Abstract
Hyaluronan (HA) is a linear polysaccharide with disaccharide repeats of D-glucuronic acid and N-acetyl-D-glucosamine. It is evolutionary conserved and abundantly expressed in the extracellular matrix (ECM), on the cell surface and even inside cells. Being a simple polysaccharide, HA exhibits an astonishing array of biological functions. HA interacts with various proteins or proteoglycans to organize the ECM and to maintain tissue homeostasis. The unique physical and mechanical properties of HA contribute to the maintenance of tissue hydration, the mediation of solute diffusion through the extracellular space and the lubrication of certain tissues. The diverse biological functions of HA are manifested through its complex interactions with matrix components and resident cells. Binding of HA with cell surface receptors activates various signaling pathways that regulate cell function, tissue development, inflammation, wound healing and tumor progression and metastasis. Taking advantage of the inherent biocompatibility and biodegradability of HA, as well as its susceptibility to chemical modification, researchers have developed various HA-based biomaterials and tissue constructs with promising and broad clinical potential. In this article, we illustrate the properties of HA from a matrix biology perspective by first introducing principles underlying the biosynthesis and biodegradation of HA, as well as the interactions of HA with various proteins and proteoglycans. We next highlight the roles of HA in physiological and pathological states, including morphogenesis, wound healing and tumor metastasis. A deeper understanding of the mechanisms underlying the roles of HA in various physiological processes can provide new insights and tools for the engineering of complex tissues and tissue models.
Keywords: Hyaluronan, Synthase, Hyaluronidase, Hyaluronan Receptors, Hyaluronan Binding Proteins, Morphogenesis, Wound Healing, Cancer, Tissue Engineering
1. Introduction
HA was purified first from the vitreous humor of bovine eyes by Karl Meyer in 1934 [1]. He named the molecule “hyaluronic acid” owing to the hyaloid appearance of the substance when swollen in water and the probable presence of hexuronic acid as one of the components. In the 1950’s, Meyer and colleagues determined that HA was a linear polysaccharide composed of repeating β-1,4-linked D-glucuronic acid (GlcA) and β-1,3-linked N-acetyl-D-glucosamine (GlcNAc) disaccharide units (Figure 1A) [2]. The various names of HA reflect the properties of the molecule under various conditions. When first isolated, HA behaved like a mild acid; therefore, Meyer named it “hyaluronic acid” [1]. Under physiological conditions, HA exists as a polyelectrolyte with associated cations, frequently as a sodium salt; therefore, the name sodium hyaluronate. The name was later amended to “hyaluronate” in reference to its salt form or “hyaluronan,” a term used to encompass all forms of the molecule [3].
Figure 1.
Chemical structure of HA (A) and schematic illustration of HA biosynthesis (B) and biodegradation (C). (A): HA is a linear polysaccharide with disaccharide repeats of D-glucuronic acid and N-acetyl-D-glucosamine. (B): HA is synthesized by transmembrane proteins HAS1, 2 and 3 and is extruded into the extracellular space as the polymerization proceeds. (C): HA is internalized by binding to HA receptors and Hyal2 within caveolae. Hyal2 cleaves HA into intermediate fragments that are transported to lysosomes. Lysosomal Hyal1 cleaves HA into tetramers that are finally cleaved into HA monomers by exoglycosidases. (A, B): Reproduced with permission [125], Copyright 2008, The Japanese Biochemical Society (C): Reproduced with permission, Copyright 2013, Glycoforum.
HA is found ubiquitously in the ECM of all vertebrate tissues, although its concentration and binding partners vary. In bodily fluids, the concentration of HA ranges from 0.01–0.1 µg/g in blood serum to 1400–3600 µg/g in synovial fluid; HA content in soft connective tissues ranges from 8.5–18 µg/g in the thoracic lymph to 140–338 µg/g in the vitreous body [4]. HA also is present on some cell surfaces as a pericellular sugary coat, a feature thought to be involved in cell differentiation and morphogenesis. In the cumulus cell-oocyte complex, the HA concentration can be as high as 0.5–1.0 mg/mL [5, 6]. Classically considered an extracellular molecule, the presence of HA in the cytoplasm and the nucleus was suggested as early as the 1970’s [7, 8] and has been convincingly confirmed in the 1990’s [9–12]. Although intracellular HA has been suggested to play important roles in inflammation, its intracellular functions remain largely unknown [13].
HA is not branched, nor does it contain any sulfate groups [14]. Despite its simple chemical composition, HA fulfills several distinct molecular functions that contribute not only to the structural and physiological characteristics of tissues but also to the mediation of cell behaviors during morphogenesis, tissue remodeling, inflammation, and diseases. Owing to its unique biophysical properties, HA contributes directly to the maintenance of tissue homeostasis and biomechanics. Through its interactions with proteoglycans and link proteins, HA organizes and maintains the structural integrity of extracellular and pericellular matrices. As a signaling molecule, HA interacts with a variety of cell surface receptors and HA-binding proteins to activate intracellular events to mediate cell functions [15].
After more than two decades of intense study, the molecular details of the role of HA in normal and pathophysiological processes are finally emerging. The fascinating characteristics of HA have motivated two distinct groups of scientists to investigate HA related phenomena and applications. While biologists continue to unravel the complex biological functions of HA and its receptors in various cell signaling processes, biomedical engineers are creating a range of HA-based hydrogel materials with increasing complexity and diverse functions for tissue regeneration purposes [16–18]. In this article, we highlight the essential biological functions of HA, with the goal of motivating the biomaterials community to investigate HA both as a synthetic building block and a biological signaling motif. This is not an all-inclusive review and the readers are referred to in-depth reviews in an edited book for further reading [15].
2. Biosynthesis and degradation
Unlike other glycosaminoglycan (GAG) molecules that are synthesized in the Golgi apparatus, HA is synthesized at the plasma membrane by a group of highly specialized membrane proteins, HA synthases (HAS) [19]. There are three well-conserved HAS isozymes present in mammalian species, HAS1, HAS2, and HAS3 [20], each possessing two distinct binding domains for UDP-sugars (Figure 1B). Polymerization of HA occurs at the inner face of the plasma membrane where HAS alternatively adds UDP-GlcA and UDP-GlcNAc monomers to the reducing end of the growing polymer. As the polymerization is occurring, the non-reducing end of the sugar chain is translocated into the extracellular space through a pore in the HAS structure [21]. An intriguing question is why nature uses three different isozymes for the synthesis of HA with such a simple repeating unit. Although these three enzymes share a structural identity of about 55–70%, they differ in terms of their ability to synthesize HA. HAS1 has a significantly higher Michaelis constant (Km) value, the substrate concentration where the reaction rate is half of its maximum, for both UDP-GlcA and UDP-GlcNAc compared to HAS2 and HAS3, suggesting that HAS1 has a slower rate of HA synthesis compared to the other synthases [22, 23]. As discussed below, HA of different sizes exhibits distinctly different, sometimes conflicting biological functions. Therefore, the expression of various HAS isozymes is likely to be a fine control system critical for the effective mediation of diverse cell behaviors. While HAS1 and HAS2 are able to produce large-sized HA (up to 2,000 kDa), HA produced by HAS3 is of a lower molecular mass (100–1,000 kDa) [22, 24]. McDonald and coworkers was the first to recognize the isoform specificity for HA production in embryogenesis; they discovered that HAS2 (but not HAS1 or HAS3) knock-out mice died at day 9.5 from incomplete atrioventricular septum formation [25].
The expression levels of HAS isozymes differ during morphogenesis and in disease states [26]. Thus, the differential distribution of HA in tissues varies at individual developmental stages and in pathological conditions, and is controlled by the spatio-temporally regulated transcription of the three different synthases. HA is abundant in fetal tissues, but is partially replaced by collagen fibers and proteoglycans during development, so that the mature tissues can fulfill more stringent mechanical tasks [27]. For example, the newborn vocal fold is composed of a loose connective tissue rich in HA. As the vocal fold develops and matures, HA content is reduced and the fibrous proteins are deposited across the lamina propria in a gradient fashion. Overall, HA is indispensable for the vocal fold development and maturation [28] and its presence in vocal fold is evolutionary beneficial for the tissue to cope with constant trauma [29]. As discussed below, HA is enriched in tumors and tumor-associated stromal tissues, possibly as a result of increased expression or activity of HAS isozymes.
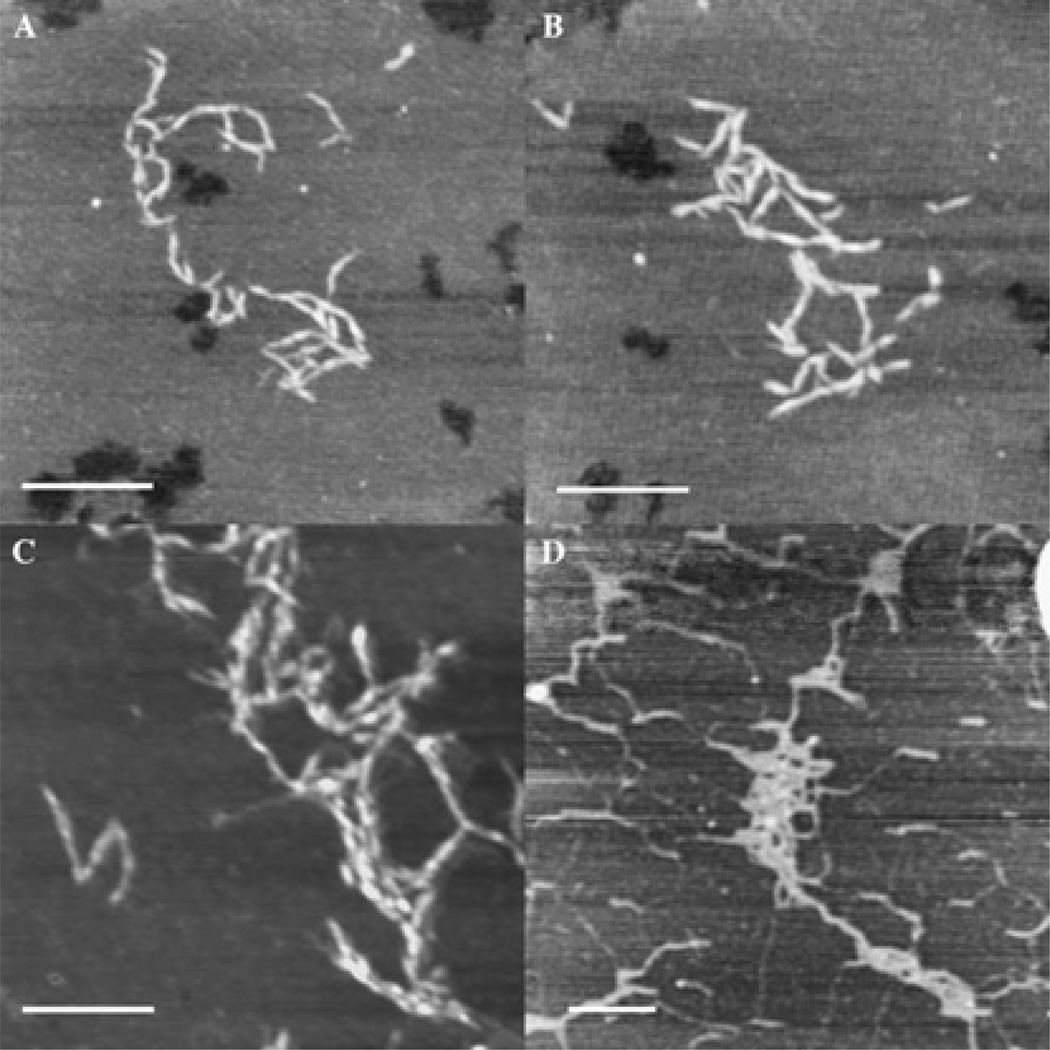
The diverse functions of HA originate from its primary and secondary structures [30, 31]. Connected by glycosidic links, individual saccharide units in HA are relatively rigid, adopting a 4C1 chair with the bulky substituents located in sterically favorable equatorial positions. X-Ray diffraction [32] and NMR [33] characterizations suggest that HA can adopt stiff helical structures in solid state, possibly as a result of the chemical structure of the disaccharide, extensive hydrophobic patch and internal hydrogen bonds. The presence of multiple dynamically formed and broken hydrogen bonds between adjacent saccharides is thought to contribute to the semiflexibility of the polymer chain in solution [33–35]. Using tapping mode AFM, Cowman and coworkers observed extended, relaxed and condensed conformations of HA (Figure 2) that had been deposited on mica surfaces under various conditions [36]. The researchers suggested that in connective tissue ECM, HA may adopt a relaxed coil or partially condensed conformation, whereas HA tethered to the cell surface or in cytosol may exist as fully condensed rods. When subjected to shear flow in tight intercellular spaces or in protein-HA complexes, tissue HA may become fibrous.
