Abstract
Background
Although the cytokine, interleukin-31 (IL-31), has been implicated in inflammatory and lymphoma-associated itch, the cellular basis for its pruritic action is yet unclear.
Objective
To determine whether immune cell-derived IL-31 directly stimulates sensory neurons, and to identify the molecular basis of IL-31-induced itch.
Methods
We used immunohistochemistry and qRTPCR to determine IL-31 expression levels in mice and humans. Immunohistochemistry, immunofluorescence, qRTPCR, in vivo pharmacology, western blotting, single cell calcium and electrophysiology were used to examine the distribution, functionality and cellular basis of the neuronal IL-31 receptor (IL-31RA) in mice and humans.
Results
Among all immune and resident skin cells examined, IL-31 was predominantly produced by TH2 and to a significantly lesser extend by mature dendritic cells. Cutaneous and intrathecal injections of IL-31 evoked intense itch, and its concentration increased significantly in murine atopic-like dermatitis skin. Both human and mouse DRG neurons express IL-31RA, largely in neurons that co-express TRPV1. IL-31-induced itch was significantly reduced in TRPV1- and TRPA1-deficient mice, not c-kit or PAR-2 mice. In cultured primary sensory neurons, IL-31 triggered Ca2+-release and ERK1/2 phosphorylation, Inhibition of which blocked IL-31 signaling in vitro and reduced IL-31-induced scratching in vivo.
Conclusion
IL-31RA is a functional receptor expressed by a small subpopulation of IL-31RA+/TRPV1+/TRPA1+ neurons, and is a critical neuro-immune link between TH2 cells and sensory nerves for the generation of T cell-mediated itch. Thus, targeting neuronal IL-31RA may be effective in the management of TH2-mediated itch, including atopic dermatitis and cutaneous T cell lymphoma.
Keywords: cytokine, atopic dermatitis, sensory nerve, skin, TRP channel
Introduction
Cytokines are critical contributors to various inflammatory skin diseases and cutaneous malignancies that are also pruritic, notably atopic dermatitis (AD) and cutaneous T cell lymphoma 1–4. How cytokines exert their pruritic effects and the extent to which there is direct or indirect involvement of sensory nerves that express specific cytokine receptors is currently unclear. Because many inflammatory and malignant pruritic skin diseases have an associated TH2 cell signature, analysis of the interplay between TH2 cells and sensory neurons will significantly enhance our understanding of the mechanisms underlying the communication between the adaptive immune and the nervous system to induce itch, and therefore how to treat recalcitrant itch in humans.
Interleukin-31 (IL-31), a TH2-cell-derived cytokine, is increased in pruritic atopic skin and cutaneous T cell lymphoma in humans 3–7 and induces severe pruritic atopic-like dermatitis (AD-like) in an IL-31 transgenic mouse model 5. Moreover, neutralization of IL-31 in NC/Nga mice, an AD-like mouse model, reduced scratching and improved wound healing 8. IL-31 binds to a IL31RA, which exists as a short, non-signaling/inhibitory, or a long signaling subunit 9. To date, no long or short form of the IL31-RA has been described in mice.
Although IL-31RA is expressed in murine neuronal tissue 10, detailed information about the neuro-immune link and functional relevance of IL-31RA+ neurons is lacking
Recent studies focused on the function of sensory neurons in itch 11–13. Thus far, TRPV1+ and a subpopulation of TRPV1+/TRPA1+ sensory neurons have been implicated to be required for pruritogen-induced itch signaling 14–18. Whether cytokine-induced itch has a comparable neuronal basis is unknown. Therefore, the aim of our study was determine the cellular basis of IL-31-induced itch.
Results
Production of IL-31 by human TH2 helper cells
Several studies demonstrated IL-31 is expressed by skin-homing TH2 cells during inflammation, most notably in AD 5, 7, 19, 20. No study has systematically compared expression levels of IL-31 in all potentially relevant immune and permanent skin cells involved in AD. Using qPCR 21, we compared expression levels of IL-31 and its receptor IL-31RA in various immune and permanent skin cells of patients with atopic dermatitis and psoriasis. Skin specimen were obtained from healthy donors (n=35), AD patients (non-lesional n=13, lesional n=50), and psoriasis patients (non-lesional n=14, lesional n=49). IL-31 mRNA transcript was increased (approx. 4-fold) in lesional skin of AD compared to non-lesional or healthy skin (Fig 1a). Lesional AD skin showed significantly higher levels of IL-31RA long isoform compared to healthy skin (Fig. 1a, p<0.001), while no statistical differences were observed for the inhibitory short isoform, which was largely expressed in healthy skin. In psoriasis, IL-31RA long was also upregulated but to a lesser extent as in lesional AD skin. Of note, while IL-31 was siginificantly upregulated in AD, this was not the case for psoriasis. Compared to AD, neither lesional nor non-lesional psoriasis specimens showed upregulation of either subforms. OSMR-β was equally expressed in all examined samples (Fig. 1a). We next co-localized IL-31 (green) with skin-infiltrating CLA+ cells (red) in human lesional AD skin (Fig. 1b, colocalization, yellow cells and arrows). As expected in AD, TH2 cells were found almost exclusively in the dermis. Quantitative analysis of immunofluorescence revealed that 62 +/− 8.2% of skin-homing CRTH2+ TH2 cells to be positive for IL-31 (n≥10 patients per group).
