Abstract
Chronic ethanol ingestion mildly damages liver through oxidative stress and lipid oxidation, which is ameliorated by dietary supplementation with the anti-inflammatory β-amino acid taurine. Kidney, like liver, expresses cytochrome P450 2E1 that catabolizes ethanol with free radical formation, and so also may be damaged by ethanol catabolism. Sudden loss of kidney function, and not liver disease itself, foreshadows mortality in patients with alcoholic hepatitis [J. Altamirano, Clin Gastroenterol Hepatol. 2012, 10:65]. We found ethanol ingestion in the Lieber-deCarli rat model increased kidney lipid oxidation, 4-hydroxynonenal protein adduction, and oxidatively truncated phospholipids that attract and activate leukocytes. Chronic ethanol ingestion increased myeloperoxidase-expressing cells in kidney and induced an inflammatory cell infiltrate. Apoptotic Terminal deoxynucleotidyl transferase Nick End Labeling (TUNEL)-positive cells and active caspase-3 increased in kidney after ethanol ingestion, with reduced filtration with increased circulating Blood Urea Nitrogen and creatinine. These events were accompanied by release of albumin, myeloperoxidase, and the Acute Kidney Injury biomarkers Kidney Injury Molecule-1 (KIM-1), Neutrophil Gelatinase-associated Lipocalin (NGAL), and Cystatin c to urine. Taurine sequesters HOCl from myeloperoxidase of activated leukocytes, and taurine supplementation reduced renal lipid oxidation, reduced leukocyte infiltration, and reduced the increase in myeloperoxidase-positive cells during ethanol feeding. Taurine supplementation also normalized circulating BUN and creatinine levels, and suppressed enhanced myeloperoxidase, albumin, KIM-1 and cystatin c in urine. Thus, chronic ethanol ingestion oxidatively damages kidney lipids and proteins, damages renal function, and induces Acute Kidney Injury through an inflammatory cell infiltrate. The anti-inflammatory nutraceutical taurine effectively interrupts this ethanol-induced inflammatory cycle in kidney.
Keywords: Kidney, Inflammation, Reactive Oxygen Species, Taurine, Acute Kidney Injury, Oxidized phospholipid, Free radicals
Introduction
Excessive alcohol use is common, with an estimated 8% of adult Americans abusing or depending on alcohol [1]. A significant subset of these individuals progress to alcoholic steatohepatitis and then to severe alcoholic hepatitis where 6-month mortality is nearly 40% [2].
Ethanol is oxidized in liver by three pathways, but metabolism by cytochrome P450 2E1 (CYP2E1) monooxygenase that forms superoxide (O2•−) and then H2O2, is the path that instigates tissue oxidative stress and damage [3]. Genetic ablation and transgenic rescue [4] show CYP2E1 participates in ethanol toxicity, while inhibition and genetic targeting to reduce reactive oxygen species (ROS) formation from type 2 NADPH oxidase (NOX2) shows this enzyme also is critical in ethanol toxicity [5, 6]. Thus, both tissue produced ROS from CYP2E1 and ROS from the NOX2 of invading inflammatory cells contribute to liver damage from ethanol catabolism.
The syndrome of Acute Kidney Injury (AKI) describes the sudden loss of kidney filtration with the retention of nitrogenous waste products in blood (i.e. blood urea nitrogen, BUN). Kidney expresses 5 to 10% of total body CYP2E1 [7–10] and while chronic alcoholism associates with chronic kidney disease [11–13], it is the development of AKI that associates with the mortality of alcoholic hepatitis [14]. This chronic ethanol use is modeled in rats by feeding relevant amounts of ethanol for 28 days [15]. Ethanol feeding well beyond this time structurally damages kidney [16, 17], but little is known about ethanol damage to this organ.
Free radical oxidation of cellular phospholipids generates a host of oxidatively truncated phospholipids. Leukocytes express the single receptor for the inflammatory phospholipid Platelet-activating Factor (PAF) [18], and some oxidized phospholipids sufficiently resemble the phospholipid PAF that they are ligands and agonists of this G protein coupled receptor [19]. This is relevant because biologic PAF metabolism is controlled [20], while formation of oxidatively truncated phospholipids is not [21], so oxidation can induce unregulated inflammation and leukocyte activation.
PAF attracts and activates neutrophils [22] that are the primary repository of myeloperoxidase. Myeloperoxidase uses H2O2 to form HOCl [23] that is primarily responsible for oxygen-dependent bacterial killing [24], but also damages host tissue.
The non-metabolizable β-amino acid taurine suppresses inflammation [25] and reduces hepatic lipid oxidative stress [26], protecting liver function during ethanol metabolism [27, 28]. Because it is non-metabolizable, the way(s) taurine is protective is ill-define, but its anti-inflammatory and anti-oxidative activity primarily [25] results from its sequestration of HOCl and HOBr [29]. However, taurine also regulates osmolarity, membrane stability, aids Ca++ homeostasis [25], and, in neurons, protects mitochondria and endoplasmic reticulum from stress [30].
We hypothesized ethanol catabolism imposes damaging oxidative stress in kidney since CYP2E1 is present in this organ. Because taurine protects against several kidney diseases [31] and has anti-inflammatory/anti-oxidant activity(ies), we also determined whether this β-amino acid would protect kidney from potential damage imposed by ethanol catabolism.
Material and Methods
Materials
Adult male Wistar rats (~170–180 g) were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). Lieber-deCarli liquid ethanol diet was from Dyets (Bethlehem, PA) and taurine from Sigma (St. Louis, MO). Anti-albumin antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), anti-4-hydroxynonenal antibody from Alpha Diagnostics (San Antonio, TX), while anti-myeloperoxidase, and anti-neutrophil gelatinase associated lipocalin (NGAL) antibodies were from Abcam (Cambridge, MA). Kits for blood urea nitrogen (BUN), and creatinine were purchased from Arbor Assays (Ann Arbor, MI). Anti-KIM-1 antibody, TUNEL assay kits, KIM-1 ELISA kits and cystatin C ELISA kits were from R&D Systems (Minneapolis, MN). Caspase-3 Apo-ONE Homogeneous caspase-3/7 fluorescence assay kits were the product of Promega (Madison, WI) and myeloperoxidase activity assay kits were from Cell Technology (Mountain View, CA).
Animal model and ethanol feeding
The chronic ethanol feeding model used in this study has been previously described [28] using animals that received humane care in a protocol approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Briefly, age-matched rat litters were randomly assigned to be ethanol-fed or pair fed a control diet where maltose dextrin isocalorically substituted for ethanol in the liquid diet to maintain equal body weights. For the first two days of the protocol, rats in the ethanol group were fed with a liquid diet with 17% of the calories supplied as ethanol and then were provided an ad libitum liquid diet for 4 weeks containing 6.4% (v/v) ethanol, which constitutes 36% of the total caloric content. The amount of ethanol in the circulation of these animals is not excessive, being in the clinically relevant 0.1–0.15% range. Rats were anesthetized and exsanguinated, with serum isolated and stored at −80°. Kidneys were isolated and stored in RNAlater™ for RNA quantification, or at −80°C until homogenization. A small portion of the kidney was immediately fixed in 10% formalin for histology. For taurine supplementation, Lieber-deCarli ethanol diets or pair-fed diets were supplemented with 30 g of taurine per Liter of diet [32].
Collection of urine
Urine from was collected by syringe withdrawal from the urinary bladder after anesthesia injection just prior to sacrifice. Samples were immediately centrifuged (300 × g, 5 min) to remove debris or casts before storage at −80°C.
