Abstract
Ritonavir (RTV), a prototypical protease inhibitor currently used as a key component for anti-HIV therapy, is known for its endothelial and hepatic toxicity. The effects of RTV and Mg-supplementation on cultured bovine endothelial cells (EC) and rat hepatic endothelial nitric oxide synthase (eNOS) status were investigated. RTV dose-dependently (5–30µM) decreased EC viability after 48hrs; high Mg (2 mM) significantly attenuated the lost viability. ECs incubated with 15 µM RTV for 6 to 24 hrs. resulted in 2–4-fold elevation of oxidized glutathione and a 25% loss of total glutathione. At 24 hrs., EC superoxide production due to RTV was detected by dihydroethidium staining, and increased 41% when quantified by flow cytometry; both altered glutathione status and superoxide levels were substantially reversed by 2 mM Mg. RTV reduced eNOS mRNA (−25% at 24 hrs.), and led to decreased eNOS dimer/monomer ratios; nitric oxide (NO)-derived products decreased 40%; both changes were attenuated by Mg-supplementation. In male LewXBrown-Norway rats, RTV administration (75 mg/kg/day, 5 weeks) resulted in an 85% increase in plasma 8-isoprostane, a 30% decrease of hepatic eNOS mRNA; concomitantly, eNOS protein decreased 72%, whereas plasma nitrite level was reduced 49%. Dietary Mg-supplementation (6-fold higher than control) prevented the eNOS mRNA decrease along with lowering 8-isoprostane, and restored the eNOS protein and plasma nitrite levels comparable to controls.
Conclusion
Mg attenuates RTV-mediated EC oxidative eNOS dysfunction, and down-regulation of hepatic eNOS expression; we suggest that Mg can serve as a beneficial adjunct therapeutic against RTV-mediated eNOS toxicity.
Keywords: Protease inhibitor ritonavir, Endothelial cell oxidative stress, Decreased eNOS dimer/monomer ratio, Rat hepatic eNOS down-regulation, Plasma 8-isoprostane, Decreased plasma nitrite, Mg-supplementation
INTRODUCTION
Ritonavir (RTV) is one of the original prototypical protease inhibitors (PI), which has been continuously used since its approval by FDA in 1996. The original therapeutic goal of RTV was to inhibit HIV protease, a key enzyme required to cleave viral polypeptide to functional units; because of its severe side effects, RTV is now seldom used as a monotherapy [1]. Rather, due to its potent ability to inhibit host hepatic cytochrome P450 3A4 isoenzyme [2], RTV is currently used at a reduced dosage universally as a booster to improve bioavailability and lower frequent dosing of the co-administered protease inhibitors [1,3]. However, it was found that even at a reduced dose, RTV still caused significant metabolic effects in healthy volunteers [4]. RTV associated endothelial and hepatic toxicities are major concerns for long term use [5–9]. In isolated porcine coronary arteries, in vitro RTV exposure was shown to induce endothelial contractile dysfunction through oxidative stress and decreased NO bioavailability associated with down-regulation of eNOS expression [10–12]; these effects were attenuated by certain antioxidants such as ginsenosides, seleno-1-methionine [11], and dihydroxylbenzyl alcohol [12]. Another structurally related PI, indinavir, was shown to induce impaired relaxation in aortic rings isolated from the drug treated rats and mice [13, 14]. However, PI-mediated down-regulation of eNOS, including that by RTV, was observed only in vitro and has never been reported in vivo.
Magnesium (Mg) is known to have direct anti-radical [15, 16], anti-calcium properties [17]. High Mg-supplementation was found to have anti-hypertensive [18] and beneficial effects on diabetes and cyclosporine induced nephrotoxicity [19,20]. Oral magnesium therapy for 6 months in coronary artery disease patients led to a significant improvement in brachial artery endothelial function [21]. Recently, we demonstrated that Mg-supplementation suppressed AZT-induced systemic oxidative stress and cardiac inflammation in rats [22]. The following study was designed to examine if high Mg is able to provide protective effects against RTV-mediated endothelial cell oxidative stress with specific emphasis on eNOS regulation and functional activity in cultured endothelial cells. As RTV is metabolized mainly by the liver, and was well known to cause hepatotoxicity, the effects of RTV administration and Mg-supplementation on liver eNOS status were assessed further in rats.
METHODS
Materials
Purified RTV was obtained from NIH AIDS Reagent Program, Germantown, MD. Concentrated RTV oral solution was purchased from Abbott Laboratories, Chicago, ILL. MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide), endothelial cell growth supplement, heparin, Griess reagent, 2,3-diaminonaphthalene (2,3-DAN), N-acetylcysteine (NAC), N-acetylcysteine amide (NACA) were purchased from Sigma (St. Louis, MO). Primers were from Integrated DNA technologies (Coralville, IA). Dulbecco's Modification of Eagle's Medium/Ham's F-12 50/50 Mix (DMEM/F12) and fetal bovine serum were obtained from Cellgro (Hemdon, VA).
Cell Culture and MTT Assay
Bovine aortic endothelial cells (BAECs) were received from Coriell Institute for Medical Research (Camden, NJ), catalog ID AG08132. Cells were grown in DMEM/F12, plus 15% fetal bovine serum, 0.02mg/ml endothelial cell growth supplement, 0.05mg/ml heparin sodium salt, 0.01ml/ml complete media with Penicillin-Streptomycin in 37°, 5% CO2 incubator. Cell viability following exposure to different combination of drug treatments with normal (0.8mM) or high (2mM) MgSO4, was measured by using the MTT method [23]. BAECs at exponential phase between passages 3–6 were seeded at 104 cells/wells in 24-well plates until it reached 85–95% confluence. The cells were incubated with 5–30µM RTV with 0.8 mM or 2 mM Mg levels. At 24 or 48 hours, 20ul of 5mg/ml MTT added to each well and incubated for 4 hours. The final blue crystals were dissolved in 200µl DMSO at 37°C, 5min. The plates were read at 490nm using a Synergy HT Microplate Reader (Bioteck, Winooski, VT). Each condition was performed in triplicate.
Glutathione Status
RTV-induced changes in total (GSH+GSSG) and oxidized (GSSG) glutathione levels in a separate set of cells grown in 6-well plates in normal or high Mg were determined enzymatically by the DTNB-glutathione reductase method as described [22–24]
Superoxide anion detection in BAECs
Superoxide anion generation was detected according the method of Zhao et al [25] in which dihydroethidium (DHE) reacts with superoxide anions to form oxyethidium which is bright red detectable by fluorescent microscope or flow cytometry. For visualization, after drug treatment, cells were rinsed twice with PBS, incubated with 2µM DHE at 37° C for 30min, and were washed with PBS and mounted using Fluoroshield with DAPI (4’,6’ diamino-2-phenylindole-2HCl Sigma); images were captured by Olympus DP72 fluorescent microscope at 540/570nm for DHE and 345/455nm for DAPI. For quantifying the emission intensity of samples, FACSCalibur DxP8 (BD, Franklin Lakes, NJ) was used by the same procedures at 488/585nm for DHE, plus live/dead Fixable Far Red Dead Cell Stain (Invitrogen, NY) at 637/660nm for checking cell viability. Population comparison was analyzed using Super-Enhanced Dmax Subtraction algorithm from FlowJo 7.6.4 (Tree Star Inc., Ashland, OR) [26].
