Summary
Mechanistic target of rapamycin (mTOR) regulates cell growth, metabolism and aging in response to nutrients, cellular energy stage and growth factors. mTOR is frequently up-regulated in cancer including hepatocellular carcinoma (HCC) and is associated with bad prognosis, poorly differentiated tumors, and earlier recurrence. Blocking mTOR with rapamycin and first generation mTOR inhibitors, called rapalogs, has shown promising reduction of HCC tumors growth in preclinical models. Currently, rapamycin/rapalogs are used in several clinical trials for the treatment of advanced HCC, and as adjuvant therapy in HCC patients after liver transplantation and TACE. A second generation of mTOR pathway inhibitors has been developed recently, and is being tested in various clinical trials of solid cancers and has been used in preclinical HCC models. The results of series of clinical trials using mTOR inhibitors in HCC treatment will emerge in the near future.
Introduction
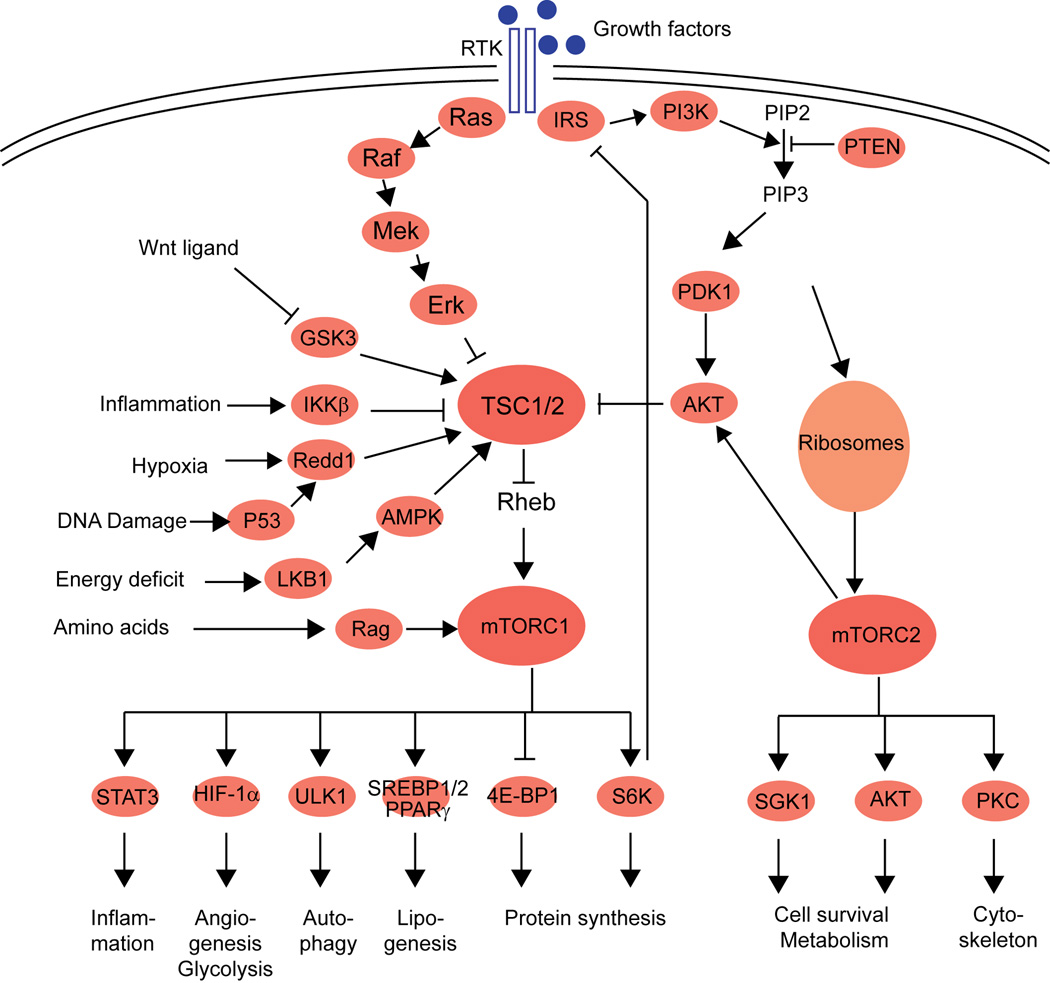
Target of rapamycin (TOR) is an evolutionary well conserved serine/threonine protein kinase that belongs to the phosphoinositide 3-kinase (PI3K)-related kinase family. Mechanistic TOR (mTOR; originally called mammalian TOR) has a broad range of action and is involved in regulation of cell growth, aging and metabolism1. mTOR can be divided into two structurally and functionally distinct complexes named mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2)1. mTORC1 is composed of mTOR, mLST8, DEPTOR, RAPTOR and PRAS40. mTORC2 consists of mTOR, mLST8, DEPTOR, PROTOR, RICTOR and mSIN11. mTORC1 is a nutrient and energy sensor at both cellular and whole-body levels2. When nutrients are available, mTORC1 is activated and stimulates anabolic processes such as protein synthesis, lipogenesis, and energy metabolism, whereas autophagy and lysosome biogenesis is inhibited1 (for more details see Figure 1). mTORC1 is activated by a myriad of inputs such as growth factors, energy status, proinflammatory cytokines, oxygen levels, amino acids, and the canonical Wnt pathway1 (Figure 1). Growth factors, e.g. insulin and insulin-like growth factor 1 (IGF1), exert their action on mTORC1 through receptor tyrosine kinases (RTK) and the well-characterized PI3K-AKT and Ras-Raf-Mek-Erk signaling pathways. These pathways activate mTORC1 by phosphorylating and thereby inhibiting the tumor suppressor TSC1-TSC2 (tuberous sclerosis 1 and 2) complex. The TSC1-TSC2 complex is a key regulator of mTORC1 and functions as a GTPase-activating protein (GAP) that negatively regulates Rheb by converting it into its inactive GDP-bound state3, 4. In contrast, down-regulation of mTORC1, is accomplished via activation of the TSC1-TSC2 complex by AMPK, LKB1 and REDD1 in situations of low energy (high AMP), low oxygen levels5 and DNA damage6.
Figure 1.
Schematic overview of the mTOR signaling pathway with the most important factors and their action.