Figure 2.
Tapping Mode AFM images of HA deposited on freshly cleaved mica. HA with moderate (A, B) or high molecular weight (C, D), produced in bacteria (A-C) or extracted from rooster comb (D), was deposited from 10 µg/mL solution in H2O (A, B), 5 µg/mL solution in 10 mM MgCl2 or 500 ug/mL solution in 0.15 M NaCl (D). Scale bar: 250 nm, Z range: 2.5–6 nm. Reproduced with permission [36], Copyright 2005, Elsevier.
At physiological pH, HA is a highly charged molecule containing associated counter ions, such as Na+, K+, Ca2+ and Mg2+. Solutions of high molecular mass HA are highly viscous because of polymer chain entanglement [37]. Such entangled networks display time-dependent viscoelasticity, exhibiting elastic properties when subjected to rapid and transient fluid flow and behaving as viscous liquid when exposed to slow fluid flow of a longer duration. Again, take the vocal fold as an example, HA is a major modulator of the tissue viscosity, providing shock absorbing properties to the tissue [38, 39]. Moreover, the shear-thinning properties of HA create optimum conditions for phonation by decreasing the tissue stiffness while vibrating [40]. The entangled HA network in the extracellular space also effectively controls the solute/protein diffusion. Obviously, the viscoelastic properties of HA and its hydration capacity depend on the molecular weight of HA. During rapid growth and tissue remodeling, HA fulfills the requirements to fill the vacant space, to undergo deformation, to maintain tissue hydration and to buffer the local environment. Such an HA-rich environment can keep cells partially localized or provide cells with a substrate on which to migrate [41, 42].
HA synthesis and degradation is tightly regulated during embryonic development and homoeostatic processes. The half-life of HA varies from less than a day in rapidly turning over skin and serum to typically 2–3 weeks in cartilage. HA is removed from the ECM as a consequence of local catabolism and/or drainage into the lymphatic system for catabolism in regional lymph nodes. HA can be catabolized by a number of enzymes in the hyaluronidase (HAase) family. Hyal1 and Hyal2 are the two most common and ubiquitously important HAases. Both enzymes are found in almost all somatic tissues [43]. Hyal1 is present in two isoforms, the first being a 57 kDa glycosylated protein and the second being a 45 kDa form with approximately 100 amino acids deleted [44, 45]. Both in vivo and in vitro studies have demonstrated that the larger isoform likely is secreted by the cell while the smaller isoform is retained in acidic intracellular vesicles [46]. Hyal2 often is found in a glycosylphosphatidylinositol (GPI)-anchored form, tethered to the extracellular side of the plasma membrane [47, 48]. Hyal3 and PH-20 are more specialized HAases. Hyal3 has been poorly studied, but has been shown to be an intracellular HAase expressed in specific tissues [49]. PH-20 is classically known as the sperm HAase involved in fertilization, and is rare in other human tissues. Like Hyal1, PH-20 has two forms, a larger, GPI-linked isoform that is anchored to the plasma membrane and a smaller, soluble isoform caused by removal of 56 amino acids at the C-terminus [50].
The HAases have differential activities in the HA fragment sizes they generate and the pH at which they show optimal activity. Hyal1 is only active at very low pH values from 3.5 – 3.8. The enzyme cleaves large or small molecular weight HA into tetramers [51]. Hyal2 shows optimal activity at pH 6.0 – 7.0, but is active over a large pH range. This enzyme cleaves high molecular weight HA into intermediate size fragments of approximately 20 kDa [52]. PH-20 is active over a relatively wide pH range between 3.0 and 9.0. PH-20 degrades high molecular weight HA into small fragments although some intermediate size fragments also are present [51].
Hyal1 and Hyal2 work in concert to degrade HA in somatic cells (Figure 1C). GPI-anchored Hyal2 binds HA extracellularly, likely in concert with HA receptors, then internalizes HA and performs preliminary cleavages on the full length HA polymer in acidic endocytic vesicles [53]. From there, Hyal1 can further process HA oligomers in these vesicles with the help of p-exoglycosidases, which can cleave sugar groups off each terminus [46]. Gene knockout studies have supported this theory, demonstrating that the action of Hyal1 can be largely compensated for by p-exoglycosidases [54], whereas Hyal2 deficient mice are either embryonic lethal or have severe defects [55].
In addition to the enzymatic degradation, HA can be fragmented by reactive oxygen species (ROS) generated by many types of cells under stressed conditions [56] and HA degradation by superoxide and peroxynitrite in various injury models has been studied [57–62]. Interestingly, HA and its degraded fragments have extraordinarily wide-ranging and often opposing biological functions, owing to the activation of different signal transduction pathways. This variation might be a mechanism by which nature diversifies the functions of a simple polysaccharide [63]. High molecular weight HA species with >1000–5000 saccharide repeats are space-filling, anti-angiogenic, and immunosuppressive; they impede differentiation, possibly by suppressing cell–cell interactions, or ligand access to cell surface receptors. HA chains up to 20 MDa are involved in ovulation, embryogenesis, wound repair and tissue regeneration [63]. Studies have shown that, in response to HA of 40–400 kDa, the NF-kB-mediated gene expression is activated by HA binding with HA receptor for endocytosis (HARE) [64]. Malignant cells produce HA polysaccharides in order to co-opt normal cellular functions. On the other hand, the ability of the naked mole rat to synthesize high molecular mass HA (5 times larger than human HA) is correlated to the cancer resistance and longevity of this species [65]. Contrarily, HA fragments of lower molecular weight are inflammatory (1000 repeats), immunostimulatory and pro-angiogenic (8–32 saccharide repeats), and they competitively bind HA receptors on cell surfaces. Under certain conditions, low-molecular-weight HA species (20–200 kDa) function as endogenous “danger signals”, while even smaller fragments can ameliorate these effects [66].
3. HA-protein interactions
While HA alone has distinct biophysical and biomechanical properties, the biological functions of HA manifest through its interactions with a large number of HA-binding proteins (HABPs, or hyaladherins) that exhibit significant differences in their tissue expression, cellular localization, specificity, affinity and regulation [67, 68]. A number of HABPs bind HA through binding motifs with the sequence B(X7)B where B is a basic residue, arginine or lysine, and the X’s contain at least one basic amino acid but can be any other non-acidic amino acids [69]. Additionally, a second HA binding motif, known as the link module, consists of a span of about 100 amino acids which binds HA when oriented in the correct tertiary structure [70]. A third possible binding motif is an arginine-arginine (R-R) sequence that has been shown biochemically to bind HA, but has not been thoroughly studied in full length proteins [71].
HABPs can be classified based on the binding motif that is used to bind HA. Members of the family that use the link module in binding HA include cluster of differentiation 44 (CD44), hyaluronectin, aggrecan, versican, lymphatic vessel endothelial receptor 1 (LYVE-1), and tumor necrosis factor-a stimulated gene 6 (TSG-6). The family of proteins that use the B(X7)B motif includes receptor for hyaluronan mediated motility (RHAMM), cdc37, P-32, and sialoprotein associated with cones and rods (SPACR). Given that HABPs generally interact with a minimal of 6–10 sugar repeats of HA [63], a single chain of high molecular weight HA can theoretically accommodate in the order of 1000 protein molecules [72]. In general, cellular signaling responses induced by HA/HABP interactions are strongly dependent on HA molecular weight and the cell phenotype [73].
CD44 is a multi-domain, ubiquitous HA receptor protein that spans the plasma membrane of the cell. It contains highly conserved membrane spanning, cytoplasmic, and HA binding domains while the membrane proximal region is poorly conserved among mammalian species. Ten alternatively spliced exons reside in the poorly conserved, extracellular region. The standard version of CD44 (CD44s) has all ten of these variant exons removed. Estimated mathematically, over 800 variant forms of CD44 (CD44v) could be created with the ten alternatively spliced exons, although not all of these combinations are expressed. Over 20 unique forms of CD44v have been identified to date [74]. The core protein of CD44s is only 37 kDa in size, but is increased to between 80 to 90 kDa through the addition of multiple oligosaccharide and GAG additions. CD44v can contain additional modifications because many of the variant exons contain sites for additional oligosaccharide and GAG additions [75]. These variant exons therefore affect the affinity of CD44 for HA binding based on the concept that the N-linked glycosylation pattern dictates the activation state of CD44. Specifically, a high degree of N-linked glycosylation activates CD44 to bind HA, while a low degree inactivates CD44 so that it binds HA poorly or not at all [75]. The activation patterns of CD44 also are affected by the type of cell, phosphorylation state of the cytoplasmic tail, and clustering in the membrane [76]. CD44/HA binding is involved in diverse functions including attachment, organization, and turnover of the ECM at the cell surface, as well as the mediation of lymphocyte migration during inflammation.
Another HABP, RHAMM, also known as CD168, was originally discovered as a soluble protein that altered migratory cell behavior [77]. In contrast to CD44 and other cell-surface receptors that contain the classical membrane spanning domain and signal sequence for secretion from the endoplasmic reticulum (ER)/Golgi complex, RHAMM does not contain a membrane spanning domain nor does the mRNA transcript contain a signal sequence. RHAMM normally is localized inside the cell and is only released by certain, poorly defined stimuli. The transport of RHAMM to the extracellular space is still unclear but may involve transport channels proteins, flippase activity, or exocytosis [78]. Intracellularly, RHAMM associates with microtubules and, working with breast cancer-2 susceptibility protein (BRCA2) and BRCA1-associated RING domain protein 1 (BARD1), plays a role in the regulation of mitosis [79]. Extracellularly, RHAMM associates with CD44, and upon binding to HA, activates intracellular signaling pathways [80]. Variants of RHAMM caused by alternative splicing have been observed, but not thoroughly studied. Preliminary reports have suggested that alternatively spliced forms of RHAMM may be upregulated in some tumor types, promoting tumor progression [81].
Expressed on the endothelial cells of the lymphatic sinus and in reticular cells in the lymph nodes, LYVE-1 binds HA via the link module to mediate the transport of HA from tissue ECM to lymph for cell uptake and degradation [82]. Based on the amino acid sequence, LYVE-1 is predicted to be a type I transmembrane glycoprotein, with a single copy of CD44-like link module located at the N-terminus of the extracellular domain that binds both soluble and immobilized HA [83, 84]. LYVE-1 has been widely used as a lymphatic vessel specific marker and offers a prognostic parameter for head and neck squamous cell carcinomas. Although the HA-binding capacity of LYVE-1 is highly regulated, HA homeostasis in LYVE-deficient mice is not perturbed [85]. Further investigations are necessary to identify the role of LYVE-1 in normal lymphatic development and function, as well as its involvement in lymphatic HA metabolism or HA-mediated cell migration.