Animal Model, RTV treatment and Diets
All rat experiments were guided by the principles of use and care of animals recommended by USDHHS and approved by the GWU Animal Care and Use Committee. Male Lewis-Brown Norway F1 (LBN-F1) rats (160 gm, 3–4 week old) were custom ordered from Harland Lab (Indianapolis, IN). The rats were fed a Mg-sufficient (0.1% Mg/kg food, Mg derived from MgO) or a Mg-supplemented (0.6% Mg) diet [22] containing extracted casein as the diet base and essential vitamins and nutrients (Harlan Teklad, Madison, WI) for 5 weeks. RTV administration (75mg/kg/day) was performed by daily gavage; time matched controls were given identical volumes of vehicle solution (ethanol/DMSO/propylene glycol/water=10/5/50/35%v/v). Blood samples were collected by cardiac puncture and plasma samples isolated immediately by centrifugation (200g × 10 min) and stored at −80°. Liver samples were rapidly removed, frozen in liquid nitrogen and stored at −80°. Plasma Mg levels were determined by flame emission atomic absorption (AA) spectroscopy [22]. Total plasma 8-isoprostane contents were determined after alkalization treatment (2N NaOH, 45°C, 2hrs) followed by neutralization (by HCl) before using an ELISA kit (Cayman Chemical, Ann Arbor, MI) [22].
Total EC NO products and Plasma Nitrate, Nitrite measurement
Total NO products (nitrite plus nitrate) released over 24 hours in the cell medium was determined by the Griess reagent method using E. coli nitrate reductase to convert nitrate to nitrite as described [23,24]. To measure rat plasma nitrite levels, the method of Nussler et al [27] using fluorometric measurement of nitrite by 2,3-diaminonaphthalene (DAN) was slightly modified as following. Briefly, 70µl plasma aliquots were diluted by 70µl H2O, followed by adding 30µl 10% ZnSO4 plus 30µl 0.5N NaOH (for protein precipitation); the mixture was centrifuged at 10000g (10min, 4°C). To 100µl supernatants (or standards) placed into a 96-microwell plate, 50µl 1.5N HCl and 50µl freshly prepared DAN (0.025mg/ml 0.625N HCl) were added and incubated at RT for 5min in the dark; the reaction was stopped by 200µl 3N NaOH. Immediately the microplate was placed in a fluorescence photometer (Gemini™ XPS Fluorescence Microplate Reader from Molecular Devices, Sunnyvale, California) and measured at 365nm/410nm. Plasma nitrate + nitrite levels were determined by the E. coli nitrate reductase/Griess reagent method as described [24]
Real-Time quantitative PCR—BAECs & LBN-F1 rat liver eNOS
Total EC RNA was extracted by Trizol Reagent (Invitrogen, CA) according to the manufacture’s procedures [28]. cDNA was synthesized and amplified from total RNA using the iScript cDNA Synthesis Kit (Bio-Rad, CA) and quantitative real-time PCR was performed using SybrGreenER qPCR Super Mix (Invitrogen, CA). EC eNOS mRNA expression values were normalized to 18s ribosomal RNA and performed on Applied Biosystems 7300 Real-Time PCR System. Bovine eNOS primers were: forward 5’-CGGAGGGGCCCAAGTTCCCT-3’ and reverse 5’-GGACTGCGCGCACAGAGTGT-3’. Bovine 18s rRNA primers were: forward 5’-AAACGGCTACCACATCCAAG-3’ and reverse 5’-TACAGGGCCTCGAAAGAGTC-3’. For the rats, 30mg liver samples were used and homogenized, followed by Trizol extraction of total RNA. All the later steps were the same as the previous EC RT-PCR procedure. Rat eNOS mRNA expression values are normalized to GAPDH mRNA. Rat eNOS primers were: forward 5’-CATGGGCACGGTGATGGCGA-3’ and reverse 5’-CCGGGGGTCAAACGCCTTCC-3’. Rat GAPDH primers were: forward 5’-GGGGCTCTCTGCTCCTCCCTG-3’ and reverse 5’-GAGACGAGGCTGGCACTGCAC-3’.
Western Blot analysis
Western blot analysis for eNOS protein was performed following the published procedures [29,30] with minor modifications. Total protein from BAECs or rat livers were obtained by homogenizing with NP-40 lysis buffer (150mM NaCl, 1.0% NP-40, 50mM Tris pH 8.0 and 1X ULTRA protease inhibitor cocktail tablet (Roche, Mannheim, Germany). Protein concentration was determined by BCA Protein Assay Kit (Pierce, Rockford, IL) according to manufacturer’s instruction. Equal amounts of total protein (30µg) were separated by electrophoresis in 4–15% precast gradient polyacrylamide gels (Bio-Rad, Hercules, CA) for approximately 75 minutes at 100V and then transferred at 4° C 45V overnight to polyvinylidene difluoride (PVDF) membranes in transfer buffer (25mM Tris, 192mM glycine, and 10% methanol, pH 8.3). PVDF was blocked by 5% nonfat dried milk in PBS with 0.05% Tween 20 for 1 hour, 4°C and incubated overnight at 4°C with primary eNOS 49G3 rabbit mAb (Cell Signaling Technology, Danvers, MA) at 1:1000 dilution and β-actin 13E5 rabbit mAb at 1:1500 dilution. The membrane was washed in TBST, incubated with HRP conjugated anti-rabbit IgG for 1 hour at RT. The membrane was then washed again, developed in SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL) and analyzed with Bio-Rad Image Lab System. To assess eNOS dimer levels, the gel was run at low temperature according to Klatt et al.[31]. The rest of the steps were identical as above.
Statistical Analysis
Data were checked by F-test for equality of groups’ variation and paired Student t-test for each subgroup versus reference controls. Data represent means of 3–7 determinations ±SEM; significance was considered at p<0.05; selected data were analyzed using two-way ANOVA followed by Tukey’s test.
