruitment and activation of platelets into a growing clot.
12-LOX activity is important for haemostasis and thrombosis in the mouse
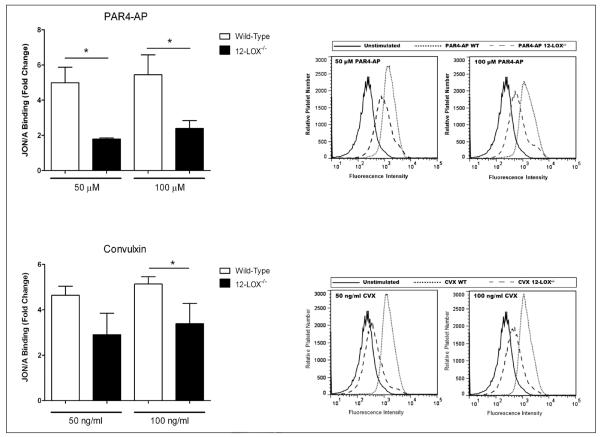
While murine 12-LOX is highly homologous to human, its enzymatic activity is less selective and is known to form at least two products from AA, 12-HETE and 15-HETE. Due to its altered enzymatic activity, the 12-LOX inhibitors directed toward the human enzyme do not bind with high efficacy to mouse 12-LOX. To determine if the observed 12-LOX effects identified in human platelets with the 12-LOX inhibitor are due to inhibition of 12-LOX activity, platelets from 12-LOX−/− and strain-matched wild-type mice were assessed for αIIbβ3 activity. Washed platelets were stimulated with either PAR4-AP or convulxin (▶ Figure 5), and the level of αIIbβ3 was measured. Mice deficient in 12-LOX showed a significant attenuation of αIIbβ3 activation for both agonists compared to wild-type mice. This data confirms an important role for 12-LOX in regulating platelet activation, thrombosis, and haemostasis.
Figure 5. 12-LOX−/− mice have impaired platelet activation and haemostasis.
Platelet function was measured in wild-type and 12-LOX−/− mice. Washed platelets were stimulated with either PAR4-AP (50 μM and 100 μM) or convulxin (CVX) (50 ng/ml or 100 ng/ml) for 10 min, and αIIbβ3 activity was measured by flow cytometry using the JON/A antibody (N=3).
Agonist-dependent regulation of platelet activation through COX-1 and 12-LOX
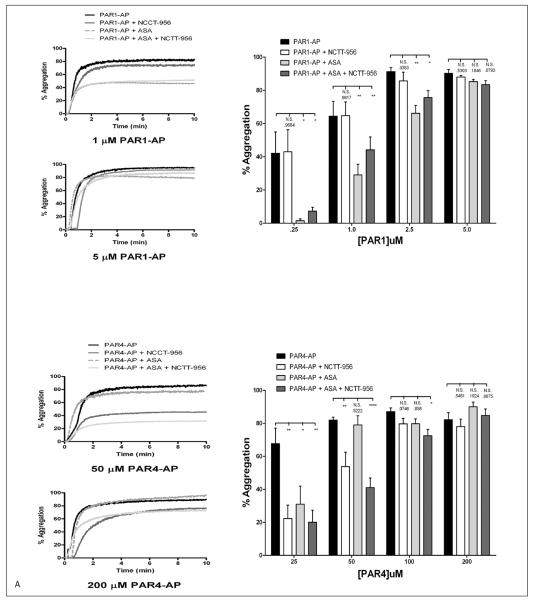
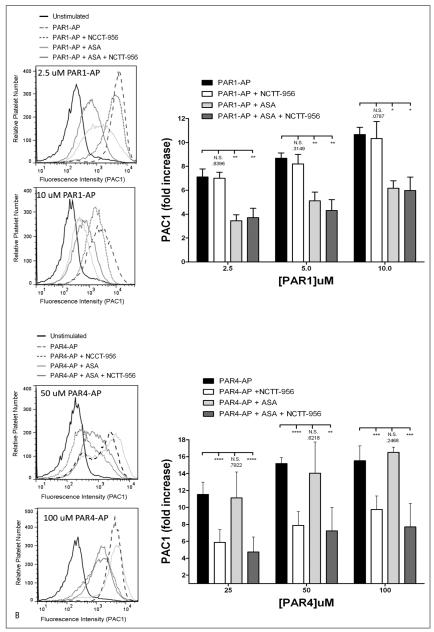
Previously, we have shown that inhibition of thromboxane formation perturbs PAR1-mediated platelet activation to a much larger extent compared to PAR4 suggesting that PAR4 may rely on a 12-LOX signal for normal platelet activation as opposed to COX-1 (34). To assess if this is the case, platelet aggregation and αIIbβ3 activation were measured in response to PAR1-AP or PAR4-AP in the presence of aspirin, NCTT-956, or both (▶ Figure 6). PAR1-AP showed no sensitivity to NCTT-956 even at low agonist concentrations but aggregation was significantly shifted to the right in the presence of aspirin. Treatment with both aspirin and NCTT-956 was not significantly different from aspirin alone. PAR4 showed no significant shift in the presence of aspirin at any concentration tested while treatment with NCTT-956 significantly shift the dose-response for aggregation to the right. Treatment with both aspirin and NCTT-956 was no different than NCTT-956 alone (▶ Figure 6A). To confirm the observed selective inhibition of PAR1 and PAR4 signalling with aspirin and NCTT-956, respectively, integrin αIIbβ3 activation was measured under the same conditions (▶ Figure 6B). Similar to what was observed for aggregation, PAR1-AP-induced αIIbβ3 activation was significantly attenuated in the presence of aspirin, but not NCTT-956, while PAR4-AP-induced αIIbβ3 activation was significantly attenuated in the presence of NCTT-956, but not aspirin. Dual treatment in either case had no additional inhibitory effect on either PAR-AP suggesting these signalling pathways are not synergistic in nature.
