Abstract
Cell-based immunotherapy has been gaining interest as an improved means to treat HIV/AIDS. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) could become a potential resource. Our previous studies have shown hESC and iPSC-derived natural killer (NK) cells can inhibit HIV-infected targets in vitro. Here, we advance those studies by expressing a HIV chimeric receptor combining the extracellular portion of CD4 to the CD3ζ intracellular signaling chain. We hypothesized that expression of this CD4ζ receptor would more efficiently direct hESC- and iPSC-derived NK cells to target HIV-infected cells. In vitro studies showed the CD4ζ expressing hESC- and iPSC-NK cells inhibited HIV replication in CD4+ T cells more efficiently than their unmodified counterparts. We then evaluated CD4ζ-hESC- and iPSC-NK cells in vivo anti-HIV activity using a humanized mouse model. We demonstrated significant suppression of HIV replication in mice treated with both CD4ζ-modified and unmodified hESC-/iPSC-NK cells compared to control mice. However, we did not observe significantly increased efficacy of CD4ζ expression in suppression of HIV infection. These studies indicate that hESC/iPSC-based immunotherapy can be utilized as a unique resource to target HIV/AIDS.
Keywords: human embryonic stem cells, induced pluripotent stem cells, Natural killer cells, HIV-1 infection inhibition, in vitro, in vivo
INTRODUCTION
Highly active antiretroviral therapy (HAART) has significantly decreased the morbidity and mortality of HIV/AIDS, but latent virus still persists in cellular reservoirs. Restoration of cellular immunity in treated patients proceeds slowly and may never return to pre-infection status [1, 2], Cell-based therapy for HIV has gained more attention for its potential of long-term virus control or cure (reviewed in [3, 4]). Initially, studies were designed to inhibit HIV transcription or translation, which is less efficient in controlling the late steps of the viral life cycle [5]. More studies were then focused on early step inhibition before HIV integration into host genome. For instance, several groups have demonstrated the resistance against R5-tropic HIVs in vitro using shRNAs to knock down CCR5 in hematopoietic stem cells (HSC) [6-8]. Holt and colleagues [9] used zinc finger nuclease (ZFN) to disrupt the CCR5 gene in human cord blood and fetal liver CD34+ cells, which protected reconstituted NSG mice from R5 HIV infection. The use of a CCR5 −/− donor for a hematopoietic stem cell transplant of an HIV infected patient with acute myelogenous leukemia has provided a novel impetus for other potential cell-based curative approaches for patents with HIV/AIDS [10, 11].
Alternatively, there are also efforts to help redirect specific immune cell subsets to target and kill HIV. Studies have engineered immune cells with “chimeric antigen receptors” (CAR) to provide enhanced antigen recognition and cellular activation. By fusing the antigen specific portion of an antibody to intracellular signaling domains of the T cell signaling framework, several groups have shown promising results in different cancer clinical trials [12-15]. A similar strategy has also been used on HIV treatment by modifying peripheral T cells with a molecularly cloned T cell receptor (TCR) to redirect cells to HIV targets[16-18]. Using a lentiviral approach, Kitchen et al successfully expressed an HIV-specific TCR into HSCs and developed CD8 T cell with response to HIV in vivo [19]. Other groups have also demonstrated anti-HIV activity by expressing a functional neutralizing antibody in B cells derived from human HSC in vitro [20] and in vivo [21].
Human embryonic stem cells (hESCs), and induced pluripotent stem cells (iPSCs), are becoming an alternative promising source for gene or immunotherapy [22]. Several groups, including our own, have reported that hESCs and iPSCs can give rise to different lymphoid and myeloid lineages [23-29]. Some studies have also demonstrated that hESC- and/or iPSC-derive immune cells are either susceptible to HIV [25, 30] or capable of targeting HIV infected cells [31]. More recently, TCR-specific T cells derived from T-iPS cells [28] further demonstrated the potential of using either hESC or iPSCs-based gene/immunotherapy. We have previously shown that NK cells derived from hESCs and iPSCs have potent anti-HIV activity [31] and these innate immune cell do not posses any antigen specific recognition receptors [1, 32, 33]. Therefore we hypothesized that engineering hESC- and iPSC-derived NK cells with chimeric receptors would enhance their anti-HIV activity. In addition, we have recently demonstrated large-scale production of hESC/iPSC-derived NK cells, which could provide an unlimited cellular therapeutic for off-the-shelf use [34, 35]. We now advance these studies using the CAR strategy to direct NK cell effector function to HIV-infected cells [36].
As the CD4 protein is an absolute requirement for HIV entry, it is plausible to utilize this as an effective “antigen recognition” domain with human leukocyte antigen restriction. June and others pioneered this approach to both basic research and clinical trials [36-40], however this had varying efficacy in vivo when transduced into patients autologous T cells. Here, we modified both hESCs and iPSCs with a CD4ζ construct to generate NK cells that express the specific HIV CD4ζ chimeric receptor. We then tested these NK cells for HIV suppression both in vitro and found that both CD4ζ-hESC- and CD4ζ-iPSC-NK cells were able to suppress HIV replication more efficiently than their unmodified counterparts. We also determined CD4ζ -hESC-/iPSC-NK cells mediate in vivo anti-HIV activity in a PBL-NSG mouse xenograft model. We found that CD4ζ-hESC- and CD4ζ-iPSC-NK cells were able to inhibit HIV replication and prevent CD4 T cells depletion but no difference compared to regular hESC and iPSC-NK cells. These studies establish a novel system to understand and direct innate immunity against HIV-1 infection. Eventually, hESC- or iPSC-based immune therapy could be utilized as a unique resource for HIV/AIDS treatment.
MATERIALS AND METHODS
Maintenance of hESCs/iPSCs and generation of CD4ζ expressing hESC (CD4ζ-hESC) and iPSC (CD4ζ-iPSC) cells
hESCs (H9) and iPSCs (UCBiPS7, derived from umbilical cord blood CD34+ cells) were maintained on mouse embryonic fibroblasts (MEF) as described previously [41]. The plasmid pCCL.PPT.hPGK 1.9.IRES.eGFP containing CD4ζ chimeric receptor was kindly provided by Drs. Scott Kitchen and Otto Yang from UCLA [36, 38]. Lentiviral production was produced in 293T cells using the Invitrogen ViraPower Lentiviral Expression System (Invitrogen). hESC and iPSC cell lines were then infected with CD4ζ lentivirus and FACS sorted for GFP+ cells. These two lenti CD4ζ-modified cell lines were used in all in vitro studies. Because the expression of CD4ζ/GFP in hESCs and iPSCs was readily silenced during differentiation, we generated more stable CD4ζ-hESC and CD4ζ-iPSC lines using the sleeping beauty system [42, 43] and developed NK cells for all in vivo experiments.
NK cell derivation from CD4ζ-hESC and CD4ζ-iPSC cells
We have been using both stromal-based systems and stroma-free embryoid body (EB)-based systems for hematopoietic differentiation of hESCs and iPSCs. Most recently, we have shifted to using “Spin-EBs” for hematopoietic differentiation of hESCs and iPSCs [34, 44-46]. Briefly, 3000 single cells were seeded per well of 96-well round bottom plates in BPEL media with stem cell factor (SCF, 40ng/ml), vascular endothelial growth factor (VEGF, 20ng/ml) and bone morphogenic protein 4 (BMP4, 20ng/ml). BPEL media was in 200ml volumes and contained Iscove's Modified Dulbecco's Medium (IMDM, 86mL, Invitrogen), F12 Nutrient Mixture with Glutmax I (86mL, Invitrogen), 10% deionized Bovine Serum Albumin (BSA, 5mL, Sigma), 5% Polyvinyl alcohol (10mL, Sigma), Linolenic acid (20uL of 1gm/mL solution, Sigma), Linoleic acid (20uL of 1gm/mL solution, Sigma), Synthecol 500x solution (Sigma), a-monothioglyceral (Sigma, 3.9ul/100ml), Protein-free hybridoma mix II (Invitrogen), ascorbic acid (5 mg/ml, Sigma), Glutamax I (Invitrogen), Insulin-transferrin-selenium 100x solution (Invitrogen), Penicillin/streptomycin (Invitrogen). At day 11 of hematopoietic differentiation, spin EBs were directly transferred into 24-well plates with or without EL08-1D2 stromal cells in NK media supplied with cytokines [34]. After 4-5 weeks of culture, single cell suspensions were stained with APC-, PE-, FITC- and PerCP-cy5.5-coupled IgG or specific antibodies against human blood surface antigens: CD45-PE, CD56-APC, CD56-PE, CD16-PerCP-cy5.5, NKG2D-PE, NKp44-PE, NKp46-PE, CD158b-FITC, CD158e1/2-FITC (all from BD Pharmingen), CD158a/h-PE and CD158i-PE (Beckman Coulter) as shown in Figure 1. All analyses were performed with a FACS Calibur (BD Biosciences) and analyzed with FlowJo software (Tree Star). NK cells isolated from peripheral blood (PB-NK) using an NK negative selection kit (Miltenyi Biotech.) were used as controls for phenotyping characterization and all following experiments.