RESULTS
Concentration-effects of Ritonavir and MgSO4 on BAECs viability
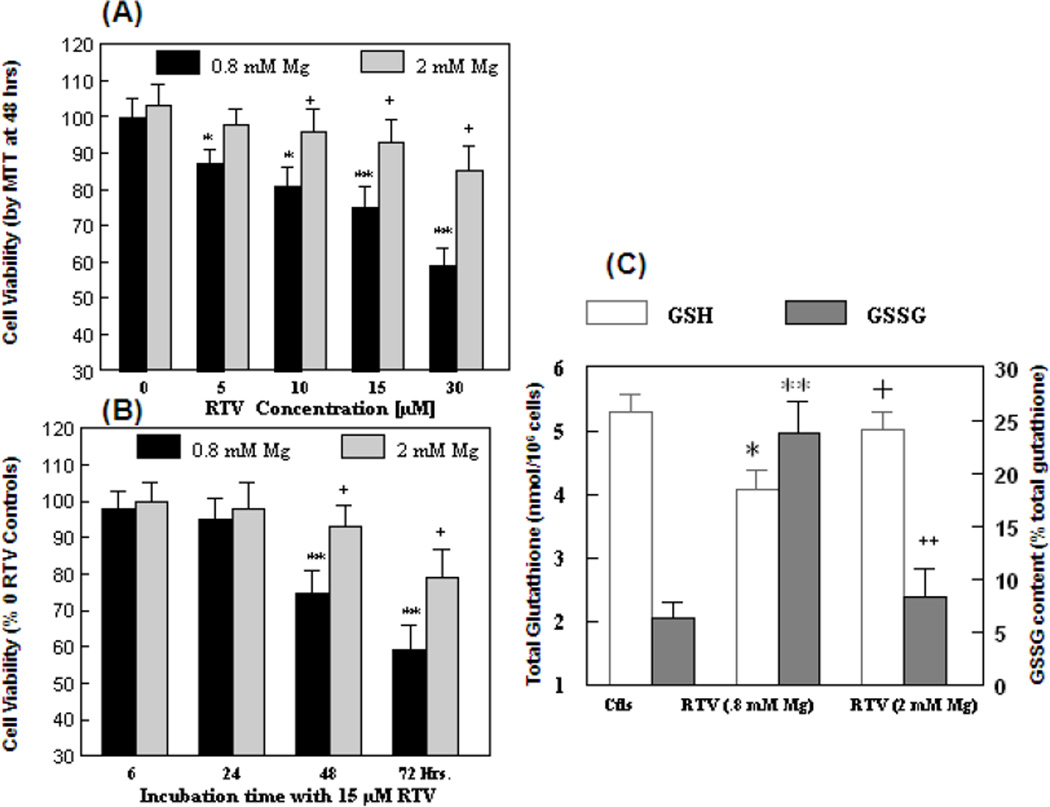
To examine the cytotoxic effects of ritonavir (RTV) and Mg, we used the well-established MTT assay [23,32]. As illustrated by Fig 1, RTV concentration-dependently caused moderate but significant losses of cell viability which were −13%, −19%, −24% and −41% respectively from 5 to 30 µM of RTV (Fig 1A). When 2mM of Mg derived from MgSO4 was present, the losses of cell viability were almost completely prevented for the 5–15µM RTV samples; partial recovery was observed for 30µM RTV. A time course study for the effect of 15µM RTV revealed that significant losses of viability were noted only at/after 48 hrs, but not up to 24 hrs (Fig. 1B); partial to complete protective effects were afforded by high Mg up to 72 hrs. In data not shown, the effects of certain thiol antioxidant reagents were assessed; 100µM NACA or 1mM NAC moderately improved the cell viability 10–20% above that of RTV alone.
Figure 1. Effects of RTV and high extracellular Mg, on BAEC viability, and on changes in GSH and GSSG levels.
(A) Cell viability was determined by the MTT assay after 48 hrs. of incubation with RTV at 0–30 µM in normal (0.8 mM) Mg or in 2 mM MgSO4 with DMSO as drug vehicle control. (B) Time course of RTV (15 µM)-mediated loss of cell viability and the effects of 2 mM Mg; cell viability was normalized to controls without RTV. (C) A separate set of cells in 6-well plates were incubated with 15 µM RTV in 0.8 or 2 mM Mg for 24 hrs; total glutathione (GSH+GSSG) and GSSG were determined. Data represent means±SEM of 5–6 separate determinations; *p<0.05, ** p<0.01 vs. Ctls. +p<0.05, ++ p<0.01 vs RTV in 0.8 mM Mg.
GSH/GSSG changes
Glutathione is the major water soluble intracellular antioxidant available to detoxify oxidative drugs. To examine earlier events leading to cell death, we assess if the glutathione status might be altered by RTV over a 24 hrs period. Incubation of ECs with 15 µM RTV in normal Mg level (0.8 mM) resulted in significant increases in GSSG level at 6 hrs (2.7-fold, p<0.05) and proceeded to 4.2-fold higher than controls at 24 hrs; concomitantly, the total glutathione (GSH+GSSG) level decreased significantly by 23% (p<0.025) at 24 hrs (Fig. 1C). In the same experiment, the RTV-induced elevated GSSG levels were substantially reduced by 2 mM Mg at 6 hrs as well as 24 hrs of incubation; the total glutathione level was maintained close to controls (Fig. 1C).
Effects of RTV and Mg on BAEC superoxide production
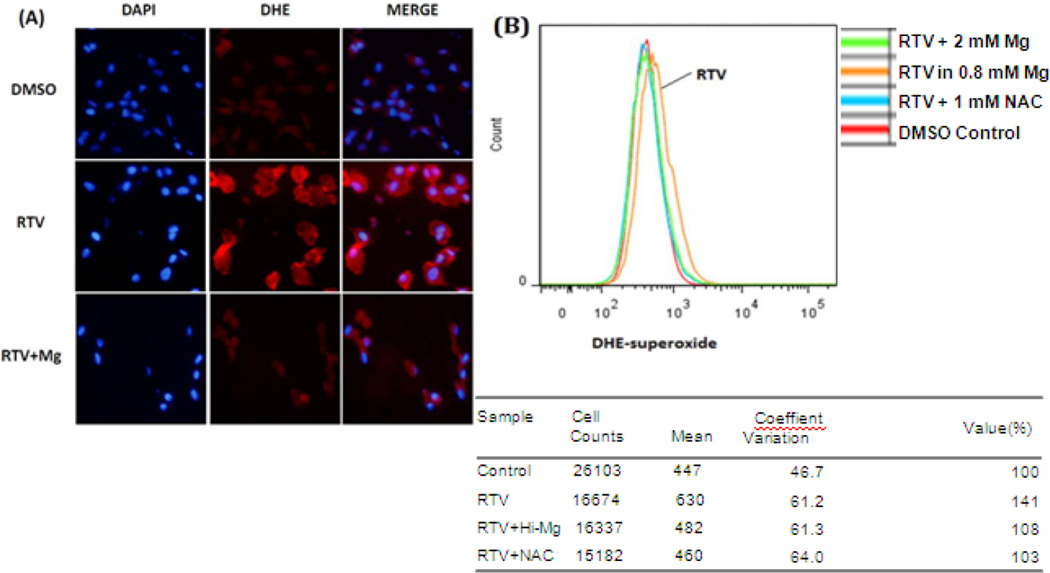
Superoxide is the primary reactive oxygen species of oxidative stress and would eventually promote the increment of GSSG/GSH ratio [33]. To evaluate whether intracellular superoxide anion was enhanced and the effect of high Mg, BAECs, after 24 hours of RTV (15µM) treatment in normal (0.8 mM) or high (2mM) Mg, were stained with 2µM DHE. Under microscopy, cells treated with RTV-alone displayed a relatively intense peri-nuclear red fluorescent image at 540/570nm suggesting enhanced level of superoxide anion, and this red fluorescence intensity was substantially diminished in the RTV+ high Mg group (Fig 2A). To eliminate the bias of subjective field selection, a relative quantification was performed by flow cytometry. Cell viability was distinguished by live/dead Fixable Far Red Dead Cell Stain. Dead cells represented < 5% of the total EC population; only live cells were used for the quantification. A population comparison plot was represented by Fig 2B, showing BAECs with oxidized DHE signal intensity for each condition. The orange curve representing RTV-alone shifted to the right indicative of higher level of superoxide exposure. Based on the computer-calculated average signal intensity for individual cells, RTV promoted superoxide production to 141% of control, which was mostly inhibited by high Mg (2 mM) treatment (108% of control). Alternatively, RTV-alone was scored positive at 40% by Super-enhanced Dmax Subtraction algorithm compared with RTV+ high Mg group. Substantial inhibitory effect was also revealed by incubation with 1 mM NAC.
Figure 2. High Mg suppresses RTV-induced superoxide production.
