Abstract
Rationale
Although both folic acid intake and vitamin D levels are hypothesized to be contributors to increased incidence of allergic diseases, prospective studies of these relationships have not been done in adults.
Objectives
To determine whether serum folate or vitamin D levels are associated with incident mouse sensitization among new workers at a mouse facility.
Methods
Subjects started employment at the Jackson Laboratory between June 2004 and July 2007. Skin testing to mouse and other allergens, and collection of questionnaire data, was performed at baseline and every 6 months. Serum folate and vitamin D levels were assessed on baseline samples stored at −80°C. Folate was categorized into tertiles (2.5–10.5 ng/ml, 10.5– 16.2ng/ml and 16.2–78.4ng/ml). Vitamin D was categorized as <20 ng/ml, 20–29 ng/ml or ≥30 ng/ml. This was a nested case/control study in which 5 controls were matched to each case on baseline atopy and type of employment. Multivariate analyses controlled for age, sex, education, smoking, season, personal mouse exposure, serum folate and vitamin D levels.
Measurements and Main Results
35 cases and 47 controls were included. The odds of incident mouse sensitization were higher in the intermediate and highest tertiles of serum folate, compared to the lowest tertile of serum folate (OR: 10.5 [95% CI: 1.8–61.5], p=0.009, and OR: 5.6 [95% CI: 1.8–31.3], p=0.049, respectively in the multivariate model). Serum vitamin D was not associated with incident mouse sensitization.
Conclusions
These findings support a role for higher serum folate levels in increased risk of incident allergic disease, even during adulthood.
Keywords: Folate, folic acid, vitamin D, allergy, mouse allergy, sensitization
Introduction
Among the hypothesized causes of the recent increase in allergic diseases are changes in micronutrients among the U.S. population. Two leading contenders for this hypothesis are folate and vitamin D. Specifically, increased folic acid intake, due to supplementation and fortification of foods, and decreased vitamin D production, due to more time indoors, have both been linked to increased risk of allergic sensitization(1–11). However, data supporting either hypothesis are mixed. Studies have generally either been cross-sectional or birth cohorts, and, for folate, have shown different associations with sensitization in studies addressing prenatal and early life exposure(1–3) than in those studying older children or adults(12, 13). It has been hypothesized that the increased risk of allergic outcomes with higher prenatal folate seen in some birth cohorts is due to epigenetic effects, and that this mechanism is relevant at critical developmental times only(5, 14). To our knowledge, there are no published studies that specifically examine whether either folate or vitamin D levels are associated prospectively with new allergen sensitization in adults.
The Jackson Laboratory cohort offers a unique opportunity to explore predictors of de novo sensitization in adults. This longitudinal cohort is composed of new employees at the Bar Harbor, Maine, Laboratory, a mouse research and production facility that sells over 2 million mice per year. Enrolled subjects are assessed at baseline and every six months for mouse sensitization, and detailed information, including direct measurement of personal mouse allergen exposure, are collected periodically. Using this cohort, we sought to determine whether baseline serum folate or vitamin D levels were associated with incident mouse sensitization.
Methods
Description of cohort
As has been previously described(15), this cohort was drawn from subjects who started non-temporary, full-time employment at The Jackson Laboratory between July 2004 and December 2007. Eligibility criteria included age of at least 18 years, provision of written informed consent, and, for this analysis, absence of mouse sensitivity by skin prick test at baseline. This study was approved by Institutional Review Boards at the Johns Hopkins Medical Institution and the Jackson Laboratory.
Assessment of outcomes and confounders
Briefly, skin prick testing was performed at baseline and every 6 months using the Multi-Test II device (Lincoln Diagnostics, Decatur, Ill). A positive result was defined as an orthogonal wheal size of at least 3 mm more than that elicited by the negative control. At the baseline visit the following allergens were tested using two Multi-testers and atopy was defined as a positive result to at least one: mouse, rat, cat, dog, Dermatophagoides pteronyssinus, Dermatophagoides farinae, pine, birch, oak, orchard grass, Alternaria species, Aspergillus species, Penicillium species, and ragweed. At follow-up visits the following allergens were tested using one Multi-tester: mouse, rat, cat, dog, dust-mite mix, and pine.
Baseline demographics were captured by a questionnaire at the first visit administered by the study staff. A follow-up questionnaire was administered at subsequent visits and captured interval allergic and occupational history. Subjects were followed for up to 36 months.