Much less is known about the later discovered mTORC2 signaling pathway. mTORC2 is insensitive to nutrients but does respond to growth factors such as insulin in association with ribosomes7. Besides its initial described role in actin cytoskeleton organization, mTORC2 also activates cell metabolism, survival, and growth. TORC2-ribosome interaction is a likely conserved mechanism of TORC2 activation that is physiologically relevant in both normal and cancer cells.
Involvement of mTOR pathway in hepatocellular carcinoma (HCC)
Given its importance in cell growth and metabolism it is not surprising that mTOR plays a pivotal role in HCC. mTORC1 and mTORC2 pathways, including pRPS6, p-AKT, IGF-1R and RICTOR are up-regulated in 40-50% of HCCs8–10. A similar upregulation is observed in other common cancer types such as breast, colon and lung carcinomas11. Moreover an up-regulation is frequently observed in cholangiocarcinoma, the second most common primary cancer of the liver12. Activation of the mTOR pathway in HCC is associated with less differentiated tumors, bad prognosis, and earlier recurrence independently of the underlying etiology of liver cancer9, 13, 14. Furthermore, it is associated with deregulation of EGF, IGF and PTEN pathways9 and, as expected, with increased lipogenesis in the tumor15. Surprisingly, alterations in copy number or somatic mutations of PTEN, PIK3CA, and PIK3B were not identified as major mechanisms of mTOR pathway deregulation in HCC by PCR9. In accordance, more recent studies using next-generation sequencing technique revealed a low frequency of mutations in the mTOR pathway including mTOR, PIK3CA and PTEN among others16–18. The most frequently mutated gene, found in one study in 9.6% of HCC was RPS6KA3, a serine/threonine kinase involved in regulating PI3K/RAS signaling16. Therefore, mutations in the mTOR pathway is a rare event in HCC and activation of the mTOR pathway appears to result largely from ligand dependent receptor activation.
Genomic studies in the past have identified multiple molecular classifications of HCC and demonstrated deregulated signaling pathways unique to subgroups of patients19–24. These studies indicated that the mTOR pathway and its upstream pathways PI3K and AKT occupy a central position in the network of deregulated signaling pathways in HCC. With the specific aim to identify driver genes associated with HCC prognosis, we have used an integrative approach combining data obtained from somatic copy number analysis and transcriptomics25. Fifty driver genes were recognized and were linked to the mTOR, AMPK or EGFR pathways. In the molecular HCC classification by Boyault et al., 6 robust subgroups (G1-G6) were identified and the G1/G2 subgroup showed AKT activation with overexpression of IGF2, IGF1R and GSK3β as well as PIK3CA, and AXIN1 mutations19. The G1/G2 patient subgroup was further confirmed in a large meta-analysis using integrative transcriptomics of 9 HCC data sets including a total of 603 patients26. This analysis assigned the patients into three subclasses (S1-S3), and the G1/G2 subgroup was enriched in the subclass S2, characterized again by activation of the upstream regulator of mTOR, AKT, in combination with MYC.