(A) BAECs were incubated with 15µM RTV in 0.8 or 2mM Mg (RTV+Mg) for 24 hours; RTV was replaced by equal amount of DMSO in control group. (A) The cells were then stained with 2µM DHE as indicator of superoxide anion production for 30 min at 37° and detected at 540/570nm for DHE adducts and 345/455nm for DAPI (4’,6’ diamino-2-phenylindole2HCl for DNA stain). (B) Relative quantification by flow cytometry was performed at 488/585nm for DHE adducts, and at 637/660nm for Far Red Dead Cell Stain. Mean values represented average oxidized DHE signal per cell from a total of 15,000 to 26,000 live cells per condition and were compared intergroups by using cells+DMSO as control (100%). Super-enhanced Dmax Subtraction (SED) algorithm showed that the RTV population was scored positive at 41.4% when compared with control population.
RTV and Mg effects on NO products, eNOS mRNA and eNOS dimer/monomer ratio
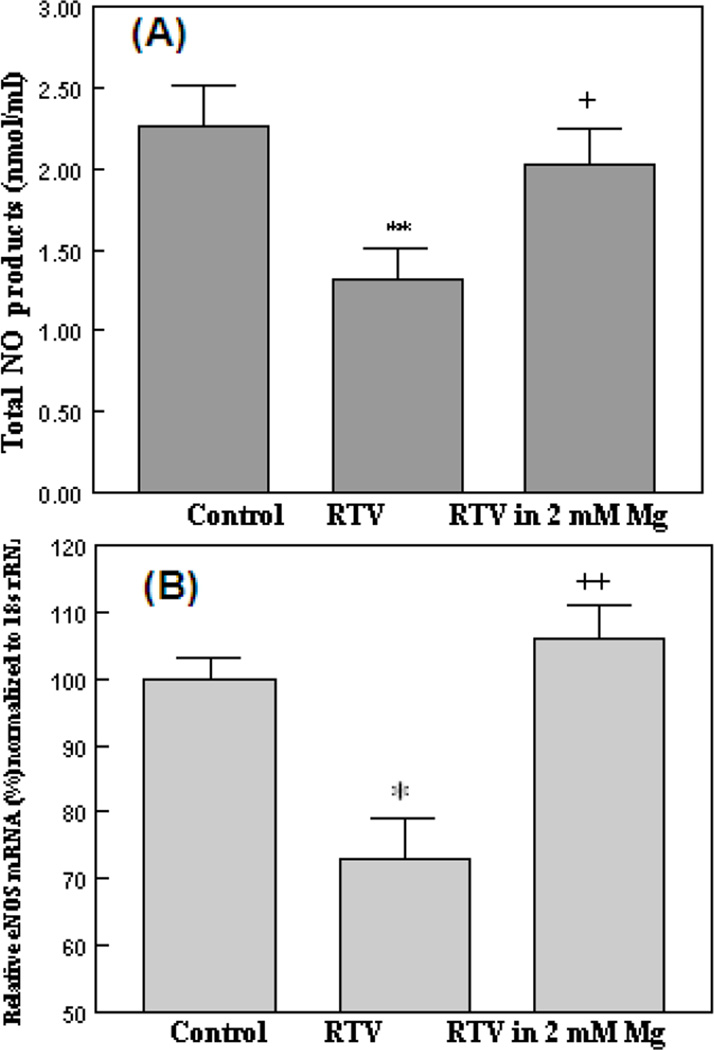
To determine if enhanced superoxide production may be associated with reduced NO availability, we used the traditional Griess Reagent method, to measure the total NO products (nitrite+nitrate) accumulated in the endothelial cell culture media. As represented by Fig 3A, after 24 hours, 15µM RTV caused a substantial (−39%, p<0.01) reduction of the NO-derived product level; in the presence of high Mg (RTV+2mM Mg), the NO product release was recovered to 90% of control (Fig 3A). To determine if alteration in NO production might reflect changes in eNOS status [34], the effects of RTV treatment and Mg on BAECs eNOS mRNA expression were investigated by RT-PCR (Fig. 3B). ECs incubated with 15µM RTV for 24 hours displayed a 25% decrease (p<0.05) in the eNOS mRNA level; when 2mM MgSO4 was present, the eNOS expression was maintained at 107 ± 4% of control (p<0.01 vs RTV alone).
Figure 3. Effects of RTV and high Mg on (A) BAEC nitrite+nitrate levels and on (B) eNOS mRNA expression.
BAECs were incubated with 15µM RTV with normal (0.8mM) or high Mg (2 mM) for 24hr. Supernatants were retrieved for total NO products (nitrite + nitrate) determination by the Griess Reagent. (B): Real time quantitative-PCR was performed to measure eNOS mRNA level in BAECs. Bovine 18s rRNA was used as the reference gene. Expression levels were compared to control group. Each assay was repeated in triplicate and data represent means ±SEM, n=4–5.; *p<0.05, **p<0.01 vs controls; +P<0.05, ++P<0.01 vs RTV (in 0.8 mM Mg).
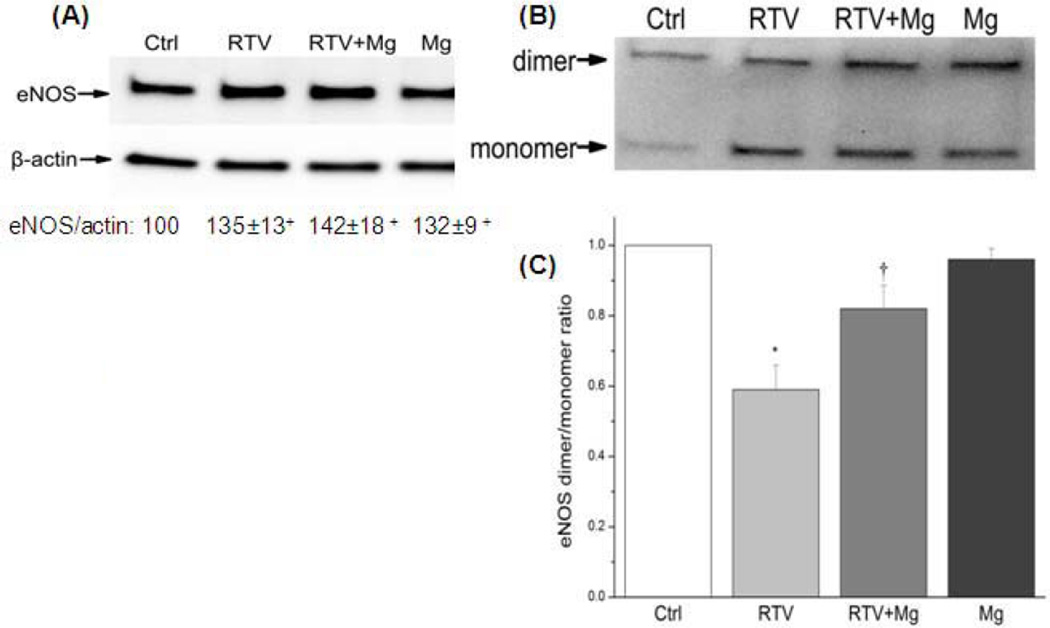
To quantify corresponding changes in the eNOS protein level, we initially used the traditional high temperature western blot procedure [29]. Interestingly, it was observed that the total eNOS protein was moderately increased (~35%, p<0.05) after the RTV treatment (Fig. 4A). Further analysis using the low temperature running procedure [31] which separates the eNOS protein into its homodimer and monomer moieties revealed that under RTV treatment, the eNOS protein existed predominantly in the monomeric form with the dimer/monomer ratio reduced to 58% (p<0.01) of control (Fig. 4B); this ratio was partially but significantly shifted back to 85% (p<0.05) of control. High Mg alone did not change the dimer/monomer ratio (98% of control).
