Abstract
Background
Atopic dermatitis (AD) is a common inflammatory skin disease with global prevalence ranging from 3% to 20%. AD patients have an increased risk for complications following viral infection (e.g., herpes simplex virus), and vaccination of AD patients with live vaccinia virus is contraindicated due to a heightened risk of eczema vaccinatum, a rare but potentially lethal complication associated with smallpox vaccination.
Objective
To develop a better understanding of immunity to cutaneous viral infection in AD patients.
Methods
In a double-blind, randomized study, we investigated the safety and immunogenicity of live attenuated yellow fever virus (YFV) vaccination of non-atopic (NA) subjects and AD patients following standard subcutaneous (SC) inoculation or transcutaneous (TC) vaccination administered with a bifurcated needle. Viremia, neutralizing antibody, and antiviral T cell responses were analyzed for up to 30 days post-vaccination.
Results
YFV vaccination by either route was well tolerated. SC vaccination resulted in higher seroconversion rates than TC vaccination but elicited similar antiviral antibody levels and T cell responses in both NA and AD groups. Following TC vaccination, both groups mounted similar neutralizing antibody responses, but AD patients demonstrated lower antiviral T cell responses by 30 days after vaccination. Among TC-vaccinated subjects, a significant inverse correlation between baseline IgE levels and the magnitude of antiviral antibody and CD4+ T cell responses was observed.
Conclusions
YFV vaccination of AD patients by the TC route revealed that high baseline IgE levels provides a potential biomarker for predicting reduced virus-specific immune memory following TC infection with a live virus.
Keywords: yellow fever virus, antibody, T cell memory, IgE, atopic dermatitis
Introduction
Atopic Dermatitis (AD) is a common inflammatory skin disease1 that causes substantial morbidity with costs of therapy, loss of work and disability estimated at $1-4 billion/year in the US2. Clinical observations indicate that AD patients have more severe cutaneous viral infections including herpes simplex virus and molluscum contagiosum3. Moreover, AD is a formal contraindication for smallpox vaccination due to risk of eczema vaccinatum, a rare but potentially life-threatening disease4, 5. Based on recommendations that individuals with AD or those who have family members with AD not be exposed to vaccinia virus, >30% of US military personnel refrained from smallpox vaccination in 20026.
Little is known about the immune deficit predisposing AD patients to viral infections but we anticipated that immune defects might occur in the skin. The goal of this study was to vaccinate AD patients with an attenuated live virus vaccine to assess clinical and immunological deficits that might explain difficulties patients have with controlling viral infections. To better understand cutaneous and systemic antiviral immunity of AD patients, we examined immune responses following vaccination with the FDA-approved live-attenuated yellow fever-17D (YFV-17D) vaccine. Unlike most live virus vaccines, YFV-17D vaccination/infection can be performed by either subcutaneous (SC) vaccination (thus bypassing the skin) or by transcutaneous (TC) vaccination, performed similarly to traditional smallpox vaccination. Transcutaneous vaccination (referred to as, “scarification”) was practiced in Africa during the 1950’s and studies of mass vaccinations totaling >130,000 recipients documented that TC vaccination with YFV-17D was safe and immunogenic7-9. Also, from a public health cost perspective, our studies indicate that a single 0.5 mL SC dose of YFV-17D could be reconstituted in a small volume to provide up to 50 doses when administered by the TC route. This report documents a randomized, double-blind multicenter study of YFV-17D vaccination administered by either the TC or SC routes in patients with AD and in non-atopic (NA) controls.
Methods
Study Design
The protocol design was a randomized, double-blind, multicenter pilot study performed in the US (NCT00723489: Immune Response to Yellow Fever Vaccination in Adults with Atopic Dermatitis). From September 2008 through March 2011, we enrolled 82 subjects 27-43 years of age who were evenly divided into 4 groups: AD and NA controls who received SC vaccination or TC vaccination with YFV-17D. This age range was selected because there had been no cases of viscerotropic disease from YFV-17D vaccination in that age range10. Subjects were informed of the risks involved with YFV vaccination including risk of yellow fever vaccine-associated viscerotropic disease (YEL-AVD). Because of potential risk for YEL-AVD, enrollment was divided into 3 stages with interim safety analysis performed by an independent NIAID Data and Safety Monitoring Board after each stage. In the first stage, enrollment was limited to non-atopic (n=10) and mild AD subjects (n=10). In stage 2, enrollment was limited to non-atopic (n=15) and moderate AD subjects (n=15). In stage 3, enrollment was limited to non-atopic subjects (n=15) and moderate and severe AD subjects (n=15). Subjects were enrolled at three sites, Oregon Health & Science University (OHSU), National Jewish Health (NJH) and University of California San Diego (UCSD). Subjects were required to be off systemic corticosteroids and other immunosuppressive medications for 30 days and topical corticosteroids/calcineurin inhibitors for 1 week before vaccination. Inhaled steroids were restricted to no more than 440 mcg per day within 6 months prior to vaccination Subjects provided written informed consent and the studies were conducted according to the Declaration of Helsinki principles and approved by Institutional Review Boards at each center.