Taken together, activation of mTOR plays a central role in HCC and blocking this pathway is an attractive strategy for HCC treatment. The main goal of this review is to offer the rationale for the use of mTOR inhibitors in HCC and provide an overview of the current and prospective clinical trials with mTOR inhibitors in HCC.
Rapamycin and first generation mTOR inhibitors
mTOR is targeted by rapamycin, a natural compound discovered from the bacterium Streptomyces hygroscopius more than 30 years ago. The two mTOR-containing complexes have different sensitivities to rapamycin. mTORC1 is inhibited by a complex formed by rapamycin and FKBP12 protein27. In contrast, mTORC2 is generally resistant to rapamycin, however, in certain cell types, mTORC2 may show sensitivity after prolonged rapamycin treatment28. Rapamycin (sirolimus) was first approved as an immunosuppressant for the prevention of graft rejection in kidney transplant recipients more than a decade ago2. A few years later rapamycin obtained approval for its use as an anti-restenosis agent following balloon angioplasty in coronary arterial stents. The early success of rapamycin has encouraged the development of derivative compounds with improved bioavailability, called rapalogs: everolimus (RAD001), temsirolimus (CCI-779), and deforolimus (AP23573). Due to the important role of mTOR in cell growth and metabolism the primary interest shifted to anti-cancer therapy, and in 2007 temsirolimus (CCI779) was approved for the treatment of renal cell carcinoma and shortly thereafter for mantle cell lymphoma. Meanwhile, everolimus (RAD001), has received approval for treatment of pancreatic neuroendocrine tumours, subependymal giant cell astrocytoma, renal cell carcinoma and HER2-negative breast cancer in combination with Exemestane.
In general, first generation mTOR inhibitors are well tolerated. The major toxicities include, stomatitis, headache, diarrhea, vomiting and thrombocytopenia. Due to their role in metabolism they can cause hyperglycemia, hyperlipidemia and hypophosphatemia. As for every immunosuppressive drug, the risk for infections is increased. Furthermore, reactivation of HBV is a serious complication and has been described in renal cell carcinoma patients under everolimus treatment29, 30. EASL Clinical practice Guidelines recommend in which situation patients undergoing immunosuppressive therapy should receive prophylactic treatment against HBV-reactivation with a nucleoside analogue31.
Currently, neither rapamycin nor rapalogs have gained approval for HCC treatment. However, several clinical trials are ongoing or have recently been completed using rapamycin/rapalogs for the treatment of advanced HCC and as adjuvant therapy after transarterial chemoembolization (TACE) or after liver transplantation of HCC patients. Furthermore, several studies suggest that mTOR inhibition may even prevent HCC development in patients at risk. The application of mTOR inhibitors in these different settings will be discussed in more detail.
1. Prevention of liver cancer by mTOR inhibition
Several studies indicate that mTOR activation is involved in the initiation of liver cancer and plays a role in the malignant transition of hepatocytes to HCC. A gradual activation of the AKT/mTOR pathway was progressively induced from non-tumorous liver tissue toward the HCC thus supporting this concept15. Interestingly, increased mTOR activity conferred a preneoplastic phenotype to HepaRG, considered a terminally differentiated hepatic cell line32. Also, treatment with mTOR inhibitor everolimus prevented proliferation of hepatocytes suffering DNA damage in the fumarylacetoacetate hydrolase-deficient mouse model of chronic liver injury and HCC development33. In transgenic mice, mTOR activation by itself was shown to be sufficient for HCC development. Two independent studies analyzed liver-specific knockout of TSC1 and the resulting chronic activation of mTOR34, 35. Both studies demonstrated the development of liver tumors, however, only one was associated with inflammation34. Likewise constant activation of mTOR in PTEN-deficient mice induced steatohepatitis and development of liver tumors36. mTOR inhibitors may also prevent HCC indirectly by reducing liver fibrosis, a risk factor for the development of HCC. Studies in rats have demonstrated that sirolimus and everolimus attenuated progression of fibrosis, in contrast to cyclosporine A and tacrolimus, two other immunosuppressive drugs37, 38.