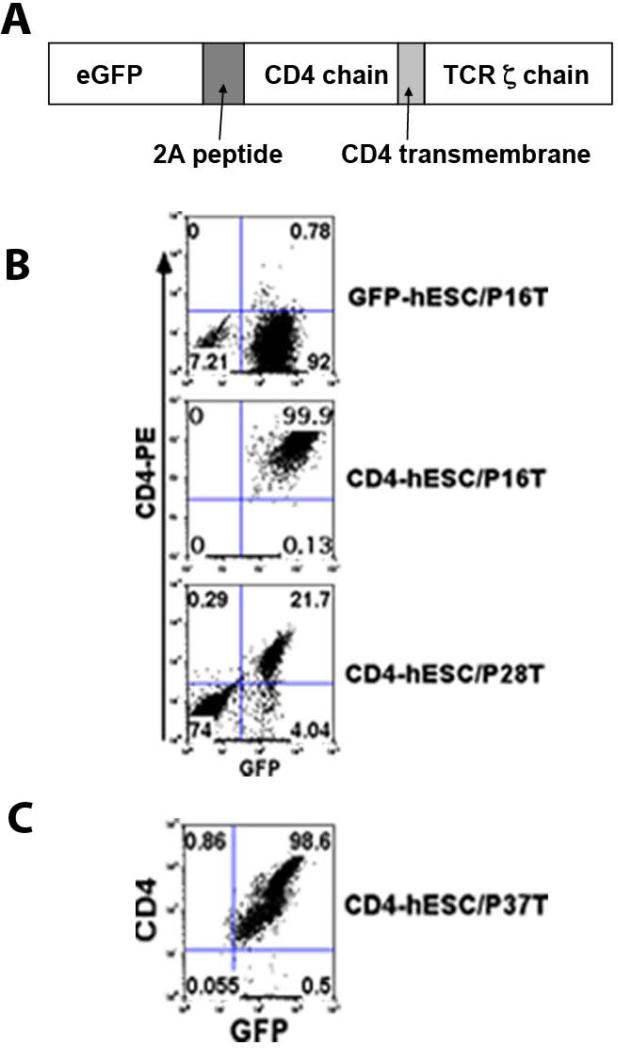
Figure 1. Expression of CD4ζ chimeric receptor in hESCs and iPSCs.
(A) Diagram of CD4ζ cloned in lentiviral vector or Sleeping Beauty transposon vector. (B) Transduced hESC cells were analyzed by flow cytometry for expression GFP and CD4ζ receptor (upper and middle lanes). Both GFP and CD4ζ expression getting silenced during culture maintain (lower lane). (C) SB-transduced hESCs stably express GFP-CD4ζ.
NK cell stimulation
CD4ζ-hESC-, CD4ζ-iPSC-NK cells and GFP only controls were starved in RPMI 1640 media overnight. As previously described [36], cells were spun down and stimulated with anti-CD4 mAb (OKT4A) for 15 min at 4°C, then washed off unbound Ab and crossed linked by goat anti-mouse IgG F(ab’)2 fragments (Jackson Immunoresearch) for 3 min at 37°C. Cells were then fixed and stained for the tyrosine phosphorylation using mouse anti-human mAb4G10 (Millipore) and PE-donkey anti-mouse IgG (Jackson Immunoresearch) following the instruction of BD phosflow kit (BD Biosciences).
CD4ζ-hESC and CD4ζ-iPSC NK cell anti-HIV activity in vitro
As in our previous studies, CEM-GFP cells infected with HIV-1 NL4-3 were used as targets to test the suppression of HIV of CD4ζ-hESC- and CD4ζ-iPSC-NK cells by comparison with their unmodified hESC and iPSC-NK cells. Briefly, CEM-GFP cells were infected with HIV-1 NL4-3 (MOI=0.1) for 4h at 37°C and then were washed twice with fresh medium. 1×105 cells were plated with CD4ζ-hESC-, hESC-, CD4ζ-iPSC- and iPSC-NK cell at effector: target ratios 1:1 and 5:1 or alone for 14 days in the presence of 100 IU/ml interleukin 2 (IL-2). Cells were collected on day 4, 7, 11, 14 for GFP expression by flow cytometry. Loss of GFP expression in the CEM-GFP cells indicates the suppression of HIV replication. To detect the anti-HIV activities of CD4ζ-hESC- and CD4ζ-iPSC-NK cells to HIV–infected primary human cells, CD4+ T cells enriched from peripheral blood were stimulated with phytohaemagglutinin (PHA from Sigma-Aldrich) in RMPI 1640 with 10% FBS, 2mM L-glutamine supplemented with 100 IU/ml IL-2 for 48-72 hr. At day 3, expanded CD4+ T cells were infected with a lab-adapted strain HIV-1 SF2 (X4R5, MOI=0.05) (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) [47]. At day 10, 1×105 of HIV-1-infected CD4 T cells were respectively mixed with same number of CD4ζ-hESC-, hESC-, CD4ζ-iPS- and iPSC-NK cells at 37°C for 5 hours, the activation of NK cells were evaluated by CD107a surface expression. PB-NK cells were used as positive controls for all experiments.
Generation of human peripheral blood-reconstituted NSG (PBL-NSG) mouse
Eight- to 10-week-old NSG mice were reconstituted with 1×107 freshly isolated human PBLs [48]. Two weeks later, blood was bled from the retro orbital venous plexus for engraftment before HIV NL4-3 infection.
HIV infection and NK cell treatment
HIV-1 NL4-3 virus was grown in 293 T cells. Virus infectivity was determined by limiting dilution titration on 293 T cells. HIV-1 NL4-3 stocks were prepared as described [49]. Two weeks after PBL reconstitution, mice were infected by intraperitoneal injection of 100ul cell-free HIV stocks containing 30,000 50% tissue culture infectious doses (TCID50) [50]. Mice were then ip injected with 2×106 hESC-, CD4ζ-hESC-, iPSC-, CD4ζ-iPSC- or PB-NK cells the day after HIV-1 NL4-3 infection. As previously demonstrated in our lab, mice received ip injection of IL-15 and IL-2 every day for the first 7 days following NK cell treatment and then IL-2 every other day for another week to enhance NK cell proliferation and function [51]. Day 6, 9 and 12 after NK cell injection, blood was collected for human CD4 T cell levels, HIV gag protein p24, viral RNA and proviral DNA detection. Day 13 after NK cell treatment (day 14 of HIV infection), mice were killed and collected for spleens and cells from peritoneal cavity for proviral DNA and intracellular p24 detection.
Measurement of human CD4 T cell levels in PBL-NSG mice
The levels of hCD4 in peripheral blood were monitored every 3 days after NK cell treatment. Whole blood was collected in EDTA-coated tubes and red blood cells were lysed by ammonium chloride for twice, 5mins each time if necessary. Cells were then stained for hCD45-APC, hCD3-PECY7 and hCD4-PE (BD Pharmingin). CD4+ T cell levels were determined as a ratio of CD4+CD3+/CD4−CD3+. To establish baseline CD4+CD3+/CD4−CD3+ratios, all mice were analyzed prior to HIV infection. To detect hESC-, iPSC- and PB-NK cells in peripheral blood, peritoneal cavity and spleen, cells were stained with hCD45-PE, hCD56-APC (BD Pharmingin) after treatment.