Western blots
Excised rat kidneys were washed with PBS, and homogenized 10 times in a Potter-Elvehjem glass homogenizer with a tight pestle containing 1× lysis buffer (Cell Signaling Technology) with protease inhibitor (Sigma) on ice before centrifugation (30 min, 14,000 × g). The resulting supernatants were mixed with Laemmli gel loading buffer containing 10% SDS and 200 mM DTT, followed by boiling. For western blotting urine samples, equal volume of urine samples were mixed with 6× Laemmli buffer containing 10% SDS and 200 mM DDT, followed by boiling. SDS-PAGE, unless otherwise stated, occurred in 10–12% gels that were blotted onto nitrocellulose membranes (Bio-Rad) and blocked with 5% nonfat dry milk (Bio-Rad). Detection used anti-CYP2E1 (Abcam, 1:2000), anti-myeloperoxidase (Abcam, 1:2000), anti-KIM-1 (R&D Systems, 1:2000), anti-NGAL (Abcam, 1:2000), or anti-albumin (Santa Cruz, 1:10000) antibodies (Abcam, 1:5000) incubated overnight at 4°C. The conjugates were then ligated by HRP-conjugated anti-rabbit (1:5000) or anti-mouse (1:10000) or anti-goat (1:20000) antibody before detection with Amersham Biosciences ECL Prime. Blots were reprobed with anti-β-actin (Santa Cruz Biotechnology).
TUNEL assay
Kidneys were fixed in 10% buffered formalin and embedded in paraffin. Kidney sections (5 μm) were deparaffinized in Safeclear II Xylene substitute and consecutively hydrated in 100%, 95%, 85% and 70% ethanol followed by two washes in PBS. TUNEL staining was performed according to the manufacturer’s (R&D Systems) protocol. Briefly, kidney sections were treated with proteinase K (30 min) for antigen retrieval before peroxidase activity was blocked with methanol and hydrogen peroxide. The sections were washed with PBS and incubated with labeling buffer followed by the reaction mix containing TdT, dNTP, and TdT enzyme with Mn2+ for 60 min at 37°C. Sections were again washed with PBS before incubation with Strep-HRP solution for 10 min at 37°C. After washing in PBS, the sections were treated with diaminobenzidine chromogen solution for 5 min at room temperature, washed, and immersed in 1% methyl green for 1 min. The sections were air dried and mounted with mounting medium. Images acquired with a 60X objective.
Caspase-3 activity
Caspase-3 activity was measured with Apo-ONE Homogeneous Caspase-3/7 assay kits (Promega) using crude kidney homogenates according to the manufacturer’s protocol. Briefly, 50 μl of Apo-ONE Caspase-3/7 reagent and 50 μl of kidney homogenate were co-incubated for 1 hr at 37°C before the fluorescence intensity was measured (SpectraMax 100) with excitation at 499 nm and emission at 515 nm. The background fluorescence was corrected by subtracting the value derived from the no-enzyme control.
RNA isolation and quantitative reverse transcription-PCR
Total RNA was extracted from kidney tissues preserved in RNAlater (Qiagen, Germantown, MD) using RNeasy mini kits (Qiagen). RNA content was measured in a NanoDrop ND-1000 spectrophotometer. mRNA was quantified by SYBR Green (Qiagen) one-step reverse transcription-PCR for CD18, CD64, MPO and S18 with the Bio-Rad MyiQ real-time PCR detection system. The primers for rat CD64 (sense, 5′-GCTGAAAGGGAAGATGATGG-3′; antisense, 5′-ATGACTGGGGACCAAATAGC-3′), rat CD18 (sense-5′-ACCCATAAGGCAGCTGAATG-3′; sense-5′-AGGGGGTCACCAAACTTTTC-3′) and rat MPO (sense, 5′-TCTTCGTGCGAGAGCATAAC-3′; antisense-5′-AAAGCTTCTCCCCATTCCAG-3′) were purchased from IDT (Coralville, IA). mRNA expression was normalized to 18S mRNA content and 2−ΔΔCT was used to calculate the fold change.
Immunohistochemistry
Kidneys were fixed in 10% buffered formalin, embedded in paraffin, and sectioned before deparaffinization in Safeclear II Xylene substitute and then consecutively hydrated in 100%, 95%, 85% and 70% ethanol followed by two washes in PBS. Sections were stained with periodate-Schiff and hematoxylin by the Cleveland Clinic Imaging core. 4-Hydroxynonenal and myeloperoxidase: Sections were treated with 10 mM citrate buffer (pH 6.0) at 95–100°C for 15 min for antigen retrieval, treated with peroxidase block (Pierce) for 30 min, washed and blocked with 10% donkey serum and 0.1% Triton X-100 in PBS before incubation with polyclonal anti-rabbit 4-hydroxynonenal antibody (1:200) or anti-myeloperoxidase antibody (1:200) for 1 hr. After 3 washes in PBS, the sections were incubated with SP-conjugated streptavidin for 30 min followed by HRP for 30 min. After washing in PBS, the sections were treated with substrate-chromogen solution (diaminobenzoate/Metal concentrate) (Pierce) for 2–5 min at room temperature. Sections were washed and mounted with mounting medium. Images acquired with a 60X objective. KIM-1: Sections were treated with proteinase K (20 μg/ml, 15 min) for antigen retrieval. Washed sections were blocked with 10% donkey serum and 0.1% Triton X-100 in phosphate-buffered saline and incubated overnight with 1:200 polyclonal anti-rat KIM-1 antibody. Washed sections were incubated with 1:1000 Alexa Fluor488-conjugated donkey-anti-rat IgG (Invitrogen). Sections were washed and mounted with VECTASHIELD (Vector Laboratories). Images were acquired at 40X. E06: Deparaffinized and hydrated kidney sections were treated with proteinase K (20 μg/ml, 15 min) for antigen retrieval. Washed sections were blocked with 10% goat serum and 0.1% Triton X-100 in phosphate-buffered saline and incubated overnight with 1:200 polyclonal anti-mouse E06 monoclonal antibody. Washed sections were incubated with 1:1000 Alexa Fluor568-conjugated goat anti-mouse IgG (Invitrogen). Sections were washed and mounted with VECTASHIELD (Vector Laboratories). Images were acquired at 60X.
Blood Urea Nitrogen (BUN), creatinine and albumin
BUN, and serum and urinary creatinine were quantified by kits according to the manufacturer’s (Arbor Assays) protocol. Quantichrom BCG Albumin Assay Kits (BioAssay Systems) were used to measure serum and urinary albumin. KIM-1 and cystatin c ELISA kits (R&D Systems) were used to measure urinary proteins.
Mass spectrometric analysis of oxidized phospholipid
The phospholipid 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine was extracted with [2H]PAF as an internal standard and purified over an aminopropyl column. This material was quantified by liquid chromatographic electrospray ionization in the positive ion mode with multiple reaction monitoring as before [33]. The phosphocholine m/z 184 product ion and the precursor to product ion transition of m/z 667 → 184 defined this phospholipid.
Myeloperoxidase activity
Myeloperoxidase chlorination and peroxidation activities were determined with EnzChek Myeloperoxidase activity assay kits from Invitrogen. Briefly, the cell free supernatant was prepared as described above and 50 μl of supernatant was mixed with 2× 3′-(p-aminophenyl) fluorescein and incubated at room temperature for 1 h, protected from light. This reaction was stopped with 10× chlorination inhibitor (10 μl) and the fluorescence intensity was measured by excitation at 485 nm with emission at 530 nm. For peroxidation assays, 50 μl of sample was mixed with 50 μ of 2× Amplex UltraRed reagent. The samples were incubated for 1 h, stopped with 10 μl peroxidation inhibitor, and the fluorescence intensity measured by excitation at 530 nm with emission at 590 nm.