Figure 4. RTV-induced changes in EC eNOS dimer/monomer ratio and Mg effects.
(A), BAECs were harvested after 24 hrs incubation with 15 µM RTV in normal or high Mg (RTV+Mg), or 2 mM Mg alone; total protein was extracted and separated by (A) a normal high temperature SDS-PAGE for total eNOS and expressed as % compared with controls; + p<0.05 vs. Ctl; or (B) by a low-temperature SDS-PAGE to separate the dimer and monomer forms of eNOS. (C), the same images were used to measure the densitometric ratio of eNOS dimer and monomer. RTV reduced the eNOS dimer/monomer ratio to 59% comparing to controls (*P<0.01, n=4) while RTV+Mg reversed the ratio to 82% of controls (†p<0.05 vs. RTV, n=4).
Alternations in hepatic eNOS mRNA, protein and plasma nitrite and 8-isoprostane levels in RTV treated rats: Effects of Mg-supplementation
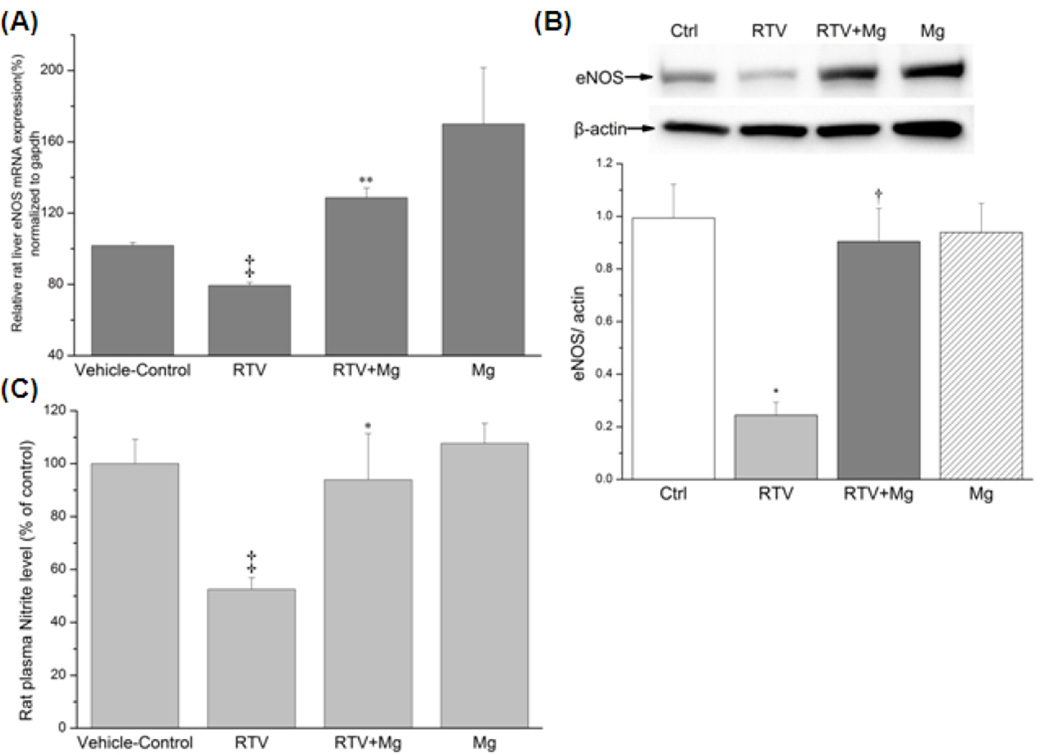
To examine chronic RTV effects on hepatic eNOS status in vivo, we employed a male LBN-F1 rat model. The rats received RTV treatment (75mg/kg/day) for 5 weeks and were placed on a normal Mg or a 6-fold higher Mg-supplemented diets. Treatment of the rats with RTV for 5 weeks resulted in a moderate weight loss (−13.3%,p<0.05) compared with vehicle controls (Table 1), suggesting some degrees of toxicity; this loss was only partially attenuated by Mg-supplementation (−9%, N.S.). Similar to observed earlier [22], Mg-supplementation (±RTV) increased the blood Mg levels by +33% As represented by Fig 5A, the hepatic eNOS mRNA level of RTV-group was reduced to 78 ± 2% of control (p < 0.01) whereas that of RTV plus Mg-supplement group (RTV+Mg) was elevated to 129 ± 5.4% (p< 0.01 vs. RTV groups). We noted that Mg-supplementation alone resulted in an increase of the eNOS mRNA (~170% of Control, Fig.5A). Hepatic eNOS protein was determined by the standard Western blot procedure. As represented by Fig. 5B, 5wks of RTV treatment resulted in a substantial reduction (−75%) of the eNOS total protein; and such reduction was completely reversed by Mg-supplementation. Mg-supplementation alone did not change the level of eNOS protein. The plasma nitrite levels were assessed by the DAN assay, the vehicle-control group displayed a relative fluorescence unit (RFU) of 224 ± 23; the RTV group showed a RFU of 117 ± 9 and which was only 52% (p< 0.01) of the controls. However, the RTV+Mg group revealed a RFU of 210 ± 39, which was 94% of control (p<0.05 vs RTV group). Mg-supplementation alone (240±16, or108%) resulted in no change in the nitrite level. The corresponding nitrite levels are summarized in Table 1. However, the total plasma nitrate+nitrite levels determined by the Griess reagent method [24], were unchanged among groups. In additional experiments, we found that RTV treatment alone enhanced the total circulating 8-isoprostane levels by +85% (Table 1). This increase was reduced +15% by Mg-supplementation. Mg-supplementation alone did not change the isoprostane content comparing to vehicle controls.
Table 1.
RTV treatment (5 Wks) and Mg-supplementation on Plasma Total 8-isoprostane, Nitrate and Nitrite Levels
| Rat Groups | Body wt. (gm) |
Plasma Mg [mmol/L] |
Total 8-isoprostane [pg/ml] |
Nitrate+Nitrite [uM] |
Nitrite [nM] |
|---|---|---|---|---|---|
| Veh. Ctl | 329±6 | 0.80±0.05 | 106±13 | 28.0±1.5 | 376±35 |
| RTV | 285±11* | 0.71±0.06 | 196±23** | 26.3±2.0 | 198±15** |
| RTV+Mg-supp. | 296±12* | 1.06±0.06** | 122±19+ | 28.6±2.2 | 356±66+ |
| Mg-supp. alone | 326±8 | 1.04±0.07** | 89±13 | 27.7±2.0 | 407±27 |
Plasma Mg was determined by AA spectroscopy, total 8-isoprostane levels by an ELISA Kit; plasma nitrate+nitrite contents were measured by the Griess reagent, nitrite levels by the DAN assay as described in Methods.
Data are means ±SEM, n = 5–7;
p<0.05,
p<0.01 vs Veh. Ctl;
p<0.05 vs.RTV alone.