Measurement of HIV viral load
Mouse peripheral blood was collected by facial bleeding in accordance with the university of Minnesota IRB. Plasma was separated by spin at 400rcf/min for 10 mins and frozen at −80C for viral RNA isolation. Viral RNA was extracted from less than 50ul of EDTA-treated plasma with the QIAamp Viral RNA kit (Qiagen, Valencia, CA). Quantitative RT-PCR was performed using the Taqman one-step RT-PCR Master Mix Reagents kit (Applied Biosystem, Branchburg, NJ) with a set of primers specific for the HIV long terminal repeat (LTR) sequence and an LTR-specific probe as described [52-54]. Viral RNA was expressed as the number of HIVRNA copies per milliliter plasma. To detect integrated provirus, cellular DNA was extracted from peripheral blood, peritoneal cavity and spleens using the high pure PCR template preparation kit (Roche, Mannheim, Germany) and subjected to Q-PCR with the same set of primers above and SYBR Green PCR Master Mix (Applied Biosystems).
Statistical analysis
Experiments were analyzed utilizing prism 5 software the Student's T test and the Wilcoxon Rank Sum Test. Results are shown as means and SD and the value of P<0.05 was determined as significant.
RESULTS
Expression of CD4ζ in hESCs and iPSCs Using Lentivirus or Sleeping Beauty Transgenesis
The CD4ζ construct contains the fused extracellular and transmembrane domains of CD4 and the cytoplasmic domain of T-cell receptor CD3ζ chain, linked to green fluorescent protein (eGFP) by a 2A self-cleaving peptide (Figure 1A). The CD4ζ lentivirus was made in 293 T cells and infected hESCs and iPSCs. We then performed flow cytometry sorting for GFP positive cells to get pure CD4ζ expressing hESCs and iPSCs (CD4ζ-hESCs or CD4ζ-iPSCs ). hESCs and iPSCs expressing GFP-only were used as controls (Figure 1B). During the maintaining of CD4ζ-hESCs and CD4ζ-iPSCs, we found that the expression of GFP and CD4ζ was commonly silenced after 10-15 passages (Figure 1B). Our previous studies and others have demonstrated that the Sleeping Beauty (SB) transposon system is a more stable means to transfer genetic information to hESCs [42, 43]. We then re-transduced the CD4ζ-GFP fused protein into hESCs and iPSCs using the SB system with puromicine antibiotic selection and did not find CD4ζ-GFP silencing even till passage 37 (Figure 1C). Here we used NK cells derived from CD4ζ-GFP-SB transduced-hESCs or iPSCs all in vivo studies.
NK Cells Derived from CD4ζ-hESCs and CD4ζ-iPSCs
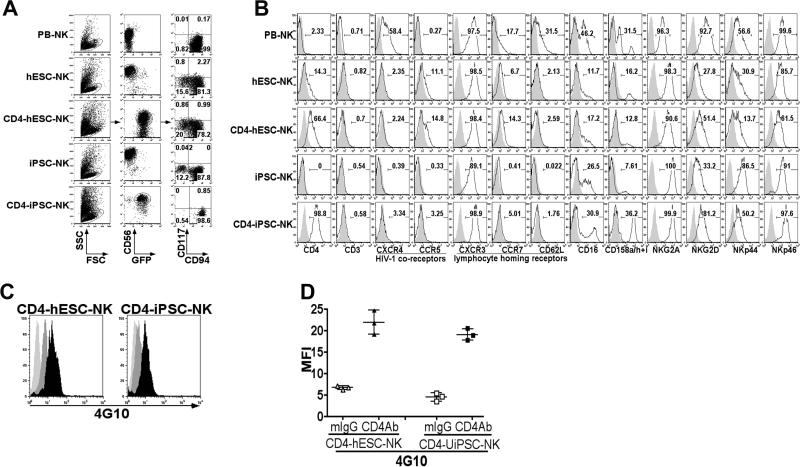
Previous studies by our group to derive NK cells from both hESCs and iPSCs have utilized stromal-based systems [31, 51]. More recently we shifted to use of defined serum-free conditions that can be effectively scaled to produce potentially clinical-scale quantities of NK cells [44, 45, 55]. Briefly, in this system, undifferentiated hESCs or iPSCs are dissociated as single cell suspension and seeded into 96-well round bottom plates by briefly spinning to form embryoid bodies (EBs). After 11 days of culture in serum-free media with defined cytokines, differentiated spin EBs containing hematopoietic progenitors CD34+/CD45+ were transferred to NK cell differentiation media supplemented with a combination of cytokines with or without EL08 stromal cells routinely generates a lymphocyte population where more than 90% of the cells are CD45+CD56+ (Figure 2A). Both CD4ζ-hESC- and CD4ζ-iPSC-derived CD45+CD56+ populations expressed the CD4 receptor and GFP. Similar to unmodified hESC-, iPSC- or PB-NK cells [31, 51], these CD45+CD56+ cell populations are mostly CD117−CD94+, which has been demonstrated to be a more cytotoxic subset of NK cells [51, 56, 57]. We have previously demonstrated extensive phenotypic analysis of hESC and iPSC-derived NK cells expressing similar surface makers including the Fc receptor CD16, killer immunoglobulin receptors (KIRs), NKG2A, NKG2D, NKp44 and NKp46 as PB-NK cells [31]. CD4ζ-hESC- and CD4ζ-iPSC-NK cells also had a similar phenotype as their unmodified counterparts and PB-NKs (Figure 2B). We then examined chemokine/cytokine receptors expression on CD4ζ-hESC- or CD4ζ-iPSC-NK cells. Expression levels of CCR5 and CXCR4, also known as HIV co-receptors [58] were not observed to high levels expression on both CD4ζ-modified hESC- and iPSC-NK cells compared to their unmodified counterparts or PBNKs (Figure 2B). The chemokine receptors CXCR3, CCR7 and adhesion molecule CD62L are all involved in NK cell homing to second lymphoid organs [59]. We found that CD4ζ-hESC or iPSCNK cells expressed similar levels of CXCR3 as PB-NKs, but less CCR7 and CD62L (Figure 2B). Next, to evaluate the function of the CD4ζ chimeric receptor in hESC- and iPSC-NK cells, addition of anti-CD4 mAb OKT4A followed by goat F (ab)’ anti-mouse IgG was used to cross-link and stimulate cells. Stimulation of effector function through the CD4 chimeric receptor is dependent on tyrosine phosphorylation [60], which can be determined by phospho-flow cytometry (Figure 2C). We found tyrosine phosphorylation is rapidly induced in both CD4ζ-hESC- and CD4ζ-iPSC-NK cells by cross-linking of the CD4ζ chimeric receptors (Figure 2D), indicating this chimeric receptor is functionally active following differentiation of pluripotent stem cells into NK cells.
Figure 2. Generation of NK cells from CD4ζ-hESCs and CD4ζ-iPSCs.
(A) Flow cytometric analysis of CD56+CD45+ NK cells derived from hESC, CD4ζ-hESC, iPSC and CD4ζ-iPSC. Expression of lymphocyte activating receptors and homing receptors on NK cells as indicated. These cells are compared to NK cells isolated from peripheral blood (PB-NK). (B) CD56+ NK cell from hESC, CD4ζ-hESC, iPSCs, CD4ζ-iPSCs are all CD3- as are PB-NKs. Expression of surface marker CD16, KIRs, NKG2A, NKG2D, NKp44, NKp46, HIV co-receptor CCR5, CXCR4 and homing receptor CXCR3, CCR7 and CD62L. (C) Activity of CD4ζ in NK cells derived from CD4ζ-hESCs and CD4ζ-iPSCs. Both CD4ζ-hESC- and CD4ζ-iPSC-NK cells were stimulated with ( ) or without (
) or without ( ) anti-CD4 and goat anti-mouse IgG F(ab’)2 to initiate receptor cross-linking. Cells were then intracellular stained by tyrosine phosphorylation Ab 4G10 followed by PE- anti-mouse IgG. Cross-linked cells were stained with mouse IgG and PE- anti-mouse IgG were used as isotype controls (
) anti-CD4 and goat anti-mouse IgG F(ab’)2 to initiate receptor cross-linking. Cells were then intracellular stained by tyrosine phosphorylation Ab 4G10 followed by PE- anti-mouse IgG. Cross-linked cells were stained with mouse IgG and PE- anti-mouse IgG were used as isotype controls ( ). Flow cytometry plots represented 1 of at least 3 independent experiments. (D) Trysine phosphorylation measured by flow cytometry for the mean fluorescent intensity (MFI). The solid lines represent the mean +/− the SD.
). Flow cytometry plots represented 1 of at least 3 independent experiments. (D) Trysine phosphorylation measured by flow cytometry for the mean fluorescent intensity (MFI). The solid lines represent the mean +/− the SD.