Figure 1. IL-31 derives from human TH2 cells, and IL-31RA is expressed on human DRG neurons.
(a) QPCR of IL-31, IL-31RAlong, IL-31RAshort, and OSMR-β. (b) Co-localization of CLA (red) and IL-31 (green) in AD skin (scale bar = 100 μm). (c) Human TH2 cells express IL-31 mRNA. (d) Immature and mature dendritic cells (iDC/mDC) express IL-31 mRNA. (e) IL-31RA immunostaining in human DRG neurons (scale bar = 50 μm, (f) control). *P<0.05, **P<0.01, ***P<0.001, Mann-Whitney U test.
Next, we used qPCR from isolated human T cell subtypes to compare IL-31 mRNA expression in the subsets of T cells and found that IL-31 was predominantly expressed by TH2 cells and derives very unlikely from TH0, TH1 or TH17 cells (Fig. 1c). We did not detect IL-31 mRNA in other immune or resident skin cells (keratinocytes, endothelium, fibroblasts) (Fig. 1d). The only other source in human skin appears to be mature dendritic cells, albeit at significantly lower levels compared to TH2 cells (appr. 100-fold, Fig. 1d). Therefore, we identified TH2 cells as the major, if not exclusive, source of IL-31 in human atopic skin. Whether mature dendritic cells can also generate physiologically relevant quantities of IL-31 in certain diseases is unknown.
Human dorsal root ganglia express IL-31RA
Given the importance of IL-31 in pruritic skin diseases 3, 5, 19, 20, 22, 23, and the detection of IL-31RA mRNA in human skin 5, 6, we next used immunohistochemistry to analyze the distribution of IL-31RA in DRG neurons obtained from human cadaver (Fig. 1e). We found 50.6% of small-diameter DRG neurons (<30 μM) were IL-31RA+ while all large diameter DRG neurons (>50μM) were IL-31RA−. Preabsorption control verifies specificity of the IL-31RA staining in human DRG (Fig. 1f).
Upregulation of IL-31 in murine atopic-like dermatitis
We used topical application of superantigen Staphylococcus aureus enterotoxin B (SEB) (Fig. 2a) to produce an AD-like phenotype in mice (Fig. 2b) 7, 21. The inflammatory infiltrate consisted of high numbers of CD3+ T cells (Fig. 2c) and eosinophils (Fig. 2d), comparable to human AD. IL-31 mRNA was significantly upregulated in the skin of SEB-treated mice compared to vehicle-treated mice (Fig. 2e). IL-4, a second TH2-associated cytokine, was also significantly upregulated in the skin (p<0.001) after SEB treatment (Fig. 2f). In a second AD-like model, we used ovalbumin (OVA) (Supplemantary Fig. 1a-d), and also observed upregulation of IL-31 and IL-4, as observed in the SEB model. Thus, IL-31 in both human AD and AD-like mouse models derives from cutaneous TH2 cells and may activate IL-31RA on sensory nerves.
Figure 2. Superantigen-induced upregulation of IL-31 in AD-like mouse model.
(a) Treatment regimen. (b) HE-staining of vehicle (PBS)- and SEB-treated skin (scale bar = 200 μm). Number of CD3+ T cells (c) and eosinophils (d) in vehicle- vs. SEB-treated skin. (e-f) qPCR from skin samples reveal increased mRNA levels for IL-31 (e) and IL-4 (f) in SEB-treated skin. N=8 mice/group. **p<0.01, ***p<0.001, Student’s t-test, error bars indicated as SEM.
Intradermal IL-31 induces itch, but not pain in murine skin
The underlying mechanism of IL-31-induced itch and effects of IL-31 on itch versus pain have not been studied as of yet. Figure 3a illustrates that IL-31-produces dose-dependent scratching after intradermal injection into the nape of neck (50±6.89 bouts/30 min with 1.575 nmol/40 μl, 90.67±10.36 bouts/30 min with 3.15 nmol/40μl and 121.1±12.79 bouts/30 min with 6.3 nmol/40 μl); vehicle: produced only 15.2±1.2 bouts/30min, p≤0.0001.
Figure 3. In vivo effects of IL-31 in mice.
(a) Injection of IL-31 into the nape of neck induced profound scratching. (b) Intraplantar IL-31 significantly increased paw-licking. (c) Cheek injection of IL-31 only produced scratching but no wiping (d). (e) Intrathecal injection of IL-31 induced significant dose-dependent scratching compared to vehicle. N=8 mice/group. **p<0.01, ***p<0.001, Student’s t-test, error bars indicated as SEM.
