Abstract
Fibrinogen is one of the primary components of the coagulation cascade and rapidly forms an insoluble matrix following tissue injury. In addition to its important role in hemostasis, fibrin acts as a scaffold for tissue repair and provides important cues for directing cell phenotype following injury. Because of these properties and the ease of polymerization of the material, fibrin has been widely utilized as a biomaterial for over a century. Modifying the macroscopic properties of fibrin, such as elasticity and porosity, has been somewhat elusive until recently, yet with a molecular-level rational design approach can now be somewhat easily modified through alterations of molecular interactions key to the protein’s polymerization process. This review outlines the biochemistry of fibrin and discusses methods for modification of molecular interactions and their application to fibrin based biomaterials.
Introduction
Fibrin is one of the classical biomaterials and has been widely utilized for a variety of applications [1–4] since it was first purified in large quantities in the 1940s [5]. Fibrin is at the crux of the coagulation cascade and is formed through the polymerization of the soluble precursor molecule fibrinogen, a process that is initiated by the serine protease thrombin, which is activated in response to injury (Reviewed by [6]). In addition to its important role in hemostasis, following cessation of bleeding, fibrin serves as a scaffold for tissue repair following injury. Furthermore, fibrinopeptides, which are released during polymerization of fibrin, are bioactive themselves and contribute to tissue repair due to their mitogenic, chemotactic and proangiogenic activities [7–9]. Fibrin degradation products are also known activators of wound repair [10–12]. Because of these properties, fibrin serves as a seminal “matrikine”, such that the molecule forms an insoluble matrix and also provides chemokines to stimulate surrounding cells.
The fibrin network provides a physical support for neutrophil, macrophage, and fibroblast infiltration, which will ultimately lay down fibronectin, collagen and other extracellular matrix (ECM) components to rebuild the damaged tissue. However, like all ECM components, fibrin is not merely a scaffold and provides a rich wealth of signals and cues to direct cell behaviors following injury through its numerous binding sites for growth factors, integrins and additional ECM components, such as fibronectin, fibulin, thrombospondin [13], and SPARC (secreted protein, acidic and rich in cysteine) [14]. Because of its role in hemostasis and wound repair, fibrin has been used extensively in hemostatic materials, such as fibrin glues, and wound dressings [15–18]. Fibrin has also been utilized for the development of cell instructive scaffolds and is widely utilized for differentiation of stem cells [19, 20], stem cell delivery [21] and induction of angiogenesis [22, 23].
This wide range of applications of fibrin is due to its rich bioactivity as well as the ease of manipulation of material properties of resulting fibrin gels. Modification of fibrin polymerization dynamics directly affects the porosity, fiber thickness and degree of branching of the polymerized gel, which in turn affects the mechanical properties [24, 25]. Fibrin polymerization is a mostly well-understood phenomenon and many groups have exploited the molecular details of this process in order to obtain biomaterials with desired properties for specific applications. Alteration of fibrin polymerization at the molecular level has been achieved through a number of methods ranging from alterations in pH, salt and thrombin concentration [26, 27] to incorporation of PEG [28] and other polymers [29, 30] that can be simply space filling or interact directly with the fibrin molecule [31]. This review will discuss the biochemistry of fibrin structure and polymerization and highlight approaches to exploiting the molecular mechanisms of fibrin gelation to create biomaterials with a wide range of material properties and applications.
Fibrin structure and polymerization
Biochemistry
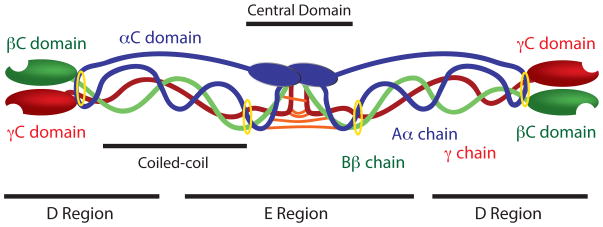
Fibrinogen, the circulating inactive precursor of fibrin monomers, is a 340 kD dimeric glycoprotein, with each dimer being comprised of three distinct chains designated as the Aα, Bβ, and γ chains (Figure 1). Thrombin selectively cleaves fibrinogen to release two sets of peptides 16 and 15 amino acids long, known as fibrinopeptides A and B respectively, from the N termini of the Aα and Bβ chains, resulting in a fibrin molecule now comprised of two α, β, and γ chains [32]. Each set of α, β and γ chains meet in the central domain of fibrinogen and are linked by disulfide bonds at their N-terminal regions. The α chains are linked by one interchain disulfide bond and the γ chains are linked by two interchain disulfide bonds [33]. The two β chains are not directly linked to one another; a disulfide bond links the β chain of one subunit to the α chain on the other subunit while the second β chain is linked to the γ chain of the opposite subunit through an additional disulfide bond. This region where the chains are linked is known as the central domain and is found within the “E” region of fibrinogen. The central domain is flanked on either side by α-helical coiled-coil regions, each stabilized by a pair of disulfide ring structures at their ends, which extend into the distal “D” regions of the molecule. There are two “D” and one “E” region per fibrinogen molecule, with these regions corresponding the proteolytic fragments obtained through complete plasmin degradation [34]. The coiled-coil regions are split approximately equally between the E and D domains. The distal portions of the D regions are comprised of the C-termini of the β and γ chains (known as the βC and γC domains, respectively) [35]. The C-termini of the α chains (known as the αC domain) account for approximately two thirds of the α chains and are comprised of a globular and a linear portion. The αC domains are located near the E region of the molecule and can interact both intramolecularly and intermolecularly [36]. These interactions are discussed subsequently in further detail.
Figure 1. Fibrinogen Structure.
Aα chains are shown in blue, Bβ chains are shown in green, and γ chains are shown in red. Interchain disulfide bridges connecting the six polypeptide chains in the central domain are shown in orange and disulfide rings stabilizing the coiled coil regions are shown in yellow.
Polymerization: knob A/knob B
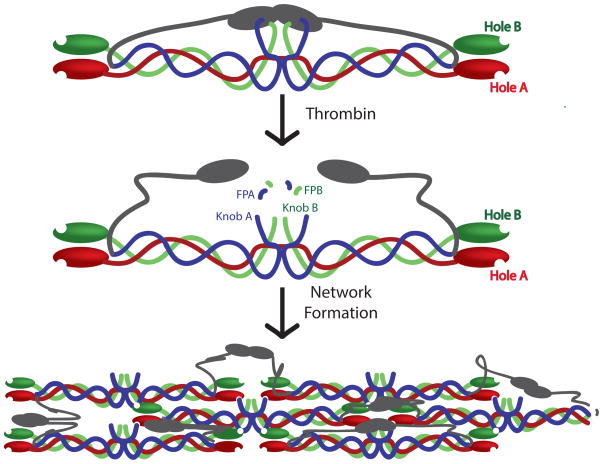
Fibrin polymerization begins when thrombin cleaves fibrinopeptides A and B from the central domain of fibrinogen. This enzymatic cleavage exposes peptide sequences at the N-termini of the α and β chains, termed knobs ‘A’ and ‘B’, respectively, which are then available to interact with the complementary holes ‘a’ and ‘b’ located at the C termini of the γ and β chains, respectively (Figure 2). The holes a and b do not require enzymatic cleavage to bind to their respective peptides and are always available for interactions. Knob A is comprised of the N-terminal Gly-Pro-Arg (GPR) motif and is complementary to hole ‘a’ located in the γ chains [37]. These knob A:hole a interactions are high affinity and appear to be the primary contributor to fibrin polymerization [38]. Indeed, fibrin polymerization is inhibited in the presence of high concentrations of a synthetic knob A peptide variant of the sequence Gly-Pro-Arg-Pro (GPRP) [39]; for reference, human knob A contains the sequence Gly-Pro-Arg-Val (GPRV). The knob B motif is comprised of the N-terminal Gly-His-Arg-Pro (GHRP) motif and is complementary to hole b located in the β chains. These knob B:hole b interactions appear to be less crucial than knob A:hole a interactions in clot formation and fibrin clots can be formed in the absence of knob B cleavage [40]. Studies using snake venom enzymes which only cleave the knob A while leaving knob B intact demonstrate that clots formed in the absence of knob B interactions displayed thinner fibers than control clots [41]. There is some debate over the physiological relevance and specific functional role of knob B:hole b interactions, however these and other studies suggest that knob B:hole b interactions promote lateral aggregation and play a role in determining clot stability and susceptibility to degradation [42, 43].
Figure 2. Fibrin Polymerization.
α chains are shown in blue, β chains are shown in green, and γ chains are shown in red. αC domains are shown in gray.
The energetics of knob A:hole a and knob B:hole b interactions vary greatly. In particular, the affinity of knob B for fragment D is approximately 5 times lower than knob A, and the strength of knob B:hole b interactions, as determined through laser tweezing based experiments, is approximately 6 times lower than that of knob A:hole a interactions [44, 45]. Furthermore, the initial release of fibrinopeptide A (and thus exposure of knob A) by thrombin cleavage is significantly faster than the release of fibrinopeptide B and exposure of knob B [46], but as polymerization proceeds, the rate of knob B exposure increases, a process which is thought to be driven by conformation changes within fibrinogen. Interestingly, studies utilizing surface bound fibrinogen rather than soluble fibrinogen found that fibrinopeptide B was cleaved faster than fibrinopeptide A [47]. In addition to the classical knob A:hole a and knob B:hole b interactions, there is evidence that knob A:hole b interactions occur while knob B:hole a interactions do not [38].
Upon initial cleavage by thrombin, knob A:hole a interactions occur between two adjacent molecules to form non-covalent bonds between the E region of one molecule and the D region of another molecule, resulting in the formation of half-staggered dimer, to which additional molecules are added to give rise to double-stranded protofibrils [48]. It is currently debatable whether knob B:hole b interactions occur within or between protofibrils [49] however, recent studies support the hypothesis that B:b occur within protofibrils [50, 51]. Protofibrils extend to a length of approximately 600–800 nM and then associate laterally with other protofibrils to form fibrin fibers. The process of lateral aggregation is enhanced by interactions between αC domains of adjacent molecules. Fibers then go on to branch, leading to the formation of a space-filling gel. The process of fiber branching is poorly understood, however thrombin concentrations are known to affect the degree of branching. Low thrombin concentrations lead to less branched networks compared to clots formed in the presence of high thrombin concentrations [52, 53]. Recent in situ studies by Fogelson et al. propose that branch formation is dependent on monomer (activated fibrinogen) supply rate, therefore this effect is due to a higher rate of monomer formation in the presence of high thrombin concentrations [53]. Several different types of branch points have been observed, however the intersection of three fibers at a node is most common [54]. Two distinct molecular mechanisms have been described which give rise to two distinct types of junctions known as bilateral and trilateral junctions [6]. Bilateral junctions arise when two protofibrils laterally aggregate to form a four-stranded fibril, which then goes on to diverge again into two separate protofibrils [55]. Trilateral junctions occur when a fibrin molecule binds to only one end of a protofibril, therefore both the attached molecule and the original protofibril can continue to elongate [6].
Formation of monomer assembly through knob:hole interactions is rapid, however these noncovalent interactions alone do not contribute significant mechanical strength or resistance to fibrinolysis. Covalent crosslinks that are introduced at a slower rate, minutes to hours, through the transglutaminase Factor XIIIa, enhance the stability of fibrin clots. Recent studies by Weisel et al. utilizing FRAP techniques demonstrate that prior to FXIIIa crosslinking, these knob:hole interactions are not as stable as previously thought and large rearrangements of clot structure can occur during early clot formation [56].