Data Analysis
All data are presented as mean ± S.E.M. Independent Student’s t test (two groups) or two-way analysis of variance (for multiple groups) was performed by Prism4 statistics software (Supplemental material). Statistical significance was considered to be p < 0.05.
RESULTS
Kidneys of ethanol-fed rats are oxidatively damaged
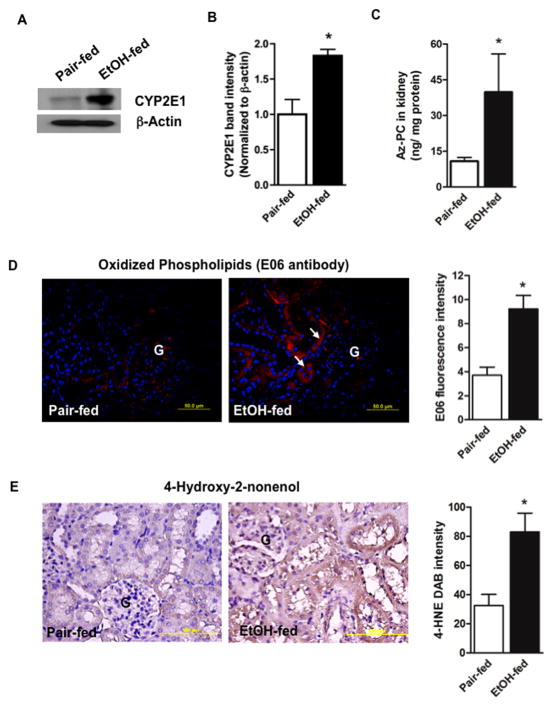
Kidney expresses the ethanol catabolic enzyme CYP2E1 [7–10] and, as in liver, ethanol ingestion induced renal CYP2E1 expression (Fig. 1A), which was significant (Fig. 1B). CYP2E1 metabolism of ethanol generates free radicals [3] that attack polyunsaturated fatty acyl residues, with oxidative fragmentation that uniquely generates the truncated phospholipid azelaoyl-phosphatidylcholine [19]. Quantitative mass spectrometry showed this PAF-like leukocyte agonist [34] increased in kidney of ethanol fed rats (Fig. 1C). Correspondingly, staining by a monoclonal antibody, E06, that recognizes just oxidized phospholipids [35] also significantly increased in the kidney of ethanol fed animals (Fig. 1D). These oxidized phospholipid-protein adducts were primarily present in tubules, not in glomeruli (denoted by “G”). Fragments of fatty acyl residues are released during this oxidation of cellular phospholipids and some of these, including 4-hydroxynonenal, are chemically reactive and adduct cellular proteins. Kidneys of ethanol-fed animals, relative to those from rats ingesting a control diet, were significantly modified by the 4-hydroxynonenal fragment (Fig. 1E). Again, tubular epithelial cells, and not Glomeruli, contained these adducts.
Figure 1. Chronic ethanol feeding induces oxidative stress in rat kidney.
A) Kidney expresses ethanol-inducible CYP2E1. Western blotting shows CYP2E1 expression (55 kDa) in the crude homogenates (20 μg) of kidneys from pair-fed control and ethanol-fed rats. Equal loading of protein was confirmed by re-probing the blot with anti-β-actin antibody. B) Densitometry of CYP2E1 immunoblots. Band densities were determined with a Kodak Image Station and are expressed as mean ± SEM, n=3 with p<0.05 (*) considered significant. C) The oxidized phospholipid azelaoyl phosphatidylcholine increases in kidney after ethanol feeding. Quantitative mass spectrometry of palmitoyl azelaoyl phosphatidylcholine (Az-PC) using [2H]PAF as an internal standard was identified by elution time and the precursor/daughter ion transition m/z 667 → 184. n=6, p<0.05 (*). D) Oxidized phospholipid epitopes accumulate in the kidney of ethanol fed rats. Antigens were retrieved from formalin-fixed and paraffin-embedded kidney sections and stained for the E06 epitope of oxidized choline phospholipid adducts using Alexa Fluor568-conjugated secondary antibody (red fluorescence) with DAPI (blue) nuclear stain. n=3. “G” glomerulus; arrows, tubular wall red fluorescence. E06 immunofluorescence was quantified in 4 to 5 sections per kidney of pair-fed and ethanol-fed rats using ImageJ 1.47v software (NIH), and the data are expressed as mean ± SEM, n=3 p<0.05 (*). E) Reactive fatty acyl fragments adduct kidney protein after chronic ethanol feeding. Formalin-fixed and paraffin-embedded kidney sections were stained for the oxidative injury marker 4-hydroxynonenal (4-HNE) and developed using diaminobenzidine as the chromogen that generates a brown precipitate. The figure is representative of four to five sections from kidney of pair-fed and ethanol-fed rats (n=4) chronically ingested ethanol or pair-fed a control diet for 28 days. The 4-HNE DAB brown intensity was quantified in 4 to 5 sections per kidney using ImageJ and the data are expressed as mean ± SEM, n=4. p<0.05 (*).
Ethanol ingestion induces leukocyte infiltration into kidney
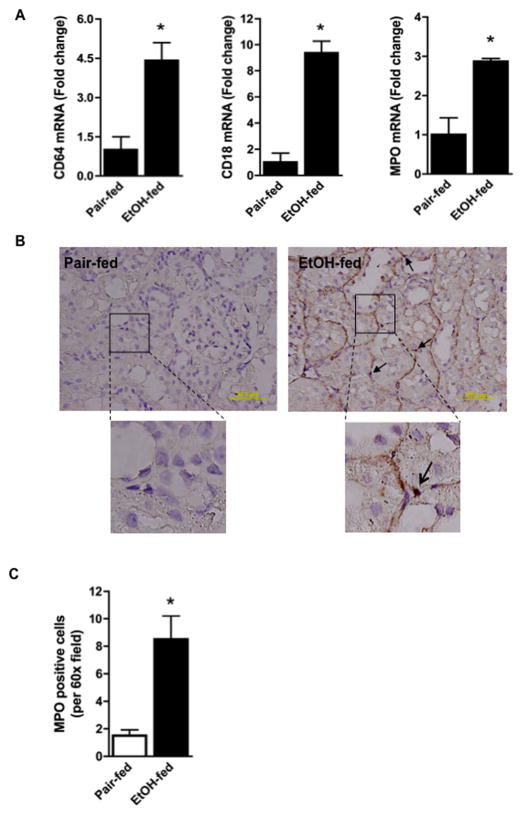
Ethanol feeding induced an influx of inflammatory cells into kidney as shown by the increase in mRNA encoding the CD64 (FcγR1) marker of activated leukocytes, and the leukocyte specific integrin CD18 (Fig. 2A). Approximately 95% of all myeloperoxidase accumulates within neutrophils, and ethanol feeding significantly increased the content of this mRNA in kidney as well. Immunohistochemistry revealed myeloperoxidase-positive cells adjacent to kidney tubular epithelium accompanied by strong peri-tubular staining after ethanol feeding (Fig. 2B). The result of this ethanol feeding regimen was that the number of myeloperoxidase-positive cells in kidney increased by five-fold (Fig. 2C).
Figure 2. Chronic ethanol feeding increases renal inflammation.