Intraplantar hindpaw injection (Fig. 3b) of IL-31 (3.15 nmol/5 μl) evoked profound paw licking (156.2±11.39 sec/30 min vs. 22.6±4.55 sec/30 min with vehicle; p≤0.0001). IL-31 injection into the cheek 24, 25(Fig. 3c) provoked robust scratching (100.4 ±4.16 bouts/30 min for 3.15 nmol/10 μl and 132.4±8.13 bouts/30 min for 6.3 nmol/10 μl vs. 18.8±6.4 bouts/30 min for vehicle, p=0.002). No differences were obtained for IL-31-induced wiping behavior compared to control (8.25±6.93 bouts/30 min, 3.15 nmol/10 μl IL-31 vs. 4.25±3.84 bouts/30 min vehicle) (Fig. 3d). As expected, capsaicin (a positive control for a painful stimulus) evoked significant wiping (54.25±5.32, 10 μg/10 μl) (Fig. 3d).
Intrathecal IL-31 evokes itch in mice
We next asked whether itch can be provoked with an approach that bypasses the skin (Fig 3a-c). To assess a possible direct action on CNS circuitry, including the central terminals of primary afferents, we injected IL-31 intrathecally (i.t, directly into the cerebrospinal fluid) at the lumbar level in mice, which induced caudally directed scratching (Fig. 3e). This was dose-dependent, ranging from 69.83±4.47 bouts/30 min (6.3 fmol/5 μl) to 152.3±17.63 bouts/30 min (6.3 pmol/5 μl; p< 0.0001). These findings suggest that IL-31 can induce itch by directly targeting spinal cord circuits, including the central terminals of primary afferents.
IL-31RA is localized in TRPV1+ peptidergic murine DRG neurons
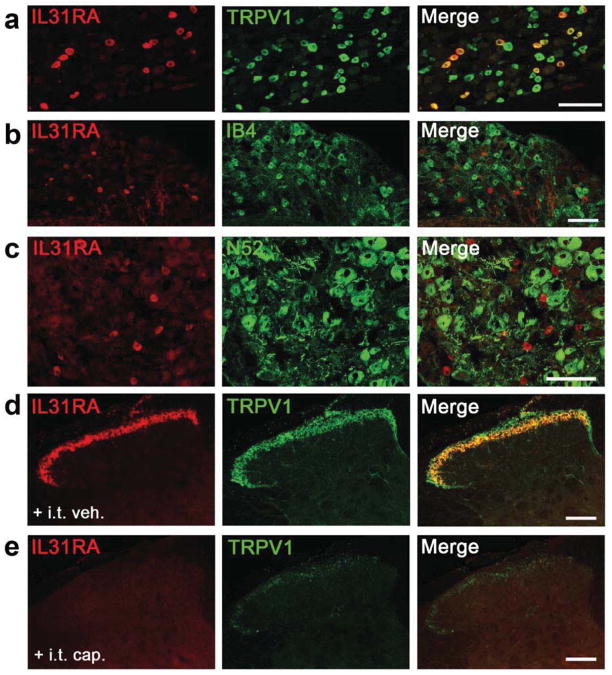
We used immunohistochemistry to localize IL-31RA in the DRG, trigeminal ganglion (TG) and SC. Consistent with our results from human DRG (Fig 1e), we found IL31RA immunoreactivity predominantly in small- to medium-sized diameter murine DRG neurons (Fig. 4a), equivalent to about 3.4 % of the total neuron population; expression in the TG was comparable (Supplementary Fig. 3). Importantly, there is complete coexpression of IL-31RA and TRPV1, a marker for capsaicin-responsive, peptidergic DRG neurons (Fig. 4a). However, only 16.2±0.7% of TRPV1+ neurons are IL-31RA+ and 6.7±0.4% bound the lectin IB4, which marks the non-peptidergic subpopulation of unmyelinated sensory neurons) (Fig. 4b). We found no overlap of IL-31RA+ neurons with N52 (a marker of cell bodies with myelinated axons; Fig. 4c). In the SC (Fig. 4d), we found a complete overlap of IL-31RA and TRPV1 in axon terminals, and no evidence for post-synaptic expression of IL-31RA. The IL-31RA-immunoreactivity was concentrated in outer lamina II, corresponding to the most ventral distribution of TRPV1 terminals. As expected, i.t. injection of capsaicin - a neurotoxin that ablates central TRPV1 terminals 26, 27 – produced a significant loss of both TRPV1+- and IL-31RA+-immunoreactive terminals in the dorsal horn (Fig. 4e). Importantly, specificity of the IL-31RA antibody was demonstrated by the absence of IL-31RA immunoreactivity in DRG neurons obtained from IL-31RA KO mice (Supplementary Fig. 2). Thus, a small subset of unmyelinated peptidergic (TRPV1+) primary sensory neurons in DRG and TG express IL-31RA (Fig 4; Supplementary Fig. 3).
Figure 4. Localization of IL-31RA in murine DRG and spinal cord.