HA can be organized into supramolecular assemblies via its association with multiple binding proteins [72, 86]. In the ECM of connective tissues, HA binds certain proteoglycans, such as aggrecan (Figure 3), versican, and brevican, to form large complexes that provide the structural integrity and mechanical functions to the tissues [87–89]. The protein product of TSG-6, secreted in response to inflammatory stimuli, also binds HA via a single link module. Binding of TSG-6 with HA results in the formation of fibrils or “cables” (Figure 4A) that are pro-adhesive to lymphocytic cell lines. Adhesion of leukocytes to the HA complexes prevents the direct contact of inflammation-promoting receptors to the underlying tissues, thereby maintaining leukocytes in a non-activated state. In response to ER stress or when exposed to high concentrations of glucose, a variety of cells produce HA cables [90]. TSG-6 catalyzes the covalent transfer of heavy chains (HCs) from inter-alpha-trypsin inhibitor (IocI) and pre-α-inhibitor (PαI) to HA, and HC-HC crosslinks results in stabilized HA cables [91–93]. Certain HA cables, for example, those synthesized by airway smooth muscle cells, can form without TSG-6 and are independent of HC attachment [94]. With an overall length greater than 200 µm long, these cables are capable of supporting the binding of a large number of leukocytes. Versican is found to associate with the cables, extending and hydrating these structures, as well as providing a means of sequestering proinflammatory chemokines through interactions with its chondroitin sulfate and dermatan sulfate chains [95].
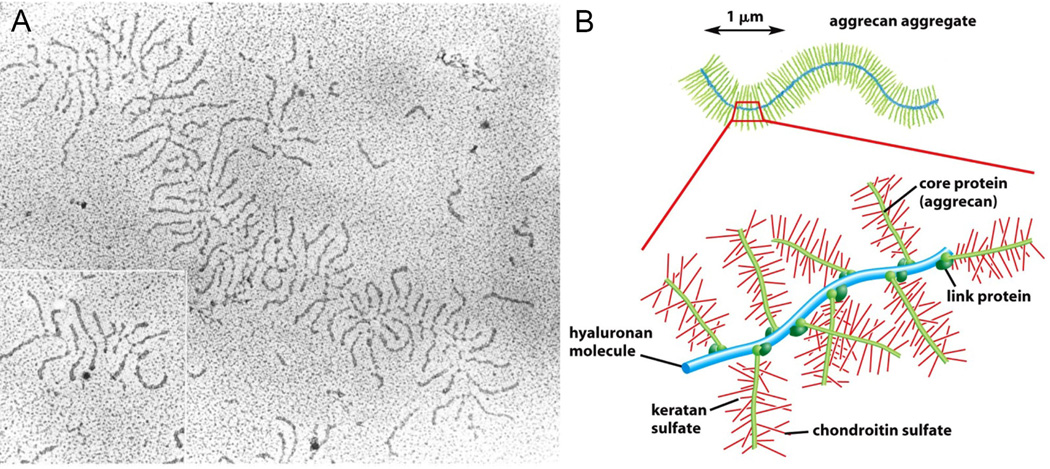
Figure 3.
HA organizes the cartilage ECM via noncovalent binding with aggrecan. (A): An electron micrograph of an aggrecan/HA aggregate (from fetal bovine cartilage) shadowed with platinum. (B): A drawing of the aggrecan/HA aggregate, showing the non-covalently binding of aggrecan via the link proteins. Each aggregate consists of approximately 100 aggrecan monomers bound to HA. With a molecular weight >108, such a complex occupies a volume equivalent to that of a bacterium. Reproduced with permission [206], Copyright 2002, Garland Science.
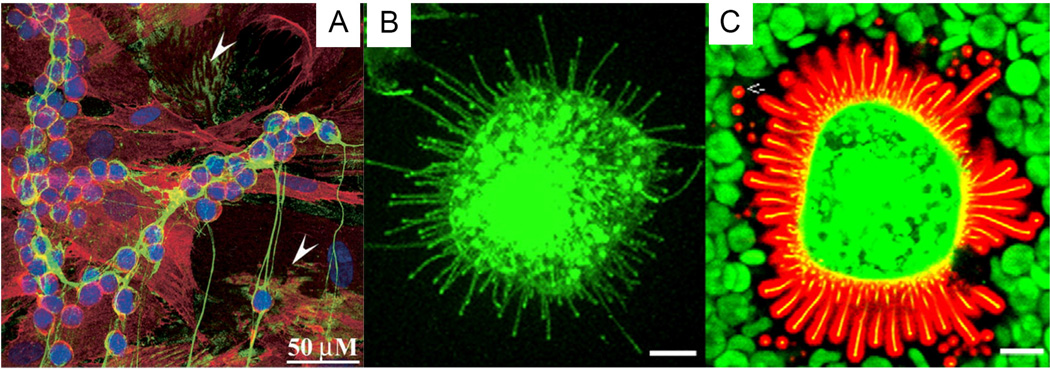
Figure 4.
HA organized as monocyte-adhesive cables (A) or pericellular coat (B, C). (A): Monocyte-adhesive HA cable, produced by treating human colon smooth muscle cells with poly I:C for 17 h. Green: HA; Red: CD44 and Blue: nuclei. Scale bar: 50 µm. Arrowheads point to areas without any leukocytes. (B, C): HA coat around a MCF-7 cell, as revealed by confocal imaging a probe made of aggrecan G1 domain and link protein tagged with Alexa Fluor 594® (red). Green staining represents green fluorescent protein tagged HAS3 (GFP-HAS3). The HA coat was visualized particle exclusion using red blood cells (green). Scale bar: 10 µm. (A): Reproduced with permission [90], Copyright 2003, Elsevier. (B, C): Reduced with permission [96], Copyright 2008, Elsevier.
The pericellular coat (Figure 4B–C), an HA-rich, 5–10 µm thick gel-like layer surrounding many types of cells, is also organized and stabilized by HABPs. The HA coat is organized by aggregating proteoglycan and cell surface HA receptors (CD44) and is crosslinked by various proteins such as tenascin, TSG-6, IαI, pentraxin (PTX) and thrombospondin 1 (TSP-1). The pericellular coat plays complex roles in cell adhesion/de-adhesion and cell shape changes associated with proliferation and locomotion, thereby contributing to the regulation of inflammation, morphogenesis, tissue regeneration and healing. The elastomeric pericellular matrix has also been implicated in cellular mechanotransduction [27, 96]. The HA coat, formed between the cumulus cells surrounding the oocyte during ovulation, is responsible for the integrity of the cumulus-oocyte complex, providing protection and facilitating the transport of the oocyte into the oviduct for fertilization. Sperm-associated hyaluronidases allow penetration of this matrix at fertilization [5, 97].
4. HA in morphogenesis and wound healing
Mounting evidence points to the involvement of HA in morphogenesis. HA-rich matrix can either facilitate cell migration by creating hydrated and non-adhesive milieu or inhibit cell migration via the increased binding of proteoglycans to pericellular HA [98]. HA exerts a profound effect on the “stemness” of hematopoietic stem cells (HSCs), human embryonic stem cells (hESCs) and mesenchymal stem cells (MSCs). All three types of stem cells reside in specific HA-rich microenvironments that maintain cells in a quiescent state with low levels of proliferation. The maintenance and differentiation of stem cells are also intimately mediated by HA/HABP interactions. Specifically, HA/CD44 and HA/RHAMM interactions are essential for the trafficking of HSCs and their homing into the bone marrow where they are maintained in an undifferentiated state [99]. In embryonic tissues, RHAMM plays an important role in the maintenance of hESC pluripotency, viability, and cell cycle. During stem cell differentiation, RHAMM expression is significantly downregulated, at the same time, HAS2 expression is markedly enhanced, resulting in a 13–24-fold increase in HA production [100]. MSCs are resident stem cells in adult tissues and CD44 long has been used as a marker for MSCs [101]. In response to platelet-derived growth factor (PDGF) stimulation, MSCs express high levels of CD44 standard isoform, which facilitates cell migration through interaction with extracellular HA. Such a migratory mechanism could be critical for the recruitment of MSC into wound sites for the preparation of tissue regeneration [102]. From a biomedical engineering perspective, delivering stem cells in HA matrices not only improves cell survival, but also allows the cells to be released to contribute to tissue regeneration owing to the susceptibility of the HA matrices to enzymatic degradation.
HA, alone or through its interaction with its binding partners, has been shown to be crucial for the morphogenesis of many tissues/organs. For example, HA produced by endocardial cells during embryonic development contributes to the formation of endocardial cushions and facilitates the endothelial-to-mesenchymal transformation in the development of cardiac valves. The maintenance of HA homeostasis, thereby the size of the endocardial cushions, is achieved through balanced activity of positive and negative regulators of HAS2 [103]. HA is also widely distributed throughout the developing central nervous system (CNS), playing a role in regulating neural crest cell migration from the dorsal neural tube. Specifically, HA promotes the separation of neural crest cells from the dorsal neural tube. In addition to regulating neuronal function and cell migration, HA may influence progenitor cell differentiation and maturation. It has been reported that the presence of HA either in vivo or in vitro blocked the maturation of oligodendrocyte progenitor cells into myelin-forming oligodendrocytes, suggesting that HA blocks remyelination by maintaining progenitor cells in an undifferentiated or immature state. Neural stem cells (NSCs) may be influenced similarly by exposure to HA in stem cell niches and in injury microenvironments, where they may synthesize their own pericellular HA [68]. Delivery of NSCs in a HA-containing hydrogel into stroke cavity promotes NSC maturation and proliferation [104]. Being a major component of the ECM of the developing limb bud, HA is involved in various aspects of limb morphogenesis. Importantly, down-regulation of HA is necessary for the cell positioning, cell-cell interaction and cartilage differentiation during condensation. The overexpression of HAS2 in the mesoderm of the chick limb bud in vivo results in the formation of shortened and severely malformed limbs that lack one or more skeletal elements [105–107].
Tissue injury and repair process is characterized by the turnover the matrix components, and HA plays important and multifaceted roles in this dynamic process [108]. Interactions between HA and its signaling receptors initiate inflammatory responses, maintain structural cell integrity, and promote recovery from tissue injury. Studies have shown that RHAMM is critical for the recruitment of macrophage to areas of tissue injury [109], whereas CD44 is critical for abrogation of inflammation [110]. Moreover, Toll-Like receptors cooperate with HA receptors, particularly with CD44, to activate the innate immune system [111–115]. The ability of HA to facilitate tissue repair and wound healing depends on its molecular weight and tissue location, as well as the specific cell population HA interacts with. The HA rich matrix, both in the early inflammatory phase of wound repair and in the granulation tissue, facilitates cell migration into the provisional wound matrix by providing an open hydrated matrix and through direct interaction with cells via HABPs [68]. Fibroblasts recruited to the wound bed produce proinflammatory cytokines that in turn, stimulate endothelial cells to produce HA, further promoting the adhesion of cytokine-activated lymphocytes through the HA-binding variant of CD44 [116]. HA also facilitates the cell detachment from the matrix and cell mitosis, thereby fostering cell proliferation [117]. Through its interaction with HABPs and aggregating proteoglycan, HA contributes to the organization of the granulation tissue matrix. Experimental results suggest that CD44 may contribute to the organization and/or stability of developing endothelial tubular networks [118]. Finally, the physiological role of HA and its oligosaccharide are central to angiogenesis, an important step in wound healing [108]. As mentioned above and consistent with this idea, high molecular weight HA has been shown to inhibit angiogenesis, while the low molecular weight counterparts promote angiogenesis and enhance the production of collagens by endothelial cells [119]. Noteworthy, intact, high molecular weight HA, not the fragmented HA, promotes the induction of regulatory T-cells [120]. Thus, the induction signals can be recapitulated using HA-containing synthetic matrices.
In the later stage of wound healing, HA may function as a moderator of inflammation by protecting against free radical damage to cells [121]. The TSG-6/IaI complex, through its interaction with HA both on the cell surface and in the ECM, may serve as a potent negative feedback loop to moderate inflammation and to stabilize the granulation tissue as healing progresses. The final stages of wound healing include re-epithelialization and remodeling. In healing wounds, HA is expressed in the wound margin, in the connective tissue matrix, and collocating with CD44 expression in migrating keratinocytes [122]. Interestingly, fetal wound healing is characterized by a lack of fibrous scarring. HA in fetal wounds remains high for longer periods than in adult wounds, probably reducing the deposition of disorganized collagen matrix. The persistent HA-rich environment can affect cell-cell and cell-matrix interactions, ultimately contributing to scarless wound healing as can occur in the HA-rich young organism [108]. In adult tissues, studies show that blocking signaling of HA fragments by using a RHAMM-mimetic peptide results in skin wound healing with reduced fibrosis [123].