The methods for assessing personal mouse allergen exposure have been described in detail elsewhere(15). Briefly, personal air samples were collected during 2 full 8-hour shifts within one week period every 6 months using Buck VSS−12 personal sampling pumps (A.P. Buck, Inc, Orlando, FL). Mus m 1, the major mouse allergen, was quantified using sandwich ELISA. Personal exposure was defined as the mean of each participant’s repeated Mus m 1 measurements and was categorized into tertiles of exposure to capture the non-linear relationships with mouse sensitization that were previously reported(15).
Serum folate and vitamin D levels were measured on sera from the baseline visit that were stored at −80°C. Serum folate was quantified by a paramagnetic particle, chemiluminescent immunoassay (Access Immunoassay Systems, Beckman Coulter, Brea, CA). Serum folate was categorized into tertiles (2.5–10.5 ng/ml, 10.5–16.2 ng/ml and 16.2–78.4 ng/ml). Vitamin D was quantified by LIAISON 25 OH Vitamin D assay from DiaSorin, Inc. (Stillwater, MN). The assay is a direct competitive chemiluminescent immunoassay (CIA) for the quantitative determination of total 25 OH vitamin D in serum. Based on prior literature, vitamin D levels were categorized as <20 ng/ml, 20–29ng/ml, and ≥30 ng/ml, which some consider insufficient, potentially insufficient and optimal, respectively, although there is considerable controversy about these labels, particularly for consideration of conditions apart from bone health(16). This resulted in groups of 26, 28 and 28 subjects respectively.
Selection of cases and controls
Cases and controls were selected using a nested case control method. Cases were defined as subjects with incident sensitization to mouse on skin prick testing. Each case subject was individually matched to five control subjects using incidence density sampling according to baseline atopic status and whether their employment at The Jackson Laboratory included mouse handling. With incidence-density sampling, each subject’s eligibility to be a control was assessed at the same follow-up time as each incident case; thus, each subject could be a control for more than one case, and cases who became mouse sensitized at later follow-up dates could serve as controls for cases presenting earlier(17, 18). With this method, time to development of sensitization is incorporated into the outcome. Most importantly, incidence density sampling is more robust to bias introduced by loss to follow-up than other methods.
Statistical analysis
Analysis of predictors of case and control status was done using conditional logistic regression except for the demographic characteristics shown in Table 1, which were analyzed by chi square statistics for dichotomous variables and Wilcoxon rank sum statistics for continuous variables and which categorized subjects by whether they ever became cases or remained controls. Power calculations, assuming a standard deviation of 8 ng/ml in each serum micronutrient level and 35 cases and 35 matched controls, found 80% power to detect a difference of at least 3.9 ng/ml in serum vitamin D or folate levels between cases and controls. Potential confounders included in the analysis were baseline age, sex, education, smoking status, and season, average personal Mus m 1 exposure, and either baseline serum vitamin D or folate levels, for analyses of serum folate and vitamin D levels, respectively. When used as a confounder, serum folate was divided into tertiles, and serum vitamin D was categorized as described above. Education was classified as either completing less than a college degree versus completing at least a college degree. Smoking history was classified as never, former or current. Confounders were selected based on prior plausibility; only season was significantly associated with sensitization in the multiple regression model. Analyses were limited to subjects with data on personal Mus m 1 exposure, which excluded 6 control subjects. All analyses were performed using STATA SE/11 (College Station, TX).
Table 1.
Characteristics of cases and controls.
| Characteristic | Cases (n=35) n (%) |
Controls (n=47) n (%) |
p value |
|---|---|---|---|
| Age, median (IQR) | 29 (22–35) | 32 (24 – 40) | 0.15 |
| Female sex | 19 (54%) | 25 (53%) | 0.89 |
| Caucasian race | 32 (89%) | 43 (86%) | 0.69 |
| Education (at least college graduate) | 23 (66%) | 25 (53%) | 0.18 |
| Smoking History | 0.02 | ||
| Never | 25 (71%) | 20 (43%) | |
| Former | 3 (9%) | 13 (28%) | |
| Current | 7 (20%) | 14 (30%) | |
| Tertile of Personal Mus m 1* | 0.59 | ||
| 1 (0.01 – 0.45 ng/m3) | 9 (26%) | 15 (32%) | |
| 2 (0.45 – 6.51 ng/m3) | 16 (46%) | 16 (34%) | |
| 3 (6.58 – 629.39 ng/m3) | 10 (29%) | 16 (34%) | |
| Sensitized to cat or dog | 11 (31%) | 12 (26%) | 0.62 |
| Previously worked with mice | 9 (26%) | 13 (28%) | 0.84 |
| Handle mice† | 19 (54%) | 29 (62%) | 0.65 |
| Atopic† | 27 (77%) | 36 (76%) | 0.95 |
| Folate level (ng/ml), median (IQR) | 15.1 (9.5–20.6) | 14.1 (6.7–78.4) | 0.03 |
| Vitamin D level (ng/ml), median (IQR) | 27 (20–36) | 23 (14–33) | 0.09 |
Personal Mus m 1 exposure was defined as the mean of all of the personal Mus m 1 exposure assessments for each subject.