Study Groups and Vaccinations
Subjects with normal clinical laboratory tests and no history of flavivirus vaccination or infection and who had not traveled to Africa or South America were recruited for the study. Subjects with egg allergy or acute hypersensitivity to vaccine components were excluded. Women were required to have a negative pregnancy test. Subjects were vaccinated with YFV-17D (YF-VAX®, Sanofi Pasteur Inc.) by the standard SC route or the experimental TC route on non-lesional skin on the right or left deltoid or thigh. TC vaccination consisted of performing 5-15 jabs with a bifurcated needle using 5-fold concentrated vaccine (i.e., reconstituted in 1/5th standard volume), estimated to be equivalent to ~1×103 PFU and similar to the lowest effective dose used in previous trials8. Using a double-dummy design, each subject received SC vaccination on one arm/thigh and TC vaccination on the contralateral arm/thigh with one inoculum containing YFV-17D and one containing placebo (vaccine diluent). TC vaccination sites were covered with an adhesive patch for 2 days and the site was swabbed at day 3 post-vaccination to assess virus shedding.
Primary Outcome: Neutralizing antibody responses
YFV-specific neutralizing antibody was measured by two approaches; the log10 neutralization index (LNI) which refers to a constant-serum-varying-virus-reduction test11 and the neutralizing titer 50 (NT50) which refers to a constant-virus-varying-serum-reduction test12.
Secondary Outcome: YFV Viremia and RNAemia
Infectious YFV was measured by plaque assay and YFV RNA was isolated from 500 μl serum obtained at 3-4, 5-6, 7-8, 10-11, 13-15, and 28-35 days post-vaccination13. Although quantitative real-time PCR was not performed, this was not necessary to determine duration of RNAemia.
Secondary Outcome: YFV-specific T cell quantitation
Intracellular cytokine staining was used to measure T cell responses14 using YFV-17D purified by ultracentrifugation through 25% glycerol. PBMC, with or without YFV, were cultured for 18 hours with Brefeldin A added for the last 6 hours to block cytokine secretion and cells were stained and analyzed on an LSR Fortessa.
Serum IgE
Total serum IgE was measured using the ImmunoCAP System (Phadia/Thermofisher Scientific) according to manufacturer’s directions.
Randomization and Masking
Subjects were randomized to receive YFV by SC or TC administration in a 1:1 ratio. Randomization took place according to a fixed schedule using a permuted block design. The randomization schedule was stratified by disease classification in study stage 1 (block sizes of 4 and 2), and by disease classification and gender in study stages 2 and 3 (block size of 2). Subjects were randomized via a centralized, automated, web-based randomization system that distributed subject vaccination route assignments on an individual basis after entry of stratification information by the site study coordinator. Site pharmacy personnel responsible for preparing the vaccine and vaccine administrators were unblinded but all other study personnel, including the subjects, physicians, and laboratory staff were blinded to vaccine designation.
Statistical Methods
Nonparametric survival curves for the duration of detectable YFV were estimated by maximum likelihood for interval-censored data, and differences in viral clearance rates between AD and NA controls were compared using generalized log-rank tests15. Mean Day 30 antibody levels (log10 Neutralization Index and log10 transformed NT50 levels) and seroconversion rates between AD and NA controls were compared using two-independent sample t-tests and Fisher’s exact tests, respectively. Longitudinal cytokine responses (log10 transformed INFγ+TNFα+CD4+ and INFγ+TNFα+CD8+) were modeled using mixed effects models with unstructured residual variance-covariance matrices. Study visit, atopic status, and their interaction were included as classification factors and baseline cytokine response as covariate, and linear contrasts were used to compare differences between AD and NA controls at Day 14 and Day 30. Associations between pre-vaccination log10 transformed total IgE levels and log10 transformed NT50 and cytokine levels were estimated by Pearson correlations.