5. HA in cancer
Tumor progression and metastasis are accompanied by the alteration of organ microenvironment. HA is enriched in many types of tumors and has been implicated in the progression and metastasis of carcinomas [27]. Enrichment of HA in tumors can be attributed to increased production by tumor cells themselves or by tumor associated stromal cells through tumor-stroma crosstalk [124]. For example, normally absent in healthy epithelial tissues, HA is upregulated when the epithelial cells undergo malignant transformation [98]. The involvement of HA in the growth and spreading of cancers of epithelial origin is complex and multifaceted. During tumorigenesis, epithelial cells can undergo epithelial-to-mesenchymal transition and detach themselves from the epithelial compartment for invasion, accompanied by enhanced HA synthesis. HA, in turn, supports cell proliferation, prevents apoptosis, maintains intercellular space to facilitate nutrient diffusion, and enhances cell locomotion that stimulates invasion. In addition, cancer cells disguise themselves with a coat of HA from the cytotoxic effects of T-lymphocytes. HA cables, on the other hand, bind tissue macrophages and modulate their activity to favor tumor growth [98].
The upregulation of HAS expression results in the accumulation of HA in tumor tissues and consequently, the creation of a pro-metastatic microenvironment [125]. Using a clone of breast cancer cell line MDA-MB-231 that forms bone metastases in an in vivo-like basement membrane model, researchers discovered that the increased expression of HAS2 in metastatic cells resulted in a 7-fold higher HA-synthesizing capacity compared with MDA-MB-231 cells [126]. Further, knockdown of HAS2 completely suppressed the invasive capability of these cells by the induction of tissue metalloproteinase inhibitor 1 (TIMP-1) and dephosphorylation of focal adhesion kinase (FAK). HAS2 knockdown-mediated inhibition of basement membrane remodeling was rescued by HAS2 overexpression, transfection with TIMP-1 siRNA, or addition of TIMP-1-blocking antibodies. Moreover, knockdown of HAS2 suppressed the EGF-mediated induction of the FAK/PI3K/Akt signaling pathway [126]. HAS2 has also been shown to be critical for the interaction of cancer stem cells (CSCs) with tumor-associated macrophages (TAM), leading to enhanced secretion of platelet-derived growth factor-BB from TAMs. This secretion could then activate stromal cells and enhance CSC self-renewal. Loss of HAS2 in CSCs or inhibition of HAS activity drastically reduced the incidence and growth of metastatic lesions in vitro or in vivo, respectively. [127]
It is noteworthy that, while a moderate increase in HA production correlates with tumor growth and metastasis, a large excess of HA can suppress tumor growth [128]. Because HA accumulation is the result of a balance between the activities of HAS isozymes and HAases, the presence of HAases may promote HA turnover in the cancer cells and overcome the tumor suppression by excess amounts of HA. By degrading the HA-rich matrix surrounding the tumor, HAases help the cancer cells escape from the primary tumor mass and play a major role in intravasation by allowing degradation of the basement membrane of the lymph or blood vessel. HAases play roles in the establishment of a metastatic lesion by helping with extravasation and clearing the ECM of the secondary site [129]. Finally, HAases produce HA fragments to stimulate endothelial cell proliferation and budding of new capillaries that promote angiogenesis to allow tumor expansion [130]. Interestingly, hypoxia also increases production of HA and activity of HAases [131].
Each of the HAases discussed above can play a role in cancer progression, although the roles they play and the cancers they contribute to may differ. Earlier in vitro cell culture experiments suggested that Hyal1 expression prevented tumor growth from the results of cells cultured in vitro [132]. Furthermore, when directly injected, Hyal-1 was found to inhibit tumor formation in vivo [133]. Subsequently, it was discovered that context matters in the case of the role of Hyal1 in cancer progression. Hyal1 plays a role in metastasis mainly via interactions with the ECM, and a lack of this tumor microenvironment would likely complicate the data in an in vitro setting. Additionally, Hyal1 functions predominantly in an intracellular fashion, explaining why direct injection of this enzyme may produce conflicting results. Clinically, there is considerable interest in using Hyal1 levels in blood or urine to predict a patient’s prognosis. Studies among bladder cancer patients show that Hyal1 levels correlated positively with poor prognosis, muscle invasion, and recurrence [134, 135]. In prostate cancer (PCa) patients, high Hyal1 levels correlate with progression 84% of the time [136]. Hyal1 is the predominant HAase responsible for lung cancers as well [137]. Laboratory studies have shown similar results. PCa cells that over-express Hyal1 form significantly more metastases than do controls that express lower levels of the enzyme [138].
Some research has been performed to examine the role of Hyal2 in cancer metastasis, however the accumulated data indicates that Hyal2 likely plays a role. A clinical study found that endometrial cancer cells express more Hyal2 than Hyal1 pointing to its importance in that type of cancer [139]. Additionally, a cell based study found that highly invasive breast cancer cell lines express more Hyal2 than poorly invasive lines [140]. When Hyal2 was over-expressed in astrocytoma cells, these cells formed more aggressive, invasive tumors in the cranium compared to control. Interestingly, the same result was not found when the cells were injected subcutaneously, indicating that the microenvironment matters for the role of Hyal2 in cancer progression [141].
The role of Hyal3 in cancer progression is poorly studied and the few studies that have investigated this HAase report contradictory results. A study of breast cancer cell lines demonstrated that poorly invasive lines express more Hyal3 than do highly invasive lines [140]. On the other hand, a study of endometrial cancer found that Hyal3 was expressed at levels 1000 times higher than Hyal1 and 33 times higher than Hyal2 suggesting that Hyal3 is the main HAase necessary for endometrial cancer progression [139]. The finding that PH-20 is normally expressed only in the testes is of interest to the medical community because it could provide a good prognostic indicator of the existence of cancer and the likelihood to metastasize. Two studies on laryngeal cancer have shown that PH-20 expression increased in late stage tumors, or those likely to metastasize [142, 143]. A similar correlation was shown for breast cancer [144, 145]. African-American women with breast cancer were shown to have a higher expression of PH-20 compared to Caucasian women, an interesting observation considering that African-Americans have a higher likelihood of breast cancer metastasis [146]. Collectively, the existing data demonstrates that PH-20 plays a strong role in cancer metastasis, at least for some cancers.
HA also interacts with its receptors to modulate cell behaviors during tumor progression and metastasis. Among the HA receptors, CD44 has been best studied. Numerous clinical studies have shown that increased expression of CD44 correlates with increased metastasis of a variety of different tumors [147]. In addition to a wealth of clinical data, the pathways by which CD44 affects cancer metastasis also have been largely determined. Collectively, CD44 affects adhesion of cancer cells, rearranges of the cytoskeleton through activation of Rho GTPases, and increases the activity of ECM degrading enzymes.
When a cell remains epithelial in nature by expressing E-cadherin, CD44/HA binding is low, preventing the activation of metastatic signaling pathways [148]. When a cell becomes cancerous, E-cadherin is down-regulated, leading to higher CD44/HA binding. After binding with HA, CD44 signals for the activation of a number of ECM degrading proteins, which allow cancer cells to detach from the primary tumor mass and migrate. CD44 increases matrix metalloproteinase-9 (MMP-9) localization to the plasma membrane and optimizes Hyal-2 activity by adjusting extracellular pH through activation of a Na+/H+ exchanger [149, 150]. CD44 itself can be cleaved by MMPs, again helping cancer cells release themselves from the tumor mass [151, 152]. An intracellular cleavage product of CD44 produced by y-secretase can participate in transduction leading to increased migration [153].
CD44 activates a number of Rho GTPases that remodel the actin cytoskeleton to allow for migration to occur. Binding of CD44 to HA controls activation of RhoA, Rac1, and Cdc42 GTPases, and subsequently the downstream targets of these GTPases [154–156]. Along with remodeling the actin cytoskeleton, CD44 also activates FAK, which allows for focal adhesion formation and turnover, another key step in the process of cell motility [157]. Another molecule that promotes invasion that CD44 activates is Snail2, through the NFkB pathway [158]. Other downstream targets of CD44 likely exist, but have not yet been discovered. While it is important that CD44 molecules be cleaved for the cancer cells to release from the primary tumor mass, interactions between the receptor and HA and other ECM molecules during the metastatic process also are important. Several isoforms of CD44v are necessary for cell motility by binding with HA and other ECM components [159–161]. Additionally, CD44 is indispensable during the processes of intravasation and extravasation because it regulates binding of the cancer cell to endothelial cells [162].
While RHAMM has been less well studied than CD44 in the progression of cancer metastasis, the research that has been produced shows that it is likely just as important in this process and probably plays a larger role in cell motility than does CD44. Increased RHAMM expression is correlated with metastases in colrectal cancer, among others [163, 164]. Mechanistically, RHAMM promotes cell motility through a number of different pathways. As is seen with CD44, RHAMM can promote focal adhesion turnover by controlling FAK phosphorylation and by cooperating with the α4β1 and α5β1 integrins [165, 166]. RHAMM also activates a number of downstream kinases including Erk 1/2 through the mitogen activated protein kinase (MAPK) pathway, pp60 (c-src), and the downstream targets of Rho kinase (ROK) [167–169]. Finally, once a metastatic lesion has been established, RHAMM can cooperate with CD44 to promote angiogenesis by promoting migration of neighboring endothelial cells towards the tumor [170].
The understanding of HA in cancer biology has led to successful clinical usage of HA for cancer treatment. For example, to improve the treatment of tumors, Halozyme Therapeutics [171] uses recombinant human of HAase (PH-20) to temporarily degrades HA, thereby facilitating the penetration and diffusion of other drugs and fluids that are injected under the skin. The use of HA by Tracey Brown and her team as drug carriers[172] for the treatment of cancer has been successfully demonstrated in clinical trials [173].
6. HA in biomedical applications
The ubiquitous presence of HA in various tissues, combined with its inherent biocompatibility and biodegradability, has motivated researchers to explore the utility of HA-based materials for tissue growth, repair and regeneration [18, 174]. In this special issue, Segura and colleagues have summarized strategies for preparing HA-based hydrogel scaffolds with desired cell-instructive features for tissue engineering and regenerative medicine applications. Many HA-derived medical products have been developed and tested and the readers are referred to recent reviews [17, 175] for in-depth analyses. In this article, we highlight our own experiences in the application of HA-based hydrogels for the repair and regeneration of healthy functional tissues, such as vocal folds [176–179], cartilage [180, 181] and salivary glands [182, 183], as well as the creation of pathological tissue models, such as tumor spheroids [184–186].
Using chemically modified HA derivatives as modular building blocks, we have engineered HA hydrogel particles (HGPs) and complex networks with defined biological functions and robust mechanical properties[18]. For example, nanoporous, micron-sized HA HGPs were synthesized using different inverse emulsion systems and crosslinking chemistries. The resultant particles either contained residual functional groups or were rendered reactive by subsequent chemical modifications. HA-based doubly crosslinked networks (DXNs) were synthesized via covalent crosslinking of HA HGPs with soluble HA macromers carrying mutually reactive functional groups. These hybrid matrices are hierarchical in nature, consisting of densely crosslinked HGPs integrated in a loosely connected secondary matrix [178]. Their mechanical properties and degradation kinetics can be readily tuned by varying the particle size, functional group density, and intra- and interparticle crosslinking. Using a custom-designed torsional wave apparatus [187], we demonstrated that the viscoelastic properties of HA DXNs can be matched to that of the vocal fold tissue samples [188] at frequencies close to human phonation. Thus, these materials are attractive injectables for the elimination of vocal fold scarring [178, 189]. Separately, HA hydrogels containing self-assembled collagen fibrils provide instructive matrices for the 3D culture of primary vocal fold fibroblasts (PVFFs). PVFFs are found to attach and spread in the matrix and proliferate readily. TWA analysis suggests that PVFFs residing in gels alter the matrix organization, chemical compositions and viscoelasticity through cell-mediated remodeling processes [179].