CD4ζ-hESC- and CD4ζ-iPSC-NK Cell Inhibition of HIV Replication in Vitro
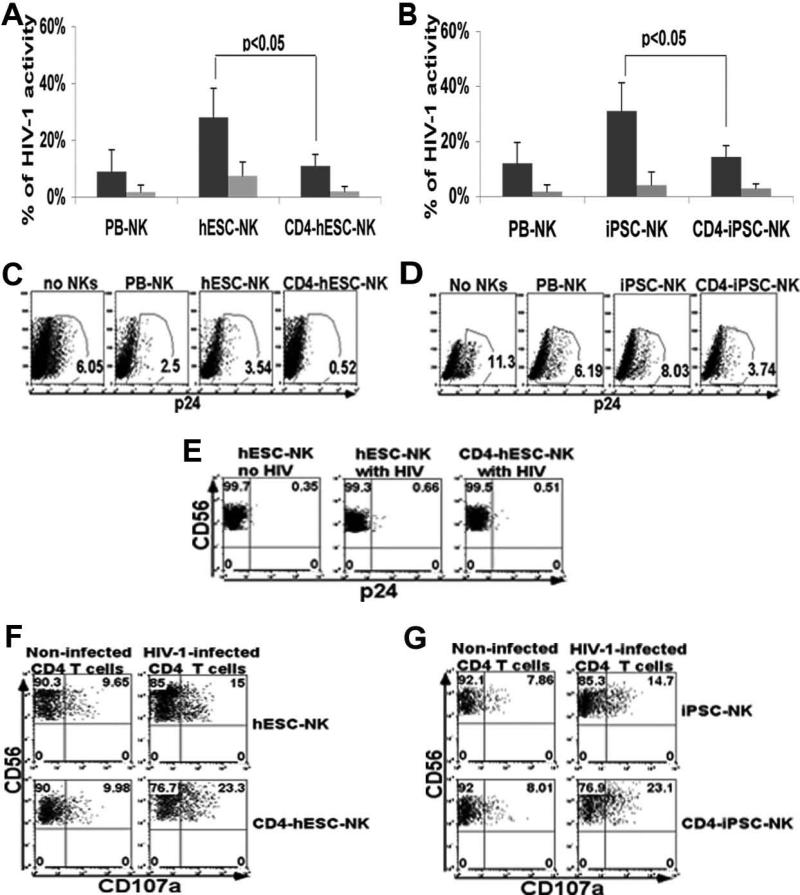
Our previous studies demonstrated that both hESC- and iPSC-NK cells have potent ability to inhibit HIV infection [31]. The CEM-GFP T cell line infected with HIV-1 NL4-3 leads to GFP expression, which provides accurate and reliable quantification of HIV infection and the effects of our NK cell-based inhibition to HIV replication [61]. To determine whether the expression of CD4ζ enhance anti-HIV activity, CD4ζ-hESC- or CD4ζ-iPSC-NK cells and their unmodified counterparts were co-cultured with NL4-3-infected CEM-GFP cells at different effector/target (E/T) ratios and monitored for HIV replication for two weeks [31, 62]. As we have previously demonstrated, unmodified hESC- and iPSC-NK cells both inhibit HIV replication in a dose dependent manner (Figure 3A and 3B). Notably, CD4ζ modified hESC- and iPSC-NK cells lead to 90% inhibition of HIV replication, significantly greater than unmodified hESC- and iPSC-NK cells (P<0.05). These studies indicate CD4ζ expression on hESC and iPSC-derived NK cells effectively directs NK cells to better target HIV-infected cells.
Figure 3. CD4ζ-hESC- and CD4ζ-iPSC-NK cells inhibit the replication of HIV in vitro.
(A to B) CEM-GFP cells were incubated with HIVNL4-3 virus for 4 hours. Cells were then co-cultured with CD4ζ-modified hESC-, CD4ζ-iPSC- or PB-NK cells for 14 days. HIV infection was assessed by flow cytometry for GFP expression. Activity of HIV-1 was measured by the percent GFP+ of CEM-GFP cells co-cultured with (A) PB-, hESC- and CD4ζ-hESC-NK cells or (B) PB-, iPSC-, and CD4ζ-iPSC-NK cells at day 11 with E:T ratios of 1:1( ) and 5:1 (
) and 5:1 ( ). Cells were CEM gated. The error bars represent the mean +/− the standard deviation (SD). Statistical comparison of % GFP+ between CD4ζ-hESC-/iPSC-NK vs. hESC-/iPSC-NK cells was performed using the Student's t test. (C-F) NK cells function against HIV-1-infected human CD4+ primary T cells. (C and D) NK cells were co-cultured with SF2-infected CD4+ T cells at E:T rations of 5:1 for two weeks. HIV infection was evaluated by intracellular staining for gag p24 in all CD4 T cells. The percentage of p24+ CD4+ in the co-cultures of (C) no NKs, PB-, hESC-, and CD4ζ-hESC-NK cells or (D) no NKs PB-, iPSC- and CD4ζ-iPSC-NK cells with HIV-infected CD4 T cells at day 11. Cells were CD56− gated. (C) and (D) demonstrate statistically lower % p24+ in CD4ζ-hESC-/iPSC-NK culture compared to hESC-/iPSC-NK cells respectively. (E) NK cells were evaluated for HIV infection in all CD56+ cells at day 11 of co-culture. Either CD4ζ-hESC-NKs or hESC-NKs were negative for p24 staining. hESC-NKs with no HIV as negative controls. (F and G) Surface expression of CD107a was evaluated to measure NK cell cytolytic activity. Flow cytometric analyses of CD107a expression on (F) hESC- and CD4ζ-hESC-NKs or (G) iPSC- and CD4ζ-iPSC-NKs following stimulation with HIV-1-infected CD4+ T cells for 5 hours. Uninfected CD4+ T cells were used as controls. Cells were all CD56+ gated. Both CD4ζ-hESC- and CD4ζ-iPSC-NK cells populations stimulated by HIV-1-infected CD4+ T cells show significantly increased CD107a expression compared to hESC- and iPSC-NK cells (P<0.05). The data represent one of at least 3 independent experiments.
). Cells were CEM gated. The error bars represent the mean +/− the standard deviation (SD). Statistical comparison of % GFP+ between CD4ζ-hESC-/iPSC-NK vs. hESC-/iPSC-NK cells was performed using the Student's t test. (C-F) NK cells function against HIV-1-infected human CD4+ primary T cells. (C and D) NK cells were co-cultured with SF2-infected CD4+ T cells at E:T rations of 5:1 for two weeks. HIV infection was evaluated by intracellular staining for gag p24 in all CD4 T cells. The percentage of p24+ CD4+ in the co-cultures of (C) no NKs, PB-, hESC-, and CD4ζ-hESC-NK cells or (D) no NKs PB-, iPSC- and CD4ζ-iPSC-NK cells with HIV-infected CD4 T cells at day 11. Cells were CD56− gated. (C) and (D) demonstrate statistically lower % p24+ in CD4ζ-hESC-/iPSC-NK culture compared to hESC-/iPSC-NK cells respectively. (E) NK cells were evaluated for HIV infection in all CD56+ cells at day 11 of co-culture. Either CD4ζ-hESC-NKs or hESC-NKs were negative for p24 staining. hESC-NKs with no HIV as negative controls. (F and G) Surface expression of CD107a was evaluated to measure NK cell cytolytic activity. Flow cytometric analyses of CD107a expression on (F) hESC- and CD4ζ-hESC-NKs or (G) iPSC- and CD4ζ-iPSC-NKs following stimulation with HIV-1-infected CD4+ T cells for 5 hours. Uninfected CD4+ T cells were used as controls. Cells were all CD56+ gated. Both CD4ζ-hESC- and CD4ζ-iPSC-NK cells populations stimulated by HIV-1-infected CD4+ T cells show significantly increased CD107a expression compared to hESC- and iPSC-NK cells (P<0.05). The data represent one of at least 3 independent experiments.