Fibrin crosslinks
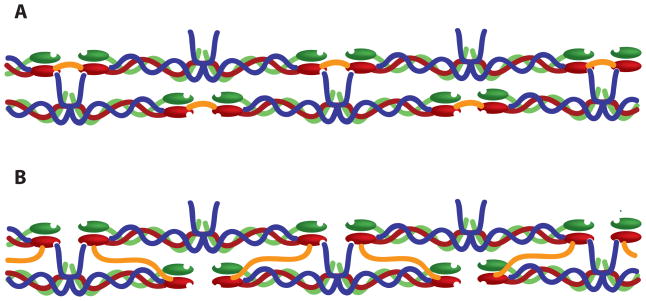
Crosslinking of fibrin clots by Factor XIIIa significantly enhances clot stability through increasing clot stiffness and resistance to deformation as well as through decreasing susceptibility to degradation [24, 57–59]. Factor XIII, the inactive precursor, circulates in plasma at a concentration of 14–28 μg/mL and, like fibrinogen, is activated through proteolytic cleavage by thrombin and thereafter denoted Factor XIIIa. This cleavage however, is approximately 40 times slower than cleavage of fibrinopeptide A [60], therefore release of fibrinopeptides seem to play a larger role in overall fibrin structure than Factor XIIIa crosslinks. Electron microscopy experiments demonstrate that FXIIIa crosslinking is not detectable under electron microscopy and does not appear to affect overall clot organization [52]. The zymogen form of FXIII has been shown to have a high affinity for fibrinogen (KD ~ 10−8 M) and is found mainly in association with the molecule [61]. Fibrinopeptide release enhances FXIII activation, leading to an interesting positive feedback loop. FXIIIa crosslinks both the α and γ chains of both fibrinogen and fibrin through intermolecular ε(γ-glutamyl)lysyl bonds to form γ-γ, α-α and α-γ crosslinks (Reviewed by [57]). γ-γ crosslinks are formed almost instantly following activation of FXIII, while α-α and α-γ form much slower. FXIIIa mediated γ-γ crosslinking of fibrin occurs much faster than γ-γ crosslinking of fibrinogen molecules. The precise contribution of each type of crosslink remains a topic of investigation, but several studies suggest that α-α crosslinks contribute more to an increase in clot rigidity than γ-γ crosslinks. For example, clots formed in the presence of antibodies which inhibit α chain crosslinking but permit γ chain crosslinking were found to exhibit a twofold decrease in elastic modulus [62]. In another study, clots formed from recombinant fibrinogen lacking C-terminal α chain FXIIIa crosslinking sites were found to be less stiff and have a higher loss modulus than control clots [63]. However, studies utilizing mutated γ chain crosslinking sites, demonstrate that γ-γ crosslinks also contribute to clot stiffness and may be required for the formation of higher-order crosslinked species (γn, αn, αm-γn) which further contribute to overall stiffness as the clot matures [62, 64]. γ-γ dimers have been the subject of much controversy and there is much debate as to whether the crosslinks between the chains occur longitudinally or transversely (Figure 3). Reviews by Weisel [65] and Mosesson [66] summarize evidence for both arguments, however recent stretching experiments by Guthold et. al on individual fibrin fibers support longitudinal crosslinking [67]. Regardless of the precise mechanism of crosslinking, we know that FXIIIa crosslinking significantly increases the mechanical stability of fibrin clots, which has far reaching implications in biomaterial design.
Figure 3. Potential FXIII mediated γ- γ crosslinks.
Longitudinal (A). Transverse (B). Crosslinks are shown in orange. Note that crosslinks are not to scale and are drawn to illustrate domain crosslinking. α chains are shown in blue, β chains are shown in green, and γ chains are shown in red. For simplicity, αC interactions are excluded in this illustration.
αC interactions
The role of αC interactions in fibrin polymerization has been extensively studied in the last ten years. The αC regions are comprised of a globular (Aα392–610) and an extended portion (Aα221–391) known as the αC domain and connector, respectively. The complete crystal structure of the αC domain has not been deduced, presumably because of its flexibility, however use of electron microscopy and NMR have provided insight into the structure of this region [68–70]. The αC domains interact with one another to form noncovalent inter- and intramolecular bonds. Prior to thrombin cleavage, αC domains interact intramolecularly with one another along with the central domain of the E region. Thrombin cleavage of fibrinopeptide B is thought to result in a large conformational change that allows the αC domains to swing out and interact with αC domains on adjacent molecules [13, 68]. These intermolecular interactions have been proposed to play a large role in lateral aggregation. However, lateral aggregation still occurs in clots formed from monomers lacking this region, suggesting that while the αC domains greatly enhance lateral aggregation, they are not required for it to occur [71–73]. Studies have demonstrated that protofibrils formed from recombinant fibrinogen lacking αC domains have thinner fibers, greater density of fibers, increased branch points and are lysed more rapidly than control clots [63]. These results differ from studies by Collet et al. which demonstrated that clots formed from native fibrinogen comprised of numerous thin fibers lysed slower than clots comprised of loose networks of thick fibers [74], indicating that additional factors besides fiber diameter and network conformations influence rates of fibrinolysis. In particular, these results demonstrate that the αC domains play a critical role in regulating fibrinolysis. Additional studies performed with fibrinogen fragment X, a proteolytic fragment which lacks the αC domain, or with dysfibrinogenemias with alterations of the αC domain, showed similar alterations in clot morphology and degradation as well as impaired fibrin polymerization [71].
Macroscopically, fibrin gels are known to exhibit nonlinear elasticity and strain hardening [75, 76], behaviors that are governed by interactions at the molecular level. Seminal work by John Ferry in the 1970’s first described the viscoelastic properties of fibrin clots [77–81]. Subsequent analysis of fibrin films through optical and x-ray scattering analysis suggested that stress could induce conformational changes within the individual fibrin monomers comprising the clot [82, 83]. While it is accepted that the macroscopic mechanical properties of fibrin networks depend on the mechanical properties of the individual fibrin monomers, the precise regions of fibrin involved in this strain-hardening phenomenon have been the topic of much investigation in recent years, and in addition to the coiled coil regions and the γ chains of fibrinogen, recent studies, including our own, have highlighted the importance of the αC domain in contributing to the elastic extensibility of fibrin fibers [63, 84–86]. AFM experiments demonstrate that fibrin fibers formed from fibrinogen variants containing exclusively α-α crosslinks resulted in fibers that were 2.5 times stiffer and had an elastic limit, defined as the strain to which a fiber can be stretched, without any discernible permanent deformation upon the release of the stress, 1.5 times higher than uncross-linked fibers, compared to fully cross-linked fibers which were 3.75 times stiffer, had an elastic limit 1.2 times higher and were 1.2 times less extensible than uncross-linked fibers [85], indicating that the αC domain contributes to fibrin fiber stiffness and elasticity. Our recent molecular dynamic simulations also suggest that αC region is largely responsible for the elasticity and strain hardening of fibrin fibers [86].
Degradation Mechanisms
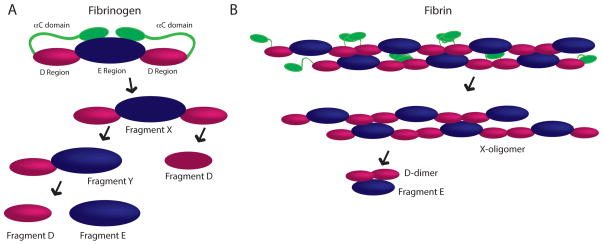
Fibrin degradation, termed fibrinolysis, is mediated by cleavage at specific sites by the serine protease plasmin. Plasmin can act on either fibrinogen or cross-linked fibrin to yield a number of fibrin degradation products (FDP); some common FDPs are shown in Figure 4. Cleavage of the αC domains from fibrinogen results in a fragment known as Fragment X (Figure 4A). Fragment X is subsequently cleaved into Fragment Y, comprised of an E and D domain, and one fragment D. Fragment Y is then further cleaved resulting in the generation of one fragment D and one fragment E molecules, therefore complete plasmin-mediated degradation of one fibrinogen molecule leads to the formation of two fragment D and one fragment E molecules. Plasmin’s action on fibrin ultimately leads to the production of the previously mentioned products, but due to the crosslinking of the network, several additional degradation products can be produced (Figure 4B), including a larger number of high molecular weight intermediates (X-oligomers) and the D-dimer, which are produced primarily through cleavage of the αC domain and lysis in the coiled-coiled region between the D and E domains [87]. The D-dimer consists of two cross-linked D domains from adjacent fibrin molecules, which are commonly found non-covalently bound to an E domain. Levels of circulating D-dimer have been used clinically to evaluate a number of thrombosis related diseases as increased levels of D-dimer are typically observed in patients with pulmonary thromboembolism, deep vein thrombosis, atherosclerosis [88–90].
Figure 4. Fibrin degradation products.
Plasmin-mediated degradation products produced from fibrinogen (A) and cross-linked fibrin (B). αC chains are shown in green, D regions are shown in pink and E regions are shown in blue.
Initiation of coagulation also initiates fibrinolysis through the activation of the plasminogen by tissue plasminogen activator (tPA), yielding plasmin. Conversion of fibrinogen to fibrin results in a conformational change that allows plasminogen and tPA to bind to the molecule through attachment to lysine residues on the C-terminus of the α chain of fibrin (reviewed in [91]). This binding is required for activation of plasminogen by tPA. Upon activation, plasmin begins to specifically cleave peptide bonds formed by the carboxyl group of lysine, thereby exposing additional lysines residues for plasminogen and tPA binding. This process results in an amplification of the rate of degradation. It should be noted that fibrinolysis is a highly orchestrated event and the rate of fibrinolysis is fine tuned by the presence of various inhibitors including aprotinin, ε-aminocaproic acid (ACA), plasminogen activator inhibitors (PAI-1 and PAI-2), α2-macroglobulin and thrombin activatable fibrinolysis inhibitor (TAIF) [92]. Furthermore, in addition to tPA, plasminogen can also be activated through urokinase (uPA), streptokinase (SK) and staphylokinase (SAK).
Fibrin structure is known to affect rates of fibrinolysis but the relationships are not straightforward. Several studies that modulated fiber thickness through variation in thrombin concentration suggest clots comprised of thick fibrin fibers are lysed more quickly than thin fibrin fibers [58, 93–96]. However, this relationship is also related to clot density and examination of fibrinolysis through confocal microscopy demonstrates that while clots comprised of a loose network of thick fibers are lysed more quickly than a dense network of thin fibers, at the individual fiber level, thin fibers are actually lysed more quickly than thick fibers [74]. This phenomenon is related to the more rapid diffusion of tPA through a loose fibrin network compared to a dense fibrin network. Further complicating the relationship between clot structure and fibrinolysis, fiber thickness also affects cellular expression of proteins involved in fibrinolysis; cells cultured on thick fibers increase expression of plasminogen (specifically the PGI2 isoform), tPA and PAI-1 while thinner fibers result in an increased production of von Willebrand Factor (vWF) by endothelial cells [97]. Degree of clot crosslinking has been suggested to affect fibrinolysis rates, however, like the role of clot structure and fiber thickness in clot degradation, this relationship does not appear to be straightforward [64] [98]. The presence of platelets also greatly affects the rate of fibrinolysis and platelet rich clots are lysed slower than platelet poor clots. Clots formed from platelet rich plasma are extremely heterogeneous, containing dense regions of fibrin associated with aggregates of platelets. These dense regions result in heterogeneous lysis within the clot, with lysis proceeding slower around the regions of platelet aggregation [99].
Fibrinolysis is extremely important in the consideration of biomaterial design, as it is the mechanism of biodegradability of fibrin materials. Fibrinolysis is also an important process in wound healing, mediating cell infiltration and tissue regeneration, and therefore modification of this process through or within fibrin-based biomaterials is an important design criteria. The fibrin degradation products, fragments E, fragments D and D-dimer have potent pro-healing activity. For example, fragment E has been shown to induce angiogenesis in a chick chorioallantoic membrane assay of angiogenesis, stimulate proliferation [10], migration and differentiation of human microvascular endothelial cells in vitro [100] and enhance the outgrowth of smooth muscle cells from rabbit aortic explants [101]. Furthermore, fragment D and D-dimer have been shown to induce neutrophil chemotaxis [102].