HA-based complex networks also have been evaluated for the repair of the cartilage and the regeneration of cartilage-bone interface. In this context, the nanoporous HA HGPs are ideal growth factor depots for chondrogenic cytokines and growth factors. To improve the biological functions of HA HGPs, perlecan/HSPG2 domain I (PlnDI), a basement membrane proteoglycan that has strong affinity for various heparin binding g rowth factors (HBGFs), was successfully conjugated to the particles through the core protein via a flexible poly(ethylene glycol) (PEG) linker. The immobilized PlnDI maintains its ability to bind bone morphogenetic proteins (BMP-2) and modulates its release [190]. The chondrogenic potential of the hydrogel particles and the stimulatory effects of the injectable formulation were confirmed in vitro using micromass culture of multipotent MSCs and in vivo in a reversible animal model of osteoarthritis (OA). Finally, celladhesive HA DXNs were fabricated by encapsulating gelatin, or collagen-like peptide-decorated HA HGPs in a secondary HA matrix [191]. Human MSCs were shown to adhere to the composite matrix through the focal adhesion sites clustered on particle surfaces. The celladhesive composite matrices supported hMSC proliferation and migration into the gels. Human MSCs were undifferentiated during the early time points of culture. However, they differentiated into osteoblast phenotype after 28 days of culture [192].
Cartilage is routinely exposed to regular compression during locomotion. Integrating mechano-responsive elements in HA hydrogels is a novel strategy to harvest the compressive forces in the tissue to effectively direct cellular behaviors. To achieve this goal, self-assembled block copolymer micelles (BCMs) containing a hydrophobic, rubbery core and a hydrophilic shell with chemically addressable groups were utilized as the dynamic building blocks and microscopic crosslinkers [193, 194]. Covalent integration of dexamethasone (DEX)-loaded BCMs in HA gels significantly reduced the initial burst release and provided sustained DEX release over a prolonged period. Importantly, DEX release from BCM-HA gels was accelerated by intermittently-applied external compression in a strain-dependent manner. Culturing macrophages in the presence of DEX-releasing BCM-HA gels significantly reduced cellular production of inflammatory cytokines, possibly through the synergistic actions of HA and the released DEX [195]. Incorporating mechano-responsive modules in synthetic matrices offers a novel strategy to harvest mechanical stress present in the healing wounds to initiate tissue repair.
Although not present at high levels in healthy salivary glands, the biocompatibility and the bioactivity of HA motivated us to investigate HA-based gels for the creation of artificial salivary glands for the treatment of xerostomia, or dry mouth. Parotid cells encapsulated in 3D HA hydrogels self-assembled into acini-like structures and expressed functional neurotransmitter receptors [183]. Structures in 3D hydrogels merged to form organized 50 µm spheroids that could be maintained in culture for over 100 days and merged to form structures over 500 µm in size. Treatment of acini-like structures with the β-adrenergic agonists increased granule production and α-amylase staining in treated structures, demonstrating regain of protein secretion. Upon treatment with the M3 muscarinic agonist acetylcholine, acini-like structures activated the fluid production pathway by increasing intracellular calcium levels. Encapsulated cells in 3D retained their spheroid structure and structural integrity, along with the salivary biomarkers and maintained viability for over three weeks in vivo. Thus, the HA hydrogels are capable of maintaining functional 3D salivary spheroid structures for long periods in vitro that retain both fluid and protein secreting functions and are suitable for tissue restoration [183].
The utility of HA-based matrices for the engineering of healthy replacement tissues has been expanded to the construction of pathological tissue models. We have developed a biologically relevant hydrogel culture system [184] that recaptures the essential feature of prostate cancer (PCa) and its associated stroma. Cell-laden hydrogels were prepared by mixing HA derivatives carrying complementary reactive groups. The resultant viscoelastic hydrogels are biodegradable and can interact with prostate cancer cells through its receptors, activating specific signaling pathways [185]. Prostate cancer (PCa) cells entrapped in HA matrices formed distinct multicellular aggregates which grew and merged, reminiscent of real tumors, whereas cells cultured on 2D monolayer adopted an atypical spread-out morphology.
The engineered tumor model was used successfully to test the efficacy of anti-cancer drugs including camptothecin, docetaxel, and rapamycin, alone and in combination, including specificity, dose and time responses. Responses of cells to anti-neoplastics differed between the 3D HA hydrogel and 2D monolayer systems [184]. The engineered tumor models also have the potential to provide predictable results for the in vivo assessment of nanomedicine. Specifically, doxorubicin (Dox) loaded NPs with an average diameter of 54±1 nm were able to diffuse into the hydrogel matrices, reach and penetrate into the tumoroids, be internalized by LNCaP PCa cells through caveolae-mediated endocytosis and macropinocytosis pathways, and finally release the drug intracellularly. A drug efficacy study revealed that LNCaP PCa cells cultured in the 3D hydrogel were more resistant to Dox in both soluble and NP-based form than were cells on 2D culture. In addition, the NP-based Dox formulation may bypass the drug efflux function of MRP1, thereby partially reversing drug resistance in 3D cultures [196].
To simulate the tumor-stroma cross-talk, a bilayer construct was developed and characterized. The top hydrogel layer contains heparin (HP)-decorated, HA-based HGPs presenting heparin-binding epidermal growth factor-like growth factor (HB-EGF) in a sustained manner. LNCaP cells were embedded within the bottom hydrogel layer and received growth stimuli from the top. We demonstrated that tumoroids grown in bilayer HA hydrogels reflect features reminiscent of native carcinoma, and exhibit promising angiogenic potential through the upregulation of pro-angiogenic factors, both at the gene and the protein levels. These structured 3D units provide a novel means to study cancer and stroma invasiveness, cell-cell interactions and drug responses [186].
To study the individual functions of HA interacting proteins in PCa motility through connective tissues, we have developed an invasion assay [185].based on the 3D HA hydrogel that provides a flexible, quantifiable, and physiologically relevant alternative to current methods. Metastatic PCa cells in these hydrogels develop fingerlike structures, “invadopodia”, consistent with their invasive properties. The number of invadopodia, as well as cluster size, shape, and convergence, can provide a quantifiable measure of invasive potential. We found that culture in the HA hydrogel triggers invasive PCa cells to differentially express and localize RHAMM/CD168 which, in the absence of CD44, appears to contribute to PCa motility and invasion by interacting with the HA hydrogel components. PCa cell invasion through the HA hydrogel also was found to depend on the activity of HAases. While HAase activity was necessary for invadopodia and inter-connecting cluster formation, activity alone was not sufficient for acquisition of invasiveness to occur. Our results suggest that development of invasive behavior in 3D HA-based systems requires development of additional cellular features, such as activation of motility associated pathways that regulate formation of invadopodia.
7. Summary and Outlook
This review highlights fundamental functions of HA in the context of biological systems, as a structural support and a signaling molecule. HA biosynthesis, tissue turnover and homeostasis are coordinately maintained by three synthases and several hyaluronidases. Traditionally known as an ECM molecule, HA is also found pericellular and intracellularly, although its intracellular function is largely speculative. HA provides structural frameworks for cells, functions as an extracellular molecule transmitting signals, and regulates a variety of cell behaviors, including cell adhesion, motility, growth and differentiation. Binding of HA to cell surface receptors activates various intracellular signaling cascades, such as c-Src, Ras and mitogen-activated protein kinases (MAPK) [73] thereby regulating cell growth and survival, cytoskeletal rearrangement, and active cell migration. The biological functions of HA depend on the molecular size of HA, the HA binding proteins, its spatial and temporal distribution in tissues, and the cellular background and tissue stages. Mounting evidence confirms the involvement of HA in morphogenesis and would healing, and its role in cancer progression and metastasis.
HA has been widely used by the biomedical community as a starting material for the fabrication of hydrogel matrices, tissue engineering tools, drug delivery vehicles or drug depot systems, and tissue filler or surgical devices [16, 197–199]. HA-based materials may impart biological activity to cells, as evidenced by changes in cellular behavior, due to the cells’ ability to interact with biomaterials based on HA compared to synthetic polymers, such as poly(ethylene glycol) (PEG). For example, the ability of HA to maintain stem cells in an undifferentiated state [200] and the involvement of HA-interacting proteins in tumor metastasis in HA gels [185] have been investigated. In drug delivery, the ability of HA to bind cell surface receptors has been explored for drug targeting purposes [201]. Notably, excessive chemical modification of HA alters its biological functions. Real-time imaging of quantum dot (QD)-tagged HA derivatives revealed that HA-QD conjugates with 35 mol% HA modification maintained the ability to bind HA receptors and were mainly accumulated in the liver, while those with 68 mol% HA modification lost much of HA characteristics and were evenly distributed throughout the body [202].
Considering the complexity of the biological functions of HA, particularly the connection with pathologies including inflammation and cancer, care must be taken to ensure long term safety of HA-based biomaterials. For example, the intra-articular injection of HA is widely used for symptomatic knee osteoarthritis. In a systematic review of randomized trials in any language that compared viscosupplementation with sham or nonintervention control in adults with knee osteoarthritis, Reichenbach and coworkers found that in patients with knee osteoarthritis, viscosupplementation is associated with a small and clinically irrelevant benefit and an increased risk for serious adverse events. The authors cautioned that trial quality was generally low and safety data were often not reported [203]. Concerns also exist for using HA-based materials for cell delivery and other modalities in tissue engineering as potentially causing/exacerbating cell migration and metastasis of the residual tumor cells following chemotherapy [204]. In a landmark paper, Khaldoyanidi’s team [205] provided experimental evidence that HA treatment does not stimulate but delays the growth of residual cancer cells, which is an important parameter in establishing whether the use of HA can enhance current chemotherapeutic strategies. As we continue to unravel the complex functions of HA under different biological conditions and disease states, advanced engineering strategies and surgical interventions will lead to the potential utility of HA-based materials in translational applications.