A) Ethanol feeding increases inflammatory cell mRNA in kidney. Total RNA was extracted from kidney and mRNA was quantified by SYBR Green onestep reverse transcription-PCR for CD64 (left), CD18 (center), myeloperoxidase (MPO, right) and ribosomal S18. mRNA expression was normalized to S18 mRNA content and 2−ΔΔCT was used to calculate change. Data are expressed as mean ± SEM (CD64, n=10; CD18, n=10; MPO, n=4). p<0.05 (*) B) Myeloperoxidase positive cells increase in kidney during ethanol feeding. Sections of kidneys from rats fed the ethanol diet or their pair-fed controls were assayed for myeloperoxidase enzymatic activity. The reaction is marked by deposition of dark brown reaction product as described in “Methods”. These images are representative of four to five sections per kidney of pair-fed and ethanol-fed rats (n=4). The 60X inset shows granular myeloperoxidase activity stain associated with tubular walls and an infiltrating cell (arrow). C) Quantification of myeloperoxidase positive cells. Myeloperoxidase positive cells were enumerated in 3 random kidney sections at 60X with the data expressed as mean ± SEM (n=4). p<0.05 (*)
Apoptosis is increased in kidneys by chronic ethanol ingestion
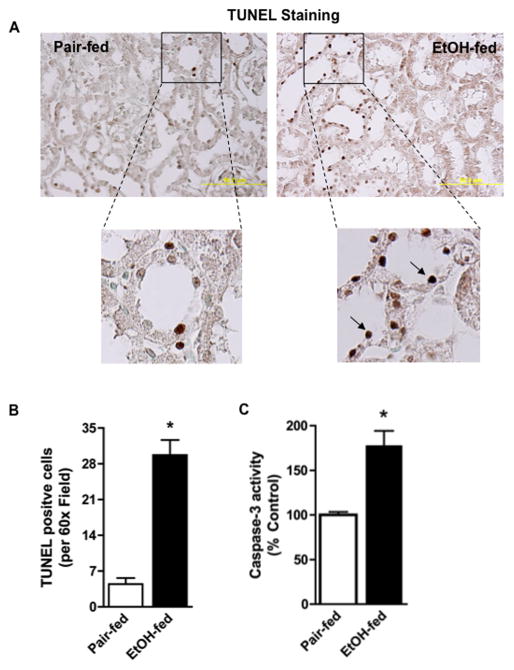
Apoptotic cell death fragments nuclear DNA, detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Immunohistochemistry shows an abundance of TUNEL positive cells in the kidneys of ethanol-fed rats (Fig. 3A). These cells were non-uniformly distributed, and primarily were epithelial cells localized to the distal tubular wall. Enumeration of these cells showed the increase was nearly 6-fold and that this increase was statistically significant (Fig. 3B). Caspase-3 is a downstream effector in the intrinsic apoptotic protease cascade, and the activated form of this enzyme also was significantly increased in kidney by ethanol feeding (Fig. 3C).
Figure 3. Chronic ethanol feeding induces renal tubular apoptosis.
A) Chronic ethanol ingestion increases kidney cell death. Kidneys of control and ethanol-fed rats were harvested, fixed, sectioned, and stained for TUNEL positive cells using a commercial kit that develops a dark brown TUNEL reaction product. The 60X inset shows these cells primarily were tubular. B) Ethanol feeding significantly increases TUNEL positive kidney cells. TUNEL positive cells were enumerated in 3 random kidney sections at 60X with the data expressed as mean ± SEM (n=4). p<0.05 (*) C) Ethanol feeding increases renal caspase-3 activity. Caspase-3 activity was determined in crude kidney homogenates of control- or ethanol-fed rats as described in “Methods”. Caspase-3 activity is given as a percent of control and data are expressed as mean ± SEM (n=6). p<0.05 (*)
Chronic ethanol ingestion decreases kidney function
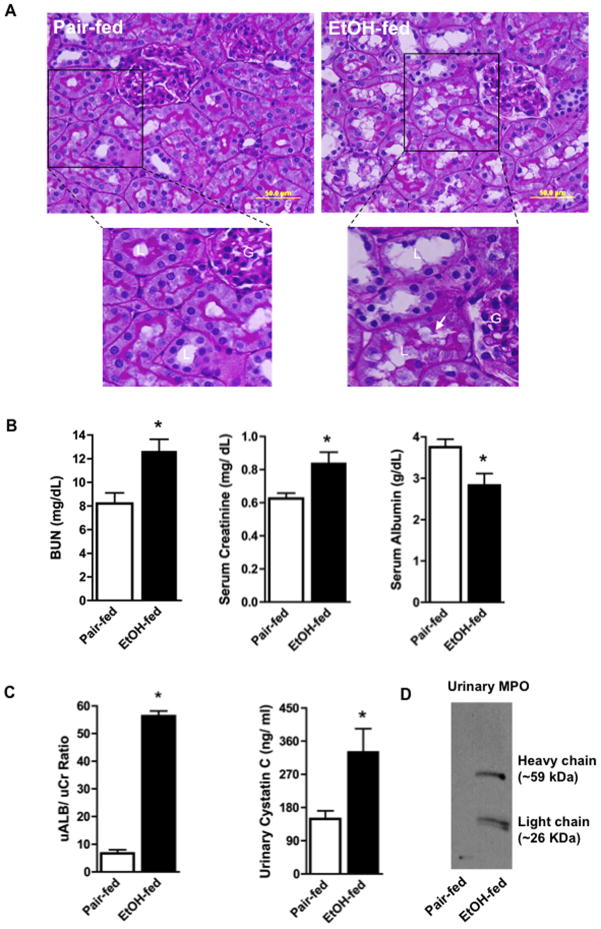
Kidneys of ethanol-fed rats displayed vacuolization, accumulation of intralumenal material, dysplastic tubular outer membranes, and loss of Periodic acid Schiff glycoprotein staining of the microvilli of proximal tubules (Fig. 4A). The kidneys of these ethanol-fed animals compared to pair-fed control animals not ingesting ethanol correspondingly displayed filtration defects. Thus, ethanol ingestion significantly increased the amounts of blood urea nitrogen (BUN) and creatinine in the circulation, which was accompanied by a decrease in circulating albumin (Fig. 4B). The urine of ethanol-fed animals contained excessive amounts of protein, with the albumin to creatinine ratio increasing nearly seven fold (Fig. 4C). The ratio of a second marker of defective filtration, urinary cystatin c, also increased two-fold in ethanol-fed animals relative to creatinine. Active myeloperoxidase is a homodimer of proteolyzed heavy and catalytically active light chain, and—uniquely—myeloperoxidase spilled into the urine of ethanol-fed rats (Fig. 4D). This protein migrated as proteolytically processed enzyme.
Figure 4. Ethanol alters renal architecture and induces renal injury.
A) Periodate-Schiff staining of kidneys from pair-fed control and ethanol-fed rats. Paraffin embedded kidney sections were deparaffinized and hydrated before being stained by periodic-acid Schiff/hematoxylin. “G”, glomerulus; “L” lumen; arrow, loss of PAS positive microvilli. B) Ethanol feeding induces filtration defects. Blood urea nitrogen (BUN), creatinine, and albumin from rats pair-fed a control diet and ethanol-fed rats were measured as described in “Methods”. Data are expressed as mean ± SEM (n=8). p<0.05 (*) C) Ethanol feeding induces albuminuria and increases urinary cystatin c. Urinary albumin, creatinine, and cystatin c were quantified by commercial kits. Data are expressed as mean ± SEM (n=4). p≤0.05 (*) D) Proteolytically processed myeloperoxidase is shed into urine of ethanol fed rats. Western blot of myeloperoxidase of urine from control and ethanol-fed rats. The lanes contained equal volumes of urine since loading controls are not appropriate.