(a) IL-31RA+ (red) and TRPV1+ (green) neurons partly co-localize. (b) Minimal overlap of IL-31RA (red) and IB4+ (green) subset of non-peptidergic nociceptors. (c) No overlap of IL-31RA+ (red) and N52+ unmyelinated neurons (green). (d) IL-31RA+/TRPV1+ in nerve terminals of the superficial dorsal horn. I.t. capsaicin (e), but not vehicle (d), ablated TRPV1+ (green) and IL-31RA (red) immunoreactivity. Scale bars = 100 μm.
Neuronal mechanisms of IL-31-mediated itch
Previous studies in mice demonstrated that TRPV1- or TRPA1-expressing DRG neurons are important contributors to scratching behavior 14–18, 26–28. Whether TRP channels are involved in IL-31-mediated itch is unknown. We found that i.t. capsaicin-treated mice markedly reduced IL31-induced scratching (6.3 pmol/5 μl; 61±13.7 bouts/30 min in i.t. capsaicin-treated vs. 133.3±14.49 bouts/30 min in i.t. vehicle-treated mice (Fig. 5a). We next injected IL-31 (6.3 nmol/40 μl) into the nape of neck of TRPV1 KO mice, and observed a significant reduction of scratching bouts (47.75± 2.56 bouts/30min in TRPV1 KO vs. 140±23.97 bouts/30min WT littermates; p=0.0086) (Fig. 5b). These findings demonstrate that TRPV1 is itself critical to IL31-evoked itch.
Figure 5. Neuronal requirement of IL-31-induced itch.
(a) Depletion of TRPV1+ neurons by i.t. capsaicin significantly decreased i.t. IL-31 induced scratching. (b) TRPV1KO and (c) TRPA1KO mice show reduction of IL-31 induced scratching compared to WT littermates. (d) c-kit mutant mice and (e) PAR-2 KO mice showed equal scratching to WT mice after IL-31 injection. N=8 mice/group. **p<0.01, ***p<0.001, Student’s t-test, error bars indicated as SEM.
Because TRPA1 is required for Mas-related G protein-coupled receptor (Mrgpr)- and ET-1-mediated itch 14, 16, 28, 29, we also studied the consequence of TRPA1 deletion. Figure 5c shows that there is a significant reduction of IL-31 (6.3 nmol/40 μl)-induced scratching, after nape of the neck injection, in TRPA1 KO mice (44.67±3.17 bouts/30 min vs. 139±11.86 bouts/30 min in WT littermates). To address the possibility that IL-31-evoked itch is amplified by a mast cell release of TRPV1/TRPA1-dependent pruritogens, e.g. histamine or tryptase, we injected IL-31 into the neck of mast-cell-deficient c-kit mutant and PAR-2 KO mice (6.3 nmol/40 μl). No differences were observed between c-kit mutant mice and their WT controls (142.8±6.48 bouts/30min vs. 109±21.57 bouts/30min, Fig. 5d). Also, we have not observed significant differences between PAR-2 KO (185.4±26.25 bouts/30min) and WT littermate mice (154±20.59 bouts/30min, Fig. 5e). Thus, IL-31-induced itch is independent of mast cell degranulation or PAR-2-mediated itch.
Functional characterization of IL-31-responsive DRG neurons
To identify the functional properties of the IL-31RA population of pruriceptors, we imaged cervical (C3–C8) DRG cells for their Ca2+-responsiveness to IL-31 (Fig. 6) 30, 31. Consistent with the anatomical analysis, we found 2.1% (4/194) responded to 0.3 μM, 3.0% (32/1,054) to 1.0 μM, and 4.0% (4/100) to 3.0 μM IL-31 in a dose-responsive manner (Fig. 6a). A detailed analysis indicates heterogeneity in the responsiveness of DRG neurons: while some DRG neurons responded to IL-31 but not histamine, others responded to histamine but not IL-31 and others responded to both, or neither. Many IL-31-responsive cells responded to capsaicin, consistent with the predominant TRPV1 expression in IL-31-responsive neurons (Fig. 6a). Moreover, we found that 11.1% of 495 tested cells responded to histamine, 3.5% of 575 cells responded to SLIGRL, 8.3% of 484 cells responded to chloroquine, and 38.6% of 484 cells responded to AITC (mustard oil). Competence of viable cells was confirmed by a robust Ca2+-influx detected in all cells exposed to capsaicin or high K+ (Fig. 6a). Thus, IL-31 induces robust Ca2+-responses in DRG that are also inducible by agonists to TRPA1, TRPV1 and chloroquine.
Figure 6. IL-31-induced calcium mobilization, and characterization of IL-31-responsive DRG neurons.
(a) Neurons responding to IL-31 only (blue), histamine only (green), IL-31 and histamine (black), neither IL-31 nor histamine (red). (b) Percentages of IL-31-responsive neurons which also respond to other compounds. (c-d) Venn diagrams for DRG neurons in percentages. (e) Percentages of IL-31-responsive neurons in different KO mice. N=193-981 cells/group. For quantification, 10–30 dishes/group were used, and 20–50 cells/dish were counted. *p<0.05, **p<0.01, unpaired t-test, error bars indicated as SEM.