Results
Study population, vaccinations and safety
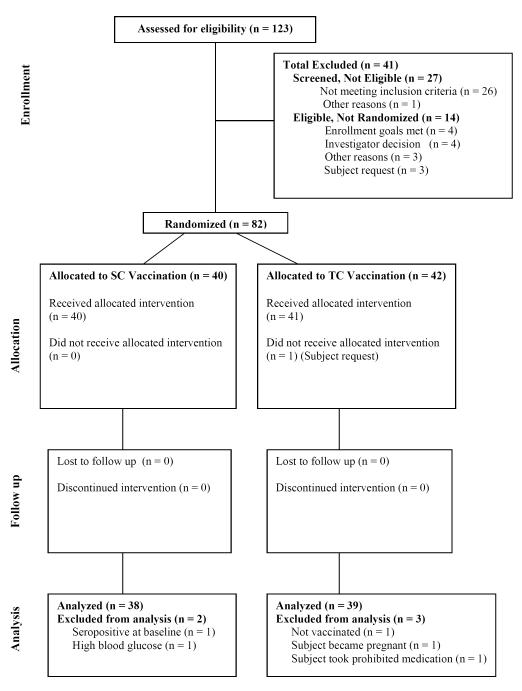
A total of 123 subjects were screened, 82 were randomized and 81 received YFV vaccination by the SC or TC route (Figure 1). Safety assessments included vital signs, vaccination site reactions, adenopathy, hematology and blood chemistry. There were no serious adverse events (AEs) among 81 vaccinated subjects. AEs occurring in >5% of subjects are listed in Table 1. The AE incident rates were generally similar between groups, but headache and fatigue were more common in AD subjects. The primary immunological and virological analysis population included 77 subjects (43% male, 83% Caucasian, 3% African American, 14% other race) with a mean age of 33 years. Total IgE at baseline was significantly higher in AD patients than non-atopic controls (p = 0.0001). Of 81 vaccinated subjects included in the safety analysis, there were 8 subjects (3 AD, 5 NA) who were non-responders, i.e., negative for YFV RNAemia, viremia, and did not seroconvert by LNI or NT50 indicating that they had not been infected by the YFV-17D inoculum. Seven of 8 non-responders were in the TC group, suggesting inadequate viral penetration and infection of the skin. All subjects were included in determining seroconversion rates but since non-responders had not been infected and did not mount an antiviral immune response, they were excluded from subsequent RNAemia and immunological analyses.
Figure 1.
Flow diagram of study subjects participating in the trial
Table 1.
Clinical adverse events experienced by >5% of subjects following yellow fever vaccination. Eighty-one subjects received SC or TC YFV vaccination. One subject withdrew consent before being vaccinated. Seventy-seven subjects were included in the overall analysis population; including 38 AD patients and 39 non-atopic controls. One subject was excluded due to pre-existing YFV-specific antibody at baseline; one subject had high blood glucose levels; one subject used a prohibited medication, and despite a negative urine pregnancy test at the time of vaccination, one woman became pregnant with a positive urine test at 34 days after vaccination and discontinued participation in the study but was included in the safety analysis. The pregnancy was uneventful and she gave birth to a healthy child. Safety assessments were made on days 3, 5, 7, 10, 14 and 30 after vaccination. Grading and attribution of AEs followed the criteria set forth in the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Vaccine Clinical Trials guidelines, ranging from Grade 1-4 (Sept 2007). Over ninety percent of AEs were mild or moderate. All but three of 13 Grade 3 AEs and each of 3 Grade 4 AE’s were transient laboratory abnormalities (mostly increased cholesterol levels), none considered relevant to vaccine. Abnormal laboratory values unrelated to vaccination are therefore not included in this table.
| SC+AD (N=19) |
SC+NA (N=21) |
TC+AD (N=23) |
TC+NA (N=19) |
|
|---|---|---|---|---|
| Adverse Event | n (%) | n (%) | n (%) | n (%) |
| Fatigue | 7 (37) | 3 (14) | 7 (32) | 0 (0) |
| Back pain | 3 (16) | 1 (5) | 5 (23) | 1 (5) |
| Headache | 5 (26) | 1 (5) | 4 (18) | 0 (0) |
| Arthralgia | 4 (21) | 3 (14) | 2 (9) | 0 (0) |
| Myalgia | 4 (21) | 3 (14) | 2 (9) | 0 (0) |
| Nausea | 4 (21) | 1 (5) | 3 (14) | 0 (0) |
| Injection site pain | 2 (11) | 4 (19) | 1 (5) | 0 (0) |
| Upper respiratory tract infection |
3 (16) | 0 (0) | 3 (14) | 0 (0) |
| Application site pain |
1 (5) | 0 (0) | 3 (14) | 1 (5) |
| Lymphadenopathy | 1 (5) | 0 (0) | 3 (14) | 1 (5) |
Measurements of yellow fever virus replication
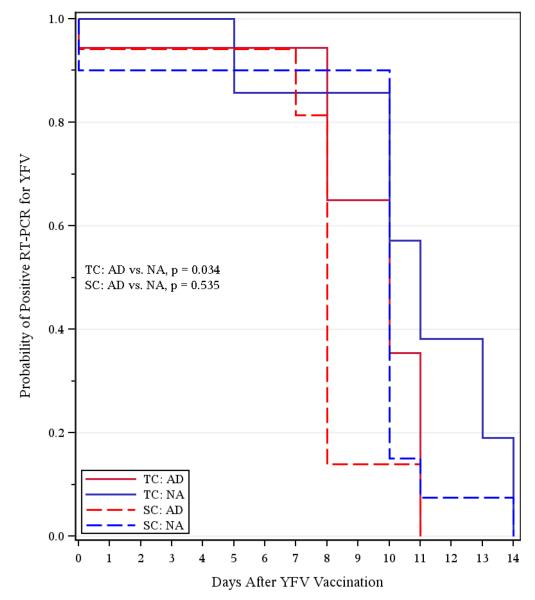
Viremia levels were low (<20 PFU/mL) and infrequent, with detection of live virus observed between 4-7 days post-vaccination in only 16% (6/38) and 10% (4/39) of subjects following SC or TC immunization, respectively and the few viremic samples that were identified were equally distributed among AD patients and NA controls. Swabs of TC vaccination sites were negative for infectious virus by plaque assay. Analysis of viral replication by RT-PCR was more informative. Although there were 4 subjects who seroconverted without detectable YFV-17D RNA in serum (i.e., RNAemia), the other 65 analyzable subjects demonstrated measurable levels of RNAemia of varying duration (Figure 2).