As a more rigorous in vitro test, we next studied the ability of each cell population to limit infection of primary CD4+ T cells. To compare the anti-HIV activity of CD4ζ -hESC or CD4ζ -iPSC-NK cells versus unmodified hESC- or iPSC-NK cells, primary CD4+ T cells infected with HIV-1SF2 (X4R5) [47] were used as targets. Again, HIV-infected CD4+ T cells were co-cultured with or without NK cells for two weeks. HIV infection was quantified by intracellular staining of CD4+T cells for the viral gag protein p24 every 3-4 days over time. Lower percentages of p24+ T cells were observed in cultures with all populations of NK cells compared to controls (Figure 3C and 3D). Additionally, the percent of p24+ T cells were significantly lower in the co-culture with CD4ζ-hESC- (P=0.034, n=3) or CD4ζ-iPSC-NK cells (P=0.029, n=3) compared to unmodified hESC-, iPSC-NK cells respectively. These findings again demonstrate CD4ζ-modified hESC- and iPSC-NK cells inhibited HIV infection more effectively than unmodified hESC-, iPSC-cells. To further confirm that the inhibition of HIV infection is due to NK cell activation, we monitored NK cell degranulation during co-culture with HIV-1-infected CD4+ T cells. Expression of CD107a has been used as a measure of NK cell activity following stimulation [62, 63]. Here, we found CD107a was significantly increased on CD4ζ-hESC- (P=0.045, n=3) and CD4ζ-iPSC-NK cells (P=0.041, n=3) than unmodified hESC- or iPSC-NK cells after co-cultured with HIV-1-infected CD4+ T cells at d7 (Figure 3E and 3F). Overall, these data demonstrate that both CD4ζ-hESC- and CD4ζ-iPSC-NK cells inhibit HIV replication more efficiently than the unmodified counterparts.
In Vivo Anti-HIV Activity of CD4ζ-hESC-NK Cells
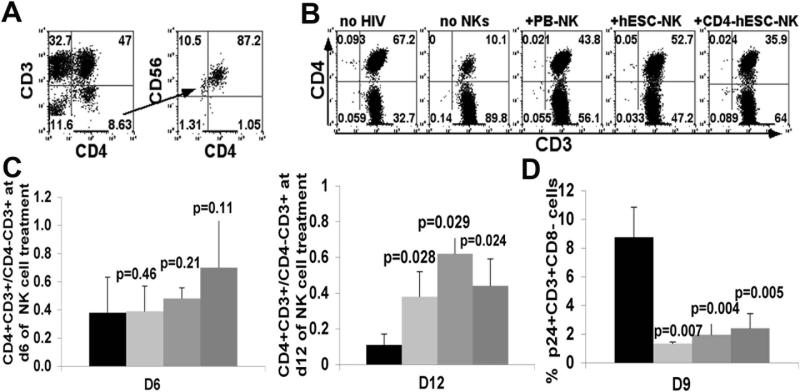
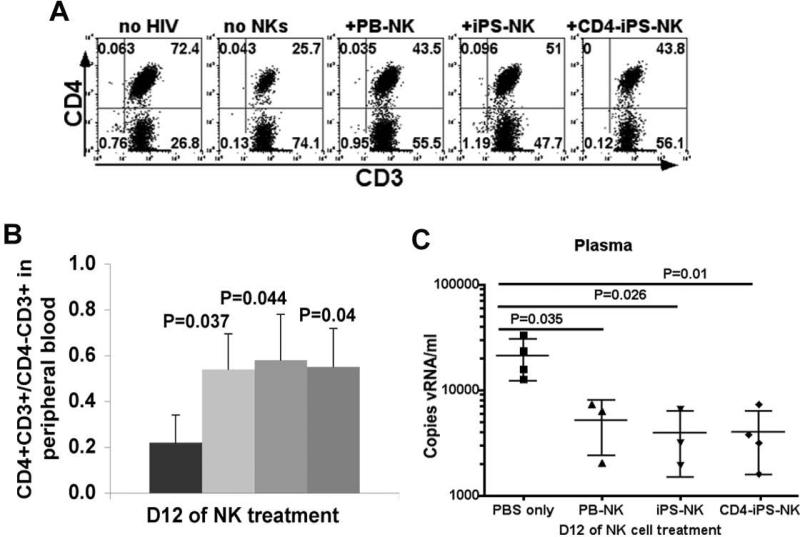
To evaluate hESC-/iPSC-NK cell anti-HIV activity in vivo, we used the hPBL-NSG mouse model of HIV-1 infection [48]. In these studies, 107 PBL are injected intraperitoneally (ip) into NSG mice. After two weeks, the mice were then infected with HIV NL4-3 at 30,000 fifty percent tissue culture infectious doses (TCID50) [50]. This leads to productive HIV infection as demonstrated by loss of CD4+ cells and production of virions [64]. Then, we injected 2 million CD4ζ-hESC-, hESC- or autologous PB-NK cells in the day following HIV infection. At days 6, 9, and 12 post-NK cell treatment, blood samples were collected and examined for CD4 T cell levels determined by human CD4+CD3+/CD4−CD3+ ratios and HIV infection determined by the percent p24+ T cells. A general pattern of higher CD4 T cell level was observed in HIV-infected mice with NK cell treatment (Figure 4), demonstrating suppression of HIV activity [19, 65]. CD4ζ-hESC-NKs cells, illustrated by their expression of CD45, CD56, CD4 and negative for CD3, were detected in peripheral blood early as day 6 after treatment (Figure 4A). At this time point, CD4+CD3+/CD4−CD3+ ratios were seen to decrease less in mice with NK cell treatment than controls (Figure 4B, Figure 4C left panel). By day 12 of NK cell treatment (day 13 of HIV infection), there was a statistically significant difference in CD4+CD3+/CD4−CD3+ ratios between mice treated with CD4ζ-hESC- (P=0.024, n=3), hESC- (P=0.028, n=3), PB-NK (P=0.029, n=3) cells and controls. (Figure 4C, right panel). As CD4 receptor on surface of HIV infected cells is decreased after HIV infection, we used CD3+CD8− instead of CD3+CD4+ to evaluate HIV infection of CD4 T cells. No significant levels of HIV-infected p24+CD3+CD8− cells were observed (less than 1%) at day 6, but by day 9 of NK cell injection, the percentages of p24+CD3+ CD8− cells were dramatically increased in control mice than those treated with either CD4ζ-hESC-, hESC- or PB-NKs (Figure 4D). Notably, we did not observe any statistical difference between CD4ζ-hESC-NK cells and unmodified hESC-NK cells on suppression of HIV infection and retaining CD4 T cell level, but these data indicate that both CD4ζ-modified and unmodified hESC-derived NK cells can inhibit HIV replication and prevent CD4+ T cells depletion in vivo.
Figure 4. hESC-NK cells and CD4ζ-hESC-NK cells suppress HIV replication in peripheral blood.
Two weeks after PBL reconstitution, mice were infected with HIV NL4-3 and treated with NK cells next day. Peripheral blood was then collected at day6, 9 and 12 with or without NK cell treatment. HIV infection was evaluated by CD4+ T cell depletion and HIV+ cell percentage in peripheral blood. CD4+ T cell level was determined by flow cytometry for CD4+CD3+/CD4−CD3+ ratios. HIV infected human cells were evaluated by intracellular staining for gag p24+. (A) CD45+CD3−CD4+ cells that were CD56+ and GFP+ detected in peripheral blood of mice treated with CD4-hESC-NK cells after day 6. (B) Human CD45+ cells that express CD3 and CD4 were assessed in peripheral blood of HIV-1-infected NSG mice treated with or without NK cells at day 6. All cells were hCD45+ gated. Flow cytometry plots are representative of 1 mouse of each condition in at least 3 independent experiments with a minimum of 3 mice in each experimental group. (C) CD4+ T cell levels in peripheral blood of HIV infected mice determined by CD4+CD3+/CD4−CD3+ ratios at day 6 (left panel) and day 12 (right panel) of NK cell treatment. All mice were analyzed prior to HIV infection to set up baseline CD4+CD3+/CD4−CD3+ratios. (D) Suppression of HIV infection was evaluated by the percentages of p24+ cells in all hCD3+CD8− from peripheral blood at day 9 of NK cell treatment. Data in (C) and (D) represent the average of one of three separated experiments with at least 3 mice in each group, the error bars indicate the mean +/− the SD. Statistical comparison of CD4+ T cell level and % p24+CD3+CD8− in NK treated mice to untreated mice was performed using Student's t test. P values are provided for each indicated comparison.