Figure 6b and the Venn diagrams in Fig. 6c and d show the proportions of IL-31-responsive DRG compared to IL-31-responsive neurons that also responded to histamine, SLIGRL, chloroquine, capsaicin and AITC (Fig 6a and c). As compared to histamine (37.5%) and SLIGRL (21%), a higher percentage of IL-31-responsive cells were activated by capsaicin (67%) and AITC (91%), respectively (Fig. 6b and d). Finally, IL-31-responsive DRG neurons were significantly reduced in TRPV1- and TRPA1 KO animals (Fig. 6e). The percentage of IL-31-responsive DRG was significantly reduced in TRPV1 KO DRG and even more in TRPA1 KO DRG (Fig. 6e). Thus, in contrast to histamine 15 or chloroquine 16, 17, 30, we show for the first time that IL-31-mediated calcium influx is, to some extent, dependent on TRPV1 and TRPA1 channels in murine DRG. As IL-31 did not elicit calcium responses in the absence of extracellular calcium, we conclude that IL-31 triggers influx of calcium through these TRP transduction channels.
Contribution of ERK1/2 to IL-31-mediated cell signaling in DRG neurons and IL31-provoked itch
Although MAPK signaling pathways have been implicated in the processing of pain message by primary afferents 31, 32, their contribution to itch has not been studied. Figs. 7a and b show that IL-31 stimulation of cultured murine DRG neurons induced phosphorylation of ERK1/2 that peaked at 5 min. The MEK-inhibitor, U0126 completely prevented IL-31-mediated phosphorylation of ERK1/2 in vitro (Fig. 7c). By contrast, IL-31 was without effect on the p38 signaling pathways in DRG (Fig. 7d). Fig. 7e illustrates that i.p. injected U0126 (30 mg/kg) 30 min prior to IL-31 injection into neck (6.3 nmol/40 μl) significantly reduced scratching bouts (31.2±7.46 vs. 151.6±9.52 vehicle treated mice; p≤0.0001). Thus, ERK1/2, but not p38, is required for IL-31-induced itch.
Figure 7. ERK1/2 phosphorylation in DRG is critical for IL-31-induced itch.
(a) Western blot and (b) densitometry analysis of murine cultured DRG for pERK1/2 illustrate peak activation of ERK1/2 after 5 min. (c) Pre-treatment with the ERK1/2 inhibitor U0126 blocked IL-31-induced ERK1/2 activation. (d) IL-31 stimulation does not lead to p38-phosphorylation in cultured DRG. (e) I.p. injection of U0126 prior to IL-31 blocked IL-31-evoked scratching. N = 8 mice/group. p<0.001, Student’s t-test, error bars indicated as SEM.
Neural responses to IL-31 in the dorsal horn of the spinal cord
To assess whether IL-31-induced itch activates pruritoceptive and/or nociceptive dorsal horn neurons, we used single unit extracellular recordings to define the properties of IL-31-activated dorsal horn neurons (Supplementary Fig. 4a-c). The majority of IL-31-responsive neurons was activated by pruritogens (histamine and SLIGRL) and noxious stimuli (heat and capsaicin; Supplementary Fig 4a and c). The fact that a common population of neurons responds to both itch- and pain-provoking stimuli suggests that a circuit downstream of the IL-31RA+/TRPV1+/TRPA1+ primary sensory neurons or a specific pattern of activity generated across subpopulations of responsive dorsal horn neurons determines the quality of the sensory perception (namely itch or pain).
Discussion
The resistance of prevalent pruritic diseases to anti-histamines, exemplified by AD, argues strongly for the existence of histamine-independent pruritic pathways that are important targets for therapy of chronic itch 11–13. We demonstrate that IL-31 induces itch by directly activating IL-31RA on TRPV1+/TRPA1+ sensory nerves in the skin. We show that TH2 cells are the predominant cellular source of IL-31, and that the number and activation of TH2 cells as well as IL-31 levels are increased in patients as well as in mouse models of AD. We conclude that TH2 cells are the source of a novel, IL-31 cytokine-triggered neuro-immune circuit that induces itch in TH2-dominated skin diseases by activating IL-31RA on sensory nerves 6, 33, 34. Whether the central terminals of primary afferents are targeted in pruritic diseases in which the blood-brain barrier is compromised, such as multiple sclerosis, allowing for penetration of IL-31 into the CNS, remains to be determined 35, 36.
Although previous studies detected IL-31RA expression in DRG neurons 10, the functional relevance of neuronal IL-31RA expression has not been explored. We found that IL-31RA is exclusively expressed by a subpopulation of TRPV1+/ TRPA1+ DRG neurons. Of note, although the subset of IL-31RA+ afferents (~4% of DRG) is relatively small (Fig. 6a), IL-31RA activation of this population by either by i.d. or i.t. IL-31 injection is clearly sufficient to evoke profound scratching in mice. These effects depend on a subset of TRPV1+ afferents (TRPV1+/IL-31RA+) as well as on TRPA1 as a signal transducer – suggesting that both TRPV1 and TRPA1 are major contributors to IL-31-induced itch 14, 16–18, 26, 29, 37.