Figure 6. COX-1 and 12-LOX regulation of PAR-mediated platelet activation.
Washed platelets were assessed for differences in sensitivity to COX-1 and 12-LOX following stimulation with either PAR1-AP or PAR4-AP. A) Platelet aggregation was measured following 10 minute stimulation with increasing concentrations of either PAR1-AP or PAR4-AP in the absence or presence of 25μM NCTT-956, 100 μM aspirin (ASA), or both (N=4–11). On the left are representative aggregation curves and on the right is the composite data for each agonist.
B) αIIbβ3 activation was measured under the same conditions (N=3–11). On the left are representative histograms and on the right is the composite data for each agonist. N.S., not significant; *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
Discussion
Regulation of platelet activation is crucial in the treatment of a number of pathophysiological conditions including cardiovascular disease and diabetes and positive feedback plays a significant role in stable clot formation in vivo (2). Although anti-platelet therapies targeting positive reinforcement by inhibition of COX-1 (aspirin) or the P2Y12 receptor (clopidogrel) have succeeded in reducing morbidity and mortality due to clot formation and stroke, new approaches are warranted to further decrease the level of platelet reactivity. We have investigated an alternative approach to attenuating platelet activation and clot formation and have shown that targeting 12-LOX activation with selective platelet 12-LOX in hibitors may represent a viable approach to anti-platelet therapy. Inhibition of 12-LOX in human platelets resulted in agonist-specific attenuation of platelet aggregation and agonist-independent inhibition of dense granule secretion (▶Figure 1 and▶ Figure 2). Since dense granule is thought to be a major reinforcement path way leading to secretion of platelet agonists such as ADP, 5-HT, and epinephrine (2, 40), 12-LOX inhibition of dense granule secretion may partially explain the observed deficit in normal platelet aggregation and adhesion as well as an inability to form stable clots under shear conditions. Further, attenuation of platelet adhesion and clot growth indicates a potential benefit inhibition of 12-LOX may present as an approach to anti-platelet therapy. Finally, while PAR1-mediated platelet activation is sensitive to inhibition of COX-1 but not 12-LOX, PAR4-mediated platelet activation is significantly attenuated in the absence of 12-LOX activity but not COX-1. Taken together, these data yield strong evidence that selective 12-LOX inhibitors have the potential to decrease the severity of platelet clot formation and minimise vessel occlusion, which in turn may lead to a reduction in the incidence and severity of cardiovascular events.