Fibrin binding species
Fibrin contains numerous binding sites for cells, ECM proteins and growth factors that contribute to its role in modulating a number of complex cellular responses. The majority of cell binding directly to fibrin occurs through integrin interactions with the molecule. Fibrin contains binding sequences for integrins αIIBβ3, αvβ3, αMβ2 and αxβ2 [103]. Platelets interact with fibrin through αIIBβ3 and αvβ3 integrins, with αIIBβ3 promoting adhesion and activation and αvβ3 promoting aggregation. Furthermore, clots formed from platelet rich plasma (PRP) undergo platelet-mediated contraction which contributes to enhanced resistance to fibrinolysis [104]. Endothelial cells and fibroblasts interact with an Arg-Gly-Asp (RGD) sequence on the C-terminal Aα chain of fibrinogen through αvβ3 integrins. αMβ2 and αxβ2 facilitate binding of leukocytes [105]. Early studies suggested that fibrinogen contained a binding site for integrin α5β1, however it seems that these interactions are actually facilitated by binding of fibronectin to fibrinogen and subsequent binding of α5β1 integrins to the attached fibronectin molecule. These interactions facilitate attachment of cell types that do not express fibrin-binding elements, such as keratinocytes [106–108]. Clots formed from fibrinogen depleted of fibronectin are not able to support keratinocyte attachment, while clots formed in the presence of fibronectin facilitates keratinocyte attachment and spreading [4]. Cells can also interact with fibrinogen through cell-surface proteoglycan, e.g. syndecan, binding to heparin binding domains on the molecule. This binding is facilitated by the syndecan’s heparan sulfate glycosaminoglycan chains. In addition to fibronectin, fibrinogen is known to bind albumin, thrombospondin, SPARC, von Willebrand Factor (vWF), fibulin, fibroblast growth factor 2 (FGF-2), vascular endothelial growth factor (VEGF) and interleukin-1 (IL-1).
Introduction to fibrin based biomaterials
Because of its role in hemostasis, directing tissue regeneration following injury, fast polymerization dynamics and ease of tunability, fibrin is a highly versatile biomaterial that has been used for a range of applications. This section will provide an overview of the range of applications in which fibrin gels have been utilized.
One of the first commercial applications of fibrin gels was in the development of fibrin sealants. The use of fibrin to augment clotting has been explored since the early 20th century. In 1909, Bergerl described the utility of fibrin powder as a hemostatic agent [109]. Some years later, in 1944, Tidrick and others reported on the use of fibrin gels as skin grafts to treat soldiers with burn injuries [110, 111]. Over a half century after these studies, the US FDA approved the first fibrin tissue sealant, Tisseel, in 1998, however use of noncommercial forms of fibrin sealant, derived from cryopreciptate and plasma, were common in the US in the 1990’s prior to this approval [112]. Furthermore, commercial fibrin sealants had been available in Europe since 1972. Since the approval of Tisseel in 1998, a number of fibrin sealants have been approved by the FDA for use as hemostats in surgery, adhesives for skin graft attachment for treatment of burn victims and as sealants in colostomy closure [16]. Currently approved fibrin sealants include those sourced from human pooled plasma (Tisseel, Artiss (Baxter) and Evicel (Johnson & Johnson)), individual units of plasma (Cryoseal (Thermogenesis)) and individual units of plasma, bovine collagen and bovine thrombin (Vitagel (Orthovita)).
A number of off label uses of fibrin sealants have also been described including use in tissue engineering and drug delivery applications as well as wide spread use as sealants and adhesives in a wide variety of surgical procedures, including neural, vascular, urological and intestinal procedures [16], [113–116]. Furthermore, formulations of fibrin sealants mixed with hydroxyaptite have been utilized in reconstructive and maxillofacial and dental surgeries to enhance bone regeneration; hydroxyapatite is incorporated into these formulations due to its osteoconductive properties [117]. Additionally, fibrin-based sealants mixed with platelets to create platelet-fibrin gels have been utilized for many clinical fields including oral and maxillofacial surgery, orthopaedic surgery, sports medicine and ophthalmology. Anecdotally, these platelet enriched constructs appear to enhance wound healing compared or fibrin only sealants, however thorough evaluations of this claim remain under investigation [118]. In more recent years, fibrin sealants have also been used to facilitate tissue engineering, biologics delivery, wound healing, tissue regeneration, stem cell delivery and angiogenesis [119–127].
Limitations and design considerations
Despite the wide spread clinical use of fibrin sealants, these sealants lack several key features to truly meet the full potential of fibrin as a hemostat, sealant and matrix for tissue regeneration. Clinically, fibrin sealants can be applied utilizing multiple techniques including application through a double barrel syringe or by spray application [128]. Aerosolized fibrin glue is widely used, particularly in plastic surgery [129], however, spraying techniques can alter the properties of the final fibrin mesh [130]. In the double barrel syringe application system, fibrin sealants are administered in a two-component system in which high concentrations of fibrinogen (80–120 mg/mL) are immediately mixed with high concentrations of thrombin (300–600 NIH U/mL) of equal volume at the delivery site. These high concentrations ensure rapid polymerization at the surgical site but are problematic for subsequent wound healing and tissue regeneration. A number of studies have demonstrated that fibrin gels formed from such high concentrations of fibrinogen and thrombin produce extremely dense networks that are inhibitory to cell infiltration and migration [131, 132]. Fibrin gels formed with low fibrinogen concentrations are more permissive to cell infiltration and facilitate better tissue repair responses. MSCs cultured in fibrin gels were found to proliferate better in gels of low fibrinogen concentration (5 mg/mL) than higher concentrations (17 and 50 mg/mL) [133]. However, gels formed from low concentrations of fibrinogen are extremely soft and do not exhibit the mechanical strength that is necessary for these materials to perform as an adhesive or tissue sealant. Furthermore, these extremely soft gels are not practical for clinical use because of their lack of robustness when manipulated.
The ideal fibrin material would be both permissive to cell infiltration and regeneration while also exhibiting mechanical properties appropriate to the specific application. In the majority of clinical applications this would require the fibrin construct to be strong but also highly porous. This could be accomplished by changing the properties of the fibrin fibers themselves by potentially making fibers stiffer, more branched or by incorporation of polymeric elements into a forming fibrin gel. The specific approach for modifying mechanical properties of fibrin materials will be determined by design goals dictated by final application but modification of fibrin polymerization and/or network properties provides a rich design space for biomaterial scientists. The molecular scale organization of fibrin fibers largely dictates the macroscopic mechanical properties and can be modified in a number of ways. The next section will discuss methods for modifying macroscopic properties of fibrin gel by modification of molecular scale properties.
Modulation of fibrin properties
The role of fibrinogen, thrombin, CaCl2, salt concentration
One of the simplest methods for altering fibrin polymerization dynamics and resulting fibrin gel properties is to simply alter the concentration(s) of fibrinogen and thrombin and/or modify Ca++ or salt concentrations. Modulation of this set of parameters allows one to fine tune fibrin network structure and mechanical properties. The role of fibrinogen, thrombin, CaCl2 and salt concentration in modulating clot physical properties is often assessed qualitatively by measuring opacity. In general, increasing fibrinogen concentration leads to formation of increasingly dense and more turbid gels [134]. If fibrin concentrations remain constant and thrombin concentrations are varied, low concentrations of thrombin lead to the formation of turbid fibrin gels consisting of thick fibers, few branch points and large pores, while higher concentrations of thrombin lead to the formation of less turbid gels consisting of tightly packed thin fibers. Increasing calcium concentration has been shown to result in the formation of more turbid gels with increased fiber size but lower rigidity [52, 135–137]. Increasing salt concentration, while utilizing low calcium concentrations (2mM) results in less turbid gels [135]. Furthermore, gel stiffness correlates with fibrinogen and thrombin concentration, with fibrinogen concentration affecting stiffness to a greater degree than thrombin concentration. Incorporation of cells into fibrin gels also influences the stiffness of the gel and fibroblasts incorporated into fibrin gels resulted in a small increase in gel stiffness [138].
Optimization of fibrinogen, thrombin, calcium and salt concentration has been used to produce fibrin gels for specific applications with the concentration of these components affecting the proliferation, migration and differentiation of cells embedded on top of (2D) or within (3D) the gels. Modulation of these components has been utilized to induce differentiation of murine embryonic stem cells into neural lineage cells [139], affect chick dorsal root ganglias (DRGs) migration [140], enhance fibroblast migration and proliferation [141] and enhance human mesenchymal stem cell proliferation [142]. Many studies utilize a combinatorial approach to determine the optimal concentrations of these components for a specific application, but modulation of a single factor has been described. Variation of fibrinogen concentration alone has been shown to affect hMSC proliferation, with proliferation being optimal on gels formed with lower concentrations (5 mg/mL) of fibrinogen [133]. Studies by Stegemann et al. describe the influence of thrombin concentration on vascular smooth muscle cell morphology; they found that cells cultured on top of fibrin gels formed from higher thrombin concentrations exhibited longer cellular protrusions than cells on gels formed from low thrombin concentrations [143]. Gugerell et al. demonstrated that high thrombin concentrations induce apoptosis of keratinocytes [144]. The effect of thrombin should be carefully considered when designing cellular constructs, because in addition to its role in fibrin network structure, thrombin itself directly influences cellular responses [145] through both proteolysis and activation of cell contractility. Studies in which NaCl concentration alone was varied from 0–4.4% (w/v) demonstrated that NaCl increased compressive modulus and fiber diameter and gels formed with 2.15% NaCl resulted in optimal alkaline phosphatase expression by hMSCs [146]. These studies demonstrate that optimization of reaction conditions is a simple yet effective method for controlling fibrin architecture and modulating cellular responses.
Modification of knob:hole interactions
The primary method we have used for engineering fibrin structure is through modulation of the knob:hole interactions, the inherent mechanism driving polymerization. As mentioned previously, the GPR sequence is the minimum knob A sequence required for engagement of hole a during polymerization, however slight modifications can be made to these sequences to enhance interactions between synthetic peptides and the polymerization holes. Numerous knob A variants have been identified from different species, including GPRP and GPRV, and these variants have been found to have various affinities for fibrinogen [39, 147, 148]. Likewise knob B mimics including GHRPY and AHRP derived from bovine and chicken fibrinogen, respectively, have been described [42, 149]. Our group has recently characterized binding kinetics of a number of synthetic knob A mimics to fibrinogen fragment D using surface plasmon resonance (SPR) to identify sequences with optimal affinity for fibrin [150]. In this study, we identify a novel knob A mimic (GPRPFPAC) with a 10-fold higher association rate (12.5 × 10−3 M−1s−1) than current mimics. These mimics can then be used alone, or in conjunction with carrier materials, to modify fibrin structure. We subsequently created a variety of knob mimic constructs including knob A-protein constructs, PEGylated knobs A and B and knob A modified elastin-like peptide (ELP) micelles and characterized their effect on fibrin properties such as polymerization, degradation and mechanical properties and network structure [31, 43, 151–153]. Knob A-protein constructs designed to display the tetrapeptide sequences GPRP, GPRV, GHRP or GSPE (non-binding control) at the N-termini of a protein fragment, specifically the cell binding 9th and 10th type III repeats of fibronectin, were found to stably bind fibrinogen via specific knob:hole interactions and were retained within fibrin matrices for 24 hours, therefore showing promise as a potential method for drug release from fibrin matrices [152]. Characterization of bivalent and tetravalent GPRP-PEG conjugates demonstrated that these constructs significantly alter network structure (Figure 5) without significantly affecting clotting time or degradation [31]. As shown in Figure 5, the addition of non-functionalized PEG at a 100:1 PEG:fibrinogen molar ratio resulted in thicker fibers compared to control clots (Figure 5B). The addition of free GPRP peptide at a 10:1 peptide:fibrinogen molar ratio (Figure 5C) produced a more fine-meshed network than clots formed in the presence of the non-binding GPSP peptide at identical ratios (Figure 5D). Clots formed in the presence of an 1:1 molar ratios of non-binding GPSP-PEG constructs:fibrinogen were appeared similar in structure to control clots (Figure 5F, H, J, L, N, P, R). However, clots formed in the presence of 1:1 molar ratios of GPRP-PEG constructs:fibrinogen altered clot structure, with the PEG conjugate size affecting the final clot structure. In particular, the addition of 2 kDa GPRP2 -PEG (Figure 5E) and 2 kDa GPRP4 -PEG (Figure 5M) resulted in thicker and straighter fibers compared to control clots, while clots formed in the presence of the presence of 3.5 kDa and 5 kDa GPRP2 -PEG (Figure 5G, I) were similar to GPSP2-PEG controls of the same size but were found to result in the formation of dense nodes of fibrin throughout the matrix. The inclusion of 7.5 kDa GPRP2 -PEG, 10 kDa and 20 kDa GPRP4-PEG resulted in networks comprised of highly branched, thin fibers (Figure 5K, O, Q), which also displayed dense nodes of fibrin throughout the matrix. These perturbations were not observed in matrices formed in the presence of 7.5 kDa GPRP2 -PEG, 10 kDa and 20 kDa GPRP4 -PEG (Figure 5L, P, R). In additional studies, our group has created PEG-knob constructs with higher affinity for fibrinogen through conjugation of a linear 5 kDa PEG to the GPRPFPAC sequence; addition of this construct to fibrin matrices was found to increase network porosity compared to the peptide sequence alone [151]. Likewise, studies utilizing a PEGylated knob B mimic, AHRPYAAC, found that this construct also enhanced the porosity of the fibrin network (Figure 6) and increased susceptibility to degradation, though interestingly, these constructs resulted in an increase in the complex modulus [43]. In recent additional studies, we have characterized the binding kinetics of this PEGylated AHRPYAAC to fragment D and have tested the effect of concentrations above and below the Kd on clot polymerization dynamics, clot structure and mechanics at both the fiber and bulk scales. The addition of increasing concentrations of PEGylated AHRPYAAC increases polymerization rates and significantly alters clot structure and mechanical properties at multiple length scales. These studies highlight the variety of applications for synthetic knob-conjugates in altering fibrin network properties.
