Abstract
Background
Neurofibromatosis Type 1 (NF1) is a genetic disorder resulting from mutations in the NF1 tumor suppressor gene. Neurofibromin, the protein product of NF1, functions as a negative regulator of Ras activity in circulating hematopoietic and vascular wall cells, which are critical for maintaining vessel wall homeostasis. NF1 patients have evidence of chronic inflammation resulting in development of premature cardiovascular disease, including arterial aneurysms, which may manifest as sudden death. However, the molecular pathogenesis of NF1 aneurysm formation is unknown.
Method and Results
Utilizing an angiotensin II-induced aneurysm model, we demonstrate that heterozygous inactivation of Nf1 (Nf1+/−) enhanced aneurysm formation with myeloid cell infiltration and increased oxidative stress in the vessel wall. Using lineage-restricted transgenic mice, we show loss of a single Nf1 allele in myeloid cells is sufficient to recapitulate the Nf1+/− aneurysm phenotype in vivo. Finally, oral administration of simvastatin or the antioxidant apocynin, reduced aneurysm formation in Nf1+/− mice.
Conclusion
These data provide genetic and pharmacologic evidence that Nf1+/− myeloid cells are the cellular triggers for aneurysm formation in a novel model of NF1 vasculopathy and provide a potential therapeutic target.
Keywords: genetics, transgenic models, aneurysm, leukocyte, statin intervention, inflammation
Neurofibromatosis Type 1 (NF1) is an autosomal dominant genetic disorder resulting from mutations in the NF1 gene. NF1 encodes the protein neurofibromin, which negatively regulates p21Ras (Ras) activity via stimulation of its GTPase function.1 Germline mutations causing NF1 affect only one NF1 allele, although loss of heterozygosity is described in primary tumor samples from NF1 patients.2 Thus, haploinsufficiency of NF1 results in disease with complete penetrance and diverse clinical manifestations in different organ systems.
Common non-neoplastic manifestations of NF1 include cognitive disorders and skeletal abnormalities,2 while cardiovascular disease (CVD) is a serious but under-recognized complication, contributing to significant increases in morbidity and premature mortality.3, 4 In particular, the aorta and proximal branches demonstrate increased aneurysm formation and exaggerated intimal hyperplasia.5 The frequency of NF1 vasculopathy is difficult to define due to a lack of routine screening; however, the prevalence of vascular lesions in large clinical series approaches 7 percent.5–7 Specifically, a study of 31 NF1 patients with a diagnosis of vascular disease identified 38 aneurysms among the group, with an average age at diagnosis of 38 years (range: 3–77).5
Studies utilizing mouse models that recapitulate NF1 vasocclusive disease revealed that neurofibromin-deficient myeloid cells and vascular smooth muscle cells (VSMCs) cooperate to induce neointima hyperplasia after arterial injury.8–10 Correlative studies demonstrate that NF1 patients have evidence of chronic inflammation and mobilization of a specific monocyte subset in their peripheral blood that is linked to vasocclusive disease progression and aneurysm formation in non-NF1 patients with CVD.8, 11 Despite these observations, the pathogenesis of NF1 aneurysm disease is unknown, partly due to a lack of animal models that mimic the human disease. Given the mostly silent presentation of aneurysms in NF1 patients and the potential for catastrophic rupture, understanding disease pathogenesis is critical for aneurysm prevention, early detection, and treatment.
In this study, we utilized an established mouse model of aneurysm formation and cell lineage-restricted transgenic mice to test the role of Nf1 haploinsufficiency (Nf1+/−) in various cell types on aneurysm formation. We provide evidence that heterozygous inactivation of Nf1 directly contributes to larger and more severe aneurysms with enhanced oxidative species production and matrix metalloproteinase-9 (MMP-9) activation. Further, lineage-restricted inactivation of a single Nf1 gene copy in myeloid cells, but not VSMCs, is sufficient for aneurysm formation, thereby implicating Nf1+/− myeloid cells as the cellular triggers for Nf1+/− aneurysm formation in vivo. Finally, we provide pharmacologic evidence that aneurysm formation in Nf1+/− mice is abrogated by daily low-dose administration of the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor simvastatin, which has antioxidant and anti-inflammatory effects. To further delineate the pharmacologic effects of simvastatin, Nf1+/− mice were treated with the antioxidant apocynin, which also reduced aneurysm formation. Thus, we generated a novel model of NF1-associated aneurysmal disease and provide genetic evidence that Nf1+/− myeloid cells are critical mediators of aneurysm formation via an antioxidant-sensitive pathway, suggesting a potential therapeutic target.
Methods
Animals
All protocols were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. Nf1+/− mice were obtained from Tyler Jacks (Massachusetts Institute of Technology, Cambridge) and backcrossed 13 generations into the C57BL/6J strain. Nf1flox/flox mice were obtained from Luis Parada (University of Texas Southwestern Medical Center, Dallas) and backcrossed 13 generations into the 129SvJ strain. LysMcre (stock 4781) and SM22cre (stock 4746) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Nf1fl/fl mice were inter-crossed with LysMcre or SM22cre mice to generate F1 C57BL/6 × 129SvJ progeny. Cre-mediated recombination was confirmed by PCR.12 LacZ lineage tracing of aortas from Nf1fl/+;Rosa26fl/+;Sm22cre mice revealed staining limited to the vessel media where VSMCs reside (data not shown). LacZ lineage tracing of aortas from Nf1fl/+;Rosa26fl/+;LysMcre mice revealed sparse staining limited to the vessel adventitia (data not shown). Nf1fl/fl 129SvJ mice inter-crossed with Nf1+/− C57BL/6 mice generated F1 Nf1+/− and WT control animals. Genotyping was performed as previously described.9
Angiotensin II-infusion Abdominal Aortic Aneurysm (AAA) model
12 week-old control and experimental male mice were infused with Angiotensin II (AngII, 1500 ng/kg/min, Calbiochem) or saline for 35 days, as described,13 with modification. Animals were anesthetized by inhalation of 2% isoflurane, and an osmotic pump (2006, Durect Corporation) containing AngII or saline was implanted subcutaenously. At indicated time points, a portion from the aortic arch to the iliac arteries was excised for analysis.
Classification and quantification of aneurysms
Images of arteries were obtained on a stereo-microscope (Carl Zeiss Inc). The maximum external arterial diameters were measured using Metamorph 6.1 (Universal Imaging Systems Corp.). Aneurysms were defined as an increase in the external aortic diameter of >/= 50% as compared to control animals. Aortic aneurysm severity wasrated from Type 0 to Type IV according to the method of Martin-McNulty et al.14 with modification: Type 0, no aneurysm; Type I, dilation 1.5 to 2 times the diameter of a normal artery; Type II, a single dilation that is more than 2 times the diameter of a normal artery; Type III, multiple dilations with the largest being 1.5 to 2 times the diameter of a normal artery; and Type IV, multiple dilations with the largest being more than 2 times the diameter of a normal artery.
Simvastatin and apocynin administration
Simvastatin (1 mg/kg/day, Besse) was administered in water via oral gavage beginning 7 days prior to AngII or saline infusion and continued throughout the experiment. Apocynin (also known as acetovanillone, 100 mg/kg/day, Acros Organics) was administered in drinking water beginning 7 days prior to AngII or saline infusion and continued throughout the experiment. Control mice received water at a similar volume.