Other studies reported co-localization of OSMRβ, a receptor subunit targeted by IL-31, in the non-peptidergic, P2X3+ neuron population 38–40. In contrast, we found IL-31RA immunoreactivity predominantly in the peptidergic TRPV1/TRPA1+ neuron population (Fig. 4). Importantly, we confirmed specificity of our IL-31RA antibody by absence of immunostaining in IL-31RA KO mice (Fig. S2a). Intriguingly, while IL-31RA mRNA was significantly increased in lesional skin of AD, this was not the case for OSMRβ mRNA indicating a pivotal role of IL-31RA but not OSMRβ in IL-31-mediated itch (Fig. 1a).
The fact that IL-31 injection into the cheek induced itch, but not pain, suggests that itch and pain are triggered by different subsets of unmyelinated afferents, and that subpopulations of afferents exist that are specialized in the itch domain 18. Indeed, single fiber recordings in humans described itch-specific unmyelinated afferents 41. We also found that chloroquine, which exerts its action via the MrgprA3 subtype of the Mrgprs activates a very large percentage (90%) of IL-31-responsive DRG neurons. Since we found that TRPA1 is also involved in IL-31-induced itch we conclude that the IL-31RA+/TRPV1+/TRPA1+ subset of DRG neurons is responsible for IL-31-induced itch. Future studies will determine whether IL-31 induces the release of BNP in murine central primary afferents or activates GRPR+ and/or NPR-A+ postsynaptic neurons 42, 43.
Our electrophysiology analyses indicate that IL-31-responsive neurons in the dorsal horn can be activated by multiple pruritogens, which is consistent with a convergent itch transmission circuit 11, 15, 18, 42, 43. Furthermore, although some pruritogen-responsive dorsal horn neurons are activated by noxious stimuli, our finding that the central terminals of the IL-31RA+/TRPV1+ afferents target the outer part of lamina II, rather than lamina I, suggests that the postsynaptic neurons engaged by the IL31RA-expressing afferents are interneurons that are part of a circuit dedicated to itch. In this context, interneurons that express GRPR and/or NPR-A are ideally positioned to receive input from the IL-31RA+/TRPV1+ afferents and presumably from the afferents that respond to other pruritogens 42–44. Together, we suggest that the IL-31RA+ population of afferents provides a major input that triggers itch, but not pain, and that GRPR+/NPR-A+ interneurons may be targets of these axons. Despite this apparent convergence, however, specificity of itch provoked by different pruritogens can be maintained because different pruritogens engage a variety of signaling pathways in the same neuron 13. The fact that itch or pain can be attenuated by inhibitors of ERK1/2 phosphorylation (31, 32 and our present findings) is also consistent with convergence of itch and pain transmission, although the locus of the ERK1/2 action could differ in itch and pain-relevant circuits.
In conclusion, our results demonstrate that TH2-derived IL-31 directly communicates with an IL-31RA+/TRPV1+/TRPA1+ subpopulation of primary afferent neurons in the skin. We suggest that IL-31RA is a functional neural cytokine receptor involved in acute and chronic itch. In this respect, IL-31RA represents the long hypothesized “missing link” in a direct neuro-immune crosstalk between T cells and sensory nerves in itch. This finding emphasizes that not only mast cells via histamine or tryptase release 45, 46, but also T cells via cytokines can directly communicate with sensory nerves to induce itch. Thus, blocking the effects of IL-31/IL-31RA may have a beneficial effect not only for the inhibition of inflammation but also to ameliorate directly the deleterious effects of T-cell-mediated itch. The exceptionally high incidence of itch and AD worldwide, and the fact that IL-31 as well as IL-31RA are elevated in both 47–49, underscores the significance of our findings for the development of IL-31-directed anti-pruritic therapies.
Materials and Methods
Materials
Recombinant mouse IL-31 was provided by ZymoGenetics, Inc. (Seattle, WA). For details, see Supplementary Material and Methods.
Patients
Patients and healthy controls were included after providing written informed consent within a study protocol approved by the ethics committees of the University Hospital Muenster, Heinrich-Heine-University Düsseldorf, and University Hospital Goettingen, Germany. For details, see Supplementary Material and Methods.
Purification of naive CD4+ T lymphocytes from adult blood and T helper cells
PBMCs were separated from buffy coats of healthy blood donor volunteers. For details, see Supplementary Material and Methods.
Cell isolation and cell culture of human cells
For details, see Supplementary Material and Methods.
Quantitative real time PCR (TaqMan®)
QPCR was performed to analyze expression of IL-31, IL31RA and OSMRβ in lesional vs. non-lesional skin from AD patients vs. healthy human subjects. For details, see Supplementary Materials and Methods.