These variables were matched on for selection of controls.
Results
Baseline Characteristics
The cohort from which this nested study was drawn consisted of 260 subjects. Overall, the cohort was predominantly female (57%), Caucasian (91%), and had positions that involved handling mice (66%). Similar to the U.S. population as a whole(19), 10% reported current asthma at baseline. For this analysis, 35 cases and 47 controls were selected. As can be seen in Table 1, cases and controls were similar except in smoking history; subjects who had incident mouse sensitization were much more likely to be never smokers than controls (p=0.02). Both cases and controls were overwhelmingly Caucasian, and were generally highly educated. (Table 1)
Predictors of serum folate levels
No demographic characteristics were statistically significant predictors of serum folate levels except smoking status; folate levels were higher among never smokers (median folate 14.0 ng/ml) compared to former (median folate 10.6 ng/ml) and current (median folate 11.0 ng/ml) smokers (p=0.046). Sex, education, race/ethnicity, season and vitamin D levels were not predictors of serum folate levels (>0.35 for all). (Table 2) Similarly, age was not a predictor of serum folate (p=0.85).
Table 2.
Predictors of serum folate and vitamin D levels.
| Predictors of serum folate levels | ||
| Median folate level (IQR), ng/ml | p value | |
| Smoking history | 0.046 | |
| Never | 14.0 (11.1 – 21.4) | |
| Former | 10.6 (8.1 – 16.2) | |
| Current | 11.0 (8.5 – 15.8) | |
| Sex | 0.52 | |
| Female | 14.1 (9.1 – 19.4) | |
| Male | 12.2 (10.3 – 15.2) | |
| Education | 0.25 | |
| Less than college graduate | 12.1 (8.7–16.2) | |
| College graduate or higher | 14.1 (9.7–18.9) | |
| Race/ethnicity | 0.88 | |
| Caucasian | 12.8 (9.6 – 17.3) | |
| Non-Caucasian | 12.4 (6.9 – 27.6) | |
| Age | 0.75 | |
| <30 y | 12.8 (10.3–16.2) | |
| ≥30 y | 13.0 (9.1–19.4) | |
| Season | 0.88 | |
| Winter | 12.9 (9.6 – 19.4) | |
| Spring | 15.2 (8.3 – 21.4) | |
| Summer | 12.2 (8.8 – 17.0) | |
| Fall | 13.0 (9.3 – 17.3) | |