Acknowledgments
Work in the authors’ collaborating laboratories has been funded by grants from the National Institutes of Health (R01 DC008965, XJ; P20 RR017716, XJ; P01 CA098912, MCFC; R01 DC01137, RLW, MCFC and XJ), the National Science Foundation (DMR 0643226, XJ), and the Department of Defense (PCRP W8 1XWH-11-1-0564, LAG). KTD thanks Robert W. Gore for the Gore Fellowship. The authors wish to acknowledge Genzyme for generously providing HA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyer K, Palmer JW. The polysaccharide of the vitreous humor. J Biol Chem. 1934;107:629–634. [Google Scholar]
- 2.Meyer K. Chemical structure of hyaluronic acid. Fed Proc. 1958;17:1075–1077. [PubMed] [Google Scholar]
- 3.Balazs EA, Laurent TC, Jeanloz RW. Nomenclature of hyaluronic acid. Biochem J. 1986;235:903. doi: 10.1042/bj2350903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhuo LS, Kimata K. Cumulus oophorus extracellular matrix: Its construction and regulation. Cell Struct Funct. 2001;26:189–196. doi: 10.1247/csf.26.189. [DOI] [PubMed] [Google Scholar]
- 6.Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Seminars in Reproductive Medicine. 2006;24:217–227. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa K, Terayama H. Isolation and identification of glycosaminoglycans associated with purified nuclei from rat liver. Biochim Biophys Acta. 1977;499:278–289. doi: 10.1016/0304-4165(77)90010-1. [DOI] [PubMed] [Google Scholar]
- 8.Margolis RK, Crockett CP, Kiang WL, Margolis RU. Glycosaminoglycans and glycoproteins associated with rat brain nuclei. Biochim Biophys Acta. 1976;451:465–469. doi: 10.1016/0304-4165(76)90141-0. [DOI] [PubMed] [Google Scholar]
- 9.Eggli PS, Graber W. Association of hyaluronan with rat vascular endothelial and smooth muscle cells. J Histochem Cytochem. 1995;43:689–697. doi: 10.1177/43.7.7608523. [DOI] [PubMed] [Google Scholar]
- 10.Evanko SP, Wight TN. Intracellular localization of hyaluronan in proliferating cells. J Histochem Cytochem. 1999;47:1331–1342. doi: 10.1177/002215549904701013. [DOI] [PubMed] [Google Scholar]
- 11.Kan FW. High-resolution localization of hyaluronic acid in the golden hamster oocyte-cumulus complex by use of a hyaluronidase-gold complex. Anat Rec. 1990;228:370–382. doi: 10.1002/ar.1092280403. [DOI] [PubMed] [Google Scholar]
- 12.Ripellino JA, Bailo M, Margolis RU, Margolis RK. Light and electron microscopic studies on the localization of hyaluronic acid in developing rat cerebellum. J Cell Biol. 1988;106:845–855. doi: 10.1083/jcb.106.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hascall VC, Majors AK, de la Motte CA, Evanko SP, Wang AM, Drazba JA, et al. Intracellular hyaluronan: a new frontier for inflammation? Biochimica Et Biophysica Acta-General Subjects. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64:1591–1596. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg HG, Hales CA. Chemistry biology of hyaluronan. 1st ed. Oxford: Elsevier Ltd; 2004. [Google Scholar]
- 16.Allison DD, Grande-Allen KJReview. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 17.Burdick JA, Prestwich GD. Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Materials. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia XQ. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter. 2012;8:3280–3294. doi: 10.1039/C2SM06463D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 20.Spicer AP, McDonald JA. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998;273:1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- 21.Prehm P. Identification and regulation of the eukaryotic hyaluronate synthase. Ciba Found Symp. 1989;143:21–30. doi: 10.1002/9780470513774.ch3. discussion −40, 281-5. [DOI] [PubMed] [Google Scholar]
- 22.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 23.Rilla K, Oikari S, Jokela TA, Hyttinen JMT, Karna R, Tammi RH, et al. Hyaluronan Synthase 1 (HAS1) Requires Higher Cellular UDP-GlcNAc Concentration than HAS2 and HAS3. J Biol Chem. 2013;288:5973–5983. doi: 10.1074/jbc.M112.443879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itano N, Kimata K. Mammalian hyaluronan synthases. Iubmb Life. 2002;54:195–199. doi: 10.1080/15216540214929. [DOI] [PubMed] [Google Scholar]
- 25.Camenisch TD, Biesterfeldt J, Brehm-Gibson T, Bradley J, McDonald JA. Regulation of cardiac cushion development by hyaluronan. Exp Clin Cardiol. 2001;6:4–10. [PMC free article] [PubMed] [Google Scholar]
- 26.Weigel PH, Hascall VC, Tammi M. Hyaluronan synthases. J Biol Chem. 1997;272:13997–4000. doi: 10.1074/jbc.272.22.13997. [DOI] [PubMed] [Google Scholar]
- 27.Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Hirano M, Nakashima T. Fine structure of the human newborn and infant vocal fold mucosae. Annals of Otology Rhinology and Laryngology. 2001;110:417–424. doi: 10.1177/000348940111000505. [DOI] [PubMed] [Google Scholar]
- 29.Gray SD. CELLULAR PHYSIOLOGY OF THE VOCAL FOLDS. Otolaryngol Clin North Am. 2000;33:679–697. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 30.Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: Preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 31.Hargittai I, Hargittai M. Molecular structure of hyaluronan: an introduction. Structural Chemistry. 2008;19:697–717. [Google Scholar]
- 32.Almond A, Brass A, Sheehan JK. Deducing polymeric structure from aqueous molecular dynamics simulations of oligosaccharides: Predictions from simulations of hyaluronan tetrasaccharides compared with hydrodynamic and X-ray fibre diffraction data. J Mol Biol. 1998;284:1425–1437. doi: 10.1006/jmbi.1998.2245. [DOI] [PubMed] [Google Scholar]
- 33.Scott JE, Heatley F. Hyaluronan forms specific stable tertiary structures in aqueous solution: A (13)C NMR study. Proc Natl Acad Sci U S A. 1999;96:4850–4855. doi: 10.1073/pnas.96.9.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowman MK, Matsuoka S. Experimental approaches to hyaluronan structure. Carbohydr Res. 2005;340:791–809. doi: 10.1016/j.carres.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Cowman MK, Feder-Davis J, Hittner DM. (13)C NMR studies of hyaluronan 2 Dependence of conformational dynamics on chain length and solvent. Macromolecules. 2001;34:110–115. [Google Scholar]
- 36.Cowman MK, Spagnoli C, Kudasheva D, Li M, Dyal A, Kanai S, et al. Extended, relaxed, and condensed conformations of hyaluronan observed by atomic force microscopy. Biophys J. 2005;88:590–602. doi: 10.1529/biophysj.104.049361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gribbon P, Heng BC, Hardingham TE. The molecular basis of the solution properties of hyaluronan investigated by confocal fluorescence recovery after photobleaching. Biophys J. 1999;77:2210–2216. doi: 10.1016/S0006-3495(99)77061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008–21. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 39.Chan RW, Titze IR. Viscoelastic shear properties of human vocal fold mucosa: theoretical characterization based on constitutive modeling. J Acoust Soc Am. 2000;107:565–580. doi: 10.1121/1.428354. [DOI] [PubMed] [Google Scholar]
- 40.Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607–614. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 41.Turley EA. HYALURONAN AND CELL LOCOMOTION. Cancer Metastasis Rev. 1992;11:21–30. doi: 10.1007/BF00047600. [DOI] [PubMed] [Google Scholar]
- 42.Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem (Tokyo) 2008;144:131–137. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- 43.Csoka AB, Scherer SW, Stern R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics. 1999;60:356–361. doi: 10.1006/geno.1999.5876. [DOI] [PubMed] [Google Scholar]
- 44.Csoka AB, Frost GI, Heng HH, Scherer SW, Mohapatra G, Stern R. The hyaluronidase gene HYAL1 maps to chromosome 3p21.2-p21.3 in human and 9F1-F2 in mouse, a conserved candidate tumor suppressor locus. Genomics. 1998;48:63–70. doi: 10.1006/geno.1997.5158. [DOI] [PubMed] [Google Scholar]
- 45.Frost GI, Csoka AB, Wong T, Stern R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem Biophys Res Commun. 1997;236:10–15. doi: 10.1006/bbrc.1997.6773. [DOI] [PubMed] [Google Scholar]
- 46.Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20:499–508. doi: 10.1016/s0945-053x(01)00172-x. [DOI] [PubMed] [Google Scholar]
- 47.Lepperdinger G, Strobl B, Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466–22470. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- 48.Rai SK, Duh FM, Vigdorovich V, Danilkovitch-Miagkova A, Lerman MI, Miller AD. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc Natl Acad Sci U S A. 2001;98:4443–4448. doi: 10.1073/pnas.071572898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemming R, Martin DC, Slominski E, Nagy JI, Halayko AJ, Pind S, et al. Mouse Hyal3 encodes a 45- to 56-kDa glycoprotein whose overexpression increases hyaluronidase 1 activity in cultured cells. Glycobiology. 2008;18:280–289. doi: 10.1093/glycob/cwn006. [DOI] [PubMed] [Google Scholar]
- 50.Meyer MF, Kreil G, Aschauer H. The soluble hyaluronidase from bull testes is a fragment of the membrane-bound PH-20 enzyme. FEBS Lett. 1997;413:385–388. doi: 10.1016/s0014-5793(97)00936-8. [DOI] [PubMed] [Google Scholar]
- 51.Hofinger ES, Hoechstetter J, Oettl M, Bernhardt G, Buschauer A. Isoenzyme-specific differences in the degradation of hyaluronic acid by mammalian-type hyaluronidases. Glycoconj J. 2008;25:101–109. doi: 10.1007/s10719-007-9058-8. [DOI] [PubMed] [Google Scholar]
- 52.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and −2. J Biol Chem. 2007;282:5597–5607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 53.Tammi R, Rilla K, Pienimaki JP, MacCallum DK, Hogg M, Luukkonen M, et al. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 54.Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S, et al. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet. 2008;17:1904–1915. doi: 10.1093/hmg/ddn088. [DOI] [PubMed] [Google Scholar]
- 55.Jadin L, Wu X, Ding H, Frost GI, Onclinx C, Triggs-Raine B, et al. Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? Faseb J. 2008;22:4316–4326. doi: 10.1096/fj.08-111997. [DOI] [PubMed] [Google Scholar]
- 56.Kogan G, Šoltés L, Stern R, Mendichi R. Pethrick RA, Ballada A, Zaikov GE, editors. Hyaluronic Acid: A Biopolymer with Versatile Physico-Chemical and Biological Properties. Handbook of Polymer Research: Monomers, Oligomers, Polymers and Composites: Nova Science Publishers, Inc. 2007:393–439. [Google Scholar]
- 57.Gao F, Koenitzer JR, Tobolewski JM, Jiang D, Liang J, Noble PW, et al. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J Biol Chem. 2008;283:6058–6066. doi: 10.1074/jbc.M709273200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moseley R, Waddington RJ, Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta. 1997;31:2–3. doi: 10.1016/s0925-4439(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 60.Osterholt HC, Dannevig I, Wyckoff MH, Liao J, Akgul Y, Ramgopal M, et al. Antioxidant protects against increases in low molecular weight hyaluronan and inflammation in asphyxiated newborn pigs resuscitated with 100% oxygen. PLoS One. 2012;7:11. doi: 10.1371/journal.pone.0038839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hrabarova E, Juranek I, Soltes L. Pro-oxidative effect of peroxynitrite regarding biological systems: a special focus on high-molar-mass hyaluronan degradation. Gen Physiol Biophys. 2011;30:223–238. doi: 10.4149/gpb_2011_03_223. [DOI] [PubMed] [Google Scholar]
- 62.Li M, Rosenfeld L, Vilar RE, Cowman MK. Degradation of hyaluronan by peroxynitrite. Arch Biochem Biophys. 1997;341:245–250. doi: 10.1006/abbi.1997.9970. [DOI] [PubMed] [Google Scholar]
- 63.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: An information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Pandey MS, Baggenstoss BA, Washburn J, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis (HARE) activates NF-kappaB-mediated gene expression in response to 40–400-kDa, but not smaller or larger, hyaluronans. J Biol Chem. 2013;288:14068–14079. doi: 10.1074/jbc.M112.442889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499 doi: 10.1038/nature12234. 346-U122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stern R. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- 67.Turley EA. HYALURONAN-BINDING PROTEINS AND RECEPTORS. Advanced Drug Delivery Reviews. 1991;7:257–264. [Google Scholar]