Figure 2. Duration of YFV RNAemia after subcutaneous or transcutaneous vaccination.
Kaplan-Meier curves show the proportion of YFV-seroconverted subjects who score positive for YFV RNA by RT-PCR during the first two weeks after vaccination. The limit of detection was <1 PFU/ml for serum spiked with purified YFV.
Abbreviations; AD, atopic dermatitis; NA, non-atopic; TC, transcutaneous; SC, subcutaneous TC-AD, n = 18; TC-NA, n = 14; SC-AD, n = 17; SC-NA, n = 20.
Following SC vaccination, there was no statistically significant difference between duration of YFV-17D RNAemia in AD patients (SC-AD) and NA subjects (SC-NA) (p = 0.535). In contrast, AD patients (TC-AD) cleared RNAemia significantly faster than normal controls (TC-NA) after TC vaccination (p = 0.034). This was an unexpected finding and indicates that route of infection (TC vs. SC) may impact systemic viral replication. From a safety perspective, this also suggests that TC vaccination of AD patients with YFV-17D does not represent an increased risk for systemic spread.
Antiviral antibody and T cell responses after vaccination
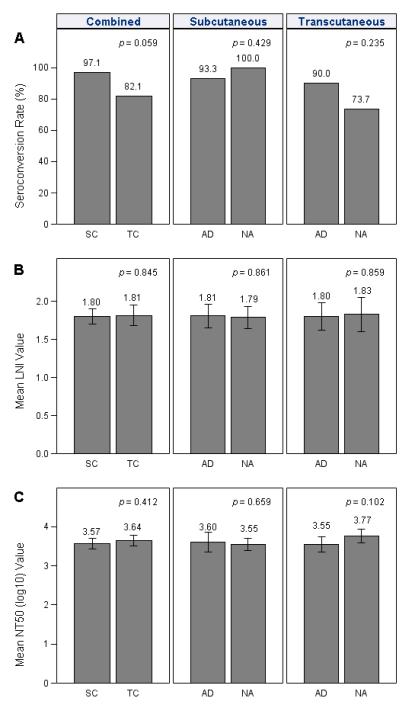
Virus-specific neutralizing antibody represents the principal mechanism of protection against YFV10, 16. Seroconversion and protection are typically defined by a neutralizing antibody titer of ≥0.7 LNI, which is roughly estimated to be greater than an NT50 of 1:10ref12. Following SC vaccination, we obtained 97% seroconversion whereas after TC vaccination, the overall seroconversion rate was 82% (p = 0.059) (Figure 3A). Lower seroconversion by the TC route may have been due to technical features of TC vaccination with a bifurcated needle; during the first stage of the protocol, TC vaccination was performed using a “5 jab” technique resulting in 67% (6/9) seroconversion whereas in subsequent stages the “15 jab” technique was used, resulting in 87% (26/30) seroconversion. There was no significant difference in antibody titers elicited after vaccination by the 5-jab or 15-jab technique when measured by LNI (p = 0.986) or by NT50 (p = 0.247). Antibody responses, measured by LNI (Figure 3B) or NT50 (Figure 3C) showed no significant differences among seroconverters, regardless of route (TC vs. SC) or group designation (SC-AD vs. SC-NA or TC-AD vs. TC-NA).
Figure 3. Induction of neutralizing YFV-specific antibody responses following subcutaneous or transcutaneous vaccination.
(A) At 30 days after YFV vaccination, seroconversion rates were determined for the different vaccine groups and was defined as a YFV-specific neutralizing titer of 10 or more. (B) Levels of YFV neutralizing antibody among seropositive subjects were measured by the log neutralizing index (LNI) in which serum is held constant and different amounts of virus are neutralized. (C) Levels of YFV neutralizing antibody among seropositive subjects were measured by determining the serum dilution required to neutralize 50% of virus plaques (held constant at 50-100 YFV plaques). Error bars represent 95% confidence intervals.