| Vitamin D category | 0.38 | |
| <20 ng/ml | 11.9 (7.9 – 15.8) | |
| ≥20–29 ng/ml | 13.0 (10.3 – 23.0) | |
| ≥30 ng/ml | 13.4 (9.6 – 19.0) | |
| Predictors of serum vitamin D level | ||
| Median vitamin D level (IQR) | p value | |
| Smoking history | 0.31 | |
| Never | 23.5 (17 – 33) | |
| Former | 22.5 (15 – 32.5) | |
| Current | 26.5 (23 – 36) | |
| Sex | 0.0006 | |
| Female | 30 (22 – 36) | |
| Male | 20 (16 – 26) | |
| Education | 0.75 | |
| Less than college graduate | 25 (20– 33) | |
| College graduate or higher | 24 (17 – 36) | |
| Race/ethnicity | 0.09 | |
| Caucasian | 25 (18 – 33) | |
| Non-Caucasian | 18 (14 – 31) | |
| Age | 0.33 | |
| <30 | 27 (17–35) | |
| ≥ 30 | 23 (18–31) | |
| Season | 0.003 | |
| Winter | 20 (16 – 26) | |
| Spring | 17 (14 – 24) | |
| Summer | 27.5 (22 – 37) | |
| Fall | 28.5 (18 – 33) | |
| Folate tertile | 0.76 | |
| 1 (2.5 – 10.5 ng/ml) | 25 (17 – 33) | |
| 2 (10.5 – 16.2 ng/ml) | 24 (17 – 32) | |
| 3 (16.2 – 78.4 ng/ml) | 24 (21 – 32) | |
Association between serum folate levels and incident mouse sensitization
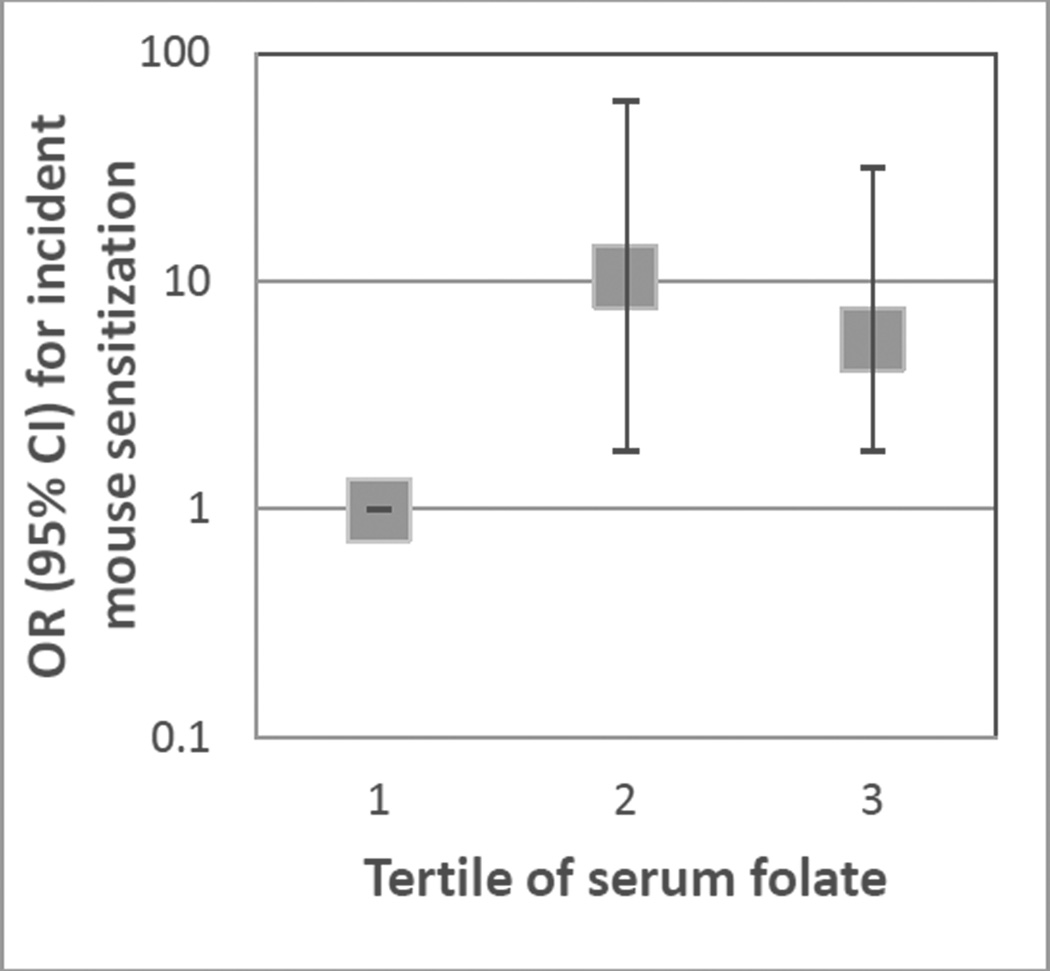
The odds of incident mouse sensitization was highest in the second tertile of serum folate in unadjusted models (OR: 5.4 [95% CI: 1.9–15.2], p=0.001 when compared to the lowest tertile of serum folate). This association persisted when analyses were adjusted for age, sex, education, smoking status, mouse allergen exposure, season and vitamin D group (OR: 10.5 [95% CI: 1.8– 61.5], p=0.009). The highest tertile of serum folate was also associated with a higher odds of incident mouse sensitization than the lowest tertile, although the odds were not as high as in the second tertile of serum folate (unadjusted analyses: OR: 2.4 [95% CI: 0.9–6.9], p=0.09, fully adjusted model: OR: 5.6 [95% CI: 1.8–31.3], p=0.049, compared to lowest tertile). When folate was treated in a continuous manner, there was not a statistically significant relationship with incident mouse sensitization, although there was a positive trend (OR: 2.0 [95% CI: 0.7–5.3], p=0.17 in fully adjusted model) (Table 3).
Table 3.