Statistical analysis
Quantitative results are shown as mean ± SEM. All statistical analyses were performed using Prism 5 (GraphPad Software). P values were obtained by the unpaired-Student’s t-test when comparing 2 groups and by one-way analysis of Variance (ANOVA) followed by Tukey’s analysis when comparing 3 or more groups. P<0.05 were considered significant. To determine significance of categorical data, Fisher’s exact test was used with Bonferonni correction.
Results
Heterozygous inactivation of Nf1 amplifies the incidence and severity of aneurysm formation in angiotensin II-infused mice
To test the hypothesis that Nf1 heterozygosity enhances aneurysm formation in vivo, wild-type (WT) and Nf1+/− mice were infused with AngII to induce aneurysms. Infusion of AngII induces inflammatory mediators and reactive oxygen species (ROS) within the arterial wall, producing abdominal aortic aneurysms (AAA) that recapitulate human lesions.15
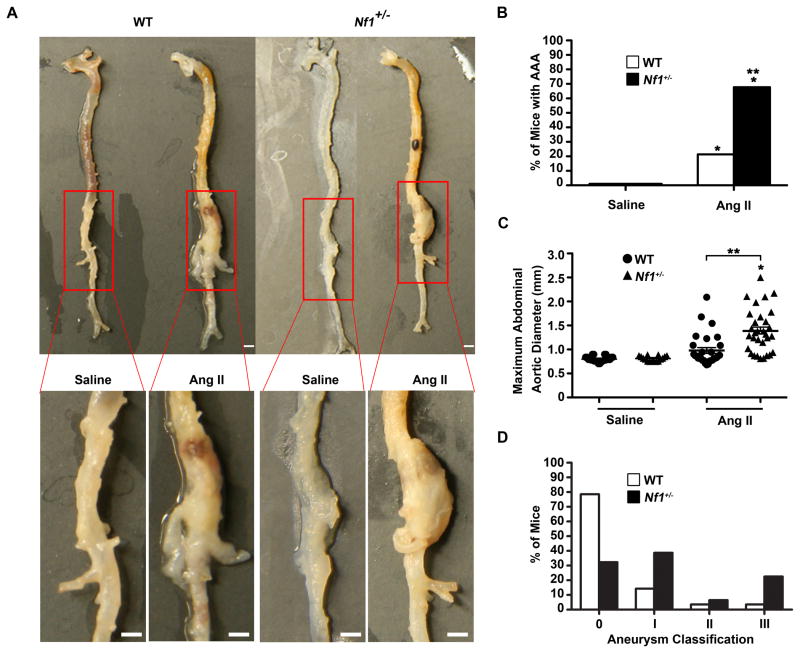
Infusion of AngII increased aneurysm formation 3-fold in Nf1+/− mice compared to WT mice (Figure 1A and 1B). Morphometric analysis of abdominal aortas from both genotypes revealed that AngII-infused Nf1+/− mice had significantly larger aneurysms (Figure 1C and 2A). Further, Nf1+/− aneurysms were more severe than WT aneurysms quantitatively, with increased degradation of the elastic lamina and disorganized architecture, which is reminiscent of lesions from NF1 patients (Figure 1D, 2B and 2D).16 Importantly, AngII infusion did not alter body weight or intra-arterial blood pressure in either genotype (data not shown). Saline-infused Nf1+/− and WT mice did not form aneurysms (Figure 1A, 1B and 2A). These data indicate Nf1 heterozygosity augments AngII-induced aneurysm formation.
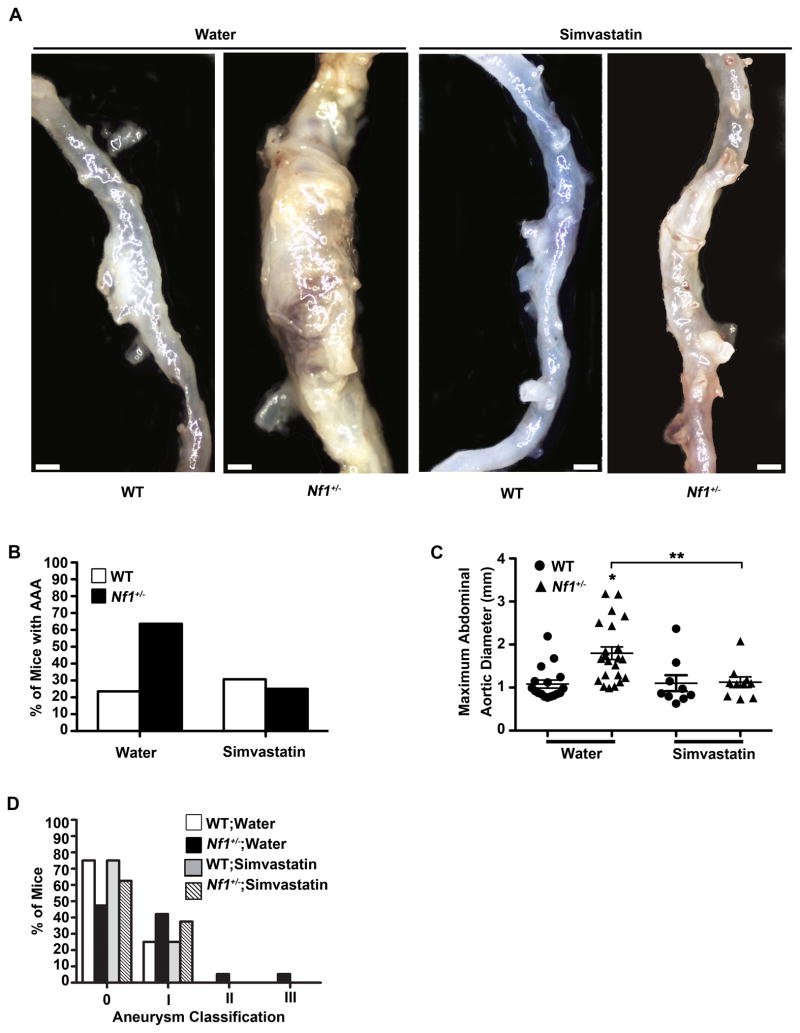
Figure 1.
Nf1+/− mice have enhanced AngII-induced AAA formation. (A) Representative photographs of the aorta and branches from saline or AngII-infused WT and Nf1+/− mice. Boxes identify area magnified in lower panel. Scale bars: 1mm. (B) Quantification of aneurysm incidence. *P<0.0083 for saline-infused WT (n=24) versus AngII-infused WT (n=29) and AngII-infused Nf1+/− (n=31). **P<0.0083 for saline-infused Nf1+/− (n=16) versus AngII-infused Nf1+/−. Analysis by Fisher’s exact test with Bonferonni correction. (C) Maximum abdominal aortic diameter of saline or AngII-infused WT and Nf1+/− mice. Clustering around 1mm represents animals without aneurysm formation. *P<0.05 for saline-infused Nf1+/− (n=16) versus AngII-infused Nf1+/− (n=31). **P<0.05 for AngII-infused WT (n=29) versus AngII-infused Nf1+/−(n=31). No statistical significance was observed for saline-infused WT (n=24) versus AngII-infused WT (n=29). Analysis by one-way ANOVA with Tukey’s test. Error bars denote the mean ± SEM. (D) Aneurysm severity for AngII-infused WT (n=29) or AngII-infused Nf1+/− (n=31) mice. No aneurysms formed in saline-infused mice of either genotype.