Randomized prospective clinical trials have not been conducted to address the question if mTOR inhibitors prevent HCC. The evidence suggesting a potential HCC prevention is solely derived from epidemiological studies with metformin, a widely used anti-diabetic drug that reduces mTOR activity but importantly also ameliorates hyperinsulinemia, which is a risk factor for HCC39. Metformin activates AMPK, which in turn suppresses the mTORC1 pathway (Fig. 2)40. A recent study showed that metformin controlled gene expression at the level of mRNA translation to an extent comparable to that of canonical mTOR inhibitors and down regulated mRNAs, which encode for proliferation and tumor-promoting proteins via the mTORC1/4E-BP pathway41. In addition, it was shown that metformin inhibited mTORC1 signaling independently of AMPK by suppressing Rag GTPase or activating REDD142, 43. A meta-analysis, including 5 clinical studies with more than 100 000 type 2 diabetes patients has been performed lately44. This study showed an overall estimated 62% reduction in the risk of liver cancer if metformin was used as an anti-diabetic treatment instead of non-metformin treatment (e.g. sulfonylurea or insulin). Although there was considerable heterogeneity between the studies, the results are encouraging. Preventive clinical trials with metformin are already underway for different cancer types such as breast cancer, colon cancer and esophagus cancer, however, for HCC they are lacking. Information of the potential preventive mechanism(s) of metformin on liver cancer is also limited. Recently, it was shown in vitro and in vivo, that metformin inhibited HCC cell growth through AMPK and LKB145, 46. Further elucidation of this mechanism(s) and defining criteria that identify individuals which are likely to benefit are therefore needed. Nevertheless, it has to be kept in mind that a few studies have shown that metformin can have tumor-promoting effects. Metformin enhanced growth of BRAF-mutant melanoma cells and ER-alpha negative breast cancer cell lines in vivo47, 48. Likewise, migration and invasion abilities of human pulmonary adenocarcinoma A549 cell lines increased under metformin treatment in vitro49.
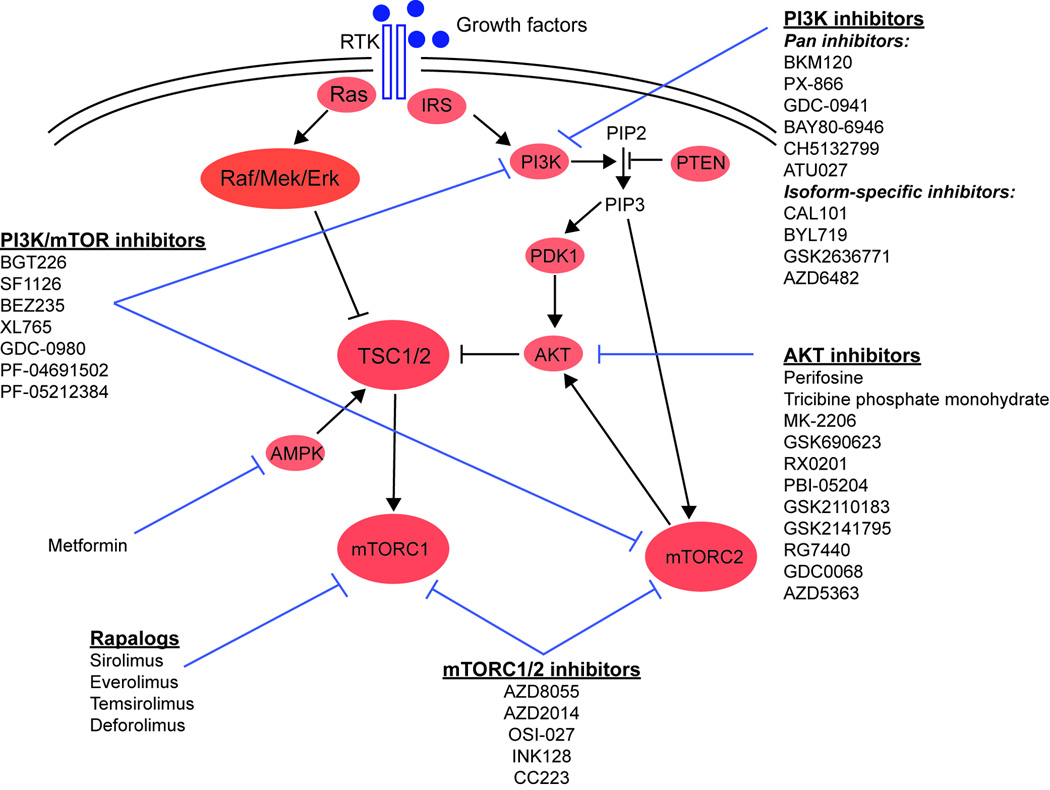
Figure 2.