Lipoxygenases play a role in a number of pathophysiological processes ranging from atherosclerosis and clot formation to cancer and essential hypertension. Elucidating the role of 12-LOX in regulation of platelet reactivity, however, has posed a significant challenge based in part on the lack of specific inhibitors (30, 41). Baicalein was initially used in human platelets to pharmacologically show that 12-LOX plays a role in calcium mobilisation and aggregation (11). This work was the first evidence that 12-LOX may play an important role in the regulation of platelet reactivity. Although baicalein is now viewed as a relatively non-specific 12-LOX inhibitor (16, 31), the observation that inhibition of 12-LOX activity resulted in attenuation of calcium mobilisation and perturbed resultant platelet activation steps helped to form the basis for the current study. The development of selective inhibitors targeting platelet 12-LOX has allowed us to investigate its potential role in the progression of platelet-mediated clot formation commonly observed in cardiovascular disease (16, 31, 32). While the current study strongly supports a primary role for 12-LOX in regulation of normal platelet function through a number of signalling pathways including collagen and PAR4, the underlying mechanisms mediating the differential regulation of PAR1 versus PAR4 by COX-1 and 12-LOX, respectively, will also need to be delineated in order to fully appreciate the unique roles these receptors play in regulating platelet activation following injury.
Secretion and positive feedback are thought to be important events in clot formation and irreversible platelet activation. Our data suggest that 12-LOX regulates dense granule secretion in an agonist-independent manner but selectively attenuates platelet aggregation by collagen and PAR4-AP, while sparing the PAR1 path way. This observation is in line with previous reports showing dense granule secretion being more important for PAR4-induced aggregation compared to PAR1 (36). Similarly, 12-LOX has recently been shown to regulate PKC activity which plays a role in dense granule secretion (16, 39, 42). The current study also showed that while aspirin does not inhibit dense granule secretion in platelets, it prevents formation of thromboxane A2 which is important for PAR1, but not PAR4-mediated platelet aggregation (34). Hence, PAR1 appears to require COX-1 for normal platelet activation while PAR4 requires 12-LOX (▶Figure 6). The complex interactions between these receptor pathways and the oxygenases in the platelet may represent unique approaches for regulating these disparate pathways and an opportunity for the development of highly targeted anti-platelet therapeutics. The mechanistic differences mediating COX-1 and 12-LOX regulation of platelet activity are currently being investigated.
While inhibition of 12-LOX blocks dense granule secretion and 12-HETE formation, our data support the hypothesis that other 12-LOX metabolites formed following platelet activation may play an important role in regulating secretion since addition of 12-HpETE, the direct product of 12-LOX, exerts an even stronger effect than its reduced form. Several products may be formed following 12-LOX metabolism of AA, including 12-HETE and hepoxilins (43, 44). Furthermore, 12-LOX can oxidise any number of fatty acids present on the platelet membrane following cleavage with cPLA2 (31, 38), suggesting that other metabolites may be contributing to the regulation of platelet activation and thrombosis.
To differentiate between regulation of dense granule secretion and α-granule secretion, P-selectin was measured as a surrogate for α-granule secretion (▶Figure 3). Our results show that 12-LOX was not required for α-granule secretion in the platelet. The potential role for 12-LOX in P-selectin secretion has been postulated by others (12) and agrees with the recently proposed model suggesting that a number of discrete pools α-granules exist in the platelet which are differentially secreted in an agonist-dependent manner (45). This is an active area of investigation which will need to be elucidated in order to understand the extent to which 12-LOX regulates platelet function.
Although 12/15-LOX knockout mice have been generated to study lipoxygenase function in mice (26), this study is the first to identify the role of 12-LOX for regulation of platelet reactivity in regulation of aggregation, secretion, and adhesion in human platelets. The mouse homolog of 12-LOX is similar in structure to that of the human protein; however, significant species differences do exist. For example, 12-LOX in the human solely produces 12-HETE from arachidonic acid, while 12/15-LOX in the mouse is known to produce both 12-HETE and 15-HETE, the latter product recently being shown to be essential for ischaemia-induced angiogenesis (46). With these limitations in mind, it is likely that the murine 12-LOX-deficient mouse shares some functional similarities to pharmacological inhibition of 12-LOX in the human as we show here that platelets isolated from 12-LOX−/− mice show significant attenuation of αIIbβ3 activation following PAR4-AP or convulxin compared to wild-type mice.