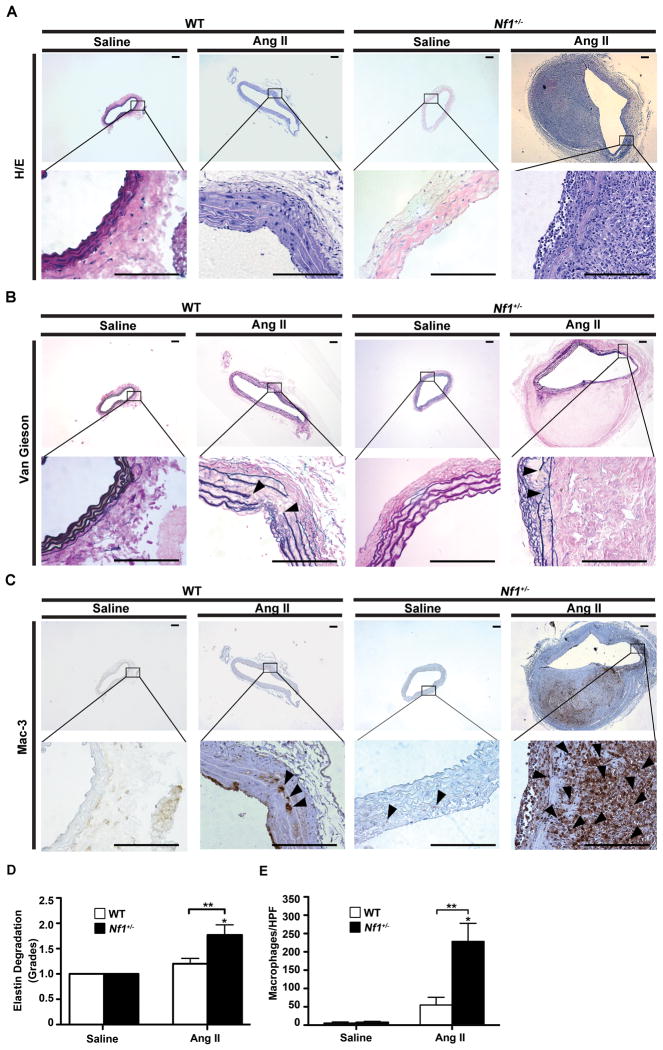
Figure 2.
Histological and morphometric analysis of abdominal aortas from WT and Nf1+/− mice. (A) Representative photomicrographs of abdominal aortic cross-sections from saline and AngII-infused WT and Nf1+/− mice stained with H&E, (B) van Gieson or (C) anti-Mac-3 antibody (arrowheads). Boxed areas magnified in lower panel. Arrowheads in B indicate elastic lamina fragmentation. Scale bars: 50μm. (D) Grading of elastic lamina degradation in saline or AngII-infused WT and Nf1+/− mice. *P<0.05 for saline-infused Nf1+/− (n=5) or WT (n=5) versus AngII-infused Nf1+/− mice (n=13). **P<0.05 for AngII-infused WT (n=15) versus AngII-infused Nf1+/− mice. No statistical significance was observed for saline-infused WT or Nf1+/− versus AngII-infused WT. (E) Quantification of Mac3-positive macrophages per high-power field (HPF) in saline or AngII-infused WT and Nf1+/− mice. *P < 0.05 for saline-infused Nf1+/− (n=5) versus AngII-infused Nf1+/− mice (n=5). **P<0.05 for AngII-infused WT (n=5) versus AngII-infused Nf1+/− mice. No statistical significance was observed for saline-infused WT (n=5) versus AngII-infused WT. Analysis by one-way ANOVA with Tukey’s test.
Nf1+/− aneurysms are characterized by inflammatory cell infiltration, VSMC expansion, and ROS production
NF1 patients and Nf1+/− mice have increased populations of circulating inflammatory monocytes and pro-inflammatory cytokines linked to aneurysm formation.8, 9 Therefore, we sought to characterize the cellular and structural composition of WT and Nf1+/− aneurysms. Histologic examination of Nf1+/− aneurysms demonstrated significant dilation and degradation of the aorta, including increased disruption of the elastic lamina and advential expansion when compared to WT aneurysms (Figure 2A through 2E). Importantly, Nf1+/− aneurysms contained 4-times the number of macrophages compared to WT (Figure 2C and 2E). Infiltrating T cells, mast cells, and neutrophils were increased in both Nf1+/− and WT aneurysms, but accounted for less than 5% of all cells and did not differ between genotypes (data not shown). Importantly, Nf1+/− macrophages co-localized to sites of elastic lamina degradation, medial rupture and adventitial expansion, indicating their potential role in aneurysm formation (Figure 2B and 2C). AngII infusion also induced a significant expansion of VSMCs in the media of Nf1+/− aortas compared to WT (Supplemental Figure 1A and 1B). Nf1+/− VMSC expansion is consistent with previous findings that Nf1+/− VSMCs exhibit increased proliferation and migration in response to cytokines secreted by macrophages and vascular wall cells implicated in CVD.9, 17 Finally, co-staining with α-smooth muscle actin and anti-Mac-3 illustrated VSMC expansion was within and near the vessel media while macrophage infiltration was primarily in the adventia (Supplemental Figure 1C).
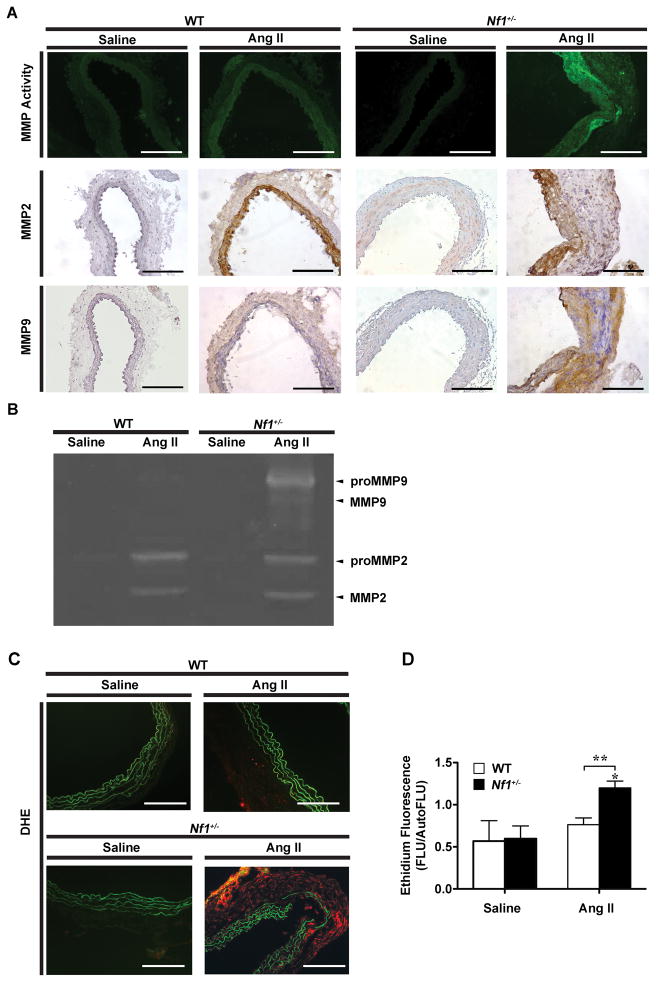
Genetic studies demonstrate that VSMC and macrophage secretion of MMPs and ROS are important molecular triggers for extracellular matrix (ECM) remodeling and aneurysm induction.18 Given the increased density of VSMCs and macrophages in Nf1+/− aneurysms, we measured expression and activation of MMP-2 and MMP-9 in aortas harvested from Nf1+/− and WT mice infused with AngII or saline. AngII infusion increased both MMP-2 and MMP-9 activity and preferentially amplified MMP-9 expression in Nf1+/− aneurysms when compared with AngII-infused WT aneurysms as determined by in situ zymography and immunohistochemistry (IHC) (Figure 3A). In addition, gelatin zymography of abdominal aortic explants from AngII-infused WT and Nf1+/− mice corroborated increases of MMP-2 and MMP-9 in both genotypes with significantly enhanced activation of MMP-9 in Nf1+/− aortas (Figure 3B). Amplified MMP-9 expression in Nf1+/− aortas is an important observation since MMP-9 is largely derived from vessel wall macrophages,19 which is consistent with the increased macrophage infiltration observed in Nf1+/− aneurysms.