Mouse model of AD and bacterial superantigen-induced skin inflammation
To determine IL-31 levels from AD-like skin lesions, we used two established mouse models, namely treatment with ovalbumin (OVA) or staphylococcus enterotoxin B (SEB), as described recently 20, 21. For details, see Supplementary Materials and Methods.
Pruritogen-induced scratching
For details, see Supplementary Materials and Methods.
Immunostaining of mouse DRG and spinal cord
Cryosections of murine SC (10 μm) and DRGs (10 μm) were used. For details, see Supplementary Materials and Methods.
Primary DRG culture
Mice were anesthetized by intraperitoneal injection of pentobarbital, perfused transcardially with Ca2+-free and Mg2+-free PBS. DRG neurons were cultured, as previously described 50. For details, see Supplementary Materials and Methods.
Calcium imaging
Upper- to mid-cervical mouse DRGs were enzymatically digested and processed for calcium imaging as described 42, 50. For details, see Supplementary Materials and Methods.
Western blotting
DRG neurons from primary cell culture were homogenized by hot lysis in protein lysis buffer containing a protease and phosphatases inhibitor mixture (Roche Applied Science, Penzberg, Germany) and sonicated. Then, cell debris was removed by centrifugation (14,000xg, 4°C, 10 min). Samples were processed as described 50. For details, see Supplementary Materials and Methods.
Supplementary Material
Clinical Implications.
We show that a functional cytokine receptor expressed by sensory nerves is involved in itch, leading to novel therapeutic strategies targeting neuronal cytokine receptors to treat T cell-mediated itch and atopic dermatitis.
Acknowledgments
This work was supported by grants from the NIH/NIAMS (AR059402), Deutsche Forschungsgemeinschaft (DFG) (Ste1014/2-2), IZKF Muenster (STE3/034/07), CE.R.I.E.S. Paris (to M.S.); NIH NS14627 (to A.B.), NIH AR057194 (to E.C.); DFG Ce165/1-1 (to F.C.); DFG Ke1672/1-1 (to C.K.), DFG (Ho2092/4-1), DFG-FOR729 (Ho2092/5-2) (to B.H.). We thank Janine Bilsborough for helpful discussions, Mirela Iodi-Carstens for assistance in electrophysiology, Christine Stadelmann-Nesser for human DRG, Stephan Seeliger, Christian Mess, Ron Manlapaz, Robert Kubitza, Sabine Kellermann, Ulrike Wiesner, Ulrich Pippirs, Andrea Poppe, and Victoria Fong for expert technical assistance.
Non-standard abbreviations
- C-kit/kit
white locus mutation (c-kit gene) resulting in mast-cell deficiency
- CD45RO
isoform of leukocyte common form antigen
- CLA
cutaneous lymphocyte-associated antigen
- DAB
diaminobenzidine
- DRG
dorsal root ganglia
- EC
endothelial cells
- ERK
extracellular signal-regulated kinases
- ET-1
endothelin-1
- Fb
fibroblast
- h
hour
- iDC
immature dendritic cell
- IL
interleukin
- IL-31RA
interleukin-31 receptor alpha
- IB4
isolectin B4
- i.t
intrathecal
- KC
keratinocyte
- KO
knockout
- mDC
mature dendritic cell
- ON
overnight
- OSMRβ
oncostatin M receptor-beta
- OVA
ovalbumin
- pERK1/2
phospho-ERK1/2
- p-p38
phopho-p38
- PBS
phosphate-buffered saline
- qPCR
quantitative real time PCR
- RT
room temperature
- SC
spinal cord
- SEB
staphylococcal enterotoxin B
- TRG
trigeminal root ganglion
- TRPA1
transient receptor channel potential cation channel ankyrin subtype-1
- TRPV1
transient receptor potential cation channel vanilloid subtype-1
- WT
wild-type
Footnotes
Author contribution:
F. C.: conducted most of the experiments, designed the study, wrote manuscript. X. W.: conducted in vivo and morphological experiments with F.C. T.A: performed single cell calcium measurement and electrophysiology recordings under supervision of E.C; T. S.: designed the study for the in vivo mouse models of AD under supervision of H.A; A.A, M.F.: performed human staining experiments of skin tissue and qPCR of cells under supervision of B.H.; C. K.: performed western blotting and wrote part of the manuscript; G. K.: performed human staining experiments of skin tissue and qPCR of cells; A. I.: assisted in cheek model assay; T. B.: stained human DRG for IL-31RA; H. A.: supervised the murine AD study; S. D.: supervised vivo mouse studies; E. C.: supervised electrophysiology study; B. H.: designed, supervised human IL-31 studies and mouse atopy models, and wrote manuscript; A.I.B.: designed, supervised the neuronal experiments, and wrote manuscript; M.S.: designed, supervised all experiments, analyzed data, and wrote manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2006;117:418–25. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 2.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. The Journal of allergy and clinical immunology. 2006;117:411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, et al. IL-31 Is Produced by the Malignant T-Cell Population in Cutaneous T-Cell Lymphoma and Correlates with CTCL Pruritus. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 4.Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625–34. doi: 10.1056/NEJMcp1208814. [DOI] [PubMed] [Google Scholar]
- 5.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nature immunology. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 6.Bieber T. Atopic dermatitis. The New England journal of medicine. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 7.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunological reviews. 2011;242:233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Exp Dermatol. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 9.Diveu C, Lak-Hal AH, Froger J, Ravon E, Grimaud L, Barbier F, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15:291–302. [PubMed] [Google Scholar]