Our data support a physiologically important role for 12-LOX in mediating a number of pro-thrombotic processes, which are key in the formation of a stable platelet clot formation. Adequate regulation of platelet activation has been a central focus in clinically treating a number of diseases including acute coronary syndrome and cardiovascular disease as well as the cardiovascular complications often associated with metabolic syndrome and diabetes. Although several inhibitors of the reinforcement pathways are currently in use including aspirin and clopidogrel, a need exists for the development of alternative strategies to control platelet reactivity and minimise the occurrence of thrombotic events in patients diagnosed with a number of cardiovascular risks (47). Hence, 12-LOX inhibition may represent a new approach to anti-platelet therapy as it not only targets platelet aggregation, dense granule secretion, and adhesion to collagen under shear, but shows significant agonist-dependence specifically targeting collagen and PAR4 while sparing PAR1 signalling (16) (▶Figure 6). Taken together, these findings provide impetus for studies to further identify how 12-LOX regulates platelet activity at a mechanistic level through PAR4 and collagen, and further probe the potential for 12-LOX in hibitors as a next generation of anti-platelet therapeutic agents either alone or in combination with existing anti-platelet approaches (48–51).
Supplementary Material
What is known about this topic?
12-lipoxygenase is highly expressed in platelets and functions primarily through production of eicosanoids to form bioactive metabolites which may function in a paracrine or autocrine manner.
12-LOX eicosanoids are known to play an important role in regulation of tumor growth, diabetes, and vascular tone.
12-LOX activity may play an important role in pro- or anti-throm botic regulation of platelet function, but the agonists affected by 12-LOX and the mechanism of action remain unclear.
What does this paper add?
We show the relative importance of 12-LOX activity in regulating a number of PAR4- and GPVI-mediated biochemical endpoints crucial to normal platelet activation and clot formation.
12-LOX regulation of thrombin signalling in the platelet is limited to the protease-activated receptor 4 (PAR4), while PAR1 appears to be primarily regulated by COX-1.
The observed differential regulation of agonist-mediated platelet activity through 12-LOX and COX-1 provides a basis for potentially targeting 12-LOX as a means to attenuate unwanted platelet clot formation.
Acknowledgments
We would like to thank Lucia Stefanini and Wolfgang Bergmeier for help with establishing the flow chamber assay, and Audra Judd for analysis of prostanoids.
Financial support: This work was supported in part by the National Institutes of Health grants HL089457 (MH), HL081009 (OB), MH081283 (TRH), the American Heart Association (MH), the Parenteral Drug Association Foundation (MH), and the Cardeza Foundation for Hematologic Research (MH).
Footnotes
Conflicts of interest None declared.
References
- 1.Verstraete M, Zoldhelyi P. Novel antithrombotic drugs in development. Drugs. 1995;49:856–884. doi: 10.2165/00003495-199549060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Elwood PC, Renaud S, Sharp DS, et al. Ischemic heart disease and platelet aggregation. The Caerphilly Collaborative Heart Disease Study. Circulation. 1991;83:38–44. doi: 10.1161/01.cir.83.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Lincoff AM. Important triad in cardiovascular medicine: diabetes, coronary in tervention, and platelet glycoprotein IIb/IIIa receptor blockade. Circulation. 2003;107:1556–1559. doi: 10.1161/01.cir.0000055653.52489.e9. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Marcus AJ. Aspirin as prophylaxis against colorectal cancer. N Engl J Med. 1995;333:656–658. doi: 10.1056/NEJM199509073331011. [DOI] [PubMed] [Google Scholar]
- 7.Hamberg M, Samuelsson B. Prostaglandin endoperoxides. Novel trans formations of arachidonic acid in human platelets. Proc Natl Acad Sci USA. 1974;71:3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus AJ. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978;19:793–826. [PubMed] [Google Scholar]