Figure 3.
AngII induces MMP-9 expression and activity and ROS production in Nf1+/− mice. (A) Representative photomicrographs of abdominal aortic cross-sections from saline and AngII-infused WT and Nf1+/− mice. MMP activity (green) was visualized by in situ zymography and expression of MMP-2 and MMP-9 was detected by IHC staining with anti-MMP-2 (brown) and anti-MMP-9 (brown) antibodies. Scale bars: 50μm. (B) Representative zymogram showing abdominal aortic MMP-2 and MMP-9 levels for saline and AngII-infused WT and Nf1+/− mice. (C) Representative photomicrographs of abdominal aortic cross-sections from saline or AngII-infused WT and Nf1+/− mice, showing superoxide production identified by in situ DHE staining (red). Auto-fluorescence of murine tissue is visible (green). (D) Quantification of ethidium fluorescence. *P<0.05 for AngII-infused Nf1+/− (n=9) versus saline-infused WT (n=5) and Nf1+/−(n=3). **P<0.05 for AngII-infused Nf1+/− versus AngII-infused WT (n=8).
We next assessed ROS production in abdominal aortic cross-sections from AngII and saline-infused Nf1+/− and WT mice with dihydroethidium (DHE), a superoxide probe. AngII infusion significantly increased superoxide production in Nf1+/− aortas when compared with WT aortas, while superoxide production was nearly undetectable in the aortas from saline-infused Nf1+/− and WT aortas (Figure 3C and 3D). Collectively, these data demonstrate that Nf1 heterozygous aneurysms have evidence of increased inflammatory cell infiltration, MMP activation, and ROS production, which are linked to abnormal arterial remodeling and disease progression.
Heterozygous inactivation of Nf1 in myeloid cells alone is sufficient to recapitulate Nf1+/− aneurysm formation
Nf1+/− mice have increased aneurysm formation characterized by increased macrophages and VSMCs and their secretory products that promote disease progression. Based on these observations, we generated transgenic mice with a single copy of the Nf1 gene ablated in VMSCs or myeloid cells alone to determine the role of Nf1 heterozygosity in VSMCs and macrophages on aneurysm formation. Briefly, Nf1fl/fl mice containing conditional Nf1 alleles susceptible to Cre-mediated recombination were inter-crossed with SM22cre or LysMcre transgenic mice, generating Nf1fl/+;SM22cre and Nf1fl/+;LysMcre progeny. Nf1fl/fl mice underwent efficient Cre-mediated recombination when crossed with LysMcre (Supplemental Figure 2F) or SM22cre mice.10 LysMcre-mediated recombination was seen in the aortic adventia, a known location for macrophages, while the aortic media showed minimal recombination.
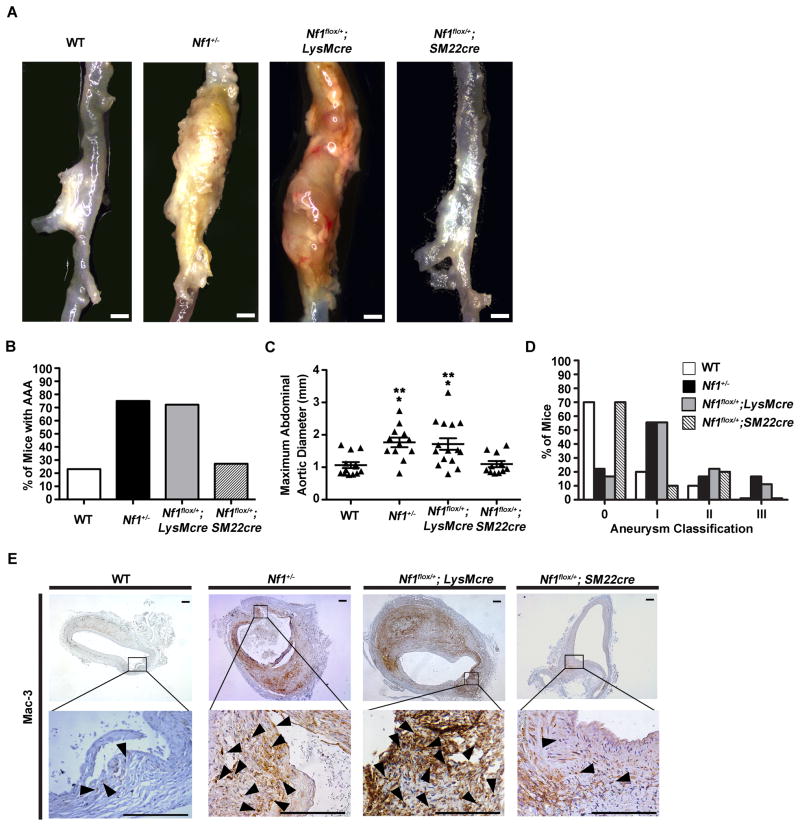
Nf1fl/+;SM22cre and Nf1fl/+;LysMcre mice were infused with AngII or saline and evaluated for aneurysm formation along with littermate controls (WT and Nf1+/− mice). Nf1fl/+;LysMcre mice infused with AngII developed large aneurysms recapitulating the phenotype of Nf1+/− mice, while Nf1fl/+;SM22cre mice produced less severe aneurysms similar to WT mice (Figure 4A through 4D). Specifically, a 2.5-fold increase in AAA incidence was observed in Nf1fl/+;LysMcre mice compared with Nf1fl/+;SM22cre and WT mice (Figure 4B). Saline infusion failed to produce aortic aneurysms in all genotypes (data not shown). Additionally, AngII-infused Nf1fl/+;LysMcre aneurysms displayed similar maximal dilation and aneurysm severity when compared to Nf1+/− mice (Figure 4C and 4D).