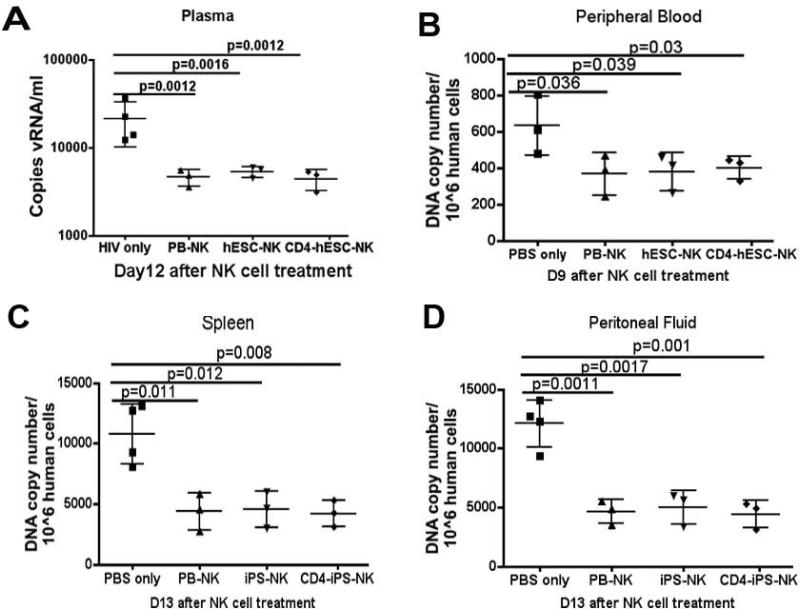
To further determine if the NK cell treatment could suppress HIV replication, the levels of viral RNA in mouse peripheral blood plasma and proviral DNA in different tissues were evaluated. We isolated viral RNA from plasma collected on day 12 of NK cell treatment and performed quantitative reverse PCR was performed to measure the RNA level of long terminal repeat (LTR) sequence [54, 65]. Standard LTR cDNA was used to calculate RNA copy numbers [19, 65]. The viral RNA in HIV-infected mice had approximately 2000 copies/ml, whereas the RNA levels were significantly lower in all mice treated with either CD4ζ-hESC-, hESC- or PB-NK cells at day 12 (Figure 5A). This confirms NK cell-mediated suppression of HIV replication in vivo. To test integrated proviral DNA, peripheral blood was collected from mice at day 6, 9 and 12 post-NK cell treatments. DNA was extracted and proviral elements were detected by specific amplification of HIV-1. We were not able to detect proviral DNA from blood samples by QPCR until day 9 (Figure 5B). Similar to the viral RNA level, the proviral DNA was decreased in peripheral blood in mice receiving NK cells. We did not observe detectable DNA levels in peripheral blood at day 12, which might be due to low levels of CD4+ T cells. To test proviral DNA in other peripheral tissues/organs, mice were then sacrificed on day 13 of NK cell treatment (day 14 of HIV infection) and mouse peritoneal washes and spleens were collected for the quantity of LTR sequences. Again, the DNA levels of HIV were also found to be significantly lower in the peritoneal cavity (Figure 5C) and spleen (Figure 5D) of HIV-1-infected mice treated with CD4ζ-hESC-NK cells, hESC-NK cells or PB-NK cells as compared to control mice. Thus, these findings suggest that NK cell treatment resulted in significant suppression of HIV replication in several tissues/organs in this hPBL NSG mouse model.
Figure 5. CD4ζ- modified and unmodified hESC-NK cells suppress HIV replication in the plasma and tissues of PBL-NSG mice.
(A) Blood plasma from HIV infected mice was collected 12 days after NK cell treatment. Viral RNA levels per sample were determined by quantitative reverse transcriptase (Q-RT)-PCR and results were calculated based on the standard LTR cDNA copy numbers. The points represent the copies of HIV RNA per milliliter of blood and the solid line represents mean per group. HIV proviral DNA was quantitatively assessed in human cells from peripheral blood collected on day 6, 9, 12 day after NK cell treatment. The DNA level was detected by Q-PCR at day 9 (B). Mice were sacrificed at day 13 of NK cell treatment and cells were collected from spleen (C) and peritoneal fluid (D) for Q-PCR. The points represent the copies of HIV proviral DNA per 106 human CD45+ cells and the solid line represents mean per group. Statistical comparison was performed using prism 5 between NK cell treated groups vs. non-treated group. The solid lines represent the mean +/− the SD. The data are representative of one of 3 experiments with at least 3 mice each group.
In Vivo Anti-HIV Activity of CD4ζ-iPSC-NK Cells
Next, we investigated in vivo anti-HIV activities of both CD4ζ-modified and unmodified iPSC-NK cells. Using the PBL-NSG mouse model, we treated HIV-infected mice with CD4ζ-iPSC-, iPSC- or autologous PB-NK cells as above. CD4+ T cell levels (measured as CD4+CD3+/CD4-CD3+ ratios again) were examined at days 6, 9 and 12 post NK cell treatment (Figure 6A). Consistent with CD4ζ-modified and unmodified hESC-NK cells, CD4ζ-iPSC- and iPSC-NK cells were able to retain CD4+CD3+/CD4−CD3+ ratios at higher levels in mice up to two weeks after HIV infection (Figure 6B). The viral load was suppressed to a significantly lower level in the plasma of mice treated with all NK cells compared to controls (Figure 6C). All of these results suggest that NK cells derived from iPSCs are also capable of inhibiting HIV replication in vivo.
Figure 6. iPSC- and CD4ζ- expressing NK cells suppress HIV replication in peripheral blood in HIV-infected mice.
Peripheral blood was collected from HIV-infected NSG mice after NK cell treatment. CD4+ T cell levels were determined by flow cytometry for CD4+CD3+/CD4−CD3+. HIV infected human cells were evaluated by gag p24+. (A) Human CD45+ cells that express CD3 and CD4 were assessed in peripheral blood of HIV-1-infected NSG mice treat with or without NK cells at day 12. All cells were hCD45+ gated. The flow cytometry plots are representative of 1 mouse of each group in at least 3 independent experiments with a minimum of 3 mice. (B) CD4+ T cell levels in peripheral blood of HIV infected mice determined by CD4+CD3+/CD4−CD3+ ratios after NK cell treatment at day 12. (C) HIV infection was evaluated by the percentages of p24+ cells in CD3+CD8−. Data in (B) and (C) represent the average of one of three separated experiments with at least 3 mice in each group, the error bars indicate the mean +/− the SD.
DISCUSSION
Recent studies based on genetic modification of HSCs or mature lymphocytes have begun to use combined gene and immune therapy against HIV/AIDS. However, this approach typically needs to be done on a patient-specific basis, which could be challenging to apply in a broad clinical setting. In contrast, hESC and iPSCs derived cells could provides a novel “universal” and “off-the-shelf” cell population for anti-HIV treatment without concerns for immune rejection. In fact, studies have demonstrated that hESC and iPSCs are able to differentiate into different hematopoietic lineages including recently TCR specific-T cells derived from direct reprogramming or T-iPSCs [23-29], indicating the potential of hESC- or iPSC-based innate and adaptive immunotherapy for HIV.
CARs have been designed and targeted to a wide-array of cancers and virally-infected targets. This system has been suggested a highly effective means to increase immunity in cancer therapy [12-15]. Investigators have also adapted this strategy to HIV treatment by genetic engineering primary T/NK cells with specific anti-HIV TCR or HIV CD4 receptor (reviewed in [4]). The CD4ζ construct has been extensively tested in multiple systems such primary T and NK cells against HIV [36-40]. Some of these trials transduced CD4ζ into primary T cells of HIV-infected patients and turned out varying effect [37, 39]. Here, we combined the unique advantages of the hESCs/iPSCs system and the specificity and efficacy of CARs together to genetically modify hESCs/iPSCs. We initially expressed CD4ζ in hESCs and iPSCs by lentivirus, which was commonly silenced after 10-15 passages (Figure 1C). Our previous studies and others have demonstrated that the Sleeping Beauty (SB) transposon system is a more stable means to transfer genetic information to hESCs [42, 43]. Therefore, we used the SB system to achieve more stable CD4ζ expressing hESC and iPSC cell lines for our in vivo studies.