Schematic overview of the mTOR signaling pathway with the target position of drugs.
Prevention of HCC by metformin and mTOR inhibitors may also involve autophagy, an important homeostatic cellular recycling mechanism, which has a dual role in carcinogenesis. Autophagy is important in limiting DNA damage caused by the accumulation of reactive oxygen species and damaged organelles through removal of dysfunctional proteins and organelles50. Especially in the context of HCC development, which arises in 80% of the cases in the background of a chronic liver inflammation with constant DNA damage, induction of autophagy by mTOR inhibitors may prevent HCC development and recurrence. In contrast, increased autophagy may promote tumor growth, especially once tumor is established, as autophagy is also a pro-survival mechanism to cellular stress and further enhances chemoresistance50, 51.
2. Adjuvant therapy with mTOR inhibitors after liver transplantation or TACE
For patients with non-resectable HCC and early stage disease, liver transplantation is recognized as the treatment of choice, with disease-free survival of 60-80% at 5 years52. Immunosuppressants, such as cyclosporine A, tacrolimus and rapamycin are crucial for the prevention of graft rejection after transplantation. However, cyclosporine A and tacrolimus are known to induce tumor growth in preclinical studies53, 54. On the contrary, rapamycin has additional anticancer function. Accordingly, a meta-analysis of 5 studies with a total of almost 3000 patients indicated that survival is significantly prolonged after liver transplantation of HCC patients if sirolimus is administered instead of non-sirolimus immunosuppressives55. Although these results indicate that sirolimus should be used as treatment of choice after liver transplantation of HCC patients, it has to be noted that none of the clinical studies conducted so far were randomized. Additional information regarding the efficacy of sirolimus to improve survival and prevent recurrence should be provided by three ongoing randomized controlled studies (Table 1). The largest trial is a multicenter, phase III study with over 500 patients (SiLVER) comparing sirolimus-based versus sirolimus-free immunosuppression in patients undergoing liver transplantation for HCC. However, results are not expected before 2014.
Table 1.
Summary of completed and ongoing clinical trials with first generation mTOR inhibitors in HCC
| Drug | Patient number |
HCC Stage | Phase | Child- Pugh Score |
Study design | Results (or ID in Clinical Trials website) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Adjuvant therapy after liver transplantation | Sirolimus vs mTOR inhibitor free | 510 | Milan criteria and extended | III | NA | Randomized | Active, not recruiting (NCT00355862, SiLVER) | ||
| Sirolimus vs FK506 | 220 | Exceeding Milan criteria | III | NA | Randomized | Recruiting (NCT00554125) | |||
| Sirolimus vs mTOR inhibitor free | 86 | Exceeding Milan criteria | II | NA | Randomized | Recruiting (NCT01374750) | |||
| Adjuvant therapy after TACE | TACE (Doxorubicin) +/− everolimus | 98 | Intermediate | I/II | A, B (≤7) | Randomized | Recruiting (NCT01009801) | ||
| TACE +/− Everolimus | 80 | Intermediate | II | A, early B | Randomized | Recruiting (NCT01379521, TRACER) | |||
| Treatment of advanced HCC | Single drug | First line therapy | Sirolimus | 21 | I to IV (TNM) | pilot | A, B, C | Non-randomized | Completed63. 1 PR Dose determined by plasma level. Median dose was 1mg/d; |
| Sirolimus | 18 | BCLC B, C, D | pilot | A, B, C | Non-randomized | Completed62. No objective response Twice daily. Dose determined by plasma level. Median dose was not reported. | |||
| Sirolimus | 25 | BCLC B or C | II | A, B | Non-randomized | Completed64. 1 CR and 1 PR, 30mg/week; | |||
| Temsirolimus | 50 | advanced | II | B | Non-randomized | Terminated: toxic events | |||
| First and second line | Everolimus | 28 | advanced | I/II | A, B | Non-randomized | Completed60. 1 PR MTD = 10mg/day; | ||
| Everolimus | 39 | III (AJCC) | I/II | A, B | Randomized | Completed61. 1 PR MTD = 7.5mg/day or 70mg/week but DLT not reach for weekly schedule; | |||
| Second line therapy | Everolimus | 546 | advanced | III | A | Randomized | Completed, not yet published, failed to demonstrate efficacy compared to placebo (NCT01035229, EVOLVE) | ||