Figure 4.
Heterozygous inactivation of Nf1 in myeloid cells alone is sufficient to recapitulate Nf1 aneurysm formation. (A) Representative photographs of abdominal aortas from AngII-infused WT, Nf1+/−, Nf1flox/+;LysMcre and Nf1flox/+;SM22cre mice. Scale bars: 1mm. Saline-infused WT, Nf1+/−, Nf1flox/+;LysMcre and Nf1flox/+;SM22cre mice did not form aneurysms (data not shown). (B) Quantification of aneurysm incidence. (C) Maximum abdominal aortic diameter of AngII-infused WT, Nf1+/−, Nf1flox/+;LysMcre and Nf1flox/+;SM22cre mice. Clustering around 1mm represents animals without aneurysm formation. *P<0.05 for AngII-infused WT (n=10) versus AngII-infused Nf1+/− (n=9), and AngII-infused WT versus Nf1flox/+;LysMcre (n=15). **P<0.05 for AngII-infused Nf1flox/+;SM22cre (n=10) versus AngII-infused Nf1+/−, and AngII-infused Nf1flox/+;SM22cre versus AngII-infused Nf1flox/+;LysMcre. Analysis by one-way ANOVA with Tukey’s test. Error bars denote mean ± S.E.M. For B and C, no statistical significance was observed for AngII-infused WT versus AngII-infused Nf1flox/+;SM22cre, or AngII-infused Nf1+/− versus AngII-infused Nf1flox/+;LysMcre. (D) Severity index of aneurysms for AngII-infused WT (n=10), Nf1+/− (n=9), Nf1flox/+;LysMcre (n=15) and Nf1flox/+;SM22cre mice (n=10). (E) Representative photomicrographs of abdominal aortic cross-sections from AngII-infused WT, Nf1+/−, Nf1flox/+;LysMcre, and Nf1flox/+;SM22cre mice stained with anti-Mac-3 (arrowheads). Boxes specify area magnified in lower panel. Scale bars: 50μm.
Histologic examination of H&E and van Gieson stained arterial cross-sections of Nf1fl/+;LysMcre aneurysms revealed increased elastic lamina degradation and adventitial expansion, similar to aneurysms harvested from AngII-infused Nf1+/− mice (Supplemental Figure 2A through 2C). Cross-sections from Nf1fl/+;LysMcre aortas demonstrated increased macrophage density similar to Nf1+/− aneurysms, while Nf1fl/+;SM22cre and WT mice contained significantly reduced macrophage numbers (Figure 4E). Similar to Nf1+/− mice, macrophages in Nf1fl/+;LysMcre mice were in close proximity to areas of advential expansion and elastic lamina degradation (Figure 4E and Supplemental Figure 2B). Additionally, Nf1fl/+;LysMcre and Nf1fl/+;SM22cre displayed similar MMP activity and DHE staining as Nf1+/− and WT mice, respectively (Supplemental Figure 2D and 2E). Collectively, these data provide genetic evidence that heterozygous inactivation of Nf1 in myeloid cells alone is sufficient to recapitulate Nf1+/− aneurysm formation in vivo, thereby implicating Nf1+/− macrophages as the cellular trigger for aneurysm formation.
Simvastatin attenuates AngII-induced AAA formation in Nf1+/− mice
HMG-CoA reductase inhibitors are clinically efficacious in the prevention of several manifestations of CVD, including aneurysm formation,20 which is in part attributable to their anti-inflammatory and antioxidant function.21 Recent studies have shown that daily statin therapy reduces arterial stenosis in Nf1+/− mice, in part by inhibiting macrophage functions central to disease progression.9 Therefore, we tested whether simvastatin would reduce Nf1+/− aneurysm formation given our experimental observations.
WT and Nf1+/− mice were treated with daily low-dose simvastatin (1 mg/kg/day) or water for 7 days prior to initiation of AngII infusion and continued for 35 days. Low-dose simvastatin reduced AAAs in AngII-infused Nf1+/− mice by greater than 2-fold compared to water-treated controls (Figure 5A and 5B) without affecting blood pressure or serum cholesterol levels (data not shown). Corresponding decreases in aortic diameter and severity in AngII-infused Nf1+/− mice were also observed (Figure 5C and 5D). There was no significant difference in AAA incidence, maximum aortic diameter, or severity in AngII-infused WT mice in either treatment group (Figure 5A through 5D). Further, simvastatin treatment reduced arterial remodeling, macrophage infiltration (Supplemental Figure 3A and 3B) MMP-9 expression and activation, and ROS production (Supplemental Figure 4A through 4D) in arterial cross-sections from Nf1+/− mice when compared to water treatment. These results demonstrate that simvastatin prevents AngII-induced AAA formation in Nf1+/− mice, providing a potential therapeutic for NF1 aneurysmal disease.
Figure 5.
Preventive effect of simvastatin on AngII-induced AAA formation in Nf1+/− mice. (A) Representative photographs of abdominal aortas from water or simvastatin-treated, AngII-infused WT and Nf1+/− mice. Scale bars: 1mm. (B) Quantification of aneurysm incidence in water or simvastatin-treated, AngII-infused mice. (C) Maximum abdominal aortic diameter of water or simvastatin-treated, AngII-infused WT and Nf1+/− mice. Clustering around 1mm represents animals without aneurysm formation. *P<0.05 for water-treated WT (n=17) versus water-treated Nf1+/− (n=22). **P<0.05 for water-treated Nf1+/− versus simvastatin-treated Nf1+/− (n=10). Analysis by one-way ANOVA with Tukey’s test. Error bars denote the mean ± S.E.M. For B and C, no statistical significance was observed for water-treated WT versus simvastatin-treated WT (n=13). (D) Severity index of AAAs of AngII-infused WT and Nf1+/− mice treated with water (WT, n=17; Nf1+/−, n=22) or simvastatin (WT, n=9; Nf1+/−, n=10). For B–D, saline-infused WT or Nf1+/− mice in either treatment group did not form aneurysms.