By using “spin EB” system, we were able to develop NK cells from CD4ζ-modified hESCs and iPSCs that express high levels of CD4 and a panel of surface markers as unmodified hESC-, iPSC- and PB-NKs (Figure 2). We found that both CD4ζ-modified hESC-NK and iPSC-NK cells are able to inhibit HIV replication more efficiently in vitro than their unmodified counterparts respectively (Figure 3). Although KIR expression is associated with NK licensing through interaction with HLA class I molecules, the activation of NK cells is ultimately determined by the balance between inhibitory and activating receptors including but not limited to KIRs [66]. Thus higher KIR expression may not necessary lead increased functional responses at a single cell level [67]. Here we noticed that CD4ζ-iPSC-NKs express higher levels of KIRs (Figure 2b), but their response (measured by CD107a) to HIV-infected targets was similar to CD4ζ-hESC-NKs that had lower KIRs expression (Figure 3B). However, fewer infected T cells (as evidenced by p24+CD4+) in CD4ζ-hESC-NKs and HIV co-culture (Figure 3C) might be secondary to other mechanisms such as increased apoptosis through TNF-related apoptosis-inducing ligand (TRAIL) pathway [68].
During the in vivo assays, we also observed both CD4ζ-modified hESC- and iPSC-NK cells and their unmodified cells were able to suppress HIV infection in PBL-NSG mice (Figure 4, 5 and 6). However, we did not find a significant difference in HIV inhibition from CD4ζ-modified NK cells as in vitro. In this case, we treated HIV-infected mice with 2 × 106 NK cells, which may be insufficient cell number for CD4ζ-hESC-/iPSC-NK cells to induce significant inhibition on viral infection in vivo. The lack of additional co-stimulatory domains present on the intracellular portion ζ chain may limit the activity [69]. As has been demonstrated in several pre-clinical cancer models, CARs containing additional signaling molecules (2nd and 3rd generation CARs) have enhanced in vivo persistence and activity [70]. Additionally, other investigators have found that addition of membrane bound cytokines can also enhance the in vivo activity of CAR containing effectors [67, 71]. Collectively, the CD4ζ receptor may not be the best choice for HIV therapy as its varying effect has been suggested in preclinical studies [37, 39]. Recently, a broadly neutralizing anti-HIV antibody b12, which recognizes gp120 CD4 binding site, has been successfully engineered into human HSPCs and produced functional IgG in culture system [20]. Interestingly, several neutralizing Abs have been identified and characterized (reviewed in [72]). It may be beneficial to engineer them as “CAR” into hESC/iPSC system and test their in vivo efficacy.
Studies by Bernstein et al showed that stimulated CD4+ NK cells had a low percentage of p24+ when infected by HIV-1-Bal (R5) [73]. As we expressed HIV CD4 receptor in hESCs and iPSCs, it is reasonable to question whether the CD4 expression would make hESC- and iPSCs-NK cells susceptible to HIV infection. Fortunately, these hESC- or iPSC-NK cells did not express the HIV co-receptor CXCR4, which is required for HIV-1 X4 or X4R5 entry into targets [74] (Figure 2B). Although a small proportion of CD4ζ-hESC-NK cells are positive for another co-receptor CCR5 (Figure 2B), NK cells cells were not infected when they were co-cultured with HIV-1 (SF2, X4R5)-infected CD4 T cells (Fig.3E).
CXCR3, CCR7 and CD62L are three major receptors involved in NK cells homing to secondary lymphoid organs [59]. We examined their expression on hESC- or iPSC-NK cells (Figure 2B) and found that CXCR3 was comparably expressed on both CD4ζ modified and unmodified hESC- and iPSC-NK cells as PB-NKs. However, CD62L and CCR7 are expressed at a lower level on hESC- and iPSC-NK cells, which may explain why hESC- or iPSC-NK cells were not detected in second lymphoid organs such as spleen in NSG mice after injection (data not shown). It is also pertinent to note that the mouse model does not recapitulate the extent of human physiology and could alter the in vivo trafficking of the infused cells. Subsequent work will aim to improve the homing and trafficking activity of hESC- or iPSC-derived NK cells by enforced expression of defined homing receptors [67, 71], which could also potentially help to clear viral reservoirs.
CONCLUSION
CD4ζ- hESC- and CD4ζ- iPSC-derived NK cells are capable of suppressing HIV replication in vitro with higher efficacy than their unmodified counterparts. Both CD4ζ- modified and unmodified hESC- and iPSC-NK cells demonstrate their inhibition of HIV inhibition in vivo, indicating the feasibility of using hESC/iPSC as a cellular source for combined immune/gene therapy of HIV treatment in vivo. These studies also provide a foundation and a model system to further investigate innate immune responses during viral infection.
Acknowledgements
The authors would like to thank Mike Depp and Dr. Louis Mansky (University of Minnesota) for providing HIV-1 NL 4-3 virus, Dr. Scott Kitchen (UCLA) for providing CD4ζ construct, technical support and suggestions, as well as Dr. Ramesh Akkina (Colorado State University) for providing standard LTR cDNA and RNA and technical support. We would also like to thank Melinda Hexum, Allison Bock and Mike Lepley for technical assistance and David Hermanson for proofreading. This work was supported by NIH grant HL77923 (DSK) and a Grand Challenges Exploration grant from the Bill & Melinda Gates Foundation (DSK), and a fellowship grant from amfAR-The Foundation for AIDS Research (ZN).
Footnotes
Author contributions: Z.N. designed and performed experiments and wrote the manuscript. D.A.K performed experiments and wrote the manuscript. L.B and J.A performed experiments. D.S.K. designed experiments, wrote and edited the manuscript.
REFERENCES
- 1.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. NATURE REVIEWS. 2005;5:835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 2.Iannello A, Boulassel MR, Samarani S, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. JOURNAL OF IMMUNOLOGY. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 3.Kiem HP, Jerome KR, Deeks SG, et al. Hematopoietic-stem-cell-based gene therapy for HIV disease. CELL STEM CELL. 2012;10:137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoxie JA, June CH. Novel cell and gene therapies for HIV. COLD SPRING HARBOR PERSPECTIVES IN MEDICINE. 2012:2. doi: 10.1101/cshperspect.a007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitchen SG, Shimizu S, An DS. Stem cell-based anti-HIV gene therapy. VIROLOGY. 2011;411:260–272. doi: 10.1016/j.virol.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson J, Akkina R. CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection. RETROVIOLOGY. 2005;2:53. doi: 10.1186/1742-4690-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SS, Peer D, Kumar P, et al. RNAi-mediated CCR5 silencing by LFA-1-targeted nanoparticles prevents HIV infection in BLT mice. MOELCULAR THERAPY. 2010;18:370–376. doi: 10.1038/mt.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu S, Hong P, Arumugam B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. BLOOD. 2010;115:1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. NATURE BIOTECHNOLOGY. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alter G, Altfeld M. NK cells in HIV-1 infection: evidence for their role in the control of HIV-1 infection. JOURNAL OF INTERNAL MEDICINE. doi: 10.1111/j.1365-2796.2008.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. THE NEW ENGLAND JOURNAL OF MEDICINE. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 12.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. THE NEW ENGLAND JOURNAL OF MEDICINE. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. SCIENCE TRANSLATIONAL MEDICINE. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. BLOOD. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: genetic modification to redirect effector cell specificity. CANCER JOURNAL (Sudbury, Mass) 2010;16:336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper LJ, Kalos M, Lewinsohn DA, et al. Transfer of specificity for human immunodeficiency virus type 1 into primary human T lymphocytes by introduction of T-cell receptor genes. JOURNAL OF VIROLOGY. 2000;74:8207–8212. doi: 10.1128/jvi.74.17.8207-8212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph A, Zheng JH, Follenzi A, et al. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. JOURNAL OF VIROLOGY. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. NATURE MEDICINE. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchen SG, Levin BR, Bristol G, et al. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLOS PATHOGNE. 2012;8:e1002649. doi: 10.1371/journal.ppat.1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo XM, Maarschalk E, O'Connell RM, et al. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. BLOOD. 2009;113:1422–1431. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 21.Joseph A, Zheng JH, Chen K, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. JOURNAL OF VIROLOGY. 2010;84:6645–6653. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. BLOOD. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vodyanik MA, Bork JA, Thomson JA, et al. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. BLOOD. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 24.Woll PS, Marcus R, Kaufman DS. NK Cells Derived from Human Embryonic Stem Cells Demonstrate More Effective In Vivo Clearance of Xenografted Human Tumor Cells Compared to NK Cells Derived from Cord Blood. BLOOD. 2007;110:Abstract 2745. [Google Scholar]