| Temsirolimus | 50 | advanced | I/II | A | Non-randomized | Active, not recruiting (NCT01251458) | |||
| Temsirolimus | 25 | advanced | II | A, B (≤9) | Non-randomized | Recruiting (NCT01567930) | |||
| Drug combination | First and second line | Sorafenib and Temsirolimus | 25 | III, IV | I | A, B (≤7) | Non-randomized | Completed70. 2 PR; MTD = temsirolimus 10mg/week + sorafenib 200mg twice daily; | |
| First line therapy | Sorafenib +/− Everolimus | 130 | Advanced liver cancer | I/II | A | Non-randomized | Terminated: everolimus MTD too low | ||
| Sorafenib +/− Everolimus | 106 | B,C BCLC | II | A, B (≤7) | Randomized | Active, not recruiting (NCT01005199) | |||
| Sorafenib and Everolimus | 30 | III, IV and liver dysfunction | I | A, B (≤7) | Non-randomized | Active, not recruiting (NCT01013519) | |||
| Sorafenib and Everolimus | 28 | AJCC II,III,IV | II | A, B (≤7) | Non-randomized | Recruiting (NCT01687673) | |||
| Second line therapy | Everolimus+ Bevacizumab | 24 | advanced | I/II | A, B | Non-randomized | Completed. Not yet published (NCT00775073) | ||
| Sirolimus + Bevacizumab | 24 | advanced | I | A, B | Non-randomized | Completed69. 1 CR and 2 PR; MTD = 4mg/day, | |||
| Sorafenib and Everolimus | 18 | advanced | I/II | A, B (≤8) | Non-randomized | Recruiting (NCT01335074) | |||
| Everolimus and Pasireotide | 30 | BCLC stage C | II | A | Non-randomized | Recruiting (NCT01488487) | |||
Recruiting; the study is currently recruiting participants,
Active not recruiting: The study is ongoing but potential participants are not currently recruited
Completed: The study has ended normally, and participants are no longer being examined or treated
Terminated: The study has stopped recruiting participants early and will not start again
PR: partial response; CR: complete response
TACE is the treatment of choice for intermediate HCC patients52. Following TACE treatment the levels of vascular endothelial growth factor (VEGF) have been shown to increase. VEGF promotes angiogenesis and is associated with bad prognosis. Therefore, TACE has been combined in clinical trials with other drugs that inhibit angiogenesis such as sorafenib, brivanib or orantinib. Interestingly, everolimus has also been shown to reduce vessel formation in preclinical HCC models56 and two ongoing clinical trials are evaluating the effect of TACE in combination with everolimus (Table 1).
3. mTOR inhibition in advanced HCC
Sorafenib is the only approved drug for HCC treatment57, 58. However, the treatment effects are small, only selected patients are eligible for therapy, and side effects often limit applicability. Therefore, developing novel and effective therapies are urgently needed. Rapalogs have been shown to inhibit liver tumor growth in a large number of in vitro and in vivo pre-clinical studies59 and have encouraged clinical trials in HCC patients (Table 1, information retrieved form www.clinicaltrials.gov). Two phase I/II dose-finding studies of 39 and 28 patients have been completed, using everolimus as a first and second line single agent and resulted in dose recommendations of 7.5mg/day and 10mg/day respectively60, 61. In both studies, complete response was not observed, however, one HCC patient in each study showed a partial response, and stable disease was observed for a short time in 71.4% and 40% of the patients. Three earlier clinical studies with 18 to 25 patients using rapamycin as a first line single agent provided interesting results with partial responses and even one complete response; however, the differences in treatment schedule prevent any firm conclusions62–64. Overall, these preliminary results using rapamycin/rapalogs are encouraging; however, further studies with more patients are needed. Besides, one study using temsiroliums as a first line agent had to be terminated due to toxic events. The recent outcome of a multicenter randomized, double blind, phase III study dashed the hope to use mTOR inhibitors as a second line therapy for advanced HCC patients (press release). This study investigated the effect of everolimus or placebo in 546 patients with Child-Pugh A cirrhosis, whose disease progressed after treatment with or who were intolerant to sorafenib. It will be interesting to see if two other phase I/II studies using temsirolimus as a second line single agent obtain similar negative results.