Figure 5. Confocal images of clots formed in the presence of synthetic A knob mimic GPRP conjugated to 5 kDa PEG.
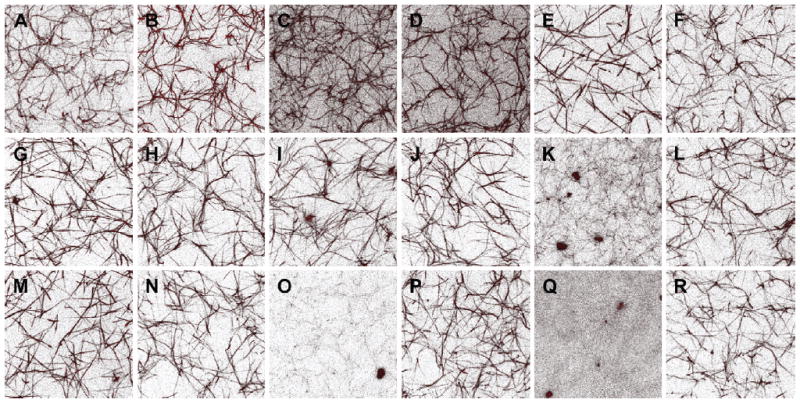
Hydrogels were formed within sealed chambers on glass slides and imaged using laser scanning confocal microscopy. Images were rendered from 45×45×10 μm slices. (A) Control clot (1 mg/mL fibrinogen, 0.25 NIH U/mL thrombin, 5 Loewy units/mL factor XIIIa). (B) Representative network structure formed in the presence of 100:1 molar ratio of PEG-to-fibrinogen. (C) Network structure in the presence of 10:1 molar ratio of GPRP-to-fibrinogen. (D) Network structure in the presence of 10:1 molar ratio of GPSP-to-fibrinogen. Subsequent paired images are of hydrogels formed in the presence of GPRPn-PEG or GPSPn-PEG conjugates at a 1:1 conjugate:fibrinogen molar ratio as follows: 2 kDa GPxP2-PEG (E, F), 3.5 kDa GPxP2-PEG (G, H), 5 kDa GPxP2-PEG (I, J), 7.5 kDa GPxP2-PEG (K, L), 2 kDa GPxP4-PEG (M, N), 10 kDa GPxP4-PEG (O, P), 20 kDa GPxP4-PEG (Q, R). From Soon AS, Lee CS, Barker TH: Modulation of fibrin matrix properties via knob:hole affinity interactions using peptide-PEG conjugates, Biomaterials, Copyright © 2011 by Elsevier. Reprinted by permission of Elsevier.
Figure 6. Confocal images of clots formed in the presence of synthetic B knob mimic AHRPYAAC conjugated to 5 kDa PEG.
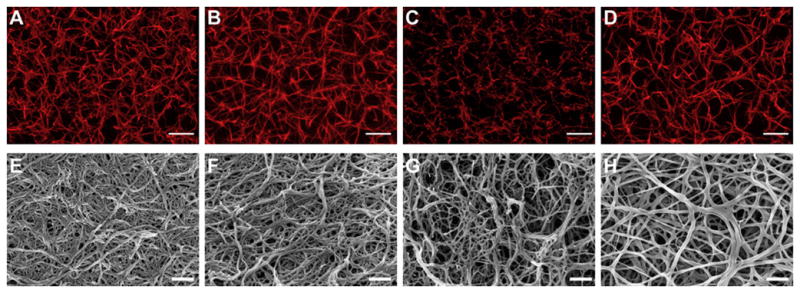
Confocal and scanning electron microscopy: Representative confocal micrograph projections (10 μm z-stacks; A–D) and SEM images (E–H) from no peptide control (A and E), control peptide conjugate, GPSPFPAC-PEG (B and F), knob ‘A’ conjugate, GPRPFPAC-PEG (C and G), and knob ‘B’ conjugate, AHRPYAAC-PEG (D and H). Confocal: Objective = 63×, Scale bar = 10 μm. SEM: Magnification = 50,000×, Voltage = 3.0 kV, Scale bar = 500 nm. From Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH: Engineering fibrin polymers through engagement of alternative polymerization mechanisms, Biomaterials, Copyright © 2012 by Elsevier. Reprinted by permission of Elsevier.
Perturbation of knob:hole interactions can also be achieved through the use of various snake venom enzymes which preferentially cleave either the A (batroxobin, ancrod) or B (venzyme) fibrinopeptides from fibrinogen. These thrombin-like serine proteases have been used to elucidate the roles of specific knob:hole interactions in fibrin network formation and resulting network structure. Batroxobin-catalyzed clots have been found to be similar to thrombin-catalyzed gels, while venzyme-catalyzed clots are highly fragile [41, 154]. Stegemann’s group has recently described the use of ancrod for creation of fibrin-collagen matrices. Ancrod-polymerized gels were found to have larger fiber diameters, decreased length between fiber nodes, increased number of fiber bundles per volume and increased maximum force to failure in uniaxial tensile testing compared to thrombin-polymerized gels [155]. These studies demonstrate the utility of modifying knob:hole interaction during clot formation to promote the desired micro and macroscale properties.
Polymeric modification: PEGylation
PEG is an extremely attractive biomaterial for modification of fibrin gels because it is biologically inert but can be easily functionalized to impart biospecificity. Furthermore, PEG can be cross-linked to form hydrogels, the properties of which can be easily modified by chain length and degree of crosslinking to obtain [156, 157]. Alteration of fibrin clot properties through some mode of PEGylation has been extensively described in the literature. As described in the previous section, our group has utilized PEGylated fibrin knobs to alter clot polymerization dynamics, network structure, degradation kinetics and mechanical properties of fibrin clots. Formation of hydrogels from PEGylated self-assembling peptides based on the coiled-coil region of fibrinogen have also been described [158]. Several groups, including ours, have PEGylated fibrinogen itself to alter network properties [159]. Sugg’s group has utilized amine-reactive PEG to conjugate fibrinogen to the PEG molecule and the resulting fibrinogen-PEG molecules [21]. They report that PEGylation of fibrinogen in this manner does not affect thrombin-catalyzed polymerization as long as the PEGylation ratio is less than 10 while our earlier study reported ~6 PEGs as a critical cut-off for maintaining full clottability of fibrinogen [21, 159]. These fibrin-PEG gels have been successfully loaded with a variety of soluble factors including antimicrobials [160], platelet derived growth factor-BB (PDGF-BB) and transforming growth factor-beta 1 (TGF-β1) [161] and have been shown to support neovascularization of adipose-derived stem cells [162], [163] and increase MSC viability [21]. In a very different approach, Seliktar’s group has created PEGylated-fibrin constructs by denaturing fibrinogen, reducing disulfide bonds and finally crosslinking free sulfhydryls using PEG-diacrylate. This approach is highly versatile and has been used to create gels with a wide range of elastic moduli (0.01 to 10 kPa), degradation rates and cellular invasion kinetics through simply modulating the size of PEG and fibrin to PEG ratios [21, 164]. Furthermore, a variety of cell types including smooth muscle, cardiac, cartilage, human foreskin fibroblasts, and human embryonic stem cell cultures have been successfully cultured in these PEG-fibrin gels [164–167]. Recently, these gels have been loaded with low doses of Bone Morphogenetic Protein-2 (BMP-2) and have shown efficacy in inducing bone formation for treatment of critical size calvarial defects in mice [168]. These studies demonstrate the utility of creating PEG-fibrin systems for tissue engineering and wound healing applications.
Fibrin-hybrid constructs
Composite fibrin gels are often constructed in order to obtain desired material, drug delivery or cell instructive properties. Fibrin gels copolymerized with polyurethane [169], polyethylene oxide [170], polycaprolactone [171], heparin [172], collagen [173], keratin [174], chitosan [175], carbon nanotubes [176], hyaluronic acid [177], poly-L-lactic acid (PLLA) and poly(lactic-co-glycolic acid) (PLGA) [178] and even “fibrin in fibrin” systems in which fibrin microspheres are impregnated in a fibrin scaffold for drug delivery [179] have been described. These composite fibrin gels can be created in a variety of ways. Recently Atala’s group described the development of a technique to create tissue constructs of 1mm thickness with viability after 1 week by alternating electrospun polycaprolactone fibers with inkjet printing of chondrocytes in fibrin-collagen hydrogels [180]. Lesman et al. demonstrated that PLLA/PLGA fibrin composites supported neovascularization in a mouse full defect injury model to a greater extent than fibrin gels alone [178] (Figure 7). Fibrin gels mixed with supplementary ECM molecules are often copolymerized with the second fibrillar molecule [173, 181, 182]. The precise mechanism by which a second fibrillar molecule changes network architecture and mechanical behavior are poorly understood, but recent studies in which each component of a fibrin-collagen hybrid gel were selectively degraded suggest that extensive end-to-end crosslinking of collagen and fibrin fibers does not occur, instead it seems that each component forms its own network that interpenetrates the second [181]. Additional studies have demonstrated that collagen-fibrin composite gels have mechanical properties that are distinct from gels constructed of only a single component, even when total protein concentrations are constant [155, 183].
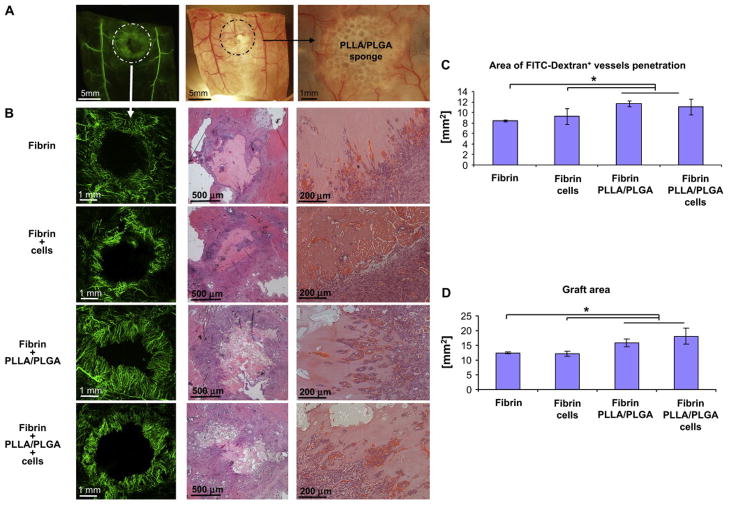
Figure 7. Fibrin PLLA/PLGA composite gels facilitate graft neovascularization and perfusion.
(A) Low power images of FITC-Dextran (green) and bright-field images highlighting the graft area. (B) Zoom at the graft area revealed intense FITC-Dextran+ neovessels penetration following 10 days postimplantation (left). Histological H&E examination demonstrated neovessels occupied with red blood cells penetrating into the graft area. Quantification of FITC-Dextran+ vessel area penetrating into the graft area (C) as well as calculation of the graft area size (D) revealed that Fibrin + PLLA/PLGA (with or without cells) constructs were most supportive of graft neovascularization and perfusion (n = 3–6). From Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S: Engineering vessel-like networks within multicellular fibrin-based constructs, Biomaterials, Copyright © 2011 by Elsevier. Reprinted by permission of Elsevier.