TC vaccination against YFV has not been previously examined in terms of antiviral T cell responses. SC vaccination with YFV-17D elicits a strong and polyfunctional virus-specific T cell response17, 18 and is an excellent model for analyzing the interplay between innate and adaptive immunity. Following in vitro stimulation of PBMC with YFV-17D, virus-specific T cells producing two antiviral cytokines, IFNγ and TNFα, were enumerated by intracellular cytokine staining analysis (Figure 4A). Although the IFNγ+TNFα- T cell response was higher than the IFNγ+TNFα+ T cell response at day 14, by day 28-35, the majority of T cells co-expressed both IFNγ and TNFα (Figure 4A and data not shown). The total IFNγ+ T cell response did not have the same specificity as the IFNγ+TNFα+ T cell response due to higher frequencies of IFNγ+ T cells at day 0 (i.e., prior to vaccination). However, the trends for the total IFNγ+ T cell response (Figure E1 in Online Repository) were similar to that observed when IFNγ+ TNFα+ T cells were measured (Figure 4). YFV-specific CD4+ T cells peaked by 14 days after SC vaccination (Figure 4B), but after TC vaccination, CD4+ T cell responses were lower at 14 days post-vaccination and either remained flat or continued to increase by day 30 (Figure 4C, Figure E2 in Online Repository). We found no significant differences in CD4+ T cell responses between AD and NA groups following SC vaccination (Figure 4B, Figure E2 in Online Repository) whereas YFV-specific CD4+ T cell responses differed significantly at 30 days following TC vaccination (Figure 4C) (p = 0.036). YFV-specific CD8+ T cell responses peaked by 14 days after SC vaccination (Figure 4D) but were delayed following TC vaccination (Figure 4E, Figure E2 in Online Repository). Similar to antiviral CD4+ T cells, SC vaccination resulted in CD8+ T cell responses that were indistinguishable between AD and NA groups (Figure 4D), but following TC vaccination antiviral CD8+ T cell responses showed a modestly significant difference (Figure 4E) (p = 0.045). Analysis of polyfunctional T cells that express three cytokines (i.e., IFNγ+TNFα+IL-2+) provided similar results; there was a significant difference in antiviral CD4+ T cell responses (p = 0.043) and CD8+ T cell responses (p = 0.0097) at 30 days after TC vaccination, but no significant difference between groups at 30 days after SC vaccination (p >0.50) (data not shown). There was also no significant effect of subject age on the outcome of antiviral T cell responses at day 14 (p >0.4) or day 30 (p >0.1) (data not shown). Together, these results demonstrate that when skin is bypassed by SC vaccination, there is no difference in antiviral T cell responses between AD patients and non-atopic controls, but if viral infection is initiated in skin via TC vaccination, AD patients demonstrate significantly lower YFV-specific T cell responses at later time points.
Figure 4. Kinetics and magnitude of vaccine-induced YFV-specific CD4+ and CD8+ T cell responses.
The frequency of virus-specific T cells was measured by stimulating PBMC with purified YFV and measuring the frequency of IFNγ+TNFα+ T cells at each time point by intracellular cytokine staining analysis. Representative flow cytometry dot plot showing the virus-specific CD4+ and CD8+ T cell response after YFV vaccination (A). The dot plots were pre-gated on CD4+CD8− and CD4−CD8+ T cells and show the number of events per 106 CD4+ or CD8+ T cells after background subtraction (medium alone). The kinetics and magnitude of YFV-specific CD4+ T cells (B and C) or CD8+ T cells (D and E) were measured in subjects vaccinated by the subcutaneous route (B and D) or the transcutaneous route (C and E).
The numbers indicate measured P values at each time point and error bars represent 95% confidence intervals.
Association between pre-existing total IgE and antiviral immunity
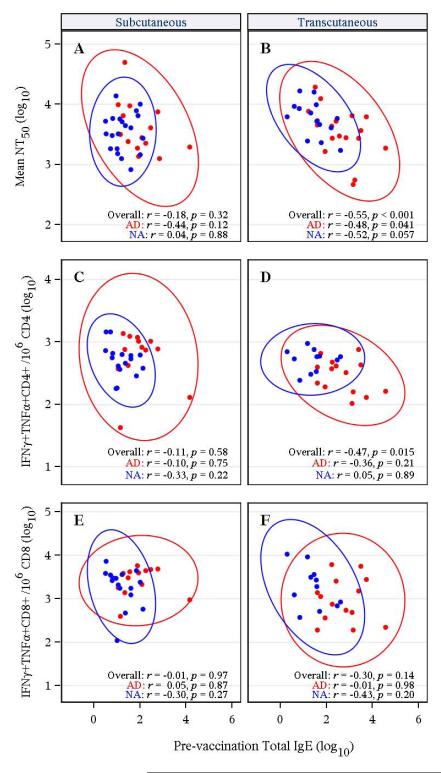
AD patients often have elevated serum IgE and for some viral infections the highest levels of IgE are associated with more severe disease manifestations19. We measured the magnitude of antiviral antibody (Figure 3) and T cell responses (Figure 4) after YFV vaccination, and compared these parameters to total serum IgE levels at baseline (Figure 5, Table E1 in Online Repository). Following SC vaccination (Figure 5A), there was no significant correlation between neutralizing antibody responses and pre-existing IgE levels for AD patients (p = 0.12) or NA controls (p = 0.88). Following TC vaccination (Figure 5B), there was a trend towards a significant inverse correlation between IgE and neutralizing antibody levels in NA subjects (95% CI: −0.82 to 0.04, p = 0.057) and a modestly significant inverse correlation between IgE and antibody titers in AD patients (95% CI: −0.77 to −0.01, p = 0.041). The results of Figure 5B are consistent with Figure 3C, in which there is a trend towards lower NT50 titers (although not significant, p=0.102) following transcutaneous vaccination of AD vs. NA subjects. When all TC-vaccinated subjects were analyzed together, the inverse correlation became highly significant (95% CI: −0.75 to −0.25, p <0.001). In contrast, combined analysis of all subjects who received SC vaccination did not alter the outcome; there was no significant correlation between IgE and antiviral antibody levels following immunization by the SC route (95% CI: −0.48 to 0.17, p = 0.32).