Apocynin attenuates AngII-induced AAA formation in Nf1+/− mice
Increased production of ROS has been demonstrated in several animal models of CVD and antioxidant therapy has shown some utility in reversing many of these processes.22 Based on our observation that Nf1+/− aortas have evidence of increased ROS in response to AngII infusion, we sought to explore the role of antioxidant therapy, using apocynin, in attenuating Nf1+/− aneurysm formation. Though generally recognized as a non-specific antioxidant, recent evidence suggests that apocynin may inhibit superoxide production in cells containing myeloperoxidase, including macrophages and monocytes.23, 24
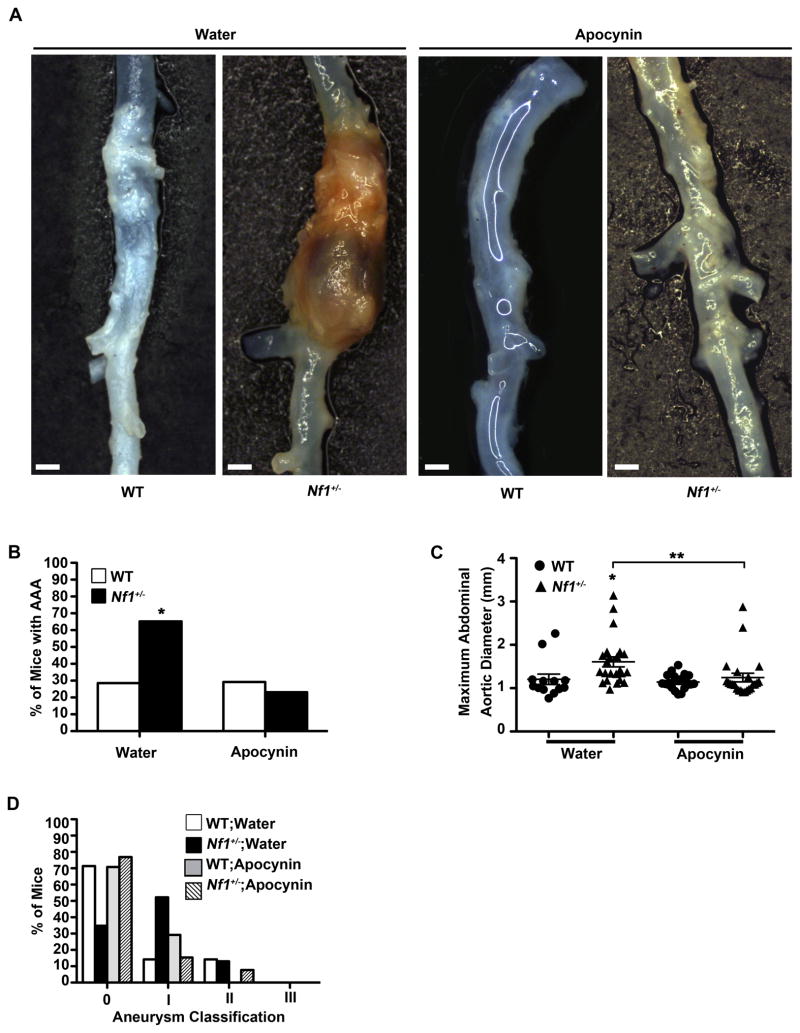
WT and Nf1+/− mice were treated with apocynin for 7 days prior to initiation of AngII infusion and continued for 35 days thereafter. Apocynin reduced AAAs in AngII-infused Nf1+/− mice by greater than 2-fold compared to water-treated controls, while apocynin did not have a significant effect on WT mice (Figure 6A and 6B). Additionally, decreases in both maximum abdominal aortic diameter and aneurysm severity were also noted in apocynin-treated Nf1+/− mice while apocynin-treated WT mice did not display a difference in either parameter (Figure 6C and 6D). Remodeling of the arterial wall, macrophage infiltration and ROS production was significantly reduced in apocynin-treated Nf1+/− mice when compared to water-treated controls (Supplemental Figure 5A–5D). These results identify overproduction of ROS as a significant contributor to Nf1+/− aneurysm formation and provide evidence that antioxidants may be a viable therapeutic option.
Figure 6.
Preventive effect of apocynin on AngII-induced AAA formation in Nf1+/− mice. (A) Representative photographs of abdominal aortas from water or apocynin-treated, AngII-infused WT and Nf1+/− mice. Scale bars: 1mm. (B) Quantification of aneurysm incidence in water or apocynin-treated, AngII-infused mice. *P<0.0083 for water-treated Nf1+/− (n=23) versus apocynin-treated Nf1+/− (n=26). Analysis by Fisher’s exact test with Bonferroni Correction. (C) Maximum abdominal aortic diameter of water or apocynin-treated, AngII-infused WT and Nf1+/− mice. Clustering around 1mm represents animals without aneurysm formation. *P<0.05 for water-treated WT (n=14) versus water-treated Nf1+/− (n=23). **P<0.05 for water-treated Nf1+/− versus apocynin-treated Nf1+/− (n=26). Analysis by one-way ANOVA with Tukey’s test. Error bars denote the mean ± S.E.M. For B and C, no statistical significance was observed for water-treated WT versus apocynin-treated WT (n=24). (D) Severity index of AAAs of AngII-infused WT and Nf1+/− mice treated with water (WT, n=14; Nf1+/−, n=23) or apocynin (WT, n=24; Nf1+/−, n=26). For B–D, saline-infused WT or Nf1+/− mice in either treatment group did not form aneurysms.
Discussion
Cardiovascular disease is a non-neoplastic manifestation in NF1 patients, which contributes to debilitating morbidities and early mortality.3, 4 Many of these vascular pathologies, including aneurysm formation and arterial stenosis, are often clinically silent until a catastrophic event, making an accurate measure of disease burden difficult to determine. Thus, understanding the pathogenesis of NF1 vasculopathy is critical to facilitate appropriate CVD screening, early recognition and targeted intervention in NF1 patients.
A major limitation in understanding NF1 aneurysmal disease has been the lack of an animal model that closely recapitulates the human disease. In this study, we present a murine model of NF1 aneurysmal disease, which provides a novel approach to examine the cellular mechanisms that regulate NF1 aneurysm formation. Analysis of Nf1+/− aneurysms revealed increased macrophage infiltration, MMP-9 expression and activation, and ROS production, which are molecular and cellular signatures of aneurysm formation observed in other experimental animal models independent of neurofibromin deficiency.19, 25 These observations suggest that vascular inflammation and macrophage secretory products are critical factors in NF1 aneurysmal disease, which is consistent with aneurysm formation in other chronic inflammatory diseases.25, 26 This is an important observation since we previously demonstrated that NF1 patients without known CVD have increased numbers of a specific subset of circulating inflammatory monocytes and cytokines,8 which have been previously linked to vascular disease in non-NF1 subjects in large population studies.11
To determine the contribution of neurofibromin-deficient myeloid cells to Nf1+/− aneurysm formation, we utilized lineage-restricted transgenic mice to specifically ablate a single Nf1 allele in myeloid cells alone. Heterozygous inactivation of the Nf1 gene in myeloid cells was sufficient to reproduce the aneurysm phenotype observed in Nf1+/− mice infused with AngII. Importantly, despite significant expansion of Nf1+/− VSMCs in arterial walls, aneurysm frequency and severity in transgenic mice harboring a single Nf1 allele in VSMCs alone were similar to WT controls. Additionally, administration of simvastatin, a statin with potent anti-inflammatory and antioxidant effects, diminished aneurysm formation in Nf1+/− mice. Finally, the antioxidant apocynin efficiently reduced Nf1+/− aneurysm formation. Collectively, these data directly implicate neurofibromin-deficient myeloid cells as the critical cellular effectors of aneurysm formation in Nf1+/− mice and indicate ROS as a therapeutic target to prevent or treat NF1 aneurysm formation.
Neurofibromin negatively regulates the Ras signaling cascade in multiple cell types by accelerating the conversion of active Ras-GTP to its inactive GDP confirmation.1 Loss of neurofibromin activates Ras and its downstream effectors, including the Ras-Mek-Erk and Ras-PI-3K pathways, rendering cells hypersensitive to diverse growth factors, contributing to the complexity of disease manifestations observed in NF1 patients.2, 8, 9, 17 Interestingly, myeloid progenitor cells are particularly sensitive to Ras activation and demonstrate multiple gain-of-function phenotypes contributing to myelo-proliferative disease, plexiform neurofibroma formation, bone disease, and vasocclusive disease in animal models of NF1 disease.8, 9, 27–29 Relevant to the current study, myeloid cells secrete growth factors and cytokines that are mediators of CVD, including vessel occlusion and aneurysm formation.9, 30 In view of our recent report that neurofibromin-deficient myeloid cells are the primary mediators of Nf1+/− arterial stenosis,9 the observation that mice with heterozygous inactivation of Nf1 in myeloid cells alone form aneurysms at a similar incidence to Nf1+/− mice highlights the global pathogenic consequences of neurofibromin-deficient myeloid cells to diverse NF1 clinical manifestations, including CVD.