The majority of these composite structures have been shown to enhance mechanical properties compared to fibrin gels alone, however many of these composites are not simply structural but impart additional biological activity to the matrix. In particular, incorporation of heparin allows for the immobilization of heparin binding growth factors, such as BMP-2, Fibroblast Growth Factor 2 and Nerve Growth Factor, and controlled release of these molecules [184–186]. Heparin incorporation into fibrin matrices has been achieved through covalent coupling to the molecule [184] as well as through the use of a bi-domain heparin-binding peptide consisting of both a transglutaminase sequence to facilitate cross-linking by Factor XIIIa and a heparin binding domain [187–190]. Furthermore, ECM components have rich amounts of bioactivity and incorporation of additional natural components, such as collagen, fibronectin and keratin, can impart cell instructive cues not found in a fibrin gel alone. For example, Clark and co-workers recently described the differential but synergistic effects of fibrin and collagen in regulating sprouting angiogenesis of human dermal microvascular endothelial cells in 3-D matrices [191]. In particular they found that fibrin is essential for promoting sprouting angiogenesis of human dermal microvascular endothelial cells in the presence of angiogenic factors, while replacement of fibrin with collagen correlated with sprout regression. They also found that integrin β3, a component of the angiogenesis-associated integrin αvβ3, was highly up regulated in vascular endothelial cells in fibrin-rich environments but not collagen-rich environments [192, 193]. Because the presence of additional matrix components, collagen, fibronectin etc., can greatly affect the phenotypes of cells embed within fibrin gels, the purity of fibrinogen utilized to construct these gels should be carefully considered.
Modulation of degradation
Fibrin gels are highly susceptible to degradation, predominantly mediated through proteolytic cleavage by plasmin and matrix metalloproteinases (MMPs). Though plasmin-mediated degradation is important for remodeling of fibrin constructs and subsequent tissue regeneration, if not controlled, fibrin gels can degrade before their desired effects can come to fruition. A number of protease inhibitors have been added to fibrin gels to slow proteolysis [194–196] including the serine protease inhibitor aprotinin and various pharmacological MMP inhibitors. Recently an engineered aprotinin has been shown to enhance longevity of fibrin matrices compared to wild type aprotinin [197]. Fibrin degradation has also been controlled by the addition of the fibrinolysis inhibitor ε-aminocaproic acid [198] [199]. Studies by Tranquillo’s group demonstrated a decrease in fibrin degradation products with increasing ε-aminocaproic acid concentration [198]. Interestingly, fibrin degradation products were found to stimulate vascular smooth muscle cell proliferation as well as collagen and elastin deposition, events that were all decreased in the presence of ε-aminocaproic acid. This study highlights the importance of fine-tuning fibrin degradation to obtain an optimal cellular response.
Additional considerations
In addition to controlling fibrin properties through modulation of gelation properties, knob:hole interactions and degradation kinetics, polymeric modification and creation of fibrin:hybrid constructs, a number of additional methods have been described to modify fibrin matrices to achieve desired properties including photo-crosslinking and conferring anisotropic mechanical properties by directing fibrin alignment. Ruthenium-catalyzed photo-crosslinking of fibrinogen can be utilized to create fibrin constructs that do not require thrombin for polymerization or to enhance the mechanical properties of fibrin constructs formed through thrombin-initiated polymerization [200, 201]. Werkmeister and colleagues utilized the later approach to create fibrin sealants, which polymerized rapidly (reaching maximum bond strength within 20 seconds) and were found to produce tissue bonds 5 times stronger than those produced by commercial tissue sealants [202]. Tranquillo and coworkers utilized ruthenium-catalyzed photochemical crosslinking to increase the mechanical stiffness of tissue-engineered scaffolds with the goal of minimizing cell-mediated fibrin gel contraction [201]. Fibroblasts were entrapped and cultured within thrombin-polymerized fibrin gels for 24 hours, at which point the constructs were photo-chemically cross-linked. This crosslinking method was found to increase the stiffness of the gels, while having minimal effects on cell viability, proliferation or collagen deposition. Furthermore, cross-linked constructs were twice as long as non-cross-linked gels after four weeks in culture. This crosslinking method is therefore useful for applications requiring rapid polymerization, such as tissue sealants, or for controlling fibrin gel size during long-term culture that is typically required for tissue engineering applications.
Controlling fibrin fiber alignment is an additional design parameter than can be utilized to impart anisotropic mechanical properties to fibrin gels and has been shown to affect cellular responses. Fiber alignment can be induced through a number of methods including cell-mediated gel contraction [203], magnetic alignment of fibrin molecules [204], flow-induced alignment [205] and electrospinning [206]. Induction of cell-mediated fiber alignment can be achieved by embedding cells within fibrin gels constrained to specific geometries [207, 208]; in this system, fiber alignment results from a combination of cell contraction forces, mechanical constraints due to the mold and gel geometry [207]. Sander et al. demonstrated that differing fiber alignment patterns can be induced by embedding fibroblasts within cruciform molds of either symmetric or asymmetric geometries. Furthermore, fiber alignment was found to correlate with increased predicted stress and strain and increased collagen synthesis [203]. In addition to increasing matrix synthesis, fibrin fiber alignment can be utilized to direct cell fate; for example, fibrin fiber alignment and has been shown to direct the alignment of internal actin stress fibers [209] and guide the direction of sprouting from endothelial spheroids in fibrin gels [210].
Summary and Conclusions
Fibrin is a rich biomaterial that can be applied in a multitude of ways to construct a wide variety of materials including hemostatic agents, bioglues, tissue engineering constructs and cell and drug delivery vehicles. Though fundamental questions remain about the polymerization, cross-linking and degradation mechanisms of fibrin, much of these processes are well characterized and offer an abundant design landscape for biomaterial scientists. Modulation of these events at the molecular level governs the resulting structural properties of the fibrin construct. These structural properties in turn govern the mechanical, degradation and cell infiltration properties of the gel. The mechanical properties of fibrin biomaterials can be supplemented by the construction of hybrid materials containing polymeric and/or additional ECM components, such as collagen. Furthermore, additional bioactivity can be easily incorporated into fibrin constructs through the additional ECM components, peptides or growth factors. The fast polymerization kinetics, biocompatibility and easy manipulation of fibrin properties make this coagulation protein an ideal candidate for an almost endless array of applications. By rationally designing interactions at the molecular level we can create fibrin materials with properties ideal for the desired application.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.James Ferguson SN, Redl Heinz. Fibrin: The Very First Biomimetic Glue — Still a Great Tool. In: Byern J, Grunwald, Ingo, editors. Biological Adhesive Systems. Springer; Vienna: 2010. pp. 225–36. [Google Scholar]
- 2.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10. doi: 10.1098/rsif.2008.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spotnitz WD, Burks SG, Prabhu R. Tissue adhesives in clinical medicine. Hamilton, Ontario, Canada: BC Decker; 2005. Fibrin-based adhesives and hemostatic agents; pp. 77–112. [Google Scholar]
- 4.Sylvia Nürnberger SW, Peterbauer-Scherb Anja, Morton Tatjana J, Feichtinger Georg A, Gugerell Alfred, Meinl Alexandra, Labuda Krystyna, Bittner Michaela, Pasteiner Waltraud, Nikkola Lila, Gabriel Christian, van Griensven Martijn, Redl Heinz. Properties and Potential Alternative Applications of Fibrin Glue. In: Byern J, Grunwald, Ingo, editors. Biological Adhesive Systems. Springer; Vienna: 2010. pp. 237–59. [Google Scholar]
- 5.Cohn EJ, Strong LE, et al. Preparation and properties of serum and plasma proteins; a system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–75. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 6.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood. 2013;121:1712–9. doi: 10.1182/blood-2012-09-306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senior RM, Skogen WF, Griffin GL, Wilner GD. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986;77:1014–9. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson DL, Pepper DS, Kay AB. Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol. 1976;32:507–13. doi: 10.1111/j.1365-2141.1976.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 9.Gray AJ, Reeves JT, Harrison NK, Winlove P, Laurent GJ. Growth factors for human fibroblasts in the solute remaining after clot formation. J Cell Sci. 1990;96 (Pt 2):271–4. doi: 10.1242/jcs.96.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Thompson WD, Smith EB, Stirk CM, Marshall FI, Stout AJ, Kocchar A. Angiogenic activity of fibrin degradation products is located in fibrin fragment E. J Pathol. 1992;168:47–53. doi: 10.1002/path.1711680109. [DOI] [PubMed] [Google Scholar]
- 11.Robson SC, Shephard EG, Kirsch RE. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994;86:322–6. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- 12.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med. 2011;17:568–73. doi: 10.2119/molmed.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70:247–99. doi: 10.1016/S0065-3233(05)70008-5. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Workman G, Chen S, Barker TH, Ratner BD, Sage EH, et al. Secreted protein acidic and rich in cysteine (SPARC/osteonectin/BM-40) binds to fibrinogen fragments D and E, but not to native fibrinogen. Matrix Biol. 2006;25:20–6. doi: 10.1016/j.matbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Spotnitz WD, Burks S. State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clin Appl Thromb Hemost. 2010;16:497–514. doi: 10.1177/1076029610363589. [DOI] [PubMed] [Google Scholar]
- 16.Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg. 2010;34:632–4. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 17.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48:1502–16. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaiman M, Krakovski D, Gavriel H. Fibrin sealant reduces pain after tonsillectomy: prospective randomized study. Ann Otol Rhinol Laryngol. 2006;115:483–9. doi: 10.1177/000348940611500701. [DOI] [PubMed] [Google Scholar]
- 19.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–97. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385–96. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 22.Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med. 2009;4:527–38. doi: 10.2217/rme.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takei A, Tashiro Y, Nakashima Y, Sueishi K. Effects of fibrin on the angiogenesis in vitro of bovine endothelial cells in collagen gel. In Vitro Cell Dev Biol Anim. 1995;31:467–72. doi: 10.1007/BF02634260. [DOI] [PubMed] [Google Scholar]
- 24.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–76. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Falvo MR, Gorkun OV, Lord ST. The molecular origins of the mechanical properties of fibrin. Biophys Chem. 2010;152:15–20. doi: 10.1016/j.bpc.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair CH, Shah GA, Dhall DP. Effect of temperature, pH and ionic strength and composition on fibrin network structure and its development. Thromb Res. 1986;42:809–16. doi: 10.1016/0049-3848(86)90117-9. [DOI] [PubMed] [Google Scholar]