Figure 5. Comparison between baseline total IgE levels and YFV vaccine-induced immune responses.
Total serum IgE levels obtained at baseline were compared to subsequent YFV vaccine-induced immune responses at 30 days post-vaccination and included YFV-specific neutralizing titers (NT50) (A and B), YFV-specific CD4+ T cell responses (C and D) and YFV-specific CD8+ T cell responses (E and F) in subjects vaccinated by the subcutaneous route (A, C, and E) or the transcutaneous route (B, D, and F). The red symbols represent AD patients and the blue symbols represent non-atopic (NA) subjects and the red or blue ellipses represent 95% confidence interval ellipses.
Analysis of antiviral T cell responses indicated a dichotomy between the two arms of the cellular immune response. As with the relationship between IgE and neutralizing antibody responses, YFV-specific CD4+ T cell responses showed a significant inverse correlation with IgE levels following TC vaccination (95% CI: −0.73 to −0.09, p = 0.015; Figure 5D) but not after SC vaccination (95% CI: −0.45 to 0.27, p = 0.58; Figure 5C). YFV-specific CD8+ T cell responses showed no significant relationship with baseline IgE titers in AD or NA groups regardless of receiving TC or SC vaccination (Figure 5E and 5F). Together, these results indicate that, unlike CD8+ T cells, the CD4+ T cell response elicited by AD patients was significantly reduced in patients with high baseline IgE, but this is found only when YFV-17D infection is initiated in the skin and this relationship no longer exists when skin is bypassed by SC vaccination.
Discussion
The goal of this study was to develop a better understanding of the immune responses of AD subjects following cutaneous viral infection. No significant differences in antiviral responses were observed between mild versus moderate/severe AD (data not shown). In contrast, comparisons between TC and SC vaccination with a live virus vaccine (YFV-17D) revealed differences in the kinetics/magnitude of antiviral T cell responses and AD patients with higher baseline IgE were more likely to develop lower antiviral antibody and CD4+ T cell responses. Further studies will be needed to determine if IgE might serve as a biomarker of potential immunogenicity in response to transcutaneous/transdermal vaccination strategies in other patient populations.
Although AD patients have higher total serum IgE levels than non-atopic controls, it does not appear that they have a substantial deficit in innate or early adaptive immune responsiveness to YFV infection of the skin since AD patients cleared YFV RNAemia more rapidly than their non-atopic counterparts (Figure 2). Virus-specific IgE levels were not measured in this study and it is unknown if non-specific total IgE is involved with immune regulation in this model. More likely, it simply represents an indicator of immune responsiveness to transcutaneous YFV vaccination. Moreover, there is little known about the levels of IgE in the skin and more studies are needed in order to learn if cutaneous IgE levels are elevated in AD patients. The rapid clearance of viremia by AD patients, however, is an important observation because a small vaccination study performed in cynomolgus macaques using recombinant strains of YFV-17D expressing other flavivirus genes (e.g., JEV envelope) showed that 3/3 animals that received chimerivax-JEV transcutaneously with a prototype microenhancer array (MEA) abrasion device had higher and more prolonged viremia than animals that received standard subcutaneous vaccination20. Fortunately, our data in 39 human subjects indicates that following non-recombinant YFV-17D vaccination there was no evidence for increased viremia following transcutaneous vaccination. Likewise, another clinical study involving intradermal YFV vaccination found no major difference in viral RNA levels at 5 days post-vaccination in comparison with subcutaneous vaccination21, further suggesting that this may be a safe approach to vaccine delivery. However, larger clinical studies will be needed to determine the safety of TC versus SC vaccination against yellow fever.
In terms of vaccine-induced cellular immunity, the initial antiviral CD4+ and CD8+ T cell responses of AD patients measured at 14 days post-infection were not significantly lower than in NA controls but responses were significantly lower at one month post-vaccination in the TC-vaccinated cohort (Figure 4). Whether lower T cell responses in AD patients observed at later time points are due to weaker antiviral immune memory or to lower antigenic load resulting in more rapid memory T cell contraction is a question that will require further study. We do not believe that genetics or overt immunosuppression are factors in the lower immune responses elicited by AD patients after TC vaccination since AD patients mount normal antiviral immune responses when vaccinated by the SC route. In any case, the strong inverse relationship between neutralizing antibody responses and antiviral CD4+ T cell responses with baseline IgE levels represents an intriguing finding – especially since it is only observed during TC infection and is not found in cases in which the skin is bypassed by SC injection/infection (Figure 5).