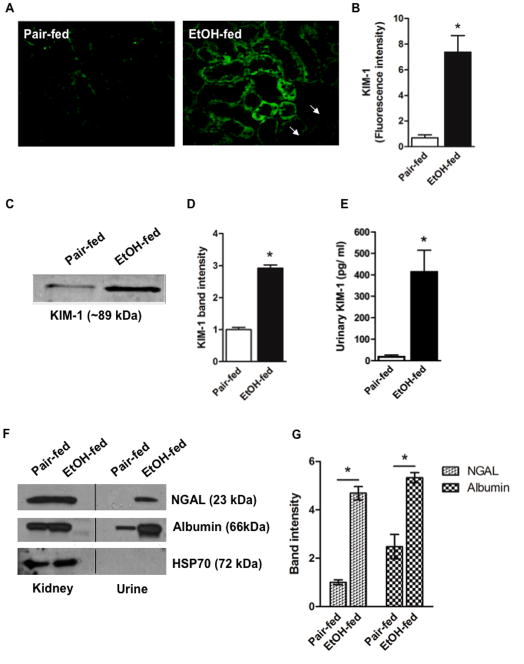
AKI biomarkers are shed to urine during ethanol feeding
The AKI biomarker KIM-1 is undetectable in normal kidney, but is highly induced in dedifferentiated tubular cells in response to diverse forms of injury [36]. We found KIM1 was largely absent in sections of control rat kidney, but was abundantly expressed in kidney tubular lumens of ethanol-fed animals (Fig. 5A). This increase from ethanol ingestion was significant (Fig. 5B). KIM1 of tubular epithelium is sloughed to urine, so urinary KIM1 marks AKI [37]. Western blotting showed KIM1 was more abundant in urine after 4 weeks of ethanol ingestion compared to pair-fed control rats (Fig 5C). Densitometry confirmed this increase was significant (Fig. 5D), while quantitative ELISA with a second antibody showed the significant increase was approximately 20-fold (Fig. 5E). A second biomarker of AKI, NGAL, also was present in the urine of ethanol-fed animals, and this increase was accompanied by excessive release of intact albumin (Fig. 5F). The increase of both NGAL and albumin in urine was significant after ethanol ingestion (Fig. 5G), as was KIM-1 (Fig. 5E).
Figure 5. Chronic ethanol feeding induces AKI.
A) Ethanol feeding induces renal KIM-1 expression. Kidney sections from pair-fed control rats and those fed ethanol were immunostained for KIM-1 and detected with Alexa Fluor488-conjugated secondary antibody. These immunofluorescent images are representative of four to five sections per kidney of pair-fed and ethanol-fed rats (n=4). B) Quantification of KIM-1 fluorescence. Image intensity was quantified using ImageJ, and expressed as mean ± SEM, n=4. p<0.05 (*) C) Urinary KIM-1 increases in response to ethanol ingestion. Western blot of KIM-1 in urine from pair-fed and ethanol-fed rats migrating at approximately 89 kDa. D) Quantification of urinary KIM-1 immunoblots. Relative band density was quantified in a Kodak Image Station, and the data expressed as mean ± SEM (n=3). p<0.05 (*) E) Immunoreactive KIM-1 increases in urine after ethanol feeding. Urinary KIM-1 was quantified by ELISA with the data expressed as mean ± SEM (n=8). p<0.05 (*) F) NGAL and albumin are shed from the kidneys of rats ingesting ethanol. Western blots of crude kidney homogenates or equal volumes of urine collected from ethanol-fed rats or their pair-fed controls were immunoblotted for NGAL, albumin, or cellular HSP70. The lack of HSP70 shows effective clearance of cellular debris from urine samples. G) Quantification of urinary AKI markers. Relative band intensities for NGAL and albumin were quantified in a Kodak Image Station, with data expressed as mean ± SEM (n=3). p<0.05 (*)
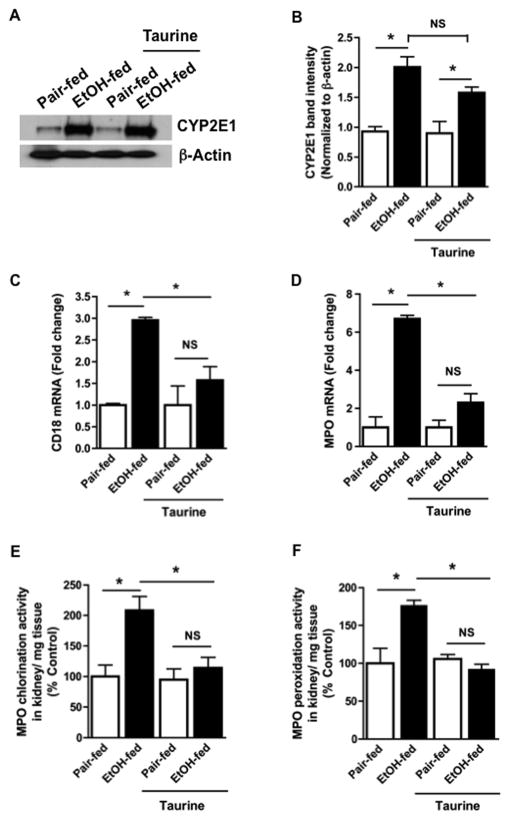
Taurine suppresses oxidative stress from chronic ethanol ingestion
Dietary taurine did not alter CYP2E1 expression in the kidney of either control or ethanol-fed mice (Figs. 6A, B), although it did effectively suppress leukocyte infiltration into kidney after ethanol feeding. Thus, mRNA encoding either leukocyte specific CD18 (Fig. 6C) or myeloperoxidase (Fig. 6D) were significantly reduced in the kidneys of taurine-supplemented, ethanol-fed rats. We found both chlorination (Fig. 6E) and peroxidation (Fig. 6F) myeloperoxidase half reactivities were significantly increased in kidney after ethanol feeding, and that both of these activities were significantly suppressed by dietary taurine. The outcomes in the absence of taurine in these separate experiments confirmed the previous results, e.g. Fig. 2, that used separate litters.
Figure 6. Dietary taurine ameliorates ethanol-induced renal inflammation and kidney injury.