- 27.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Reviews. 2007;21:131–42. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Jiang B, Waller TM, Larson JC, Appel AA, Brey EM. Fibrin-loaded porous poly(ethylene glycol) hydrogels as scaffold materials for vascularized tissue formation. Tissue Eng Part A. 2013;19:224–34. doi: 10.1089/ten.tea.2012.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao HG, Ma L, Gong YH, Gao CY, Shen JC. A polylactide/fibrin gel composite scaffold for cartilage tissue engineering: fabrication and an in vitro evaluation. Journal of Materials Science-Materials in Medicine. 2009;20:135–43. doi: 10.1007/s10856-008-3543-x. [DOI] [PubMed] [Google Scholar]
- 30.Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. Journal of Orthopaedic Research. 2002;20:16–9. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 31.Soon AS, Lee CS, Barker TH. Modulation of fibrin matrix properties via knob:hole affinity interactions using peptide-PEG conjugates. Biomaterials. 2011;32:4406–14. doi: 10.1016/j.biomaterials.2011.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 33.Henschen A, Lottspeich F, Kehl M, Southan C. Covalent Structure of Fibrinogen. Ann N Y Acad Sci. 1983;408:28–43. doi: 10.1111/j.1749-6632.1983.tb23232.x. [DOI] [PubMed] [Google Scholar]
- 34.Nussenzweig V, Grabar P, Seligmann M. Les Produits De Degradation Du Fibrinogene Humain Par La Plasmine .2. Etude Immunologique - Mise En Evidence Danticorps Anti-Fibrinogene Natif Possedant Des Specificites Differentes. Annales De L Institut Pasteur. 1961;100:490. [PubMed] [Google Scholar]
- 35.Everse SJ, Pelletier H, Doolittle RF. Crystallization of Fragment-D from Human Fibrinogen. Protein Science. 1995;4:1013–6. doi: 10.1002/pro.5560040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisel JW, Medved L. The structure and function of the alpha C domains of fibrinogen. Fibrinogen. 2001;936:312–27. doi: 10.1111/j.1749-6632.2001.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 37.Laudano AP, Cottrell BA, Doolittle RF. Synthetic Peptides Modeled on Fibrin Polymerization Sites. Ann N Y Acad Sci. 1983;408:315–29. doi: 10.1111/j.1749-6632.1983.tb23254.x. [DOI] [PubMed] [Google Scholar]
- 38.Litvinov RI, Gorkun OV, Owen SF, Shuman H, Weisel JW. Polymerization of fibrin: specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood. 2005;106:2944–51. doi: 10.1182/blood-2005-05-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978;75:3085–9. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furlan M, Seelich T, Beck EA. Clottability and cross-linking reactivity of fibrin(ogen) following differential release of fibrinopeptides A and B. Thromb Haemost. 1976;36:582–92. [PubMed] [Google Scholar]
- 41.Weisel JW. Fibrin assembly. Lateral aggregation and the role of the two pairs of fibrinopeptides. Biophys J. 1986;50:1079–93. doi: 10.1016/S0006-3495(86)83552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doolittle RF, Pandi L. Binding of synthetic B knobs to fibrinogen changes the character of fibrin and inhibits its ability to activate tissue plasminogen activator and its destruction by plasmin. Biochemistry. 2006;45:2657–67. doi: 10.1021/bi0524767. [DOI] [PubMed] [Google Scholar]
- 43.Stabenfeldt SE, Gourley M, Krishnan L, Hoying JB, Barker TH. Engineering fibrin polymers through engagement of alternative polymerization mechanisms. Biomaterials. 2012;33:535–44. doi: 10.1016/j.biomaterials.2011.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everse SJ, Spraggon G, Veerapandian L, Riley M, Doolittle RF. Crystal structure of fragment double-D from human fibrin with two different bound ligands. Biochemistry. 1998;37:8637–42. doi: 10.1021/bi9804129. [DOI] [PubMed] [Google Scholar]
- 45.Litvinov RI, Gorkun OV, Galanakis DK, Yakovlev S, Medved L, Shuman H, et al. Polymerization of fibrin: Direct observation and quantification of individual B:b knob-hole interactions. Blood. 2007;109:130–8. doi: 10.1182/blood-2006-07-033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis SD, Shields PP, Shafer JA. Characterization of the kinetic pathway for liberation of fibrinopeptides during assembly of fibrin. Journal of Biological Chemistry. 1985;260:10192–9. [PubMed] [Google Scholar]
- 47.Riedel T, Suttnar J, Brynda E, Houska M, Medved L, Dyr JE. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood. 2011;117:1700–6. doi: 10.1182/blood-2010-08-300301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferry JD, Morrison PR. Preparation and properties of serum and plasma proteins; the conversion of human fibrinogen to fibrin under various conditions. J Am Chem Soc. 1947;69:388–400. doi: 10.1021/ja01194a066. [DOI] [PubMed] [Google Scholar]
- 49.Geer CB, Tripathy A, Schoenfisch MH, Lord ST, Gorkun OV. Role of ‘B-b’ knob-hole interactions in fibrin binding to adsorbed fibrinogen. J Thromb Haemost. 2007;5:2344–51. doi: 10.1111/j.1538-7836.2007.02774.x. [DOI] [PubMed] [Google Scholar]
- 50.Lounes KC, Ping L, Gorkun OV, Lord ST. Analysis of engineered fibrinogen variants suggests that an additional site mediates platelet aggregation and that “B-b” interactions have a role in protofibril formation. Biochemistry. 2002;41:5291–9. doi: 10.1021/bi011988s. [DOI] [PubMed] [Google Scholar]
- 51.Okumura N, Terasawa F, Haneishi A, Fujihara N, Hirota-Kawadobora M, Yamauchi K, et al. B:b interactions are essential for polymerization of variant fibrinogens with impaired holes ‘a’. J Thromb Haemost. 2007;5:2352–9. doi: 10.1111/j.1538-7836.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- 52.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–26. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fogelson AL, Keener JP. Toward an understanding of fibrin branching structure. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:051922. doi: 10.1103/PhysRevE.81.051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baradet TC, Haselgrove JC, Weisel JW. Three-dimensional reconstruction of fibrin clot networks from stereoscopic intermediate voltage electron microscope images and analysis of branching. Biophys J. 1995;68:1551–60. doi: 10.1016/S0006-3495(95)80327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mosesson MW, DiOrio JP, Siebenlist KR, Wall JS, Hainfeld JF. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood. 1993;82:1517–21. [PubMed] [Google Scholar]
- 56.Chernysh IN, Nagaswami C, Purohit PK, Weisel JW. Fibrin clots are equilibrium polymers that can be remodeled without proteolytic digestion. Sci Rep. 2012;2:879. doi: 10.1038/srep00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagoly Z, Koncz Z, Harsfalvi J, Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012;129:382–7. doi: 10.1016/j.thromres.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 58.Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011;31:494–9. doi: 10.1161/ATVBAHA.110.213389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts WW, Lorand L, Mockros LF. Viscoelastic properties of fibrin clots. Biorheology. 1973;10:29–42. doi: 10.3233/bir-1973-10105. [DOI] [PubMed] [Google Scholar]
- 60.Greenberg CS, Miraglia CC. The effect of fibrin polymers on thrombin-catalyzed plasma factor XIIIa formation. Blood. 1985;66:466–9. [PubMed] [Google Scholar]
- 61.Greenberg CS, Shuman MA. The zymogen forms of blood coagulation factor XIII bind specifically to fibrinogen. Journal of Biological Chemistry. 1982;257:6096–101. [PubMed] [Google Scholar]
- 62.Ryan EA, Mockros LF, Stern AM, Lorand L. Influence of a natural and a synthetic inhibitor of factor XIIIa on fibrin clot rheology. Biophys J. 1999;77:2827–36. doi: 10.1016/S0006-3495(99)77114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collet JP, Moen JL, Veklich YI, Gorkun OV, Lord ST, Montalescot G, et al. The alphaC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood. 2005;106:3824–30. doi: 10.1182/blood-2005-05-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Standeven KF, Carter AM, Grant PJ, Weisel JW, Chernysh I, Masova L, et al. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximizes clot stiffness. Blood. 2007;110:902–7. doi: 10.1182/blood-2007-01-066837. [DOI] [PubMed] [Google Scholar]
- 65.Weisel JW. Cross-linked gamma-chains in a fibrin fibril are situated transversely between its strands. J Thromb Haemost. 2004;2:1467–9. doi: 10.1111/j.1538-7836.2004.00873.x. discussion 9–73. [DOI] [PubMed] [Google Scholar]
- 66.Mosesson MW. Cross-linked gamma-chains in fibrin fibrils bridge ‘transversely’ between strands: yes. J Thromb Haemost. 2004;2:388–93. doi: 10.1111/j.1538-7933.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu W, Carlisle CR, Sparks EA, Guthold M. The mechanical properties of single fibrin fibers. J Thromb Haemost. 2010;8:1030–6. doi: 10.1111/j.1538-7836.2010.03745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veklich YI, Gorkun OV, Medved LV, Nieuwenhuizen W, Weisel JW. Carboxyl-terminal portions of the alpha chains of fibrinogen and fibrin. Localization by electron microscopy and the effects of isolated alpha C fragments on polymerization. Journal of Biological Chemistry. 1993;268:13577–85. [PubMed] [Google Scholar]
- 69.Burton RA, Tsurupa G, Hantgan RR, Tjandra N, Medved L. NMR solution structure, stability, and interaction of the recombinant bovine fibrinogen alphaC-domain fragment. Biochemistry. 2007;46:8550–60. doi: 10.1021/bi700606v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erickson HP, Fowler WE. Electron microscopy of fibrinogen, its plasmic fragments and small polymers. Ann N Y Acad Sci. 1983;408:146–63. doi: 10.1111/j.1749-6632.1983.tb23242.x. [DOI] [PubMed] [Google Scholar]
- 71.Weisel JW, Medved L. The structure and function of the alpha C domains of fibrinogen. Ann N Y Acad Sci. 2001;936:312–27. doi: 10.1111/j.1749-6632.2001.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 72.Litvinov RI, Yakovlev S, Tsurupa G, Gorkun OV, Medved L, Weisel JW. Direct evidence for specific interactions of the fibrinogen alphaC-domains with the central E region and with each other. Biochemistry. 2007;46:9133–42. doi: 10.1021/bi700944j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ping L, Huang L, Cardinali B, Profumo A, Gorkun OV, Lord ST. Substitution of the human alphaC region with the analogous chicken domain generates a fibrinogen with severely impaired lateral aggregation: fibrin monomers assemble into protofibrils but protofibrils do not assemble into fibers. Biochemistry. 2011;50:9066–75. doi: 10.1021/bi201094v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, et al. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol. 2000;20:1354–61. doi: 10.1161/01.atv.20.5.1354. [DOI] [PubMed] [Google Scholar]
- 75.Kang H, Wen Q, Janmey PA, Tang JX, Conti E, MacKintosh FC. Nonlinear elasticity of stiff filament networks: strain stiffening, negative normal stress, and filament alignment in fibrin gels. J Phys Chem B. 2009;113:3799–805. doi: 10.1021/jp807749f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bale MD, Ferry JD. Strain enhancement of elastic modulus in fine fibrin clots. Thromb Res. 1988;52:565–72. doi: 10.1016/0049-3848(88)90129-6. [DOI] [PubMed] [Google Scholar]
- 77.Nelb GW, Kamykowski GW, Ferry JD. Rheology of fibrin clots. V. Shear modulus, creep, and creep recovery of fine unligated clots. Biophys Chem. 1981;13:15–23. doi: 10.1016/0301-4622(81)80020-8. [DOI] [PubMed] [Google Scholar]
- 78.Rosser RW, Roberts WW, Ferry JD. Rheology of fibrin clots. IV. Darcy constants and fiber thickness. Biophys Chem. 1977;7:153–7. doi: 10.1016/0301-4622(77)80008-2. [DOI] [PubMed] [Google Scholar]
- 79.Nelb GW, Gerth C, Ferry JD. Rheology of fibrin clots. III. Shear creep and creep recovery of fine ligated and coarse unligated closts. Biophys Chem. 1976;5:377–87. doi: 10.1016/0301-4622(76)80050-6. [DOI] [PubMed] [Google Scholar]
- 80.Gerth C, Roberts WW, Ferry JD. Rheology of fibrin clots. II. Linear viscoelastic behavior in shear creep. Biophys Chem. 1974;2:208–17. doi: 10.1016/0301-4622(74)80046-3. [DOI] [PubMed] [Google Scholar]
- 81.Roberts WW, Kramer O, Rosser RW, Nestler FH, Ferry JD. Rheology of fibrin clots. I. Dynamic viscoelastic properties and fluid permeation. Biophys Chem. 1974;1:152–60. doi: 10.1016/0301-4622(74)80002-5. [DOI] [PubMed] [Google Scholar]
- 82.Roska FJ, Ferry JD, Lin JS, Anderegg JW. Studies of fibrin film. II. Small-angle x-ray scattering. Biopolymers. 1982;21:1833–45. doi: 10.1002/bip.360210911. [DOI] [PubMed] [Google Scholar]
- 83.Roska FJ, Ferry JD. Studies of fibrin film. I. Stress relaxation and birefringence. Biopolymers. 1982;21:1811–32. doi: 10.1002/bip.360210910. [DOI] [PubMed] [Google Scholar]
- 84.Houser JR, Hudson NE, Ping L, O’Brien ET, 3rd, Superfine R, Lord ST, et al. Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J. 2010;99:3038–47. doi: 10.1016/j.bpj.2010.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Helms CC, Ariens RA, Uitte de Willige S, Standeven KF, Guthold M. alpha-alpha Cross-links increase fibrin fiber elasticity and stiffness. Biophys J. 2012;102:168–75. doi: 10.1016/j.bpj.2011.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Averett RD, Menn B, Lee EH, Helms CC, Barker T, Guthold M. A modular fibrinogen model that captures the stress-strain behavior of fibrin fibers. Biophys J. 2012;103:1537–44. doi: 10.1016/j.bpj.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaffney PJ. Fibrin degradation products. A review of structures found in vitro and in vivo. Ann N Y Acad Sci. 2001;936:594–610. [PubMed] [Google Scholar]