Figure 5. Chronic RTV treatment and protective effects of Mg-supplementation on hepatic (A) eNOS mRNA expression, (B) eNOS protein and (C) plasma nitrite levels in rats.
Male Lewis-Brown Norway F1 rats (160g) were administered RTV (75mg/kg/day, p.o.) for 5 weeks fed a normal (0.1% Mg) or a 6-fold (0.6% Mg) Mg diet. Liver and plasma samples were harvested and processed for the assays. Vehicle-control group received the solvent of RTV solution (10% ethanol, 5% DMSO, 50% propylene glycol, and 35% water). (A) Rat liver eNOS expression level was measured by RT-PCR using GAPDH as reference gene and compared with vehicle controls (‡p<0.01 vs vehicle-controls; **p<0.01 vs. RTV, n=4–5).
(B), RTV depresses Liver eNOS protein level and the effect of Mg-supplementation. Total liver protein for each group was isolated and boiled; eNOS protein expression was run and detected by regular western blot. After being normalized with β-actin, RTV depressed eNOS expression to 24% and RTV plus high Mg-Supplement preserved the eNOS protein level to 90% of controls (*P<0.01 vs controls, †p<0.01 vs. RTV, n=3–4)
(C) Plasma nitrite was determined by the DAN method; RFU was recorded and compared to standard samples and to controls; ‡p <0.01 vs. Controls, * p<0.05 vs RTV, n=4–5.
DISCUSSION
Our study confirms that ritonavir alone in clinically relevant concentrations [35] caused oxidative stress and eNOS dysfunction in cultured ECs. Significant losses of cell viability were observed after 48 hrs of RTV (5–30 µM) incubation in physiological Mg level (0.8 mM); Mg-supplementation as MgSO4 to 2 mM, which is within the clinical therapeutic levels reported [36, 37], attenuated the lost viability. Glutathione (GSH) is the most abundant antioxidant in aerobic cells and the GSSG/GSH ratio can often be used as a reliable indicator for cellular redox balance [38]. The initial (6 hrs) increase in GSSG/GSH ratio and a partial loss of total glutathione at 24 hrs suggest that the compromised glutathione defense status likely contributed to the eventual cell death. Increased superoxide production is often the initial oxidative event. By using the specific fluorescent probe, DHE, we obtained both visual imaging (by microscopy) and quantitative (flow cytometry) comparison of superoxide production under different conditions. The increase in superoxide production in the RTV-treated BAECs is consistent with the published observations by others [10–12]. For our pursuit, the new observations were that supplementation with higher extracellular Mg can diminish RTV-induced superoxide production along with preservation of the glutathione status and cell viability. Superoxide anions rapidly react with NO to form peroxynitrite, and thus may lower the NO bioavailability. By monitoring the NO product level which is relatively stable in tissue culture, we confirmed that RTV treatment led to decreased EC NO production. Concomitantly, we found that RTV down-regulated eNOS mRNA significantly by 24 hrs which was completely prevented by higher extracellular Mg. Unexpectedly, we initially found the total eNOS protein was moderately increased by RTV treatment (Fig 4 A). To resolve this discrepancy, we resorted to separate the total eNOS protein to its monomeric and dimeric forms using the low temperature SDS-PAGE procedure Klatt et al. [31]. Indeed it was revealed that the ratio of dimer/monomer was significantly decreased by RTV treatment. It has been recognized that the homodimeric form of eNOS represents the functionally active NO synthase; and that switching eNOS from dimer to monomer state would lead to eNOS uncoupling generating superoxide anions instead of NO [39]. Structural studies support that the presence of the co-factor tetrahydrobiopterin (BH4) is essential to maintain the eNOS dimerization [40]. However, BH4 is very sensitive to oxidative stress, especially to peroxynitrite, and that intracellular GSH is important to protect against BH4 oxidation [41]. Conceivable, RTV-induced loss of GSH may contribute to eNOS monomerization through BH4 oxidation. We hypothesize that RTV treatment resulted in sustained oxidant (e.g. ·O2− to H2O2) formation which not only promoted eNOS protein uncoupling, but also accelerated the mRNA degradation. We showed here that Mg-supplementation (in 2 mM) prevented eNOS mRNA loss, preserved the eNOS dimer/monomer ratio, and restored the EC function of NO production; all the effects can result from the primary action of suppressing superoxide formation.
Despite extensive research on RTV-mediated down regulation of eNOS in vitro; such effect has hitherto been unconfirmed in vivo. To examine the RTV effects on eNOS status in vivo, we chose to use the LBN-F1 hybrid rats which were reported to be sensitive to RTV-mediated metabolic side effects [42]. The dose of RTV used in the current study was 5–11 fold higher than the recommended human dose. However, the dosage used might be appropriate for rats as recognized in a guidance issued by the U.S. Food and Drug Administration [43]. Rodents have much higher drug metabolic rates compared with human, and require at least 6-fold higher dose levels to achieve clinically comparable circulating protease inhibitors. It has been suggested that RTV can exert both mitochondrial and cytosolic oxidative stress [6]. RTV is very lipophilic and its subcellular targets likely to be membrane associated. More recently, it was reported that RTV can induce severe endoplasmic reticulum (ER) stress which is linked to impaired lipid catabolism [44]. It was further suggested that subcellular oxidative events contributed to RTV-mediated ER stress and dysregulation of key mediators of lipid metabolism. In our rat model, RTV also promoted systemic oxidative stress as evidenced by the significantly increased circulating levels of 8-isopronstane (Table 1) which was derived from nonenzymatic peroxidation of polyunsaturated fatty acids in vivo. RTV is hepatically metabolized and may negatively affect the liver eNOS status. Indeed we found that RTV treatment for 5 weeks resulted in significant decreases in both hepatic eNOS mRNA and eNOS protein levels in the rats fed a control Mg-diet. While the decrease in eNOS mRNA level was rather modest (23%), the loss of the eNOS protein level was quite dramatic (~75%) possibly reflecting both decreased synthesis and increased oxidative degradation due to RTV treatment. Conceivably, NO is essential for regulation of normal liver function; recent literature supports that endothelial functional activity and NO generated from eNOS are clearly important to confer protection against chronic cirrhosis, portal hypertension and ischemia reperfusion injury [45–47]. PI regimens especially RTV use were reported to be associated with hepatotoxicity [8,9]; however the etiopathogenesis of PI-associated liver injury remains unclear. Our findings suggest that down-regulation of hepatic eNOS expression may at least in part contribute to the PI-mediated liver toxicity, and could explain that RTV use in full dosage was associated with higher rates of severe hepatotoxicity [9].
Due to the limited in vivo scope of current study, the RTV effects on the eNOS status in other organs e.g. the heart, the kidney, and large blood vessels are not determined. Nevertheless, in parallel with the decreased levels in the hepatic eNOS mRNA and protein levels, we found that, plasma nitrite levels from the RTV treated rats displayed a 50% decrease suggesting that systemic eNOS status might also be compromised. This notion was supported by the clinical findings that patients receiving RTV-containing PI regimens displayed decreased brachial arterial flow mediated dilation [5]. Importantly, we demonstrated that dietary Mg supplementation not only attenuated the decreased hepatic eNOS mRNA expression, but also prevented the dramatic loss of the eNOS protein due to RTV treatment; Mg-supplementation also restored the normal level of nitrite in the circulation suggesting that the systemic eNOS functional integrity was preserved. Our findings lend support to the hypothesis that plasma nitrite level can be used as a reliable biomarker to reflect acute changes in eNOS activity in various mammals, including humans, and may also reflect the degree of endothelial dysfunction [34,48].