A) Taurine does not alter CYP2E1 expression in kidney of ethanol-fed rats. Western blotting of CYP2E1 expression (55 kDa) in crude homogenates of kidneys from rats ingesting ethanol and/or taurine as shown. Equal loading of protein was confirmed by re-probing the blot with anti-β-actin antibody. B) Quantitation of CYP2E1 immunoblot band density. Densitometry for CYP2E1 accumulated as stated are expressed as mean SEM, n=3, determined by two-way analysis of variance. p<0.05 (*) C) Taurine suppresses infiltration of CD64-expressing cells into kidney of ethanol-fed rats. Taurine was included, or not, in the liquid diet fed with or without ethanol for 28 days. mRNA for CD18 in kidney homogenates was assessed as in panel 2A with data expressed as mean ± SEM (n=4) by two way ANOVA. p<0.05 (*) D) Taurine suppresses infiltration of CD18-expressing cells into kidney of ethanol-fed rats. Taurine and/or ethanol ingestion were as stated in the panel before renal myeloperoxidase mRNA in kidney homogenate was assessed as in panel 2A. The data are expressed as mean ± SEM (n=4) analyzed by two factor analysis of variance. p<0.05 (*) Dietary taurine supplementation abolishes the ethanol-induced increase in kidney myeloperoxidase activity. Chlorination (E) and peroxidation (F) activities were separately determined in the crude homogenates of rat kidney from ethanol- or control-fed rats additionally, or not, ingesting taurine. These myeloperoxidase half reactions were measured as described in “Methods’ with enzymatic activity expressed as a percent of control. The data are the mean ± SEM (n=4) by two-way ANOVA. p<0.05 (*)
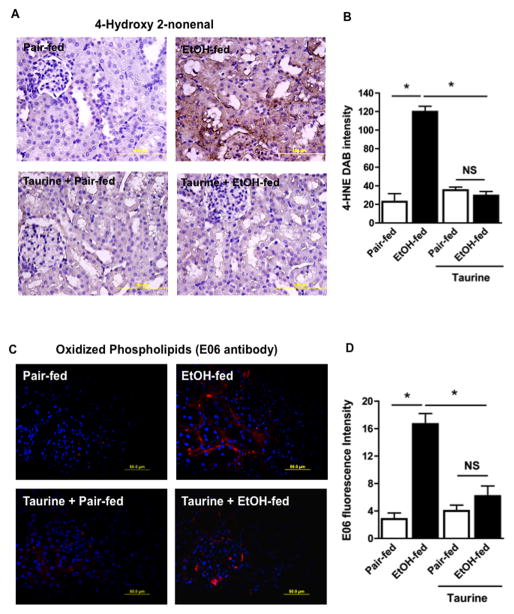
Taurine administration also effectively reduced adduction of kidney proteins by reactive 4-hydroxynonenal fragments in ethanol fed rats (Fig. 7A). This reduction was significant (Fig. 7B), resulting in equivalent, basal staining of adducted renal proteins. Dietary taurine also suppressed generation of the E06 epitope displayed by proteins adducted by oxidized phospholipids (Fig. 7C). Again, this reduction was significant (Fig. 7D).
Figure 7. Taurine supplementation ameliorates ethanol-induced renal oxidative injury.
A) Dietary taurine suppresses 4-hydroxynonenal protein modification induced by ethanol ingestion. Kidneys from rats fed as described in the panels were harvested, sectioned, fixed, and immunostained with anti-4-hydroxynonenal (4-HNE) antibody as in Fig. 1E. Panels are representative of four sections from each of four animals. B. 4-Hydroyxnonenal image quantitation. DAB staining was quantified by ImageJ in 3 random kidney sections at 60X with the data expressed as mean ± SEM using two-way ANOVA (n=4). p<0.05 (*) C) Dietary taurine suppresses the ethanol-induced increase in renal choline phospholipid oxidation adducts. Kidneys of rats fed as described were harvested, sectioned, fixed, and immunostaining with E06 antibody as in Fig. 1D. Panels are representative of four sections from each of four animals. D. E06 image quantitation. E06 fluorescence was quantified by ImageJ in 3 random kidney sections at 60X with the data expressed as mean ± SEM by two-way analysis of variance (n=4). p<0.05 (*)
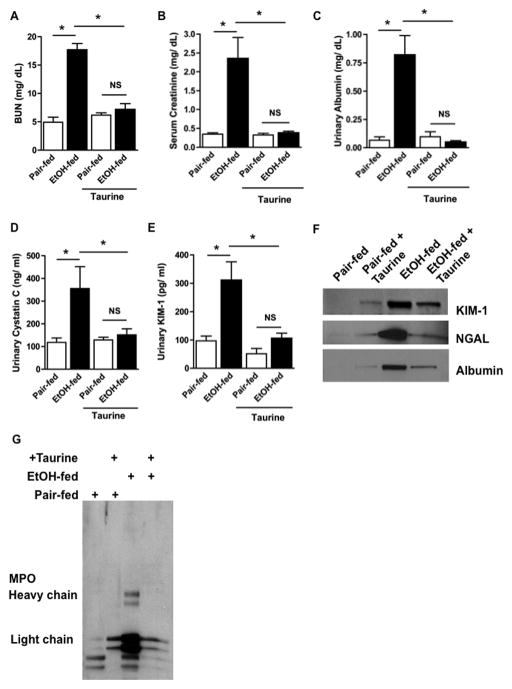
Taurine protects kidney function from ethanol ingestion
Ethanol feeding significantly increased circulating BUN, but inclusion of taurine in the ethanol diet fully normalized this measure of decreased kidney function (Fig. 8A). Supplemental taurine also completely abolished the significant increase in circulating creatinine in these animals (Fig. 8B). In addition, taurine supplementation also abolished the albuminuria of ethanol-feeding (Fig. 8C). Correspondingly, supplemental taurine prevented the appearance of the AKI biomarkers cystatin c (Fig. 8D) and KIM-1 (Fig. 8E) in the urine of ethanol-fed animals. Western blotting confirmed that the urine of the taurine-supplemented animals ingesting ethanol contained reduced levels of urinary KIM-1, NGAL, and albumin (Fig. 8F). Finally, taurine supplementation reduced the amount of tissue myeloperoxidase (not shown) and loss of the enzyme to the urine of ethanol fed rats (Fig. 8G).
Figure 8. Taurine supplementation abolishes ethanol-induced renal dysfunction and development of AKI.
Dietary taurine abolishes renal filtration defects induced by chronic ethanol ingestion. Sera of rats fed ethanol or pair-fed a control diet, received dietary taurine, or not, was assayed for A) BUN or B) creatinine as in Fig. 4B. The data are analyzed by two factor analysis of variance and shown as mean ± SEM (n=4). p<0.05 (*) C) Ethanol-induced release of albumin, D) cystatin C, and E) KIM-1 to urine was blocked by dietary taurine. Urinary markers were quantitated as in Fig. 4C and expressed as mean ± SEM (n=4) and analyzed by two-way ANOVA. p<0.05 (*) F) Taurine supplementation suppresses protein shedding to urine. Western blots of urinary KIM-1, NGAL, and albumin, using antibodies distinct from those of the preceding panel, were detected as described in Fig. 5F. G) Taurine supplementation ameliorates ethanol-induced myeloperoxidase shedding to urine. Myeloperoxidase fragments were detected in equal volumes of urine by western blotting with anti-myeloperoxidase as in Fig. 4D.
Discussion
Ethanol ingestion is common among the American population with the majority of adults using alcohol [38], and an estimated 6% and 2% of males and females, respectively, classified as alcohol dependent [39]. A significant number of individuals—perhaps a third of heavy alcohol users—progress to alcoholic hepatitis [1, 2, 40] that associates with significant mortality [2]. Kidney filtration is affected by this chronic ethanol use [11–13], but alcoholic disease is uniformly viewed as hepatic disease. Despite this, the mortality of patients hospitalized with alcoholic hepatitis correlates to the rapid development of kidney dysfunction, not the underlying hepatitis [14]. While alcoholism does associate with renal dysfunction [41, 42], alcohol-induced kidney damage is rarely studied [16, 17].
Our data show ethanol is sufficient to induce acute kidney failure after just 4 weeks of ingestion in amounts relevant to humans. This Lieber-deCarli diet has been the standard model for decades [15] to investigate the effects of alcohol on liver, but now we find a far more severe effect on kidney in this model. At a time when liver is only modestly damaged [28, 43], there was significant loss of effective renal filtration. This was accompanied by the development of Acute Kidney Injury, shown by escape of circulating albumin to urine and shedding of validated [44] AKI biomarkers to urine. One of these markers, KIM-1, is not significantly expressed in normal kidney, but is induced by any of an array of nephrotoxic drugs [37] prior to sloughing to urine. This work now identifies ethanol as a new nephrotoxin, but we also argue—below—that unlike most nephrotoxic drugs, ethanol is an indirect nephrotoxin that requires the participation of activated leukocytes.