Myeloid cell recruitment and infiltration of the vessel wall to initiate elastic lamina degradation are essential steps in aneurysm formation.25 AngII facilitates aneurysm formation via activation of monocytes and other leukocytes, which secrete cytokines and chemotactic factors leading to enhanced macrophage production of MMPs, resulting in vascular inflammation and vessel wall remodeling.31 Though several molecular ligand-receptor signaling cascades contribute to the progression of aneurysmal disease, pharmacologic inhibition or genetic disruption of monocyte chemotactic protein-1 (MCP-1) binding to its primary receptor, CCR2, reduces aneurysm formation as well as MMP-2 and MMP-9 activation.31–33 This signaling axis is particularly interesting since our laboratory recently demonstrated that myeloid cell heterozygosity mobilized Ly6Chi monocytes in peripheral blood,9 which are the murine correlate of human pro-inflammatory monocytes and co-express high cell surface levels of the CCR2 receptor.34, 35 Whether Nf1+/− macrophages are mobilized from the bone marrow via the MCP-1/CCR2 axis or proliferate locally within the aortic wall from the recently described common myeloid progenitor remains to be elucidated.31, 36
Another striking observation in our study is the increased production of ROS and MMPs in Nf1+/− vessel walls and developing aneurysms. ROS and MMP production by various cell types is critical for aneurysm formation in several model systems. Genetic or pharmacologic disruption of MMP-2 and MMP-9 in mice decreases aneurysm formation.37 These findings suggest that MMPs, released by resident and infiltrating vascular wall cells, are key molecular events in aneurysm formation. Given these observations, our study suggests that increased levels of MMP-9 observed in Nf1 heterozygous aneurysms may play a significant role in NF1 aneurysm progression, warranting further investigation to examine whether genetic modification and pharmacologic inhibition of MMP-9 activity can inhibit aneurysm progression in our Nf1 experimental system.
Increased ROS levels are also detected in human and murine cardiovascular lesions including aneurysms.38, 39 Moreover, evidence suggests that ROS overproduction facilitates MMP activation and contributes to vascular inflammation and vessel wall remodeling.40 Neurofibromin directly stimulates the adenylyl cyclase/cyclic AMP pathway, while loss of neurofibromin amplifies mitochondrial ROS production in Drosophila melanogaster.41 Conversely, constitutively active Ras mutations dramatically increase ROS production in mammalian hematopoietic progenitor cells via NAD(P)H oxidase (NOX) activation without increasing mitochondrial ROS.42 Additionally, we have found that simvastatin and apocynin, which both have antioxidant properties, reduce Nf1+/− aneurysms, implicating oxidative stress as a major contributor to neurofibromin-deficient aneurysm formation.21, 23 Further investigation using complex transgenic mice and targeted pharmacotherapies will now be needed to explore the redox balance in neurofibromin-deficient cells and to determine the source of ROS in aneurysm pathogenesis.
Previous studies have also identified increased expression of ROS-producing NOX subunits p22phox and p47phox within aortic aneurysms of non-NF1 patients and mice.38, 43 Genetic disruption of p47phox significantly diminishes oxidative stress and subsequent aneurysm formation in mice infused with AngII.26 These data suggest a role of NOX proteins as producers of ROS in aneurysm pathogenesis, indicating potential sources for observed Nf1+/− ROS production. Interestingly, Heumuller et al. extensively studied apocynin’s ability to reduce ROS in infiltrating and resident vascular wall cells, concluding that apocynin is dimerized and activated in myeloperoxidase expressing cells, including macrophages and granulocytes.23 Apocynin’s dimerized form is believed to inhibit the binding of the p47phox cytosolic subunit to the membrane-bound gp91phox subunit, therefore inhibiting the formation of the active NOX2 complex.44 These studies provide rationale for transgenic murine studies using cell specific deletion of p47phox and gp91phox within endothelial cells, VSMCs and monocytes/macrophages in Nf1+/− mice to test whether specific NOX isoform(s) contribute to ROS overproduction and aneurysm development. These studies are currently underway in our laboratory to identify specific therapeutic targets for preventing and treating NF1 vascular disease.
Statins are efficacious in reducing inflammation and oxidative stress independent of their lipid lowering capacity in both human trials and animal models.21 Additionally, statins have a safety profile that makes them advantageous for use in pediatric patients, as evidenced by recent trials in NF1 children,45 which may be important since evidence of vascular inflammation was identified in adolescent NF1 patients.8 In the current study, simvastatin reduced Nf1+/− aneurysm formation with corresponding attenuation of MMP-9 activation and ROS production, lending evidence to the role of inflammation and oxidative stress in NF1 aneurysm development. Finally, treatment with the antioxidant apocynin, which produced similar results to simvastatin, indicates that a reduction of oxidative stress may mediate the therapeutic effect simvastatin has on reducing Nf1+/− aneurysm formation. Based on our pre-clinical findings that low-dose statin treatment attenuates aneurysm formation and vasocclusive disease,9 it is possible that statins or more general antioxidants could be a viable therapeutic intervention in NF1 patients for the prevention and treatment of CVD.