Mg-supplementation suppressed the induced isoprostane formation supporting the notion the protective mechanism of Mg is in part conferred by its anti-peroxidative properties. In our own study, we reported that Mg at 2 mM protected against superoxide anion driven, Fe-catalyzed loss of total glutathione and cell viability in cultured endothelial cells [49]. We suggested that high Mg may be able to competitively displace “catalytic iron” from membrane phospholipid binding sites. In the endothelial cell model, elevated superoxide might derive from neighboring cultured cells treated with RTV and that direct anti-radical action of Mg can be mediated by a ‘site-specific’ inhibition of OH radical generation [15,16,49]. However, Mg may also provide its protective effects as ‘a natural calcium blocker’ [17]. An in vitro study indicated that Mg-sulphate can provide effective L-type calcium channel blocking ability and thus able to inhibit endotoxin-induced up-regulation of inflammatory activity in activated RAW264.7 cells [50]. Increased superoxide generation could be generated by the NADPH oxidase system [6,51,52]. Since NADPH oxidase activation may depend on calcium interaction [52,53], one may speculate that by partially inhibiting calcium influx, Mg would inhibit NADPH oxidase-dependent oxy-radical generation. Elevated superoxide formation might lead to not only neutralization of NO but also enhancement of peroxynitrite-mediated BH4 oxidation and, eNOS protein thiyl radical formation; both processes are suggested to participate in uncoupling of eNOS activity [54]. Mg-supplementation may also independently up-regulate eNOS expression as it was shown in term placentas obtained from preeclampsia patients receiving Mg-therapy [55].
In conclusion we have presented definitive evidence that Mg-supplementation can protect against RTV-mediated EC oxidative eNOS dysfunction in vitro, and down-regulation of hepatic eNOS expression in vivo. While the precise mechanism(s) remain to be further determined, the Mg protective effects likely are contributed by its antioxidant and anti-calcium properties. Decreased NO availability is regarded as the hallmark of endothelial dysfunction, and in patients receiving RTV-containing PI treatment, this dysfunction may contribute to the known vascular and hepatotoxicity. Our findings strongly support the translational benefits of using Mg-supplementation as an adjuvant therapy to reduce ritonavir-mediated systemic endothelial dysfunction and liver toxicity for HIV patients.
High Lights.
HIV protease inhibitor ritonavir (RTV) causes endothelial dysfunction & hepatotoxicity
RTV ↑ ˙O2−, GSSG, ↓ NO in endothelial cells (EC); inhibited by high medium Mg.
RTV ↓ EC eNOS mRNA, ↓ eNOS dimer/monomer ratio, both attenuated by high Mg.
RTV treatment in rats (5WKs) ↓ liver eNOS mRNA, ↓↓ eNOS protein, ↓↓ plasma nitrite.
Dietary Mg preserved hepatic eNOS mRNA, protein and function in RTV-treated rats.
ACHNOWLEDGMENTS
This study was supported by NIH-R21NR012649 (ITM). The authors wish to thank Mr. Liang Chen for his advices for the RT-PCR technique, Mr. Wenjie Bao for assistance with the DHE fluorescent imaging. We appreciate the excellent technique of Mr. Dean Brostowin for the daily RTV administration to the rats. Special thanks are to Ms. Teresa Hawley for her guidance in using the flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Both authors have no conflict of interest to declare.
REFERENCES
- 1.Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Annals of Medicine. 2011;43:375–388. doi: 10.3109/07853890.2011.572905. [DOI] [PubMed] [Google Scholar]
- 2.Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J. Antimicrobial Chemotherapy. 2004;53:4–9. doi: 10.1093/jac/dkh029. [DOI] [PubMed] [Google Scholar]
- 3.DHHS. Panel on Antiretroviral Guidelines for Adults and Adolescence. 2013 Available at http//aidsinfo.nih.gov 2/12/2013.
- 4.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 5.Stein JH, Klein MA, Bellehumeur JL, McBride PE, Wiebe DA, Otvos JD, Sosman JM. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 6.Kline ER, Sutliff RL. The role of HIV-1 proteins and antiretroviral drug therapy in HIV-1 associated endothelial dysfunction. J. Investig. Med. 2008;56:752–769. doi: 10.1097/JIM.0b013e3181788d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonfanti P, Valsecchi L, Parazzini F, Carradori S, Pusterla L, Fortuna P, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J Acquir Immune Defic Syndr. 2000;23:236–245. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Sulkowski MS. Hepatotoxicity associated with antiretroviral therapy containing HIV-1 protease inhibitors. Semin Liver Dis. 2003;23:183–194. doi: 10.1055/s-2003-39949. [DOI] [PubMed] [Google Scholar]
- 9.Nunez M. Hepatotoxicity of antiretrovirals: Incidence, mechanisms and management. J. Hepatology. 2006;44:S132–S139. doi: 10.1016/j.jhep.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–781. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–499. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 12.Weakley SM, Jiang J, Lu J, Wang X, Lin PH, Yao Q, Chen C. Natural antioxidant dihydroxybenzyl alcohol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human endothelial cells. Medical Science Monitor. 2011;17:BR235–BR241. doi: 10.12659/MSM.881926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Khandelwal AR, Rogers LK, Hebert VY, Kleinedler JJ, Zavecz JH, Shi W, Orr AW, Dugas TR. Antiretrovirals induce endothelial dysfunction via an oxidant-dependent pathway and promote neointimal hyperplasia. Toxicol Sci. 2010;117:524–536. doi: 10.1093/toxsci/kfq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbot J, Brown DG. Stabilization of iron-catalyzed hydrogen peroxide decomposition by magnesium. Can J Chem. 1990;68:1537–1543. [Google Scholar]
- 16.Mak IT, Komarov AM, Kramer JH, Weglicki WB. Protective mechanisms of Mg-gluconate against oxidative endothelial cytotoxicity. Cell. Mol. Biol. 2000;46:1337–1344. [PubMed] [Google Scholar]
- 17.Iseri LT, French JH. Magnesium: nature's physiologic calcium blocker. Am. Heart J. 1984;108:188–193. doi: 10.1016/0002-8703(84)90572-6. [DOI] [PubMed] [Google Scholar]
- 18.Sontia B, Touyz RM. Role of magnesium in hypertension. Arch. Biochem. Biophys. 2007;458:33–39. doi: 10.1016/j.abb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Soltani N, Keshavarz M, Minail B. Effects of oral Mg on plasma glucose and pathological changes in the aortic and pancreas of diabetic rats. Clin Exp Pharmacol Physiol. 2005;32:604–610. doi: 10.1111/j.0305-1870.2005.04238.x. [DOI] [PubMed] [Google Scholar]
- 20.Asai T, Nakatani T, Yamanka S. Mg supplementation prevents experimental chronic cyclosporine nephrotoxicity. Transplantation. 2002;74:784–791. doi: 10.1097/00007890-200209270-00009. [DOI] [PubMed] [Google Scholar]
- 21.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102:2353–2358. doi: 10.1161/01.cir.102.19.2353. [DOI] [PubMed] [Google Scholar]