- 25.Anderson JS, Bandi S, Kaufman DS, et al. Derivation of normal macrophages from human embryonic stem (hES) cells for applications in HIV gene therapy. RETROVIROLOGY. 2006;3:24. doi: 10.1186/1742-4690-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy M, Awong G, Sturgeon CM, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. CELL REPORTS. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. CELL STEM CELL. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. CELL STEM CELL. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Slukvin, Vodyanik MA, Thomson JA, et al. Directed differentiation of human embryonic stem cells into functional dendritic cells through the myeloid pathway. JOURNAL OF IMMUNOLOGY. 2006;176:2924–2932. doi: 10.4049/jimmunol.176.5.2924. [DOI] [PubMed] [Google Scholar]
- 30.Bandi S, Akkina R. Human embryonic stem cell (hES) derived dendritic cells are functionally normal and are susceptible to HIV-1 infection. AIDS RESEARCH AND THERAPY. 2008;5:1. doi: 10.1186/1742-6405-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ni Z, Knorr DA, Clouser CL, et al. Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity utilizing diverse cellular mechanisms. JOURNAL OF VIROLOGY. 2011 doi: 10.1128/JVI.01774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caligiuri MA. Human natural killer cells. BLOOD. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iannello A, Debbeche O, Samarani S, et al. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. JOURNAL OF LEUKOCYTE BIOLOGY. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 34.Knorr DA, Ni Z, Hermanson D, et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. STEM CELLS TRANSLATIONAL MEDICINE. 2013;2:274–283. doi: 10.5966/sctm.2012-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knorr DA, Kaufman DS. Pluripotent stem cell-derived natural killer cells for cancer therapy. TRANSLATIONAL RESEARCH: THE JOURNAL OF LABORATORY AND CLINICAL MEDICINE. 2010;156:147–154. doi: 10.1016/j.trsl.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran AC, Zhang D, Byrn R, et al. Chimeric zeta-receptors direct human natural killer (NK) effector function to permit killing of NK-resistant tumor cells and HIV-infected T lymphocytes. JOURNAL OF IMMUNOLOGY. 1995;155:1000–1009. [PubMed] [Google Scholar]
- 37.Deeks SG, Wagner B, Anton PA, et al. A phase II randomized study of HIV-specific T- cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. MOLECULAR THERAPY. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 38.Yang OO, Tran AC, Kalams SA, et al. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATEDS OF AMERICA. 1997;94:11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsuyasu RT, Anton PA, Deeks SG, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. BLOOD. 2000;96:785–793. [PubMed] [Google Scholar]
- 40.Roberts MR, Qin L, Zhang D, et al. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. BLOOD. 1994;84:2878–2889. [PubMed] [Google Scholar]
- 41.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATEDS OF AMERICA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilber A, Linehan JL, Tian X, et al. Efficient and stable transgene expression in human embryonic stem cells using transposon-mediated gene transfer. STEM CELLS. 2007;25:2919–2927. doi: 10.1634/stemcells.2007-0026. [DOI] [PubMed] [Google Scholar]
- 43.Giudice A, Trounson A. Genetic modification of human embryonic stem cells for derivation of target cells. CELL STEM CELL. 2008;2:422–433. doi: 10.1016/j.stem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Ng ES, Davis RP, Azzola L, et al. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. BLOOD. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 45.Ng ES, Davis R, Stanley EG, et al. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. NATURE PROTOCOLS. 2008;3:768–776. doi: 10.1038/nprot.2008.42. [DOI] [PubMed] [Google Scholar]
- 46.Le Garff-Tavernier M, Beziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. AGING CELL. 2010;9:527–535. doi: 10.1111/j.1474-9726.2010.00584.x. [DOI] [PubMed] [Google Scholar]
- 47.Levy JA, Hoffman AD, Kramer SM, et al. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. SCIENCE (New York, NY) 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 48.Mosier DE, Gulizia RJ, Baird SM, et al. Transfer of a functional human immune system to mice with severe combined immunodeficiency. NATURE. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 49.Dapp MJ, Clouser CL, Patterson S, et al. 5-Azacytidine can induce lethal mutagenesis in human immunodeficiency virus type 1. JOURNAL OF VIROLOGY. 2009;83:11950–11958. doi: 10.1128/JVI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks DG, Cohen MD, Jamieson BD, et al. Rapid size dependent deletion of foreign gene sequences inserted into attenuated HIV-1 upon infection in vivo: implications for vaccine development. CURRENT HIV RESEARCH. 2005;3:377–392. doi: 10.2174/157016205774370410. [DOI] [PubMed] [Google Scholar]
- 51.Woll PS, Grzywacz B, Tian X, et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. BLOOD. 2009;113:6094–6101. doi: 10.1182/blood-2008-06-165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berges BK, Akkina SR, Remling L, et al. Humanized Rag2(−/−)gammac(−/−) (RAG-hu) mice can sustain long-term chronic HIV-1 infection lasting more than a year. VIROLOGY. 2010;397:100–103. doi: 10.1016/j.virol.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neff CP, Kurisu T, Ndolo T, et al. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLOS ONE. 2011;6:e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. JOURNAL OF CLINICAL MICROBIOLOGY. 2005;43:2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beziat V, Hervier B, Achour A, et al. Human NKG2A overrides NKG2C effector functions to prevent autoreactivity of NK cells. BLOOD. 2011;117:4394–4396. doi: 10.1182/blood-2010-11-319194. [DOI] [PubMed] [Google Scholar]
- 56.Grzywacz B, Kataria N, Sikora M, et al. Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. BLOOD. 2006;108:3824–3833. doi: 10.1182/blood-2006-04-020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freud AG, Yokohama A, Becknell B, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. THE JOURNAL OF EXPERIMENTAL MEDICINE. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berkowitz RD, Alexander S, Bare C, et al. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. JOURNAL OF VIROLOGY. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moretta A, Marcenaro E, Parolini S, et al. NK cells at the interface between innate and adaptive immunity. CELL DEATH AND DIFFERENTIATION. 2008;15:226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 60.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. THE JOURNAL OF EXPERIMENTAL MEDICINE. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gervaix A, West D, Leoni LM, et al. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATEDS OF AMERICA. 1997;94:4653–4658. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. THE JOURNAL OF EXPERIMENTAL MEDICINE. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlsten M, Bjorkstrom NK, Norell H, et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. CANCER RESEARCH. 2007;67:1317–1325. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 64.Beziat V, Descours B, Parizot C, et al. NK cell terminal differentiation: correlated stepwise decrease of NKG2A and acquisition of KIRs. PLOS ONE. 2010;5:e11966. doi: 10.1371/journal.pone.0011966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neff CP, Zhou J, Remling L, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. SCIENCE TRANSLATIONAL MEDICINE. 2011;3:66ra66. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carrington M, Alter G. Innate immune control of HIV. COLD SPRING HARBOR PERSPECTVES IN MEDICINE. 2012;2:a007070. doi: 10.1101/cshperspect.a007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beziat V, Traherne JA, Liu LL, et al. Influence of KIR gene copy number on natural killer cell education. BLOOD. 2013;121:4703–4707. doi: 10.1182/blood-2012-10-461442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melki MT, Saidi H, Dufour A, et al. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLOS PATHOGENS. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beziat V, Nguyen S, Lapusan S, et al. Fully functional NK cells after unrelated cord blood transplantation. LEUKEMIA. 2009;23:721–728. doi: 10.1038/leu.2008.343. [DOI] [PubMed] [Google Scholar]
- 70.Hervier B, Beziat V, Haroche J, et al. Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-gamma production in patients with active disease. ARTHRITIS AND RHEUMATISM. 2011;63:1698–1706. doi: 10.1002/art.30313. [DOI] [PubMed] [Google Scholar]
- 71.Beziat V, Liu LL, Malmberg JA, et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. BLOOD. 2013;121:2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burton DR, Poignard P, Stanfield RL, et al. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. SCIENCE (New York, NY) 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernstein HB, Wang G, Plasterer MC, et al. CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. VIROLOGY. 2009;387:59–66. doi: 10.1016/j.virol.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nguyen S, Beziat V, Roos-Weil D, et al. Role of natural killer cells in hematopoietic stem cell transplantation: myth or reality? JOURNAL OF INNATE IMMUNITY. 2011;3:383–394. doi: 10.1159/000323935. [DOI] [PubMed] [Google Scholar]