Associations between incident sensitization and serum folate levels.
| Unadjusted Model |
Adjusted Model 1* | Adjusted Model 2† | |
|---|---|---|---|
| OR (95% CI) p value |
|||
| Folate (natural log) | 1.6 (0.9–3.1) p=0.13 |
2.3 (0.9–5.8) p=0.09 |
2.0 (0.7–5.3) p=0.17 |
| Folate tertiles | |||
| Tertile 1 (2.47–10.45 ng/ml) | REF | REF | REF |
| Tertile 2 (10.53–16.16 ng/ml) |
5.4 (1.9–15.2) p=0.001 |
10.3 (1.9–55.7) p=0.007 |
10.5 (1.8–61.5) p=0.009 |
| Tertile 3 (16.22–78.44 ng/ml) | 2.4 (0.9–6.9) p=0.09 |
6.5 (1.3–32.9) p=0.02 |
5.6 (1.8–31.3) p=0.049 |
adjusted for age, sex, education, smoking status, season, mouse allergen level
adjusted for age, sex, education, smoking status, season, mouse allergen level, and vitamin D category
Statistically significant findings are bolded
Predictors of serum vitamin D levels
Serum vitamin D levels were higher among female subjects than male subjects (medians: 30 and 20 ng/ml, respectively; p=0.0006) and in the summer and fall (medians: 28 and 29 ng/ml, respectively) compared to winter and spring (medians: 20 and 17 ng/ml, respectively; p=0.003). Vitamin D levels tended to be higher among Caucasians (median 25 ng/ml) compared to non-Caucasians (medians: 25 and 18 ng/ml, respectively; p=0.09). Smoking history, tertile of serum folate and education did not predict serum vitamin D level (p=0.31, 0.76 and 0.75, respectively). (Table 2) Similarly, age was not a predictor of serum vitamin D (p=0.17).
Association between serum vitamin D levels and incident sensitization
Category of vitamin D level was not associated with incident mouse association in either unadjusted or adjusted models, although there was a trend towards increased odds of sensitization in the intermediate category of serum vitamin D (unadjusted model: OR: 2.3 [95% CI: 0.8–6.1], p=0.09, fully adjusted model: OR: 2.6 [95% CI: 0.7–10.1], p=0.17, compared to lowest category of vitamin D). However, this trend was not evident for the highest category of vitamin D (unadjusted model: OR: 1.7 [95% CI: 0.6–4.7], p=0.27, fully adjusted model: OR: 1.6 [95% CI: 0.4–6.5], p=0.55, compared to lowest category of vitamin D). In addition, there was no linear relationship between vitamin D and incident sensitization (OR: 3.2 [95% CI: 0.7–14.1], p=0.13 in fully adjusted model).
Discussion
In this prospective cohort of new employees at a mouse facility, we found that serum folate levels were associated with incident sensitization to mouse. Specifically, we found that those in the middle tertile of serum folate had a 10-fold higher odds of sensitization compared to those in the lowest tertile, while those in the highest tertile had a 6-fold higher odds of sensitization compared to the lowest tertile. Our findings that higher levels of serum folate are associated with higher risk of incident sensitization are consistent with a small but growing literature showing higher risk of allergic outcomes with higher folate levels. Intriguing evidence from animal models suggests that folate’s role as a methyl-donor may mediate its association with allergic disease through epigenetic changes, but this mechanism has previously been hypothesized to be relevant only during prenatal and early life. Our findings suggest that folate may continue to have an effect on allergic outcomes throughout the lifespan. In contrast, although we hypothesized that lower vitamin D levels would be associated with higher risk of incident sensitization, we did not find a consistent relationship in this cohort; in fact, there was a trend towards an association between higher levels of vitamin D and increased odds of sensitization.
In the U.S., mandatory fortification of certain grain products with folic acid for the prevention of neural tube defects began in 1998, and was associated with a more than doubling in mean serum folate levels in the general U.S. population(20). Over the same period, recommendations for prenatal and preconception supplementation with folic acid were also strengthened(21). Because these interventions occurred simultaneously with increases in certain allergic diseases, the role of folate in development of atopy has received recent attention. Several birth cohorts have examined the association between prenatal or early life folate levels and atopic outcomes. Most relevantly, Okupa et al. found that early life serum folate levels were associated with higher risk of incident allergic sensitization(1). Similarly, Kiefte-de Jong et al.(2) and Haberg et al.(3), found that higher maternal folate levels were associated with higher risk of eczema and asthma, respectively, in children, although sensitization was not assessed in either cohort. Others studies examining folic acid intake but not serum folate levels have found mixed results with regard to allergic sensitization, perhaps because of the biases associated with relying on maternal report of folic acid intake (4, 22–24).