The syndrome of AKI describes the sudden loss of kidney filtration with the retention of nitrogenous waste products in blood. However, the clinical measure of this injury, the estimated glomerular filtration rate, is problematic [45]. This has led to an intense search for biomarkers shed to urine, as an easily accessible fluid, as surrogates that correlate to pathophysiologic changes in kidney [46]. KIM-1 and NGAL are now identified as robust biomarkers of renal disease and AKI [37, 47, 48] that together achieve a perfect diagnostic score for AKI [44].
Ethanol ingestion in the standard Lieber-deCarli preclinical model increased BUN and circulating creatinine levels. This evidence of poor filtration was accompanied by an increase in the release of albumin to urine. Moreover, ethanol ingestion increased urinary KIM-1, NGAL, and cystatin c, so ethanol ingestion induces AKI.
Kidney damage in ethanol fed animals correlated with the recruitment and activation of leukocytes. Neutrophils and monocytes both express the PAF receptor [49], and stimulation of this unique PAF receptor recruits and activates these leukocytes during the initial phase of acute inflammation [50]. Neutrophils robustly respond to PAF and oxidatively generated PAF-like phospholipids at concentrations as low as 10−12 M [21]. Both PAF and the PAF-like phospholipid azelaoyl phosphatidylcholine increased in kidney during ethanol feeding, so in concordance with expectations, kidneys from ethanol fed rats displayed an inflammatory infiltrate marked by increased leukocyte CD18 and CD64 mRNA. Neutrophils were prominent among the leukocytes in these kidneys as shown by the increase in myeloperoxidase mRNA and its enzymatic activity. In fact, proteolytically processed myeloperoxidase escaped to the urine of mice chronically fed ethanol, an event previously only reported in the literature once as distinguishing kidney rejection from urinary tract infection [51].
Myeloperoxidase plays a prominent role in a range of kidney disease [52, 53] that damages both glomerular function—marked by poor filtration and increased albumin leak—and tubulointerstital damage that is marked by KIM and NGAL release to urine. Neutrophils increase albumin escape to urine, and this is through their production of HOCl [54] Notably, polymorphisms in the myeloperoxidase gene associate with clinical outcomes in patients with AKI [55].
PAF directly increases glomerular permeability and decreases renal filtration [56], but we conclude that it likely was the inflammatory infiltrate, and active myeloperoxidase, that underlies the kidney damage and dysfunction that results from chronic ethanol catabolism. Taurine does not affect oxidative ethanol catabolism, nor did taurine suppress the increase in expression of CYP2E1 in the kidneys of ethanol-fed mice, so taurine would not act by reducing the amount of endogenous oxidants released during ethanol catabolism. Instead, the main [25] anti-inflammatory action of taurine results from its abundance and the availability of its primary amine function. The primary amine of taurine sequesters HOCl (and HOBr), with formation of chlorinated or brominated taurine adducts [29]. This reaction implies leukocyte–derived HOCl underlies ethanol-induced kidney damage. However, there are elements of ambiguity in this conclusion. In addition to sequestration of hypohalous acids, taurine is an abundant, non-metabolizable β-amino acid that functions as an osmolite to modulate fluid flow through the kidney [31]. Taurine also reduces the stress response in kidney [31] and restores ethanol-induced depletion of anti-oxidants by unknown mechanisms [57]. And, chlorinated or brominated taurine adducts have their own complicated roles [25]. Deletion of the myeloperoxidase gene will resolve its potential role in ethanol-induced kidney damage.
Overall, this work establishes three novel points. First, the insult of ethanol metabolism alone is sufficient to significantly damage kidney function. This is remarkable since the primary site of ethanol oxidative catabolism is liver, and liver function is only slightly affected in this ethanol feeding model [58]. Ethanol-induced kidney damage likely is relevant to patients hospitalized with alcoholic hepatitis because those who develop sudden loss of kidney function constitute nearly all of those patients who succumb from this disease [14].
The second point is that ethanol-induced dysfunction correlates with leukocyte infiltration and activation, and not primarily from oxidative ethanol catabolism by CYP2E1. CYP2E1 metabolism may be necessary to initiate inflammation in liver [4], but kidneys contain significantly less of this monooxygenase than liver, and liver is only modestly affected by ethanol in this animal model. Conversely, we find neutrophils were abundant in the kidneys after ethanol feeding. Neutrophil type 2 NADPH oxidase has a critical role in ethanol-induced liver damage [5, 6], and may contribute to the oxidative stress in the kidneys as well.
The third point we establish is that ethanol-induced kidney damage was nearly fully abolished by inclusion of taurine in the diet. While the way taurine accomplishes this may be complicated, taurine suppressed formation of an inflammatory infiltrate. So, the reduction in renal oxidative stress, the decrease in glomerular filtration, the increase in renal cell apoptosis, and the induction and release of AKI biomarkers all may be secondary to repression of neutrophil recruitment and activation into kidney during ethanol ingestion.
We conclude ethanol acts as an indirect nephrotoxin to induce Acute Kidney Injury. However, in contrast to drugs that are directly nephrotoxic, chronic ethanol metabolism induces a previously unappreciated cycle of leukocyte infiltration and activation necessary to induce its nephrotoxic effects. Taurine, potentially acting to sequester myeloperoxidase HOCl, interrupts this inflammatory cycle and ablates renal damage.
Supplementary Material
Highlights.
Ethanol ingestion increases lipid oxidation, induces leukocyte infiltration, and damages kidney tissue and function
Taurine, an anti-inflammatory nutraceutical, blocks ethanol-induced renal inflammation and loss of kidney function
Ethanol is a nephrotoxin that acts through a cycle of leukocyte infiltration, activation, and ROS production.
Acknowledgments
We greatly appreciate the advice and insight of William M. Baldwin 3rd and the animal husbandry of Jazmine Danner and Megan R. McMullen. The aid of Renliang Zhang and the Lerner Research Institute small molecule mass spectrometry core are also appreciated. This work was supported by AA017748, P20 AA017837, and R37 AA011876.