This study represents the first immunological and virological examination of transcutaneous YFV vaccination performed in >40 years. Although our study is not powered to identify rare adverse events22, 23, prior studies involving >130,000 vaccinees indicate that TC vaccination with YFV-17D is safe, with no serious adverse events recorded7-9. Using a 15-jab approach to TC vaccination, we obtained 87% seroconversion with neutralizing antibody levels well above the LNI = 0.7 level required for protective immunity. It is unclear whether TC vaccination will provide a safety benefit over SC vaccination, but it may provide a mechanism for decreasing vaccine cost and increasing supplies in times of vaccine shortage because a single SC dose of YFV-17D can provide ~50 doses when delivered by TC inoculation. This is key because YFV immunization campaigns can be hampered by vaccine shortages and intradermal YFV vaccination has also been shown to work well with a 1/5th dose typically used for subcutaneous vaccination21. There is growing interest in developing intradermal vaccination strategies24-27 and several studies have targeted the skin to enhance vaccine immunogenicity28-30. However, these protocols did not specifically include patients with skin diseases. Considering that AD is a common health problem, these patients as well as those with other skin diseases, should be evaluated during early stage trials involving cutaneous delivery. In addition, biomarkers such as total IgE may prove useful to identify population subsets that may respond differently to cutaneous infection or vaccination.
Supplementary Material
Figure E1. Vaccine-induced IFNγ+ YFV-specific CD4+ and CD8+ T cell responses. The kinetics and magnitude of YFV-specific CD4+ T cells (A and B) or CD8+ T cells (C and D) were measured in subjects vaccinated by the subcutaneous route (A and C) or the transcutaneous route (B and D). The frequency of virus-specific T cells was measured by stimulating PBMC with purified YFV and measuring the frequency of total IFNγ+ T cells. Error bars represent 95% confidence intervals. Numbers at bottom of each panel denote p values of the comparisons between AD and NA at days 7, 14, and 30.
Figure E2. Effect of vaccination route on YFV-specific CD4+ and CD8+ T cell responses. The kinetics and magnitude of YFV-specific CD4+ T cells (A) or CD8+ T cells (B) were measured in subjects vaccinated by the subcutaneous (SC) and transcutaneous (TC) routes. The frequency of virus-specific T cells was measured by stimulating PBMC with purified YFV and measuring the frequency of total IFNγ+TNFα+. Numbers at bottom of each panel denote p values of the comparisons between SC and TC for AD (red) and NA (blue) subjects at days 14 and 30.
Table E1. Baseline Total IgE, NT50, and day 30 IFNγ+TNFα+/106 CD4+, IFNγ+TNFα+CD8+/106 CD8+ counts. Geometric mean counts for each diagnostic group by route are listed in red. N.D. denotes Not Determined.
Clinical Implications.
Clinical trials involving vaccination through the skin should include AD patients – especially since this group can differ significantly in terms of their immune responses to skin-associated vaccine delivery.
Acknowledgments
We thank the study subjects for their time and their participation in this research study; R. Hamilton at Johns Hopkins University for IgE measurements; M. Dubois, P. Taylor, and J. Herrell for study coordination and sample procurement; C. Carocci for administrative support; D. Zaccaro for early statistical analysis; R. Gallo for helpful scientific discussions, and J. Reese and A. Holliday for operational support. M. Plaut, M. Gomez, J. Laurienzo Panza and J. Goldstein from NIH/NIAID provided oversight of this study. M. Plaut and D. Rotrosen reviewed the manuscript and provided comment. The authors were not paid to write this article by a pharmaceutical company or other agency. M.K.S. is the corresponding author and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Source: This project has been funded with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services contract number HHSN266200400029C (to DL, TH, JH, and MS) and HHSN26620400033C, and Oregon National Primate Research Center grant, 8P51 OD011092-53 (to MS).
Abbreviations
- YFV
yellow fever virus
- AD
atopic dermatitis
- NA
non-atopic
- TC
transcutaneous
- SC
subcutaneous
- NT50
neutralizing titer 50
- LNI
log neutralizing index
- TNFα
tumor necrosis factor-alpha
- IFNγ
interferon-gamma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure OHSU, E.H., and M.K.S. have a financial interest in Najít Technologies, Inc., a company that is developing an H2O2-inactivated yellow fever vaccine. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. G.D., H.L., and R.W. are employed by Rho, Inc, and have received research support from the National Institute of Allergy and Infectious Diseases to perform clinical trial study coordination and independent statistical data analysis. There is no financial relationship between Rho, Inc. and Najít Technologies, Inc. All other authors declare no financial conflicts of interest.