To our knowledge, prospective studies of the relationship between serum folate levels and atopic outcomes outside of early childhood have not been done. Two cross-sectional studies in general populations showed that higher serum folate levels were associated with lower risk of asthma diagnosis and exacerbation, but were mixed in their findings of allergic sensitization (12, 13). In contrast, among asthmatics, Lin et al. found that moderate levels of folate were associated with higher exhaled nitric oxide and IgE, but did not find an association with asthma symptoms(14). Serum folate may have a relationship with asthma symptoms and diagnosis that is distinct from that with incident sensitization, as many allergic and non-allergic factors play a role in asthma pathology. Our prospective data, adjusted for important confounders, suggests that higher folate levels may increase the risk for new allergic sensitization well into adulthood.
Thus, although cross-sectional data initially suggested a protective role for folate in allergic sensitization, prospective data, including this study, now suggest the opposite. Previous cross-sectional data may have been inaccurate because it did not adequately measure folate at the critical time for development of sensitization, or because of unmeasured confounding.
Epigenetic modifications have been proposed as the pathway by which higher folate may increase the risk of allergic disease. As a methyl-donor, folic acid could contribute to increased methylation of genetic areas relevant to allergy. Work by Hollingsworth et al. supports this mechanism. In seminal mouse models, they supplemented pregnant mice with a diet high in methyl donors, including folic acid, and found increased severity of allergic airways disease in their offspring, a change that was inherited trans-generationally. Moreover, they tied this outcome to excessive methylation of the Runt-related transcription factor 3, a known regulator of allergic airways disease(5). Whether this mechanism mediates the effects of folate on allergic diseases in humans has not yet been explored.
Folate has a myriad of other biological functions that could also be relevant to sensitization. Recently it was discovered that folate metabolites activate mucosa-associated invariant T cells (25), a cell type that could potentially be involved in the early steps of sensitization. Other functions include key roles in DNA turnover, which may also be important in allergic diseases. For example, it is possible that low folate levels interfere with Th2 skewing by impairing T cell differentiation rather than by epigenetic mechanisms (14). In our results, there was not a clear linear dose response curve for the association between folate and sensitization; in fact it appeared that the highest risk of sensitization occurred at intermediate levels of serum folate. This could be because of a threshold effect related to methylation, or could be due to other effects at higher levels of folate. It has been speculated that other anti-inflammatory properties of folate may become more important at higher levels, potentially explaining the relatively smaller effect of higher folate levels compared to intermediate folate levels on incident sensitization seen here (14). Further research needs to be done to clarify the mechanisms by which folate may affect the development of allergy.
Although several studies have shown cross-sectionally that children and adults with various atopic conditions have lower vitamin D levels(6, 7, 11, 26, 27), prospective studies of vitamin D in association with allergic sensitization have shown much more mixed results, with some showing higher risk of sensitization with lower vitamin D (8, 9), and others showing no association or the opposite relationship (10, 28–30). Our data do not support a role for vitamin D deficiency or insufficiency in new-onset allergic sensitization in adults, and in fact show a trend towards higher risk of sensitization with higher levels of vitamin D. Because vitamin D has clear anti-microbial properties, it is possible that previous relationships seen with respiratory outcomes are primarily due to this property of vitamin D, and not its effects on the allergic diathesis (31). However, one complication of our current analysis, and studies analyzing vitamin D in general, is that serum vitamin D levels are highly dependent on season, and because we do not know the key time-point at which vitamin D might influence development of sensitization, it is possible that seasonal variations obscure a real relationship between vitamin D and sensitization. Although we did adjust for season in our analyses, both residual confounding and the time-varying nature of this variable could have biased our results. In contrast, seasonal changes are not likely to be a problem for analyses of associations with folate, as this micronutrient is not influenced by sun exposure or other seasonal factors.
The strengths of this study are that it took advantage of the natural experiment that is new employment at The Jackson Laboratory to prospectively determine the effects of folate and vitamin D on incident sensitization in subjects with new or intensified exposure to allergen, a question that has not been studied in adults. In addition, subjects and controls were matched on the most important predictors of incident sensitization, atopic status and mouse handling status, and important confounders, including smoking status and season, were adjusted for in the analyses. However, it is possible that we missed other important confounders of the relationship between these micronutrients and incident sensitization. Another important caveat is that we measured incident sensitization but did not have a large enough sample to assess allergic symptoms here. However, allergic sensitization is a first step in developing allergic symptoms and is a reasonable surrogate for increased risk of symptomatic allergy. Despite these caveats, this cohort offers a rare opportunity to study incident sensitization in a relatively homogenous group of adults.