- 68.Knudson CB, Knudson W. HYALURONAN-BINDING PROTEINS IN DEVELOPMENT, TISSUE HOMEOSTASIS, AND DISEASE. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- 69.Yang B, Yang BL, Savani RC, Turley EA. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. Embo J. 1994;13:286–296. doi: 10.1002/j.1460-2075.1994.tb06261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, et al. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–775. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 71.Amemiya K, Nakatani T, Saito A, Suzuki A, Munakata H. Hyaluronan-binding motif identified by panning a random peptide display library. Biochim Biophys Acta. 2005;1724:94–99. doi: 10.1016/j.bbagen.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 72.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends in Immunology. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Turley EA, Noble PW, Bourguignon LYW. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 74.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Lesley J, English N, Perschl A, Gregoroff J, Hyman R. Variant cell lines selected for alterations in the function of the hyaluronan receptor CD44 show differences in glycosylation. J Exp Med. 1995;182:431–437. doi: 10.1084/jem.182.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isacke CM, Yarwood H. The hyaluronan receptor, CD44. Int J Biochem Cell Biol. 2002;34:718–721. doi: 10.1016/s1357-2725(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 77.Turley EA. Purification of a hyaluronate-binding protein fraction that modifies cell social behavior. Biochem Biophys Res Commun. 1982;108:1016–1024. doi: 10.1016/0006-291x(82)92101-5. [DOI] [PubMed] [Google Scholar]
- 78.Maxwell CA, McCarthy J, Turley E. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 2008;121:925–932. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 79.Maxwell CA, Keats JJ, Crainie M, Sun X, Yen T, Shibuya E, et al. RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol Biol Cell. 2003;14:2262–2276. doi: 10.1091/mbc.E02-07-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turley EA, Austen L, Vandeligt K, Clary C. Hyaluronan and a cell-associated hyaluronan binding protein regulate the locomotion of ras-transformed cells. J Cell Biol. 1991;112:1041–1047. doi: 10.1083/jcb.112.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crainie M, Belch AR, Mant MJ, Pilarski LM. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: identification of three distinct RHAMM variants. Blood. 1999;93:1684–1696. [PubMed] [Google Scholar]
- 82.Jackson DG. The lymphatics revisited - New perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc Med. 2003;13:1–7. doi: 10.1016/s1050-1738(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 83.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends in Immunology. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 85.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, Yancopoulos GD, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Day AJ, Prestwich GD. Hyaluronan-binding proteins: Tying up the giant. J Biol Chem. 2002;277:4585–4588. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe H, Cheung SC, Itano N, Kimata K, Yamada Y. Identification of hyaluronan-binding domains of aggrecan. J Biol Chem. 1997;272:28057–28065. doi: 10.1074/jbc.272.44.28057. [DOI] [PubMed] [Google Scholar]
- 88.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 90.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid: Polycytidylic acid - Inter-alpha-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhuo LS, Hascall VC, Kimata K. Inter-alpha-trypsin inhibitor, a covalent protein-glycosaminoglycan-protein complex. J Biol Chem. 2004;279:38079–38082. doi: 10.1074/jbc.R300039200. [DOI] [PubMed] [Google Scholar]
- 92.Sanggaard KW, Sonne-Schmidt CS, Krogager TP, Kristensen T, Wisniewski HG, Thogersen IB, et al. TSG-6 Transfers Proteins between Glycosaminoglycans via a Ser(28)-mediated Covalent Catalytic Mechanism. J Biol Chem. 2008;283:33919–33926. doi: 10.1074/jbc.M804240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baranova NS, Foulcer SJ, Briggs DC, Tilakaratna V, Enghild JJ, Milner CM, et al. Inter-alpha-inhibitor impairs TSG-6-induced hyaluronan cross-linking. J Biol Chem. 2013;288:29642–29653. doi: 10.1074/jbc.M113.477422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lauer ME, Fulop C, Mukhopadhyay D, Comhair S, Erzurum SC, Hascall VC. Airway smooth muscle cells synthesize hyaluronan cable structures independent of inter-alpha-inhibitor heavy chain attachment. J Biol Chem. 2009;284:5313–5323. doi: 10.1074/jbc.M807979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Milner CM, Tongsoongnoen W, Rugg MS, Day AJ. The molecular basis of inter-alpha-inhibitor heavy chain transfer on to hyaluronan. Biochem Soc Trans. 2007;35:672–676. doi: 10.1042/BST0350672. [DOI] [PubMed] [Google Scholar]
- 96.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Advanced Drug Delivery Reviews. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mukhopadhyay D, Asari A, Rugg MS, Day AJ, Fulop C. Specificity of the tumor necrosis factor-induced protein 6-mediated heavy chain transfer from inter-alpha-trypsin inhibitor to hyaluronan - Implications for the assembly of the cumulus extracellular matrix. J Biol Chem. 2004;279:11119–11128. doi: 10.1074/jbc.M313471200. [DOI] [PubMed] [Google Scholar]
- 98.Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 2008;18:288–295. doi: 10.1016/j.semcancer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 99.Haylock DN, Nilsson SK. The role of hyaluronic acid in hemopoietic stem cell biology. Regenerative Medicine. 2006;1:437–445. doi: 10.2217/17460751.1.4.437. [DOI] [PubMed] [Google Scholar]
- 100.Choudhary M, Zhang X, Stojkovic P, Hyslop L, Anyfantis G, Herbert M, et al. Putative role of hyaluronan and its related genes, HAS2 and RHAMM, in human early preimplantation embryogenesis and embryonic stem cell characterization. Stem Cells. 2007;25:3045–3057. doi: 10.1634/stemcells.2007-0296. [DOI] [PubMed] [Google Scholar]
- 101.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 102.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 103.Lagendijk AK, Szabo A, Merks RMH, Bakkers J. Hyaluronan: A critical regulator of endothelial-to-mesenchymal transition during cardiac valve formation. Trends Cardiovasc Med. 2013;23:135–142. doi: 10.1016/j.tcm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Zhong J, Chan A, Morad L, Kornblum HI, Fan G, Carmichael ST. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24:636–644. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Toole BP, Dealy CN, Kosher RA. Hyaluronan in limb morphogenesis. Dev Biol. 2007;305:411–420. doi: 10.1016/j.ydbio.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, et al. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development. 2009;136:2825–2835. doi: 10.1242/dev.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J, Li Q, Kuehn MR, Litingtung Y, Vokes SA, Chiang C. Sonic hedgehog signaling directly targets Hyaluronic Acid Synthase 2, an essential regulator of phalangeal joint patterning. Dev Biol. 2013;375:160–171. doi: 10.1016/j.ydbio.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen WYJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 109.Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, et al. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, et al. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23:475–484. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 111.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 112.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 113.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 114.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 115.Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, et al. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J Biol Chem. 2007;282:18265–18275. doi: 10.1074/jbc.M606352200. [DOI] [PubMed] [Google Scholar]
- 116.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 117.Abatangelo G, Cortivo R, Martelli M, Vecchia P. CELL DETACHMENT MEDIATED BY HYALURONIC-ACID. Exp Cell Res. 1982;137:73–78. doi: 10.1016/0014-4827(82)90009-x. [DOI] [PubMed] [Google Scholar]
- 118.Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, et al. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol. 2006;169:325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, et al. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 120.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, et al. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–7943. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wisniewski HG, Maier R, Lotz M, Lee S, Klampfer L, Lee TH, et al. TSG-6 - A TNF-, IL-1-, AND LPS-INDUCIBLE SECRETED GLYCOPROTEIN ASSOCIATED WITH ARTHRITIS. J Immunol. 1993;151:6593–6601. [PubMed] [Google Scholar]
- 122.Pasonen-Seppanen S, Hyttinen JMT, Rilla K, Jokela T, Noble PW, Tammi M, et al. Role of CD44 in the organization of keratinocyte pericellular hyaluronan. Histochem Cell Biol. 2012;137:107–120. doi: 10.1007/s00418-011-0883-2. [DOI] [PubMed] [Google Scholar]
- 123.Tolg C, Hamilton SR, Zalinska E, McCulloch L, Amin R, Akentieva N, et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol. 2012;181:1250–1270. doi: 10.1016/j.ajpath.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Toole BP. Hyaluronan: From extracellular glue to pericellular cue. Nature Reviews Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 125.Itano N, Kimata K. Altered hyaluronan biosynthesis in cancer progression. Semin Cancer Biol. 2008;18:268–274. doi: 10.1016/j.semcancer.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 126.Bernert B, Porsch H, Heldin P. Hyaluronan Synthase 2 (HAS2) Promotes Breast Cancer Cell Invasion by Suppression of Tissue Metalloproteinase Inhibitor 1 (TIMP-1) J Biol Chem. 2011;286:4234–4259. doi: 10.1074/jbc.M111.278598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okuda H, Kobayashi A, Xia B, Watabe M, Pai SK, Hirota S, et al. Hyaluronan Synthase HAS2 Promotes Tumor Progression in Bone by Stimulating the Interaction of Breast Cancer Stem-Like Cells with Macrophages and Stromal Cells. Cancer Res. 2012;72:537–547. doi: 10.1158/0008-5472.CAN-11-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stern R. Hyaluronan degradation in tumor growth and metastasis. Trends in Glycoscience and Glycotechnology. 2004;16:171–185. [Google Scholar]
- 129.Bharadwaj AG, Rector K, Simpson MA. Inducible hyaluronan production reveals differential effects on prostate tumor cell growth and tumor angiogenesis. J Biol Chem. 2007;282:20561–20572. doi: 10.1074/jbc.M702964200. [DOI] [PubMed] [Google Scholar]
- 130.Gao F, Yang CX, Mo W, Liu YW, He YQ. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin Invest Med. 2008;31:E106–E116. doi: 10.25011/cim.v31i3.3467. [DOI] [PubMed] [Google Scholar]
- 131.Gao F, Okunieff P, Han Z, Ding I, Wang L, Liu W, et al. Hypoxia-induced alterations in hyaluronan and hyaluronidase. Adv Exp Med Biol. 2005;566:249–256. doi: 10.1007/0-387-26206-7_33. [DOI] [PubMed] [Google Scholar]
- 132.De Maeyer E, De Maeyer-Guignard J. The growth rate of two transplantable murine tumors, 3LL lung carcinoma and B16F10 melanoma, is influenced by Hyal-1, a locus determining hyaluronidase levels and polymorphism. Int J Cancer. 1992;51:657–660. doi: 10.1002/ijc.2910510425. [DOI] [PubMed] [Google Scholar]
- 133.Wang F, Grigorieva EV, Li J, Senchenko VN, Pavlova TV, Anedchenko EA, et al. HYAL1 and HYAL2 inhibit tumour growth in vivo but not in vitro. PLoS ONE. 2008;3:e3031. doi: 10.1371/journal.pone.0003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aboughalia AH. Elevation of hyaluronidase-1 and soluble intercellular adhesion molecule-1 helps select bladder cancer patients at risk of invasion. Arch Med Res. 2006;37:109–116. doi: 10.1016/j.arcmed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 135.Kramer MW, Golshani R, Merseburger AS, Knapp J, Garcia A, Hennenlotter J, et al. HYAL-1 Hyaluronidase: A Potential Prognostic Indicator for Progression to Muscle Invasion and Recurrence in Bladder Cancer. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ekici S, Cerwinka WH, Duncan R, Gomez P, Civantos F, Soloway MS, et al. Comparison of the prognostic potential of hyaluronic acid, hyaluronidase (HYAL-1), CD44v6 and microvessel density for prostate cancer. Int J Cancer. 2004;112:121–129. doi: 10.1002/ijc.20368. [DOI] [PubMed] [Google Scholar]
- 137.Junker N, Latini S, Petersen LN, Kristjansen PE. Expression and regulation patterns of hyaluronidases in small cell lung cancer and glioma lines. Oncol Rep. 2003;10:609–616. [PubMed] [Google Scholar]
- 138.Patel S, Turner PR, Stubberfield C, Barry E, Rohlff CR, Stamps A, et al. Hyaluronidase gene profiling and role of hyal-1 overexpression in an orthotopic model of prostate cancer. Int J Cancer. 2002;97:416–424. doi: 10.1002/ijc.1638. [DOI] [PubMed] [Google Scholar]
- 139.Paiva P, Van Damme MP, Tellbach M, Jones RL, Jobling T, Salamonsen LA. Expression patterns of hyaluronan, hyaluronan synthases and hyaluronidases indicate a role for hyaluronan in the progression of endometrial cancer. Gynecol Oncol. 2005;98:193–202. doi: 10.1016/j.ygyno.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 140.Udabage L, Brownlee GR, Nilsson SK, Brown TJ. The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp Cell Res. 2005;310:205–217. doi: 10.1016/j.yexcr.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 141.Novak U, Stylli SS, Kaye AH, Lepperdinger G. Hyaluronidase-2 overexpression accelerates intracerebral but not subcutaneous tumor formation of murine astrocytoma cells. Cancer Res. 1999;59:6246–6250. [PubMed] [Google Scholar]