Because of resistance and compensatory activation of other signaling pathways the effect of rapalogs can be diminished. For example, after treatment with everolimus, an upregulation of MAPK was shown in tumor samples from breast cancer patients65. As anticipated, combination of mTOR inhibition with a MAPK inhibitor (sorafenib) showed enhanced anti-tumoral effect in vitro and in vivo in cancer models including HCC65–68. A combinatorial approach is currently performed in 9 clinical trials (Table 1). Rapalogs or rapamycin is complemented with multikinase inhibitor sorafenib, VEGF inhibitor bevacizumab or with pasireotide, a somatostatin analog which has been shown to inhibit tumor growth. Two phase I combination studies have been published recently69, 70. A combination of temsirolimus with sorafenib at the maximal tolerated dose (MTD; temsirolimus 10mg weekly and sorafenib 200mg twice daily), showed a partial response in 8% of the patients and a stable disease in 60%70. Although these results in 25 patients are promising, the median progression free survival was only 5.65 months, which is similar to outcomes from single agent sorafenib in the SHARP trial, though superior to outcomes observed in an Asia-Pacific clinical trial57, 58. In addition, a phase I study with rapamycin and bevacizumab of 24 patients at MTD (rapamycin 4mg/day and bevacizumab 5 mg/kg every 14 days), reported a remarkable complete response in one patient that lasted 4.5 months, partial response in two patients, and stable disease in 14 patients69. The ongoing clinical trials in phase I or II will reveal if a combinatorial approach improves efficacy in HCC treatment. The combination of drugs is certainly attractive, however, one drawback is that toxicities may increase, especially in cirrhotic patients. and prevent that efficacious dose can be applied. It is therefore important to obtain pharmacokinetic studies of both drugs (e.g. sorafenib and raplogs) with dose escalation of both drugs in cirrhotic patient in future clinical trials.
Second generation mTOR inhibitors
We are currently awaiting the results of several clinical trials using rapalogs in HCC treatment; however, the success of rapalogs in cancer therapy in general has not been as impressive as initially hoped. Several possible reasons may account for the limited action of rapalogs. First, mTORC1 inhibition abrogates the negative feedback loop, which in turn activates PI3K-AKT with MAPK and RAS signaling and therefore may actually increase growth of cancer cells65. Second, blocking of mTORC1 primarily leads to inhibition of cell growth and not cell death. Third, mTOR inhibition by rapalogs mainly results in inhibition of S6K, however, the second key substrate, 4E-BP1, is only insufficiently blocked71. Finally, rapalogs do not inhibit mTORC2, which is often activated as part of the PI3K-mTORC2-AKT signaling axis2. In order to overcome the shortcomings and resistance of rapalogs a second generation of mTOR inhibitors has been developed that function as ATP-competitive inhibitors of mTOR and have several advantages over rapalogs. Unlike rapalogs, which inhibit only mTORC1, the ATP analogues block the phosphorylation of all known downstream targets of mTORC1 and mTORC2. Furthermore, because of the similarity between the kinase domains of mTOR and PI3Ks, some of these new compounds additionally inhibit PI3K, leading to a broad inhibitory action with blocking of the feedback activation of PI3K-AKT signaling described before. Second generation mTOR inhibitors can therefore be divided into mTORC1/2 inhibitors and mTOR/PI3K inhibitors (Figure 2). In addition, a series of compounds have been developed that block upstream of the mTOR pathway such as AKT inhibitors and PI3K inhibitors.
1. Translational genomics and development of second generation mTOR inhibitors
Translational genomic analyses have been used to investigate the resistance to rapamycin. Jimenez et al.72 investigated rapamycin sensitivity or resistance in 13 HCC cell lines using drug-induced growth inhibition as the end point. The authors concluded that promoting or even maintaining effective drug sensitivity might be challenging, because of the molecular heterogeneity observed in the genomic profiles introduced in response to rapamycin, as well as determining a specific mechanism(s) of drug resistance. Furthermore, although the sensitivity to rapamycin was variable in all cell lines, the drug inhibited the phosphorylation of RPS6 and 4E-BP1, indicating that S6 and 4E-BP1 phosphorylation is not a useful marker for antiproliferative effect of mTOR inhibitors. Interestingly, in an attempt to identify rapamycin sensitive genes a later study determined that many of the genes which expression is altered by rapamycin are E-box containing and their regulation via mTOR was c-MYC independent73. Since the mTOR pathway is constitutively activated in a majority of diffuse large B-cell lymphoma this is an interesting disease model to study resistance mechanisms to mTOR inhibitors and to identify drugs which may compliment the action of rapamycin74. The authors compared the genomic signatures of 4 rapamycin sensitive and 4 resistant cell lines, and found that the central mechanism involved in the resistance to rapamycin was controlled by AKT. Using the genomic signatures to explore the Connectivity Map database to identify drugs which may reverse drug resistance, PI3K/AKT and HDAC inhibitors were the most likely candidates to synergize with an mTOR inhibitor. Resistance mechanisms to rapamycin and its derivates also include epigenome based reprogramming (e.g. miRs). Long-term rapamycin treatment results in an increase of the miR-17-92 cluster and inhibition of this change restored the drug sensitivity75. Likewise, in HCC the miR-216a/217 cluster is frequently upregulated, resulted in activation of the PI3K/AKT pathway and importantly resistance to sorafenib76.