- 10.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. 2006;142:1263–71. doi: 10.1016/j.neuroscience.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends in neurosciences. 2010;33:550–8. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–79. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 13.Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–18. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–94. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11330–5. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nature neuroscience. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2013;16:174–82. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nobbe S, Dziunycz P, Muhleisen B, Bilsborough J, Dillon SR, French LE, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta dermato-venereologica. 2012;92:24–8. doi: 10.2340/00015555-1191. [DOI] [PubMed] [Google Scholar]
- 20.Niebuhr M, Mamerow D, Heratizadeh A, Satzger I, Werfel T. Staphylococcal alpha-toxin induces a higher T cell proliferation and interleukin-31 in atopic dermatitis. International archives of allergy and immunology. 2011;156:412–5. doi: 10.1159/000323905. [DOI] [PubMed] [Google Scholar]
- 21.Savinko T, Lauerma A, Lehtimaki S, Gombert M, Majuri ML, Fyhrquist-Vanni N, et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, a mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. Journal of immunology. 2005;175:8320–6. doi: 10.4049/jimmunol.175.12.8320. [DOI] [PubMed] [Google Scholar]
- 22.Grimstad O, Sawanobori Y, Vestergaard C, Bilsborough J, Olsen UB, Gronhoj-Larsen C, et al. Anti-interleukin-31-antibodies ameliorate scratching behaviour in NC/Nga mice: a model of atopic dermatitis. Experimental dermatology. 2009;18:35–43. doi: 10.1111/j.1600-0625.2008.00766.x. [DOI] [PubMed] [Google Scholar]
- 23.Venereau E, Diveu C, Grimaud L, Ravon E, Froger J, Preisser L, et al. Definition and characterization of an inhibitor for interleukin-31. J Biol Chem. 2010;285:14955–63. doi: 10.1074/jbc.M109.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. Journal of neurophysiology. 2010;104:2442–50. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–7. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat Neurosci. 2013;16:910–8. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9075–80. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Escalera J, Balakrishna S, Fan L, Caceres AI, Robinson E, et al. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013;27:3549–63. doi: 10.1096/fj.13-229948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang J, Ji Q, Ji W. Role of transient receptor potential ankyrin subfamily member 1 in pruritus induced by endothelin-1. Neurosci Lett. 2011;492:175–8. doi: 10.1016/j.neulet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Johnson-Huang LM, McNutt NS, Krueger JG, Lowes MA. Cytokine-producing dendritic cells in the pathogenesis of inflammatory skin diseases. Journal of clinical immunology. 2009;29:247–56. doi: 10.1007/s10875-009-9278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. The Journal of allergy and clinical immunology. 2006;118:178–89. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 35.Hedegaard CJ, Enevold C, Sellebjerg F, Bendtzen K, Nielsen CH. Variation in NOD2 augments Th2- and Th17 responses to myelin basic protein in multiple sclerosis. PloS one. 2011;6:e20253. doi: 10.1371/journal.pone.0020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group Nature medicine. 2000;6:1176–82. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013 doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takaoka A, Arai I, Sugimoto M, Honma Y, Futaki N, Nakamura A, et al. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Experimental dermatology. 2006;15:161–7. doi: 10.1111/j.1600-0625.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 39.Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. 2011;66:845–52. doi: 10.1111/j.1398-9995.2011.02545.x. [DOI] [PubMed] [Google Scholar]
- 40.Morikawa Y. Oncostatin M in the development of the nervous system. Anatomical science international. 2005;80:53–9. doi: 10.1111/j.1447-073x.2005.00100.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–3. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 43.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–71. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10043–8. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 46.Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–80. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) Journal of immunology. 2000;164:3465–70. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Fyhrquist-Vanni N, Wolff H, Dieu-Nosjean MC, Kemeny L, Homey B, et al. Immunostimulatory sequence CpG elicits Th1-type immune responses in inflammatory skin lesions in an atopic dermatitis murine model. International archives of allergy and immunology. 2008;147:41–51. doi: 10.1159/000128585. [DOI] [PubMed] [Google Scholar]
- 49.Steinhoff M, Cevikbas F, Yeh I, Chong K, Buddenkotte J, Ikoma A. Evaluation and management of a patient with chronic pruritus. J Allergy Clin Immunol. 2012;130:1015–6. e7. doi: 10.1016/j.jaci.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nature protocols. 2007;2:152–60. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.