- 9.Funk CD, Furci L, FitzGerald GA. Molecular cloning, primary structure, and expression of the human platelet/erythroleukemia cell 12-lipoxygenase. Proc Natl Acad Sci USA. 1990;87:5638–5642. doi: 10.1073/pnas.87.15.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonlupt P, Croset M, Lagarde M. 12-HETE inhibits the binding of PGH2/TXA2 receptor ligands in human platelets. Thromb Res. 1991;63:239–248. doi: 10.1016/0049-3848(91)90287-7. [DOI] [PubMed] [Google Scholar]
- 11.Nyby MD, Sasaki M, Ideguchi Y, et al. Platelet lipoxygenase inhibitors attenuate thrombin- and thromboxane mimetic-induced intracellular calcium mobilization and platelet aggregation. J Pharmacol Exp Ther. 1996;278:503–509. [PubMed] [Google Scholar]
- 12.Ozeki Y, Ito H, Nagamura Y, et al. 12(S)-HETE plays a role as a mediator of expression of platelet CD62 (P-selectin) Platelets. 1998;9:297–302. doi: 10.1080/09537109876537. [DOI] [PubMed] [Google Scholar]
- 13.Pidgeon GP, Lysaght J, Krishnamoorthy S, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26:503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 14.Quintana LF, Guzman B, Collado S, et al. A coding polymorphism in the 12-lipoxygenase gene is associated to essential hypertension and urinary 12(S)-HETE. Kidney Int. 2006;69:526–530. doi: 10.1038/sj.ki.5000147. [DOI] [PubMed] [Google Scholar]
- 15.Zink MH, Oltman CL, Lu T, et al. 12-lipoxygenase in porcine coronary micro-circulation: implications for coronary vasoregulation. Am J Physiol Heart Circ Physiol. 2001;280:H693–704. doi: 10.1152/ajpheart.2001.280.2.H693. [DOI] [PubMed] [Google Scholar]
- 16.Yeung J, Apopa PL, Vesci J, et al. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81:420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel MI, McConnell RT, Porter NA, et al. Arachidonate metabolism via lipoxygenase and 12L-hydroperoxy-5,8,10,14-icosatetraenoic acid peroxidase sensitive to anti-inflammatory drugs. Proc Natl Acad Sci USA. 1980;77:308–312. doi: 10.1073/pnas.77.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruzicka T. The role of the epidermal 12-hydroxyeicosatetraenoic acid receptor in the skin. Eicosanoids. 1992;5(Suppl):S63–65. [PubMed] [Google Scholar]
- 19.Guo Y, Zhang W, Giroux C, et al. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbertsson H, Kuhme T, Evertsson U, et al. Identification of subunits of the 650 kDa 12(S)-HETE binding complex in carcinoma cells. J Lipid Res. 1998;39:237–244. [PubMed] [Google Scholar]
- 21.Liu B, Khan WA, Hannun YA, et al. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci USA. 1995;92:9323–9327. doi: 10.1073/pnas.92.20.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan AH, Dioszeghy V, Maskrey BH, et al. Phosphatidylethanolamine-esterified eicosanoids in the mouse: tissue localization and inflammation-dependent formation in Th-2 disease. J Biol Chem. 2009;284:21185–21191. doi: 10.1074/jbc.M109.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiba S, Murata T, Kitatani K, et al. Involvement of lipoxygenase pathway in do cosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull. 2000;23:1293–1297. doi: 10.1248/bpb.23.1293. [DOI] [PubMed] [Google Scholar]
- 24.Brune B, Ullrich V. 12-hydroperoxyeicosatetraenoic acid inhibits main platelet functions by activation of soluble guanylate cyclase. Mol Pharmacol. 1991;39:671–678. [PubMed] [Google Scholar]
- 25.Eynard AR, Galli G, Tremoli E, et al. Aspirin inhibits platelet 12-hydroxy-eicosatetraenoic acid formation. J Lab Clin Med. 1986;107:73–78. [PubMed] [Google Scholar]
- 26.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci USA. 1998;95:3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pidgeon GP, Tang K, Cai YL, et al. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res. 2003;63:4258–4267. [PubMed] [Google Scholar]
- 28.Raso E, Tovari J, Toth K, et al. Ectopic alphaIIbbeta3 integrin signaling involves 12-lipoxygenase- and PKC-mediated serine phosphorylation events in melanoma cells. Thromb Haemost. 2001;85:1037–1042. [PubMed] [Google Scholar]
- 29.Kaur G, Jalagadugula G, Mao G. RUNX1/core binding factor A2 regulates platelet 12-lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115:3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deschamps JD, Gautschi JT, Whitman S, et al. Discovery of platelet-type 12-human lipoxygenase selective inhibitors by high-throughput screening of structurally diverse libraries. Bioorg Med Chem. 2007;15:6900–6908. doi: 10.1016/j.bmc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeung J, Holinstat M. 12-lipoxygenase: a potential target for novel anti-platelet therapeutics. Cardiovasc Hematol Agents Med Chem. 2011;9:154–164. doi: 10.2174/187152511797037619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenyon V, Rai G, Jadhav A, et al. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. 2011;54:5485–5497. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenyon V, Chorny I, Carvajal WJ, et al. Novel human lipoxygenase inhibitors discovered using virtual screening with homology models. J Med Chem. 2006;49:1356–1363. doi: 10.1021/jm050639j. [DOI] [PubMed] [Google Scholar]