References
- 1.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 2.Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361–70. doi: 10.1067/mjd.2002.120528. [DOI] [PubMed] [Google Scholar]
- 3.Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–9. 9 e1–7. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. Jama. 1970;212:441–4. [PubMed] [Google Scholar]
- 5.Reed JL, Scott DE, Bray M. Eczema vaccinatum. Clin Infect Dis. 2012;54:832–40. doi: 10.1093/cid/cir952. [DOI] [PubMed] [Google Scholar]
- 6.Grabenstein JD, Winkenwerder W., Jr US military smallpox vaccination program experience. Jama. 2003;289:3278–82. doi: 10.1001/jama.289.24.3278. [DOI] [PubMed] [Google Scholar]
- 7.Cannon DA, Dewhurst F, Meers PD. Mass vaccination against yellow fever by scarification with 17D strain vaccine. Ann Trop Med Parasitol. 1957;51:256–63. doi: 10.1080/00034983.1957.11685814. [DOI] [PubMed] [Google Scholar]
- 8.Meers PD. Yellow fever vaccination by scarification with the 17D strain: an appreciation of the present position. Trans R Soc Trop Med Hyg. 1957;51:338–45. doi: 10.1016/0035-9203(57)90125-6. [DOI] [PubMed] [Google Scholar]
- 9.Meers PD. Further observations on 17D-yellow fever vaccination by scarification, with and without simultaneous smallpox vaccination. Trans R Soc Trop Med Hyg. 1960;54:493–501. doi: 10.1016/0035-9203(60)90099-7. [DOI] [PubMed] [Google Scholar]
- 10.Monath TP, Cetron MS, Teuwen DE. In: Yellow fever vaccine. 5th ed Plotkin SA, Orenstein WA, Offit PA, editors. Saunders/Elsevier; Vaccines: 2008. pp. 959–1056. [Google Scholar]
- 11.Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66:533–41. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 12.Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, et al. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–33. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 13.Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159–67. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Zhao Q, Zhao X. Generalized Log-Rank Tests for Interval-Censored Failure Time Data. Scandinavian Journal of Statistics. 2005;32:49–57. [Google Scholar]
- 16.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: How is it defined? Hum Vaccin. 2008;4 doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–30. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laske N, Niggemann B. Does the severity of atopic dermatitis correlate with serum IgE levels? Pediatr Allergy Immunol. 2004;15:86–8. doi: 10.1046/j.0905-6157.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 20.Dean CH, Alarcon JB, Waterston AM, Draper K, Early R, Guirakhoo F, et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005;1:106–11. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 21.Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS ONE. 2008;3:e1993. doi: 10.1371/journal.pone.0001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, et al. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–82. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on medicine and health in the tropics; Marseille, France. Monday 12 September 2005; Vaccine: 2007. pp. 2758–65. [DOI] [PubMed] [Google Scholar]
- 24.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ. 2011;89:221–6. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marra F, Young F, Richardson K, Marra CA. A Meta-analysis of intradermal versus intramuscular influenza vaccines: Immunogenicity and Adverse Events. Influenza Other Respi Viruses. 2012 doi: 10.1111/irv.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Ther Deliv. 2012;3:357–71. doi: 10.4155/tde.12.13. [DOI] [PubMed] [Google Scholar]
- 27.Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv. 2012;9:541–50. doi: 10.1517/17425247.2012.676038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redfield RR, Innis BL, Scott RM, Cannon HG, Bancroft WH. Clinical evaluation of low-dose intradermally administered hepatitis B virus vaccine. A cost reduction strategy. JAMA. 1985;254:3203–6. [PubMed] [Google Scholar]
- 29.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 30.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Vaccine-induced IFNγ+ YFV-specific CD4+ and CD8+ T cell responses. The kinetics and magnitude of YFV-specific CD4+ T cells (A and B) or CD8+ T cells (C and D) were measured in subjects vaccinated by the subcutaneous route (A and C) or the transcutaneous route (B and D). The frequency of virus-specific T cells was measured by stimulating PBMC with purified YFV and measuring the frequency of total IFNγ+ T cells. Error bars represent 95% confidence intervals. Numbers at bottom of each panel denote p values of the comparisons between AD and NA at days 7, 14, and 30.
Figure E2. Effect of vaccination route on YFV-specific CD4+ and CD8+ T cell responses. The kinetics and magnitude of YFV-specific CD4+ T cells (A) or CD8+ T cells (B) were measured in subjects vaccinated by the subcutaneous (SC) and transcutaneous (TC) routes. The frequency of virus-specific T cells was measured by stimulating PBMC with purified YFV and measuring the frequency of total IFNγ+TNFα+. Numbers at bottom of each panel denote p values of the comparisons between SC and TC for AD (red) and NA (blue) subjects at days 14 and 30.
Table E1. Baseline Total IgE, NT50, and day 30 IFNγ+TNFα+/106 CD4+, IFNγ+TNFα+CD8+/106 CD8+ counts. Geometric mean counts for each diagnostic group by route are listed in red. N.D. denotes Not Determined.