In sum, this study establishes the first animal model of NF1 aneurysm disease and identifies Nf1 heterozygous myeloid cells as the cellular effectors of Nf1+/− aneurysm formation. In addition, we provide significant evidence that oxidative stress partially mediates Nf1+/− aneurysm formation and may be a viable therapeutic target. We provide a new model of NF1 vasculopathy that will serve as a tractable platform for understanding disease pathogenesis, the identification of novel biomarkers of pre-clinical disease, and development of novel therapeutics for the prevention and/or treatment of NF1 aneurysm formation.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the following grants: NIH P50 NS052606 (to D.A.I), TL1 RR025759 (to B.D.D., A. Shakhar, PI), and T32 HL007919-26 (to M.R.D., H. E. Broxmeyer, PI). Brian Stansfield is a Fellow of the Pediatric Scientist Development Program and was supported by (K12 HD000850 to B.K.S.) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, Wolff RK, Culver M, Carey JC, Copeland NG, Jenkins NA, White R, O’Connell P. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–192. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 2.Ward BA, Gutmann DH. Neurofibromatosis 1: From lab bench to clinic. Pediatr Neurol. 2005;32:221–228. doi: 10.1016/j.pediatrneurol.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE, McManus B, Korf BR. Cardiovascular disease in neurofibromatosis 1: Report of the nf1 cardiovascular task force. Genet Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: An analysis using u.S. Death certificates. Am J Hum Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oderich GS, Sullivan TM, Bower TC, Gloviczki P, Miller DV, Babovic-Vuksanovic D, Macedo TA, Stanson A. Vascular abnormalities in patients with neurofibromatosis syndrome type i: Clinical spectrum, management, and results. J Vasc Surg. 2007;46:475–484. doi: 10.1016/j.jvs.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 6.Rea D, Brandsema JF, Armstrong D, Parkin PC, deVeber G, MacGregor D, Logan WJ, Askalan R. Cerebral arteriopathy in children with neurofibromatosis type 1. Pediatrics. 2009;124:e476–483. doi: 10.1542/peds.2009-0152. [DOI] [PubMed] [Google Scholar]
- 7.Rosser TL, Vezina G, Packer RJ. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;64:553–555. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 8.Lasater EA, Li F, Bessler WK, Estes ML, Vemula S, Hingtgen CM, Dinauer MC, Kapur R, Conway SJ, Ingram DA., Jr Genetic and cellular evidence of vascular inflammation in neurofibromin-deficient mice and humans. Journal Clin Invest. 2010;120:859–870. doi: 10.1172/JCI41443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stansfield BK, Bessler WK, Mali R, Mund JA, Downing B, Li F, Sarchet KN, Distasi MR, Conway SJ, Kapur R, Ingram DA., Jr Heterozygous inactivation of the nf1 gene in myeloid cells enhances neointima formation via a rosuvastatin-sensitive cellular pathway. Hum Mol Genet. 2013;22:977–988. doi: 10.1093/hmg/dds502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Ismat FA, Wang T, Yang J, Epstein JA. Nf1 regulates a ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–2156. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- 11.Schlitt A, Heine GH, Blankenberg S, Espinola-Klein C, Dopheide JF, Bickel C, Lackner KJ, Iz M, Meyer J, Darius H, Rupprecht HJ. Cd14+cd16+ monocytes in coronary artery disease and their relationship to serum tnf-alpha levels. Thromb Haemost. 2004;92:419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of nf1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev. 2001;15:859–876. doi: 10.1101/gad.862101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Police SB, Putnam K, Thatcher S, Batifoulier-Yiannikouris F, Daugherty A, Cassis LA. Weight loss in obese c57bl/6 mice limits adventitial expansion of established angiotensin ii-induced abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol. 2010;298:H1932–1938. doi: 10.1152/ajpheart.00961.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin-McNulty B, Tham DM, da Cunha V, Ho JJ, Wilson DW, Rutledge JC, Deng GG, Vergona R, Sullivan ME, Wang YX. 17 beta-estradiol attenuates development of angiotensin ii-induced aortic abdominal aneurysm in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1627–1632. doi: 10.1161/01.ATV.0000085842.20866.6A. [DOI] [PubMed] [Google Scholar]
- 15.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki T, Ohta F, Daisu M, Hoshii Y. Extracranial vertebral artery aneurysm ruptured into the thoracic cavity with neurofibromatosis type 1: Case report. Neurosurgery. 2004;54:1517–1520. doi: 10.1227/01.neu.0000125547.31328.69. discussion 1520–1511. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Munchhof AM, White HA, Mead LE, Krier TR, Fenoglio A, Chen S, Wu X, Cai S, Yang FC, Ingram DA. Neurofibromin is a novel regulator of ras-induced signals in primary vascular smooth muscle cells. Hum Mol Genet. 2006;15:1921–1930. doi: 10.1093/hmg/ddl114. [DOI] [PubMed] [Google Scholar]
- 18.Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- 19.Rizas KD, Ippagunta N, Tilson MD., 3rd Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev. 2009;17:201–210. doi: 10.1097/CRD.0b013e3181b04698. [DOI] [PubMed] [Google Scholar]
- 20.Takagi H, Matsui M, Umemoto T. A meta-analysis of clinical studies of statins for prevention of abdominal aortic aneurysm expansion. J Vasc Surg. 2010;52:1675–1681. doi: 10.1016/j.jvs.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 21.Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP. Pleiotropic effects of statins--clinical evidence. Curr Pharm Des. 2009;15:479–489. doi: 10.2174/138161209787315729. [DOI] [PubMed] [Google Scholar]
- 22.Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, Yoshimura K, Aoki H, Tsubota K, Yoshikawa T, Okada Y, Ogawa S, Fukuda K. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular nadph oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 24.Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- 25.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires mmp-9 activation by plasminogen in mice. J Clin Invest. 2008;118:3012–3024. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin ii-induced abdominal aortic aneurysm formation in apolipoprotein e-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing nf1+/−- and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, Morgan T, Li X, Yuan J, Hock J, Ingram DA, Clapp DW. Hyperactivation of p21ras and pi3k cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–2891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang T, Krisman K, Theobald EH, Xu J, Akutagawa J, Lauchle JO, Kogan S, Braun BS, Shannon K. Sustained mek inhibition abrogates myeloproliferative disease in nf1 mutant mice. J Clin Invest. 2013;123:335–339. doi: 10.1172/JCI63193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Review of current theories for abdominal aortic aneurysm pathogenesis. Vascular. 2009;17:253–263. doi: 10.2310/6670.2009.00046. [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, Charo IF, Kura S, Tsuzuki T, Takeshita A, Sunagawa K. Bone marrow-derived monocyte chemoattractant protein-1 receptor ccr2 is critical in angiotensin ii-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174–178. doi: 10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- 32.MacTaggart JN, Xiong W, Knispel R, Baxter BT. Deletion of ccr2 but not ccr5 or cxcr3 inhibits aortic aneurysm formation. Surgery. 2007;142:284–288. doi: 10.1016/j.surg.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 33.de Waard V, Bot I, de Jager SC, Talib S, Egashira K, de Vries MR, Quax PH, Biessen EA, van Berkel TJ. Systemic mcp1/ccr2 blockade and leukocyte specific mcp1/ccr2 inhibition affect aortic aneurysm formation differently. Atherosclerosis. 2010;211:84–89. doi: 10.1016/j.atherosclerosis.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 34.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in west nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psaltis PJ, Harbuzariu A, Delacroix S, Witt TA, Holroyd EW, Spoon DB, Hoffman SJ, Pan S, Kleppe LS, Mueske CS, Gulati R, Sandhu GS, Simari RD. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation. 2012;125:592–603. doi: 10.1161/CIRCULATIONAHA.111.059360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: A potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- 39.Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin a enhances vascular oxidative stress and the development of angiotensin ii-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong JJ, Schriner SE, McCleary D, Day BJ, Wallace DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in drosophila melanogaster. Nat Genet. 2007;39:476–485. doi: 10.1038/ng2004. [DOI] [PubMed] [Google Scholar]
- 42.Hole PS, Pearn L, Tonks AJ, James PE, Burnett AK, Darley RL, Tonks A. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human cd34+ hematopoietic progenitor cells. Blood. 2010;115:1238–1246. doi: 10.1182/blood-2009-06-222869. [DOI] [PubMed] [Google Scholar]
- 43.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: Nadph oxidases as therapeutic targets. Nat Rev. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krab LC, de Goede-Bolder A, Aarsen FK, Pluijm SM, Bouman MJ, van der Geest JN, Lequin M, Catsman CE, Arts WF, Kushner SA, Silva AJ, de Zeeuw CI, Moll HA, Elgersma Y. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: A randomized controlled trial. JAMA. 2008;300:287–294. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.