- 142.Christopoulos TA, Papageorgakopoulou N, Theocharis DA, Mastronikolis NS, Papadas TA, Vynios DH. Hyaluronidase and CD44 hyaluronan receptor expression in squamous cell laryngeal carcinoma. Biochim Biophys Acta. 2006;1760:1039–1045. doi: 10.1016/j.bbagen.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 143.Godin DA, Fitzpatrick PC, Scandurro AB, Belafsky PC, Woodworth BA, Amedee RG, et al. PH20: a novel tumor marker for laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2000;126:402–404. doi: 10.1001/archotol.126.3.402. [DOI] [PubMed] [Google Scholar]
- 144.Madan AK, Yu K, Dhurandhar N, Cullinane C, Pang Y, Beech DJ. Association of hyaluronidase and breast adenocarcinoma invasiveness. Oncol Rep. 1999;6:607–609. doi: 10.3892/or.6.3.607. [DOI] [PubMed] [Google Scholar]
- 145.Wang LP, Xu XM, Ning HY, Yang SM, Chen JG, Yu JY, et al. [Expression of PH20 in primary and metastatic breast cancer and its pathological significance] Zhonghua Bing Li Xue Za Zhi. 2004;33:320–323. [PubMed] [Google Scholar]
- 146.Beech DJ, Madan AK, Deng N. Expression of PH-20 in normal and neoplastic breast tissue. J Surg Res. 2002;103:203–207. doi: 10.1006/jsre.2002.6351. [DOI] [PubMed] [Google Scholar]
- 147.Ouhtit A, Abd Elmageed ZY, Abdraboh ME, Lioe TF, Raj MH. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am J Pathol. 2007;171:2033–2039. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu Y, Yu Q. E-cadherin negatively regulates CD44-hyaluronan interaction and CD44-mediated tumor invasion and branching morphogenesis. J Biol Chem. 2003;278:8661–8668. doi: 10.1074/jbc.M208181200. [DOI] [PubMed] [Google Scholar]
- 149.Peng ST, Su CH, Kuo CC, Shaw CF, Wang HS. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int J Oncol. 2007;31:1119–1126. [PubMed] [Google Scholar]
- 150.Tzircotis G, Thorne RF, Isacke CM. Chemotaxis towards hyaluronan is dependent on CD44 expression and modulated by cell type variation in CD44-hyaluronan binding. J Cell Sci. 2005;118:5119–5128. doi: 10.1242/jcs.02629. [DOI] [PubMed] [Google Scholar]
- 151.Kuo YC, Su CH, Liu CY, Chen TH, Chen CP, Wang HS. Transforming growth factor-beta induces CD44 cleavage that promotes migration of MDA-MB-435s cells through the up-regulation of membrane type 1-matrix metalloproteinase. Int J Cancer. 2009;124:2568–2576. doi: 10.1002/ijc.24263. [DOI] [PubMed] [Google Scholar]
- 152.Sugahara KN, Hirata T, Tanaka T, Ogino S, Takeda M, Terasawa H, et al. Chondroitin sulfate E fragments enhance CD44 cleavage and CD44-dependent motility in tumor cells. Cancer Res. 2008;68:7191–7199. doi: 10.1158/0008-5472.CAN-07-6198. [DOI] [PubMed] [Google Scholar]
- 153.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–1172. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 155.Bourguignon LY, Singleton PA, Zhu H, Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem. 2003;278:29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 156.Murai T, Miyazaki Y, Nishinakamura H, Sugahara KN, Miyauchi T, Sako Y, et al. Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J Biol Chem. 2004;279:4541–4550. doi: 10.1074/jbc.M307356200. [DOI] [PubMed] [Google Scholar]
- 157.Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. 2002;528:101–108. doi: 10.1016/s0014-5793(02)03262-3. [DOI] [PubMed] [Google Scholar]
- 158.Craig EA, Parker P, Camenisch TD. Size dependent regulation of Snail2 by hyaluronan: its role in cellular invasion. Glycobiology. 2009 doi: 10.1093/glycob/cwp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp Mol Pathol. 2009;86:95–100. doi: 10.1016/j.yexmp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 160.Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC. Expression of the CD44v2–10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res. 2003;63:887–892. [PubMed] [Google Scholar]
- 161.Iczkowski KA, Omara-Opyene AL, Shah GV. The predominant CD44 splice variant in prostate cancer binds fibronectin, and calcitonin stimulates its expression. Anticancer Res. 2006;26:2863–2872. [PubMed] [Google Scholar]
- 162.Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, et al. Cortactin underpins CD44-promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 163.Li H, Guo L, Li J, Liu N, Liu J. Alternative splicing of RHAMM gene in chinese gastric cancers and its in vitro regulation. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2000;17:343–347. [PubMed] [Google Scholar]
- 164.Zlobec I, Baker K, Minoo P, Jass JR, Terracciano L, Lugli A. Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein expression. Clin Cancer Res. 2008;14:143–148. doi: 10.1158/1078-0432.CCR-07-1380. [DOI] [PubMed] [Google Scholar]
- 165.Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994;126:575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gares SL, Pilarski LM. Balancing thymocyte adhesion and motility: a functional linkage between beta1 integrins and the motility receptor RHAMM. Dev Immunol. 2000;7:209–225. doi: 10.1155/2000/94616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hall CL, Lange LA, Prober DA, Zhang S, Turley EA. pp60(c-src) is required for cell locomotion regulated by the hyaluronanreceptor RHAMM. Oncogene. 1996;13:2213–2224. [PubMed] [Google Scholar]
- 168.Hamilton SR, Fard SF, Paiwand FF, Tolg C, Veiseh M, Wang C, et al. The hyaluronan receptors CD44 and Rhamm (CD168) form complexes with ERK1,2 that sustain high basal motility in breast cancer cells. J Biol Chem. 2007;282:16667–16680. doi: 10.1074/jbc.M702078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin SL, Chang D, Chiang A, Ying SY. Androgen receptor regulates CD168 expression and signaling in prostate cancer. Carcinogenesis. 2008;29:282–290. doi: 10.1093/carcin/bgm259. [DOI] [PubMed] [Google Scholar]
- 170.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem 2. 001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 171.Thompson CB, Shepard HM, O’Connor PM, Kadhim S, Jiang P, Osgood RJ, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 172.Brown TJ. The development of hyaluronan as a drug transporter and excipient for chemotherapeutic drugs. Curr Pharm Biotechnol. 2008;9:253–260. doi: 10.2174/138920108785161514. [DOI] [PubMed] [Google Scholar]
- 173.Gibbs P, Clingan PR, Ganju V, Strickland AH, Wong SS, Tebbutt NC, et al. Hyaluronan-Irinotecan improves progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer: a randomized phase II trial. Cancer Chemother Pharmacol. 2011;67:153–163. doi: 10.1007/s00280-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 174.Jia X, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol Biosci. 2009;9:140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuo JW, Prestwich GD. Ducheyne P, Healy K, Hutmacher D, Kirkpatrick J, editors. Hyaluronic Acid. Materials of Biological Origin - Materials Analysis and Implant Uses, Comprehensive Biomaterials: Elsevier. 2011:239–259. Chapter 73. [Google Scholar]
- 176.Jia X, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 177.Sahiner N, Jha AK, Nguyen D, Jia X. Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration. J Biomater Sci Polym Ed. 2008;19:223–243. doi: 10.1163/156856208783432462. [DOI] [PubMed] [Google Scholar]
- 178.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, et al. Structural Analysis and Mechanical Characterization of Hyaluronic Acid-Based Doubly Cross-Linked Networks. Macromolecules. 2009;42:537–546. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Farran AJE, Teller SS, Jha AK, Jiao T, Hule RA, Clifton RJ, et al. Effects of Matrix Composition, Microstructure, and Viscoelasticity on the Behaviors of Vocal Fold Fibroblasts Cultured in Three-Dimensional Hydrogel Networks. Tissue Eng. 2010;16:1247–1261. doi: 10.1089/ten.tea.2009.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jha AK, Malik MS, Farach-Carson MC, Duncan RL, Jia X. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter. 2010;6:5045–5055. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Srinivasan PP, McCoy SY, Jha AK, Yang WD, Jia XQ, Farach-Carson MC, et al. Injectable perlecan domain 1-hyaluronan microgels potentiate the cartilage repair effect of BMP2 in a murine model of early osteoarthritis. Biomedical Materials. 2012:7. doi: 10.1088/1748-6041/7/2/024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pradhan S, Zhang C, Jia X, Carson DD, Witt R, Farach-Carson MC. Perlecan domain IV peptide stimulates salivary gland cell assembly in vitro. Tissue Eng Part A. 2009;15:3309–3320. doi: 10.1089/ten.tea.2008.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pradhan-Bhatt S, Harrington DA, Duncan RL, Jia XQ, Witt RL, Farach-Carson MC. Implantable Three-Dimensional Salivary Spheroid Assemblies Demonstrate Fluid and Protein Secretory Responses to Neurotransmitters. Tissue Eng. 2013;19:1610–1620. doi: 10.1089/ten.tea.2012.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC. Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials. 2009;30:6076–6085. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gurski LA, Xu X, Labrada LN, Nguyen NT, Xiao LX, van Golen KL, et al. Hyaluronan (HA) Interacting Proteins RHAMM and Hyaluronidase Impact Prostate Cancer Cell Behavior and Invadopodia Formation in 3D HA-Based Hydrogels. Plos One. 2012:7. doi: 10.1371/journal.pone.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xu X, Gurski LA, Zhang C, Harrington DA, Farach-Carson MC, Jia XQ. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials. 2012;33:9049–9060. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jiao T, Farran A, Jia X, Clifton RJ. High Frequency Measurements of Viscoelastic Properties of Hydrogels for Vocal Fold Regeneration. Experimental Mechanics. 2009;49:235–246. doi: 10.1007/s11340-008-9126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Teller SS, Farran AJE, Xiao LX, Jiao T, Duncan RL, Clifton RJ, et al. High-Frequency Viscoelastic Shear Properties of Vocal Fold Tissues: Implications for Vocal Fold Tissue Engineering. Tissue Eng. 2012;18:2008–2019. doi: 10.1089/ten.tea.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jia XQ, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–3344. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 190.Jha AK, Yang W, Kirn-Safran CB, Farach-Carson MC, Jia X. Perlecan domain I-conjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–6975. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krishna OD, Jha AK, Jia X, Kiick KL. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials. 2011;32:6412–6424. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jha AK, Xu X, Duncan RL, Jia X. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32:2466–2478. doi: 10.1016/j.biomaterials.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiao LX, Liu C, Zhu JH, Pochan DJ, Jia XQ. Hybrid, elastomeric hydrogels crosslinked by multifunctional block copolymer micelles. Soft Matter. 2010;6:5293–5297. doi: 10.1039/C0SM00511H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xiao LX, Zhu JH, Londono JD, Pochan DJ, Jia XQ. Mechano-responsive hydrogels crosslinked by block copolymer micelles. Soft Matter. 2012;8:10233–10237. doi: 10.1039/C2SM26566D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xiao L, Tong Z, Chen Y, Pochan DJ, Sabanayagam CR, Jia X. Hyaluronic Acid-based hydrogels containing covalently integrated drug depots: implication for controlling inflammation in mechanically stressed tissues. Biomacromolecules. 2013;14:3808–3819. doi: 10.1021/bm4011276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu X, Sabanayagam CR, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic Acid Hydrogel-Based Tumor Models for the Efficacy Evaluation of Polymeric Nano-Therapeutics. Manuscript in preparation. 2013 [Google Scholar]
- 197.Larsen NE, Balazs EA. DRUG DELIVERY SYSTEMS USING HYALURONAN AND ITS DERIVATIVES. Advanced Drug Delivery Reviews. 1991;7:279–293. [Google Scholar]
- 198.Prestwich GD. Evaluating drug efficacy and toxicology in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 199.Fakhari A, Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomaterialia. 2013;9:7081–7092. doi: 10.1016/j.actbio.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogen for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brown TJ. The development of hyaluronan as a drug transporter and excipient for chemotherapeutic drugs. Current Pharmaceutical Biotechnology. 2008;9:253–260. doi: 10.2174/138920108785161514. [DOI] [PubMed] [Google Scholar]
- 202.Kim J, Kim KS, Jiang G, Kang H, Kim S, Kim BS, et al. In Vivo Real-Time Bioimaging of Hyaluronic Acid Derivatives Using Quantum Dots. Biopolymers. 2008;89:1144–1153. doi: 10.1002/bip.21066. [DOI] [PubMed] [Google Scholar]
- 203.Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and metaanalysis. Ann Intern Med. 2012;157:180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 204.Kosaki R, Watanabe K, Yamaguchi Y. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 1999;59:1141–1145. [PubMed] [Google Scholar]
- 205.Mueller BM, Schraufstatter IU, Goncharova V, Povaliy T, DiScipio R, Khaldoyanidi SK. Hyaluronan inhibits postchemotherapy tumor regrowth in a colon carcinoma xenograft model. Mol Cancer Ther. 2010;9:3024–3032. doi: 10.1158/1535-7163.MCT-10-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science; 2002. [Google Scholar]