- 88.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–8. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 89.Morris TA, Gerometta M, Yusen RD, White RH, Douketis JD, Kaatz S, et al. Detection of pulmonary emboli with 99mTc-labeled anti-D-dimer (DI-80B3)Fab’ fragments (ThromboView) Am J Respir Crit Care Med. 2011;184:708–14. doi: 10.1164/rccm.201104-0624OC. [DOI] [PubMed] [Google Scholar]
- 90.Marci M, Lozzi A, Miconi R, Panzini E, Monaldo M, Raffa S. D-dimer assays in patients with carotid atherosclerosis in clinical practice. Minerva Cardioangiol. 2000;48:97–102. [PubMed] [Google Scholar]
- 91.Medved L, Nieuwenhuizen W. Molecular mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost. 2003;89:409–19. [PubMed] [Google Scholar]
- 92.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–80. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 93.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost. 2007;5 (Suppl 1):116–24. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 94.Carr ME, Jr, Alving BM. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul Fibrinolysis. 1995;6:567–73. doi: 10.1097/00001721-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 95.Gabriel DA, Muga K, Boothroyd EM. The effect of fibrin structure on fibrinolysis. Journal of Biological Chemistry. 1992;267:24259–63. [PubMed] [Google Scholar]
- 96.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Reviews. 2007;21:131–42. doi: 10.1016/j.blre.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Shats EA, Nair CH, Dhall DP. Interaction of endothelial cells and fibroblasts with modified fibrin networks: role in atherosclerosis. Atherosclerosis. 1997;129:9–15. doi: 10.1016/s0021-9150(96)06003-0. [DOI] [PubMed] [Google Scholar]
- 98.Siebenlist KR, Mosesson MW. Progressive cross-linking of fibrin gamma chains increases resistance to fibrinolysis. Journal of Biological Chemistry. 1994;269:28414–9. [PubMed] [Google Scholar]
- 99.Murciano JC, Harshaw D, Neschis DG, Koniaris L, Bdeir K, Medinilla S, et al. Platelets inhibit the lysis of pulmonary microemboli. Am J Physiol Lung Cell Mol Physiol. 2002;282:L529–39. doi: 10.1152/ajplung.00112.2001. [DOI] [PubMed] [Google Scholar]
- 100.Bootle-Wilbraham CA, Tazzyman S, Thompson WD, Stirk CM, Lewis CE. Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro. Angiogenesis. 2001;4:269–75. doi: 10.1023/a:1016076121918. [DOI] [PubMed] [Google Scholar]
- 101.Naito M, Stirk CM, Smith EB, Thompson WD. Smooth muscle cell outgrowth stimulated by fibrin degradation products. The potential role of fibrin fragment E in restenosis and atherogenesis. Thromb Res. 2000;98:165–74. doi: 10.1016/s0049-3848(99)00202-9. [DOI] [PubMed] [Google Scholar]
- 102.Gross TJ, Leavell KJ, Peterson MW. CD11b/CD18 mediates the neutrophil chemotactic activity of fibrin degradation product D domain. Thromb Haemost. 1997;77:894–900. [PubMed] [Google Scholar]
- 103.Lishko VK, Podolnikova NP, Yakubenko VP, Yakovlev S, Medved L, Yadav SP, et al. Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1) Journal of Biological Chemistry. 2004;279:44897–906. doi: 10.1074/jbc.M408012200. [DOI] [PubMed] [Google Scholar]
- 104.Kunitada S, FitzGerald GA, Fitzgerald DJ. Inhibition of clot lysis and decreased binding of tissue-type plasminogen activator as a consequence of clot retraction. Blood. 1992;79:1420–7. [PubMed] [Google Scholar]
- 105.Tan SM. The leucocyte beta2 (CD18) integrins: the structure, functional regulation and signalling properties. Biosci Rep. 2012;32:241–69. doi: 10.1042/BSR20110101. [DOI] [PubMed] [Google Scholar]
- 106.Kim JP, Zhang K, Chen JD, Wynn KC, Kramer RH, Woodley DT. Mechanism of human keratinocyte migration on fibronectin: unique roles of RGD site and integrins. J Cell Physiol. 1992;151:443–50. doi: 10.1002/jcp.1041510303. [DOI] [PubMed] [Google Scholar]
- 107.Gorodetsky R, Vexler A, An J, Mou X, Marx G. Haptotactic and growth stimulatory effects of fibrin(ogen) and thrombin on cultured fibroblasts. J Lab Clin Med. 1998;131:269–80. doi: 10.1016/s0022-2143(98)90100-7. [DOI] [PubMed] [Google Scholar]
- 108.Kubo M, Van de Water L, Plantefaber LC, Mosesson MW, Simon M, Tonnesen MG, et al. Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol. 2001;117:1369–81. doi: 10.1046/j.0022-202x.2001.01551.x. [DOI] [PubMed] [Google Scholar]
- 109.Bergel S. Uber wirkungen des fibrins. Dtsch Med Wochenschr. 1909:35. [Google Scholar]
- 110.Tidrick RTWJ. Fibrin fixation of skin transplants. Surgery. 1944;15:90–5. [Google Scholar]
- 111.Cronkite EPL, EL, Deaver JM. Use of thrombin and fibrinogen in skin grafting. Journal of the American Medical Association. 1944;124:976–8. [Google Scholar]
- 112.Spotnitz WD. Fibrin sealant in the United States: clinical use at the University of Virginia. Thromb Haemost. 1995;74:482–5. [PubMed] [Google Scholar]
- 113.Chung W, Kazemi P, Ko D, Sun C, Brown CJ, Raval M, et al. Anal fistula plug and fibrin glue versus conventional treatment in repair of complex anal fistulas. Am J Surg. 2009;197:604–8. doi: 10.1016/j.amjsurg.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 114.Ruggiero R, Procaccini E, Gili S, Cremone C, Docimo G, Iovino F, et al. Fibrin glue to reduce seroma after axillary lymphadenectomy for breast cancer. Minerva Chir. 2008;63:249–54. [PubMed] [Google Scholar]
- 115.Fortelny RH, Schwab R, Glaser KS, Puchner KU, May C, Konig F, et al. The assessment of quality of life in a trial on lightweight mesh fixation with fibrin sealant in transabdominal preperitoneal hernia repair. Hernia. 2008;12:499–505. doi: 10.1007/s10029-008-0365-1. [DOI] [PubMed] [Google Scholar]
- 116.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 117.Le Guehennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cell Mater. 2004;8:1–10. doi: 10.22203/ecm.v008a01. discussion -1. [DOI] [PubMed] [Google Scholar]
- 118.Bielecki T, Dohan Ehrenfest DM. Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr Pharm Biotechnol. 2012;13:1121–30. doi: 10.2174/138920112800624292. [DOI] [PubMed] [Google Scholar]
- 119.Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Control Release. 2010;148:49–55. doi: 10.1016/j.jconrel.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chekanov VS, Zargarian M, Baibekov I, Karakozov P, Tchekanov G, Hare J, et al. Deferoxamine-fibrin accelerates angiogenesis in a rabbit model of peripheral ischemia. Vasc Med. 2003;8:157–62. doi: 10.1191/1358863x03vm491oa. [DOI] [PubMed] [Google Scholar]
- 121.Ferris D, Frisbie D, Kisiday J, McIlwraith CW. In vivo healing of meniscal lacerations using bone marrow-derived mesenchymal stem cells and fibrin glue. Stem Cells Int. 2012;2012:691605. doi: 10.1155/2012/691605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yucel EA, Oral O, Olgac V, Oral CK. Effects of fibrin glue on wound healing in oral cavity. J Dent. 2003;31:569–75. doi: 10.1016/s0300-5712(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 123.He M, Gan AW, Lim AY, Goh JC, Hui JH, Lee EH, et al. The effect of fibrin glue on tendon healing and adhesion formation in a rabbit model of flexor tendon injury and repair. J Plast Surg Hand Surg. 2013 doi: 10.3109/2000656X.2013.789037. [DOI] [PubMed] [Google Scholar]
- 124.Vinatier C, Gauthier O, Masson M, Malard O, Moreau A, Fellah BH, et al. Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J Biomed Mater Res A. 2009;89:176–85. doi: 10.1002/jbm.a.31988. [DOI] [PubMed] [Google Scholar]
- 125.Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003;89:573–82. [PubMed] [Google Scholar]
- 126.Le Nihouannen D, Guehennec LL, Rouillon T, Pilet P, Bilban M, Layrolle P, et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006;27:2716–22. doi: 10.1016/j.biomaterials.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 127.Currie LJ, Sharpe JR, Martin R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001;108:1713–26. doi: 10.1097/00006534-200111000-00045. [DOI] [PubMed] [Google Scholar]
- 128.Thompson DF, Letassy NA, Thompson GD. Fibrin glue: a review of its preparation, efficacy, and adverse effects as a topical hemostat. Drug Intell Clin Pharm. 1988;22:946–52. doi: 10.1177/106002808802201203. [DOI] [PubMed] [Google Scholar]
- 129.Fezza JP, Cartwright M, Mack W, Flaharty P. The use of aerosolized fibrin glue in face-lift surgery. Plast Reconstr Surg. 2002;110:658–64. doi: 10.1097/00006534-200208000-00044. discussion 65–6. [DOI] [PubMed] [Google Scholar]
- 130.Sawamura Y, Asaoka K, Terasaka S, Tada M, Uchida T. Evaluation of application techniques of fibrin sealant to prevent cerebrospinal fluid leakage: a new device for the application of aerosolized fibrin glue. Neurosurgery. 1999;44:332–7. doi: 10.1097/00006123-199902000-00048. [DOI] [PubMed] [Google Scholar]
- 131.Karp JM, Sarraf F, Shoichet MS, Davies JE. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J Biomed Mater Res A. 2004;71:162–71. doi: 10.1002/jbm.a.30147. [DOI] [PubMed] [Google Scholar]
- 132.Hanson AJ, Quinn MT. Effect of fibrin sealant composition on human neutrophil chemotaxis. J Biomed Mater Res. 2002;61:474–81. doi: 10.1002/jbm.10196. [DOI] [PubMed] [Google Scholar]
- 133.Ho W, Tawil B, Dunn JC, Wu BM. The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587–95. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 134.Lawrie AS, McDonald SJ, Purdy G, Mackie IJ, Machin SJ. Prothrombin time derived fibrinogen determination on Sysmex CA-6000. J Clin Pathol. 1998;51:462–6. doi: 10.1136/jcp.51.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Potier ENJ, Sprecher C, Ito K. Influencing biophysical properties of fibrin with buffer solutions. J Mater Sci. 2010;45:2494–503. [Google Scholar]
- 136.Carr MEGD, McDonagh J. Influence of Ca2+ on the structure of reptilase-derived and thrombin-derived fibrin gels. Biochem J. 1986;239:513–0. doi: 10.1042/bj2390513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yao L, Swartz DD, Gugino SF, Russell JA, Andreadis ST. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng. 2005;11:991–1003. doi: 10.1089/ten.2005.11.991. [DOI] [PubMed] [Google Scholar]
- 138.Duong H, Wu B, Tawil B. Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen-thrombin compositions and by extrinsic cellular activity. Tissue Eng Part A. 2009;15:1865–76. doi: 10.1089/ten.tea.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. Journal of Biological Chemistry. 2000;275:6813–8. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 141.Cox S, Cole M, Tawil B. Behavior of human dermal fibroblasts in three-dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 2004;10:942–54. doi: 10.1089/1076327041348392. [DOI] [PubMed] [Google Scholar]
- 142.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 143.Rowe SL, Lee S, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007;3:59–67. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gugerell A, Schossleitner K, Wolbank S, Nurnberger S, Redl H, Gulle H, et al. High thrombin concentrations in fibrin sealants induce apoptosis in human keratinocytes. J Biomed Mater Res A. 2012;100:1239–47. doi: 10.1002/jbm.a.34007. [DOI] [PubMed] [Google Scholar]
- 145.Bachli EB, Pech CM, Johnson KM, Johnson DJ, Tuddenham EG, McVey JH. Factor Xa and thrombin, but not factor VIIa, elicit specific cellular responses in dermal fibroblasts. J Thromb Haemost. 2003;1:1935–44. doi: 10.1046/j.1538-7836.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 146.Davis HE, Miller SL, Case EM, Leach JK. Supplementation of fibrin gels with sodium chloride enhances physical properties and ensuing osteogenic response. Acta Biomater. 2011;7:691–9. doi: 10.1016/j.actbio.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 147.Laudano AP, Doolittle RF. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980;19:1013–9. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- 148.Laudano AP, Doolittle RF. Influence of calcium ion on the binding of fibrin amino terminal peptides to fibrinogen. Science. 1981;212:457–9. doi: 10.1126/science.7209542. [DOI] [PubMed] [Google Scholar]
- 149.Pandi L, Kollman JM, Lopez-Lira F, Burrows JM, Riley M, Doolittle RF. Two families of synthetic peptides that enhance fibrin turbidity and delay fibrinolysis by different mechanisms. Biochemistry. 2009;48:7201–8. doi: 10.1021/bi900647g. [DOI] [PubMed] [Google Scholar]