- 22.Mak IT, Chmielinska JJ, Kramer JH, Weglicki WB. AZT-induced oxidative cardiovascular toxicity: attenuation by Mg-supplementation. Cardiovsc Toxicol. 2009;9:78–85. doi: 10.1007/s12012-009-9040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mak IT, Landgraf KM, Chmielinska JJ, Weglicki WB. Angiotensin II promotes iron accumulation and depresses PGI2 and NO synthesis in endothelial cell: effects of losartan and propranolol analogs. Can J Physiol Pharmacol. 2012;90:1413–1418. doi: 10.1139/y2012-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak IT, Komarov AM, Wagner TL, Stafford R, Dickens BF, Weglicki WB. Enhanced NO production during Mg deficiency and its role in mediating red blood cell glutathione loss. Am. J. Physiol. 1996;271:C385–C390. doi: 10.1152/ajpcell.1996.271.1.C385. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Rad. Biol. Med. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti M, d'Uscio LV, Peterson TE, Katusic ZS, O’Brien T. Analysis of superoxide anion production in tissue. Methods in Molecular Medicine. 2005;108:65–72. doi: 10.1385/1-59259-850-1:065. [DOI] [PubMed] [Google Scholar]
- 27.Nussler AK, Glanemann M, Schirmeier A, Liu L, Nussler NC. Fluorometric measurement of nitrite/nitrate by 2,3-diaminonaphthalene. Nature Protocols. 2006;1:2223–2226. doi: 10.1038/nprot.2006.341. [DOI] [PubMed] [Google Scholar]
- 28.Scanlan BJ, Tuft B, Elfrey JE, Smith A, Zhao A, Morimoto M, Chmielinska JJ, Tejero-Taldo MI, Mak IT, Weglicki WB, Shea-Donohue T. Intestinal inflammation caused by magnesium deficiency alters basal and oxidative stress-induced intestinal function. Mol Cell Biochem. 2007;306:59–69. doi: 10.1007/s11010-007-9554-y. [DOI] [PubMed] [Google Scholar]
- 29.Bolt MW, Mahoney PA. High-efficiency blotting of proteins of diverse sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Analytical Biochem. 1997;247:185–192. doi: 10.1006/abio.1997.2061. [DOI] [PubMed] [Google Scholar]
- 30.Tejero-Taldo MI, Chmielinska JJ, Gonzalez G, Mak IT, Weglicki WB. N-Methyl-D-aspartate receptor blockade inhibits cardiac inflammation in the Mg-deficient rat. J Pharm. Exp. Ther. 2004;311:8–13. doi: 10.1124/jpet.104.070003. [DOI] [PubMed] [Google Scholar]
- 31.Klatt P, Schmidt K, Lehner D, Glatter O, Bachinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. The EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mak IT, Chmielinska JJ, Nedelec L, Torres A, Weglicki WB. D-propranolol attenuates lysosomal iron accumulation and oxidative injury in endothelial cells. J. Pharmacol. Exp. Therap. 2006;317:522–528. doi: 10.1124/jpet.105.097709. [DOI] [PubMed] [Google Scholar]
- 33.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 34.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Rad. Biol. Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 35.Danner SA, Carr A, Leonard JM, Lehman LM, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian Collaborative Ritonavir Study Group. N. Eng. J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 36.Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest. 2000;118:1690–1695. doi: 10.1378/chest.118.6.1690. [DOI] [PubMed] [Google Scholar]
- 37.Jones NA, Jones SD. Management of life-threatening autonomic hyper-reflexia using magnesium sulphate in a patient with a high spinal cord injury in the intensive care unit. British J. Anaesth. 2002;88:434–438. doi: 10.1093/bja/88.3.434. [DOI] [PubMed] [Google Scholar]
- 38.Owen JB, Butterfield DA. Measurement of oxidized/reduced glutathione ratio. Methods in Mol. Biol. 2010;648:269–277. doi: 10.1007/978-1-60761-756-3_18. [DOI] [PubMed] [Google Scholar]
- 39.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J. Endocrinol. 2011;210:271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai S, Khoo J, Mussa S, Alp NJ, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 41.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am. J. Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Dahly-Vernon AJ, Blomme EA, Lai-Zhang J, Kempf DJ, Marsh KC, et al. Liver transcriptomic changes associated with ritonavir-induced hyperlipidemia in sensitive and resistant strains of rats. Veterinary J. 2010;185:75–82. doi: 10.1016/j.tvjl.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 43.USDHHS. Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Table 1: Conversion of animal doses to human equivalent doses based on body surface area. Guidance for Industry Estimation the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteer. 2005:1–27. http://www.fda.gov/cder/guidance/index.htm.
- 44.Cao R, Hu Y, Wang Y, Gurley EC, Studer EJ, Wang X, Hylemon PB, Pandak WM, Sanyal AJ, Zhang L, Zhou H. Prevention of HIV protease inhibitor-induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways. J Pharmacol Exp Ther. 2010;334:530–539. doi: 10.1124/jpet.110.168484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elrod JW, Duranski MR, Langston W, James Greer JM, Dugas TR, Kevil CG, Champion HC, Lefer DJ. eNOS Gene Therapy Exacerbates Hepatic Ischemia–Reperfusion Injury in Diabetes-A Role for eNOS Uncoupling. Circ Res. 2006;99:78–85. doi: 10.1161/01.RES.0000231306.03510.77. [DOI] [PubMed] [Google Scholar]
- 46.Hernández-Guerra M, de Ganzo ZA, González-Méndez Y, Salido E, Abreu P, Moreno M, Felipe V, Abrante B, Quintero E. Chronic intermittent hypoxia aggravates intrahepatic endothelial dysfunction in cirrhotic rats. Hepatology. 2013;57:1564–1574. doi: 10.1002/hep.26152. [DOI] [PubMed] [Google Scholar]
- 47.Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707–1717. doi: 10.3748/wjg.v19.i11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 49.Mak IT, Nedelec LF, Weglicki WB. Pro-oxidant properties and cytotoxicity of AZT-monophosphate and AZT. Cardiovasc. Toxicol. 2004;4:109–115. doi: 10.1385/ct:4:2:109. [DOI] [PubMed] [Google Scholar]
- 50.Lin CY, Tsai PS, Hung YC, Huang CJ. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br J Anaesth. 2010;104:44–51. doi: 10.1093/bja/aep336. [DOI] [PubMed] [Google Scholar]
- 51.Conklin BS, Fu W, Lin PH, Lumsden AB, Yao Q, Chen C. HIV protease inhibitor ritonavir decreases endothelium-dependent vasorelaxation and increases superoxide in porcine arteries. Cardiovas Res. 2004;63:168–175. doi: 10.1016/j.cardiores.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Berthier S, Nguyen MV, Baillet A, Hograindieur MA, Paclet MH, Polack B, Morel F. Molecular interface of S100A8 with cytochrome b558 and NADPH oxidase activation. PLoS One. 2012;7(7):e40277. doi: 10.1371/journal.pone.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunes P, Demaurex N, Dinauer MC. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic. 2013 Aug 23;:12115. doi: 10.1111/tra.12115. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J. Biol. Chem. 2011;286:29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ariza AC, Bobadilla N, Díaz L, Avila E, Larrea F, Halhali A. Placental gene expression of calcitonin gene-related peptide and nitric oxide synthases in preeclampsia: effects of magnesium sulfate. Magnes Res. 2009;22:44–49. [PubMed] [Google Scholar]