Abbreviations
- AKI
Acute Kidney Injury
- CYP2E1
cytochrome P450 isotype 2E1
- KIM-1
Kidney Injury Molecule-1
- NGAL
Neutrophil Gelatinase-associated Lipocalin
- PAF
Platelet-activating Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 2.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. New Engl’d J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 3.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49:1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005–1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- 6.Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronis MJ, Huang J, Longo V, Tindberg N, Ingelman-Sundberg M, Badger TM. Expression and distribution of cytochrome P450 enzymes in male rat kidney: effects of ethanol, acetone and dietary conditions. Biochem Pharmacol. 1998;55:123–129. doi: 10.1016/s0006-2952(97)00381-x. [DOI] [PubMed] [Google Scholar]
- 8.Cummings BS, Zangar RC, Novak RF, Lash LH. Cellular distribution of cytochromes P-450 in the rat kidney. Drug Metab Disposition. 1999;27:542–548. [PubMed] [Google Scholar]
- 9.Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Internat. 2003;63:1687–1696. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- 10.Zerilli A, Lucas D, Amet Y, Beauge F, Volant A, Floch HH, Berthou F, Menez JF. Cytochrome P-450 2E1 in rat liver, kidney and lung microsomes after chronic administration of ethanol either orally or by inhalation. Alcohol Alcohol. 1995;30:357–365. [PubMed] [Google Scholar]
- 11.Labib M, Abdel-Kader M, Ranganath L, Martin S, Marks V. Impaired renal tubular function in chronic alcoholics. J R Soc Med. 1989;82:139–141. doi: 10.1177/014107688908200307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol. 2006;164:263–271. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- 13.White SL, Polkinghorne KR, Cass A, Shaw JE, Atkins RC, Chadban SJ. Alcohol consumption and 5-year onset of chronic kidney disease: the AusDiab study. Nephrol Dialysis Transplant. 2009;24:2464–2472. doi: 10.1093/ndt/gfp114. [DOI] [PubMed] [Google Scholar]
- 14.Altamirano J, Fagundes C, Dominguez M, Garcia E, Michelena J, Cardenas A, Guevara M, Pereira G, Torres-Vigil K, Arroyo V, Caballeria J, Gines P, Bataller R. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71 e63. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Thiel DH, Williams WD, Jr, Gavaler JS, Little JM, Estes LW, Rabin BS. Ethanol--its nephrotoxic effect in the rat. Am J Pathol. 1977;89:67–83. [PMC free article] [PubMed] [Google Scholar]
- 17.Van Thiel DH, Gavaler JS, Little JM, Lester R. Alcohol: its effect on the kidney. Metabolism. 1977;26:857–866. doi: 10.1016/0026-0495(77)90004-x. [DOI] [PubMed] [Google Scholar]
- 18.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandin Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 19.McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: Formation, targets, and inactivation. Biochim Biophys Acta. 2012;1818:2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem. 2007;282:6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- 21.Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM. Activation of vascular cells by PAF-like lipids in oxidized LDL. Vascul Pharmacol. 2002;38:193–200. doi: 10.1016/s1537-1891(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 22.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 23.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch Biochem Biophys. 2006;445:214–224. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids. 2012 doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkan J, Kanbagli O, Aykac-Toker G, Uysal M. Taurine treatment reduces hepatic lipids and oxidative stress in chronically ethanol-treated rats. Biol Pharm Bull. 2002;25:1231–1233. doi: 10.1248/bpb.25.1231. [DOI] [PubMed] [Google Scholar]
- 27.Kerai MD, Waterfield CJ, Kenyon SH, Asker DS, Timbrell JA. Taurine: protective properties against ethanol-induced hepatic steatosis and lipid peroxidation during chronic ethanol consumption in rats. Amino Acids. 1998;15:53–76. doi: 10.1007/BF01345280. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, Nagy LE, Feldstein AE, McIntyre TM. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumari N, Prentice H, Wu JY. Taurine and its neuroprotective role. Adv Exp Med Biol. 2013;775:19–27. doi: 10.1007/978-1-4614-6130-2_2. [DOI] [PubMed] [Google Scholar]
- 31.Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci. 2010;17(Suppl 1):S4. doi: 10.1186/1423-0127-17-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554–1562. doi: 10.1002/hep.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latchoumycandane C, Marathe GK, Zhang R, McIntyre TM. Oxidatively truncated phospholipids are required agents of tumor necrosis factor alpha (TNFalpha)-induced apoptosis. J Biol Chem. 2012;287:17693–17705. doi: 10.1074/jbc.M111.300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen R, Chen X, Salomon RG, McIntyre TM. Platelet activation by low concentrations of intact oxidized LDL particles involves the PAF receptor. Arterioscler Thromb Vasc Biol. 2009;29:363–371. doi: 10.1161/ATVBAHA.108.178731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horkko S, Miller E, Branch DW, Palinski W, Witztum JL. The epitopes for some antiphospholipid antibodies are adducts of oxidized phospholipid and beta2 glycoprotein 1 (and other proteins) Proc Natl Acad Sci U S A. 1997;94:10356–10361. doi: 10.1073/pnas.94.19.10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol. 2009;238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenfield TK, Midanik LT, Rogers JD. A 10-year national trend study of alcohol consumption, 1984–1995: is the period of declining drinking over? Am J Public Health. 2000;90:47–52. doi: 10.2105/ajph.90.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caetano R, Cunradi C. Alcohol dependence: a public health perspective. Addiction. 2002;97:633–645. doi: 10.1046/j.1360-0443.2002.00184.x. [DOI] [PubMed] [Google Scholar]
- 40.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein M. Alcohol’s impact on kidney function. Alcohol Health Res World. 1997;21:84–92. [PMC free article] [PubMed] [Google Scholar]
- 42.Cecchin E, De Marchi S. Alcohol misuse and renal damage. Addict Biol. 1996;1:7–17. doi: 10.1080/1355621961000124656. [DOI] [PubMed] [Google Scholar]
- 43.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rule AD, Lieske JC. The estimated glomerular filtration rate as a test for chronic kidney disease: problems and solutions. Cleveland Clin J Med. 2011;78:186–188. doi: 10.3949/ccjm.78a.11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesch GH. Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrology. 2010;15:609–616. doi: 10.1111/j.1440-1797.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 47.Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6:2740–2749. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbschat A, Obermuller N, Haferkamp A. Biomarkers of kidney injury. Biomarkers. 2011;16(Suppl 1):S22–30. doi: 10.3109/1354750X.2011.587129. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu T, Mutoh H, Kato S. Platelet-activating factor receptor. Gene structure and tissue-specific regulation. Adv Exptl Med Biol. 1996;416:79–84. [PubMed] [Google Scholar]
- 50.Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler Thromb Vasc Biol. 2002;22:727–733. doi: 10.1161/01.atv.0000016153.47693.b2. [DOI] [PubMed] [Google Scholar]
- 51.Steinhoff J, Einecke G, Niederstadt C, Fricke L, Rob PM, Sack K. Myeloperoxidase in urine: a new marker for distinction between rejection and urinary tract infection after renal transplantation. Transplant Proc. 1997;29:3098. doi: 10.1016/s0041-1345(97)00797-5. [DOI] [PubMed] [Google Scholar]
- 52.Malle E, Buch T, Grone HJ. Myeloperoxidase in kidney disease. Kidney Int. 2003;64:1956–1967. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 53.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol. 2007;18:760–770. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- 54.Li JZ, Sharma R, Dileepan KN, Savin VJ. Polymorphonuclear leukocytes increase glomerular albumin permeability via hypohalous acid. Kidney Int. 1994;46:1025–1030. doi: 10.1038/ki.1994.363. [DOI] [PubMed] [Google Scholar]
- 55.Perianayagam MC, Tighiouart H, Liangos O, Kouznetsov D, Wald R, Rao F, O’Connor DT, Jaber BL. Polymorphisms in the myeloperoxidase gene locus are associated with acute kidney injury-related outcomes. Kidney Int. 2012;82:909–19. doi: 10.1038/ki.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma R, Sharma M, Li JZ, McCarthy ET, Savin VJ. Direct effects of platelet-activating factor on glomerular capillary permeability. Kidney Blood Pressure Res. 1997;20:25–30. doi: 10.1159/000174107. [DOI] [PubMed] [Google Scholar]
- 57.Devi SL, Viswanathan P, Anuradha CV. Taurine enhances the metabolism and detoxification of ethanol and prevents hepatic fibrosis in rats treated with iron and alcohol. Environ Toxicol Pharmacol. 2009;27:120–126. doi: 10.1016/j.etap.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 58.de la M, Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Jarvelainen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.