In summary, this study extends previous findings that have tied higher folate levels to higher risk of allergic outcomes in children to adults, but did not find evidence that higher vitamin D levels protect against sensitization. Although it would be quite premature to recommend reduction in folic acid supplementation based on these results, especially given the very compelling reasons for folic acid supplementation in pregnant women, this finding does add to a small but growing literature that supports folic acid fortification and supplementation as a possible cause of recent increases in incidence of allergic diseases. More research aimed at understanding the mechanisms of this association may lend insight into whether folic acid supplementation might be responsible, in part, for the relatively recent increase in allergic disease. Additional prospective studies extending this study’s findings to other allergic diseases are also needed, and will help inform the design of clinical trials aimed at determining if there is an optimal folate level for allergic disease prevention.
Figure 1.
Associations between serum folate level and odds of incident mouse sensitization. Tertile of serum folate: 1 (2.47–10.45 ng/ml, reference), 2 (10.53–16.16 ng/ml), 3 (16.22–78.44 ng/ml). Odds ratios are compared to tertile 1, and are adjusted for age, sex, education, smoking status, mouse allergen level, season and vitamin D levels. CI: Confidence interval.
Table 4.
Associations between incident sensitization and serum vitamin D levels.
| Unadjusted | Adjusted Model 1* | Adjusted Model 2† | |
|---|---|---|---|
| OR (95% CI) p value | |||
| Vitamin D (natural log) | 2.4 (1.0–5.9) p=0.06 |
3.9 (0.9–17.0) p=0.07 |
3.2 (0.7–14.1) p=0.13 |
| Vitamin D categories | |||
| Category 1 (<20 ng/ml) | REF | REF | REF |
| Category 2 (20–29 ng/ml) | 2.3 (0.8–6.1) p=0.09 |
3.4 (1.0–11.7) p=0.054 |
2.6 (0.7–10.1) p=0.17 |
| Category 3 (≥30 ng/ml) | 1.7 (0.6–4.7) p=0.27 |
1.65 (0.4–6.4) p=0.47 |
1.6 (0.4–6.5) p=0.55 |
adjusted for age, sex, education, smoking status, season, mouse allergen level
adjusted for age, sex, education, smoking status, season, mouse allergen level, and folate tertile
Statistically significant findings are bolded
Key Messages.
Higher serum folate levels may be associated with increased risk of new allergic sensitization among adults.
No evidence was found that low vitamin D levels increase the risk of new allergic sensitization among adults.
Acknowledgements
The authors acknowledge Lori Sokoll, PhD and Barbara Detrick, PhD for analysis of the folate and vitamin D levels and Shannon Seopaul for facilitating sample analysis.
Sources of support: This research was funded in part by NIAID/NIH grant number 1K23AI103187 (to C.A.K) and NIAID/NIH grant number R01AI081845 (to E.C.M.)
Abbreviations
- CI
Confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Corinne A. Keet, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, and Graduate Student, Johns Hopkins Bloomberg School of Public Health, Department of Epidemiology, Baltimore, MD, (ckeet1@jhmi.edu).
Wayne G. Shreffler, The Food Allergy Center at Massachusetts General Hospital, Division of Allergy and Immunology; Department of Pediatrics, Boston, MA, United States. (WSHREFFLER@PARTNERS.ORG).
Roger D. Peng, Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD (rpeng@jhsph.edu).
William Matsui, Johns Hopkins School of Medicine, Department of Oncology, Baltimore, MD (wmatsui@jhmi.edu).
Elizabeth C. Matsui, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, Baltimore, MD, (ematsui@jhmi.edu).