- 34.Holinstat M, Boutaud O, Apopa PL, et al. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol. 2011;31:435–442. doi: 10.1161/ATVBAHA.110.219527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holinstat M, Voss B, Bilodeau ML, et al. Protease-activated receptors differentially regulate human platelet activation through a phosphatidic acid-dependent pathway. Mol Pharmacol. 2007;71:686–694. doi: 10.1124/mol.106.029371. [DOI] [PubMed] [Google Scholar]
- 36.Holinstat M, Voss B, Bilodeau ML, et al. PAR4, but not PAR1, signals human platelet aggregation via Ca2+ mobilization and synergistic P2Y12 receptor activation. J Biol Chem. 2006;281:26665–26674. doi: 10.1074/jbc.M602174200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neeves KB, Maloney SF, Fong KP, et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J Thromb Haemost. 2008;6:2193–2201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 38.Ikei KN, Yeung J, Apopa PL, et al. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res. 2012;53:2546–2559. doi: 10.1194/jlr.M026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konopatskaya O, Matthews SA, Harper MT, et al. Protein kinase C mediates platelet secretion and thrombus formation through protein kinase D2. Blood. 2011;118:416–424. doi: 10.1182/blood-2010-10-312199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rendu F, Brohard-Bohn B. The platelet release reaction: granules' constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 41.Deschamps JD, Kenyon VA, Holman TR. Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases. Bioorg Med Chem. 2006;14:4295–4301. doi: 10.1016/j.bmc.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 42.Unsworth AJ, Smith H, Gissen P, et al. Submaximal inhibition of protein kinase C restores ADP-induced dense granule secretion in platelets in the presence of Ca2+ J Biol Chem. 2011;286:21073–21082. doi: 10.1074/jbc.M110.187138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margalit A, Granot Y. Endogenous hepoxilin A3, produced under short duration of high shear-stress, inhibits thrombin-induced aggregation in human platelets. Biochim Biophys Acta. 1994;1190:173–176. doi: 10.1016/0005-2736(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 44.Margalit A, Sofer Y, Grossman S, et al. Hepoxilin A3 is the endogenous lipid mediator opposing hypotonic swelling of intact human platelets. Proc Natl Acad Sci USA. 1993;90:2589–2592. doi: 10.1073/pnas.90.7.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Italiano JE, Jr., Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7(Suppl 1):173–176. doi: 10.1111/j.1538-7836.2009.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh NK, Kundumani-Sridharan V, Rao GN. 12/15-Lipoxygenase gene knockout severely impairs ischemia-induced angiogenesis due to lack of Rac1 farnesylation. Blood. 2011;118:5701–5712. doi: 10.1182/blood-2011-04-347468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 48.Dailey LA, Imming P. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr Med Chem. 1999;6:389–398. [PubMed] [Google Scholar]
- 49.Cannon CP. Effectiveness of clopidogrel versus aspirin in preventing acute myocardial infarction in patients with symptomatic atherothrombosis (CAPRIE trial) Am J Cardiol. 2002;90:760–762. doi: 10.1016/s0002-9149(02)02606-1. [DOI] [PubMed] [Google Scholar]
- 50.Glading A, Koziol JA, Krueger J, et al. PEA-15 inhibits tumor cell invasion by binding to extracellular signal-regulated kinase 1/2. Cancer Res. 2007;67:1536–1544. doi: 10.1158/0008-5472.CAN-06-1378. [DOI] [PubMed] [Google Scholar]
- 51.Jamieson DG, Parekh A, Ezekowitz MD. Review of antiplatelet therapy in secondary prevention of cerebrovascular events: a need for direct comparisons between antiplatelet agents. J Cardiovasc Pharmacol Ther. 2005;10:153–161. doi: 10.1177/107424840501000302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.