- 150.Stabenfeldt SE, Gossett JJ, Barker TH. Building better fibrin knob mimics: an investigation of synthetic fibrin knob peptide structures in solution and their dynamic binding with fibrinogen/fibrin holes. Blood. 2010;116:1352–9. doi: 10.1182/blood-2009-11-251801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stabenfeldt SE, Aboujamous NM, Soon AS, Barker TH. A new direction for anticoagulants: Inhibiting fibrin assembly with PEGylated fibrin knob mimics. Biotechnol Bioeng. 2011 doi: 10.1002/bit.23184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soon AS, Stabenfeldt SE, Brown WE, Barker TH. Engineering fibrin matrices: the engagement of polymerization pockets through fibrin knob technology for the delivery and retention of therapeutic proteins. Biomaterials. 2010;31:1944–54. doi: 10.1016/j.biomaterials.2009.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soon AS, Smith MH, Herman ES, Lyon LA, Barker TH. Development of Self-Assembling Mixed Protein Micelles with Temperature-Modulated Avidities. Adv Healthc Mater. 2013 doi: 10.1002/adhm.201200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shainoff JR, Dardik BN. Fibrinopeptide B and aggregation of fibrinogen. Science. 1979;204:200–2. doi: 10.1126/science.155308. [DOI] [PubMed] [Google Scholar]
- 155.Rowe SL, Stegemann JP. Microstructure and mechanics of collagen-fibrin matrices polymerized using ancrod snake venom enzyme. J Biomech Eng. 2009;131:061012. doi: 10.1115/1.3128673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Temenoff JS, Athanasiou KA, LeBaron RG, Mikos AG. Effect of poly(ethylene glycol) molecular weight on tensile and swelling properties of oligo(poly(ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429–37. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 157.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407–17. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 158.Jing P, Rudra JS, Herr AB, Collier JH. Self-assembling peptide-polymer hydrogels designed from the coiled coil region of fibrin. Biomacromolecules. 2008;9:2438–46. doi: 10.1021/bm800459v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barker TH, Fuller GM, Klinger MM, Feldman DS, Hagood JS. Modification of fibrinogen with poly(ethylene glycol) and its effects on fibrin clot characteristics. J Biomed Mater Res. 2001;56:529–35. doi: 10.1002/1097-4636(20010915)56:4<529::aid-jbm1124>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 160.Seetharaman S, Natesan S, Stowers RS, Mullens C, Baer DG, Suggs LJ, et al. A PEGylated fibrin-based wound dressing with antimicrobial and angiogenic activity. Acta Biomater. 2011;7:2787–96. doi: 10.1016/j.actbio.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 161.Drinnan CT, Zhang G, Alexander MA, Pulido AS, Suggs LJ. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Control Release. 2010;147:180–6. doi: 10.1016/j.jconrel.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 162.Ricles LM, Nam SY, Sokolov K, Emelianov SY, Suggs LJ. Function of mesenchymal stem cells following loading of gold nanotracers. Int J Nanomedicine. 2011;6:407–16. doi: 10.2147/IJN.S16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chung E, Nam SY, Ricles LM, Emelianov SY, Suggs LJ. Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications. Int J Nanomedicine. 2013;8:325–36. doi: 10.2147/IJN.S36711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496–506. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 165.Seliktar D. Extracellular stimulation in tissue engineering. Ann N Y Acad Sci. 2005;1047:386–94. doi: 10.1196/annals.1341.034. [DOI] [PubMed] [Google Scholar]
- 166.Frisman I, Seliktar D, Bianco-Peled H. Nanostructuring PEG-fibrinogen hydrogels to control cellular morphogenesis. Biomaterials. 2011;32:7839–46. doi: 10.1016/j.biomaterials.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 167.Frisman I, Orbach R, Seliktar D, Bianco-Peled H. Structural investigation of PEG-fibrinogen conjugates. J Mater Sci Mater Med. 2010;21:73–80. doi: 10.1007/s10856-009-3848-4. [DOI] [PubMed] [Google Scholar]
- 168.Ben-David D, Srouji S, Shapira-Schweitzer K, Kossover O, Ivanir E, Kuhn G, et al. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials. 2013;34:2902–10. doi: 10.1016/j.biomaterials.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 169.Lee CR, Grad S, Gorna K, Gogolewski S, Goessl A, Alini M. Fibrin-polyurethane composites for articular cartilage tissue engineering: a preliminary analysis. Tissue Eng. 2005;11:1562–73. doi: 10.1089/ten.2005.11.1562. [DOI] [PubMed] [Google Scholar]
- 170.Akpalo E, Bidault L, Boissiere M, Vancaeyzeele C, Fichet O, Larreta-Garde V. Fibrin-polyethylene oxide interpenetrating polymer networks: new self-supported biomaterials combining the properties of both protein gel and synthetic polymer. Acta Biomater. 2011;7:2418–27. doi: 10.1016/j.actbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 171.Van Lieshout M, Peters G, Rutten M, Baaijens F. A knitted, fibrin-covered polycaprolactone scaffold for tissue engineering of the aortic valve. Tissue Eng. 2006;12:481–7. doi: 10.1089/ten.2006.12.481. [DOI] [PubMed] [Google Scholar]
- 172.Collen A, Smorenburg SM, Peters E, Lupu F, Koolwijk P, Van Noorden C, et al. Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro. Cancer Res. 2000;60:6196–200. [PubMed] [Google Scholar]
- 173.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–64. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rahmany MB, Hantgan RR, Van Dyke M. A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials. 2013;34:2492–500. doi: 10.1016/j.biomaterials.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 175.Chen Z, Wang L, Stegemann JP. Phase-separated chitosan-fibrin microbeads for cell delivery. J Microencapsul. 2011;28:344–52. doi: 10.3109/02652048.2011.569764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voge CM, Johns J, Raghavan M, Morris MD, Stegemann JP. Wrapping and dispersion of multiwalled carbon nanotubes improves electrical conductivity of protein-nanotube composite biomaterials. J Biomed Mater Res A. 2013;101:231–8. doi: 10.1002/jbm.a.34310. [DOI] [PubMed] [Google Scholar]
- 177.Kang SW, Kim JS, Park KS, Cha BH, Shim JH, Kim JY, et al. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone. 2011;48:298–306. doi: 10.1016/j.bone.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 178.Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32:7856–69. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 179.Kulkarni MM, Greiser U, O’Brien T, Pandit A. A temporal gene delivery system based on fibrin microspheres. Mol Pharm. 2011;8:439–46. doi: 10.1021/mp100295z. [DOI] [PubMed] [Google Scholar]
- 180.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 181.Lai VK, Frey CR, Kerandi AM, Lake SP, Tranquillo RT, Barocas VH. Microstructural and mechanical differences between digested collagen-fibrin co-gels and pure collagen and fibrin gels. Acta Biomater. 2012;8:4031–42. doi: 10.1016/j.actbio.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of engineered vascular constructs made from collagen, fibrin, and collagen-fibrin mixtures. Biomaterials. 2004;25:3699–706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 183.Rowe SL, Stegemann JP. Interpenetrating collagen-fibrin composite matrices with varying protein contents and ratios. Biomacromolecules. 2006;7:2942–8. doi: 10.1021/bm0602233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang HS, Bhang SH, Hwang JW, Kim DI, Kim BS. Delivery of basic fibroblast growth factor using heparin-conjugated fibrin for therapeutic angiogenesis. Tissue Eng Part A. 2010;16:2113–9. doi: 10.1089/ten.TEA.2009.0673. [DOI] [PubMed] [Google Scholar]
- 185.Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, et al. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106:970–9. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 186.Yang HS, La WG, Bhang SH, Jeon JY, Lee JH, Kim BS. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225–33. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 187.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–58. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 188.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 189.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. FASEB J. 1999;13:2214–24. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 190.Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, et al. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 2009;5:959–68. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feng X, Tonnesen MG, Mousa SA, Clark RA. Fibrin and collagen differentially but synergistically regulate sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int J Cell Biol. 2013;2013:231279. doi: 10.1155/2013/231279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Feng X, Clark RA, Galanakis D, Tonnesen MG. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of alphav/beta3 mRNA by fibrin1. J Invest Dermatol. 1999;113:913–9. doi: 10.1046/j.1523-1747.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 193.Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J Cell Biol. 1998;140:709–19. doi: 10.1083/jcb.140.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ahmed TA, Griffith M, Hincke M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng. 2007;13:1469–77. doi: 10.1089/ten.2006.0354. [DOI] [PubMed] [Google Scholar]
- 195.Kupcsik L, Alini M, Stoddart MJ. Epsilon-aminocaproic acid is a useful fibrin degradation inhibitor for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2309–13. doi: 10.1089/ten.tea.2008.0400. [DOI] [PubMed] [Google Scholar]
- 196.Sperzel M, Huetter J. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007;5:2113–8. doi: 10.1111/j.1538-7836.2007.02717.x. [DOI] [PubMed] [Google Scholar]
- 197.Lorentz KM, Kontos S, Frey P, Hubbell JA. Engineered aprotinin for improved stability of fibrin biomaterials. Biomaterials. 2011;32:430–8. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- 198.Ahmann KA, Weinbaum JS, Johnson SL, Tranquillo RT. Fibrin degradation enhances vascular smooth muscle cell proliferation and matrix deposition in fibrin-based tissue constructs fabricated in vitro. Tissue Eng Part A. 2010;16:3261–70. doi: 10.1089/ten.tea.2009.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105:6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Elvin CM, Danon SJ, Brownlee AG, White JF, Hickey M, Liyou NE, et al. Evaluation of photo-crosslinked fibrinogen as a rapid and strong tissue adhesive. J Biomed Mater Res A. 2010;93:687–95. doi: 10.1002/jbm.a.32572. [DOI] [PubMed] [Google Scholar]
- 201.Syedain ZH, Bjork J, Sando L, Tranquillo RT. Controlled compaction with ruthenium-catalyzed photochemical cross-linking of fibrin-based engineered connective tissue. Biomaterials. 2009;30:6695–701. doi: 10.1016/j.biomaterials.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Elvin CM, Brownlee AG, Huson MG, Tebb TA, Kim M, Lyons RE, et al. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials. 2009;30:2059–65. doi: 10.1016/j.biomaterials.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 203.Sander EA, Barocas VH, Tranquillo RT. Initial fiber alignment pattern alters extracellular matrix synthesis in fibroblast-populated fibrin gel cruciforms and correlates with predicted tension. Ann Biomed Eng. 2011;39:714–29. doi: 10.1007/s10439-010-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Namani R, Wood MD, Sakiyama-Elbert SE, Bayly PV. Anisotropic mechanical properties of magnetically aligned fibrin gels measured by magnetic resonance elastography. J Biomech. 2009;42:2047–53. doi: 10.1016/j.jbiomech.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gersh KC, Edmondson KE, Weisel JW. Flow rate and fibrin fiber alignment. J Thromb Haemost. 2010;8:2826–8. doi: 10.1111/j.1538-7836.2010.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Carlisle CR, Coulais C, Namboothiry M, Carroll DL, Hantgan RR, Guthold M. The mechanical properties of individual, electrospun fibrinogen fibers. Biomaterials. 2009;30:1205–13. doi: 10.1016/j.biomaterials.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Barocas VH, Tranquillo RT. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J Biomech Eng. 1997;119:137–45. doi: 10.1115/1.2796072. [DOI] [PubMed] [Google Scholar]
- 208.Neidert MR, Tranquillo RT. Tissue-engineered valves with commissural alignment. Tissue Eng. 2006;12:891–903. doi: 10.1089/ten.2006.12.891. [DOI] [PubMed] [Google Scholar]
- 209.Alsberg E, Feinstein E, Joy MP, Prentiss M, Ingber DE. Magnetically-guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 2006;12:3247–56. doi: 10.1089/ten.2006.12.3247. [DOI] [PubMed] [Google Scholar]
- 210.Morin KT, Tranquillo RT. Guided sprouting from endothelial spheroids in fibrin gels aligned by magnetic fields and cell-induced gel compaction. Biomaterials. 2011;32:6111–8. doi: 10.1016/j.biomaterials.2011.05.018. [DOI] [PubMed] [Google Scholar]