References
- 1.Okupa AY, Lemanske RF, Jr, Jackson DJ, Evans MD, Wood RA, Matsui EC. Early-life folate levels are associated with incident allergic sensitization. J Allergy Clin Immunol. 2013 Jan;131(1):226–228. e1–e2. doi: 10.1016/j.jaci.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiefte-de Jong JC, Timmermans S, Jaddoe VW, Hofman A, Tiemeier H, Steegers EA, et al. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012 Apr;142(4):731–738. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- 3.Haberg SE, London SJ, Nafstad P, Nilsen RM, Ueland PM, Vollset SE, et al. Maternal folate levels in pregnancy and asthma in children at age 3 years. J Allergy Clin Immunol. 2011 Jan;127(1):262–264. doi: 10.1016/j.jaci.2010.10.004. 4 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009 Dec 15;170(12):1486–1493. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008 Oct;118(10):3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol. 2013 Feb 27; doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Bener A, Ehlayel MS, Tulic MK, Hamid Q. Vitamin D deficiency as a strong predictor of asthma in children. Int Arch Allergy Immunol. 2012;157(2):168–175. doi: 10.1159/000323941. [DOI] [PubMed] [Google Scholar]
- 8.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. 2011 Dec;38(6):1320–1327. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
- 9.Mullins RJ, Clark S, Wiley V, Eyles D, Camargo CA., Jr Neonatal vitamin D status and childhood peanut allergy: a pilot study. Ann Allergy Asthma Immunol. 2012 Nov;109(5):324–328. doi: 10.1016/j.anai.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011 Nov;128(5):1093–1099. e1–e5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011 May;127(5):1195–1202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuesen BH, Husemoen LL, Ovesen L, Jorgensen T, Fenger M, Gilderson G, et al. Atopy, asthma, and lung function in relation to folate and vitamin B(12) in adults. Allergy. 2010 Nov;65(11):1446–1454. doi: 10.1111/j.1398-9995.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsui EC, Matsui W. Higher serum folate levels are associated with a lower risk of atopy and wheeze. J Allergy Clin Immunol. 2009 Jun;123(6):1253–1259. e2. doi: 10.1016/j.jaci.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JH, Matsui W, Aloe C, Peng RD, Diette GB, Breysse PN, et al. Relationships between folate and inflammatory features of asthma. J Allergy Clin Immunol. 2013 Mar;131(3):918–920. e6. doi: 10.1016/j.jaci.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng RD, Paigen B, Eggleston PA, Hagberg KA, Krevans M, Curtin-Brosnan J, et al. Both the variability and level of mouse allergen exposure influence the phenotype of the immune response in workers at a mouse facility. J Allergy Clin Immunol. 2011 Aug;128(2):390–396. e7. doi: 10.1016/j.jaci.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011 Jan;86(1):50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernster VL. Nested case-control studies. Prev Med. 1994 Sep;23(5):587–590. doi: 10.1006/pmed.1994.1093. [DOI] [PubMed] [Google Scholar]
- 18.Essebag V, Genest J, Jr, Suissa S, Pilote L. The nested case-control study in cardiology. Am Heart J. 2003 Oct;146(4):581–590. doi: 10.1016/S0002-8703(03)00512-X. [DOI] [PubMed] [Google Scholar]
- 19.Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011 May 6;60(17):547–552. [PubMed] [Google Scholar]
- 20.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr. 2012 May;142(5):886–893. doi: 10.3945/jn.111.156919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junod SW. Folic Acid Fortification: Fact and Folly. FDA; 2001. [updated 04/14/2009; cited 2013 April 17]. Available from: http://www.fda.gov/AboutFDA/WhatWeDo/History/ProductRegulation/SelectionsFromFDLIUpdate seriesonFDAHistory/ucm091883.htm. [Google Scholar]
- 22.Bekkers MB, Elstgeest LE, Scholtens S, Haveman-Nies A, de Jongste JC, Kerkhof M, et al. Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur Respir J. 2012 Jun;39(6):1468–1474. doi: 10.1183/09031936.00094511. [DOI] [PubMed] [Google Scholar]
- 23.Magdelijns FJ, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011 Jul;128(1):e135–e144. doi: 10.1542/peds.2010-1690. [DOI] [PubMed] [Google Scholar]
- 24.Martinussen MP, Risnes KR, Jacobsen GW, Bracken MB. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am J Obstet Gynecol. 2012 Jan;206(1):72, e1–e7. doi: 10.1016/j.ajog.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012 Nov 29;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 26.van Oeffelen AA, Bekkers MB, Smit HA, Kerkhof M, Koppelman GH, Haveman-Nies A, et al. Serum micronutrient concentrations and childhood asthma: the PIAMA birth cohort study. Pediatr Allergy Immunol. 2011 Dec;22(8):784–793. doi: 10.1111/j.1399-3038.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- 27.Keet CA, McCormack MC, Peng RD, Matsui EC. Age- and atopy-dependent effects of vitamin D on wheeze and asthma. J Allergy Clin Immunol. 2011 Aug;128(2):414–416. e5. doi: 10.1016/j.jaci.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012 Nov;130(5):e1128–e1135. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 29.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013 Feb;68(2):220–228. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 30.Pike KC, Inskip HM, Robinson S, Lucas JS, Cooper C, Harvey NC, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012 Nov;67(11):950–956. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009 Jan;9(1):81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]