2. Preclinical studies of second mTOR, AKT and PI3K inhibitors in HCC
Several preclinical studies with second generation mTOR, AKT, and PI3K inhibitors were performed in HCC cell lines77–79, HCC xenograft mouse model77, 79, DEN-mouse HCC model80 and in a diabetic rat model of HCC81. These studies have demonstrated that the second generation inhibitors were able to control HCC proliferation better than everolimus or sirolimus. It has also been demonstrated that combination of a PI3K inhibitor (BKM120) with cisplatin77 or a PI3K/mTOR dual inhibitor (BEZ235) with everolimus80 could act synergistically with strong antitumor activity. The two drugs, everolimus and BEZ235 exerted tumor regression via inhibition of mTORC1 and mTORC2. This combined drug-effect was associated with an increase in autophagy independent of 4E-BP1. At low doses both drugs targeted mTORC1, however, inhibition of mTORC2 was enhanced by the drug combination. The cooperative drug-effect was further evident from the microarray analysis identifying a distinct set of genes, suggesting a phenotypic reversal similar to placebo-treated livers. Also, only the drug combination achieved significant inhibition of genes involved in cell cycle. These results have prompted a dose finding and a safety clinical trial of BEZ235 in combination with everolimus in patients with advanced solid tumors.
Moreover, promising result may be expected from the combination of second generation mTOR, AKT or PI3K inhibitors with other drugs. This may be particularly important in cases of sorafenib resistance which was recently demonstrated in a cancer stem cell subpopulation of HCC cells (i.e. label-retaining cancer cells), demonstrating sustained AKT and MAPK activation82. Also, in a study comparing the HCC cell line (Huh7) and sorafenib resistance Huh7 derivatives, the molecular alteration related to the acquired drug resistance included upregulation and activation of PI3K/AKT signaling83. The sorafenib resistance was overcome by either silencing AKT using RNAi or targeting the pathway using the novel allosteric AKT inhibitor (MK-2206). In agreement, the PI3K/mTOR inhibitors (PI103 and PKI-587) also augment the effect of sorafenib84, 85. Similarly, a recent study suggested that second-generation inhibitors (e.g. mTOR (AZD-8055), PI3K (BKM-120) or mTOR/PI3K (BEZ-235 and GDC-0980)) may be effective in sorafenib resistant HCC86. It should be noted that this study unfortunately was performed in SK-HEP1 and its drug-resistant derivative (SK-Sora) which are derived from a HCC patient with ascites but are of endothelial origin. The combination of everolimus together with an AKT inhibitor (MK-2206), mTOR/PI3K inhibitor (BEZ235) or PI3K inhibitor (BKM120) showed improved anti-tumoral activity77, 78, 80. Further, improved efficacy was also achieved in vitro and in vivo with novel ATP-competitive mTOR kinase inhibitors together with histone deacetylase inhibitors87.
3. First results of clinical trials with new mTOR pathway inhibitors
The results of several phase 1 clinical trials in advanced cancer (all types) are now available with this new generation of mTOR inhibitors. A phase 1 clinical trial with patients suffering from solid tumors and lymphoma using pan-mTOR inhibitor AZD805588, demonstrated dose limiting toxicities (DLT) of grade 3 with an increase in transaminases and no RECIST objective response88. Accordingly, AZD8055 will not be further tested. It is interesting to note that altered liver function was not described with mTOR inhibitors such as temsirolimus or everolimus. It will be important to see if other pan-mTOR inhibitors such as OSI-027, AZD2014, INK128 or CC223 also alter liver function.
Likewise, several dual mTOR-PI3K inhibitors are under evaluation and for 2 of them the phase 1 trial results have already been published. The first, BGT226 induced grade 3 diarrhea in 46% of patients at 125 mg, however, limiting the dose to 100 mg three times weekly resulted in insufficient inhibition of the PI3K pathway89. Modeling based on pharmacokinetic data predicted that BGT226 dose of >4000 mg/day would be required to achieve efficacious plasma exposure, exceeding the safety dose range. The second, SF1126 is composed of the pan-PI3K and mTOR inhibitor LY294002 conjugated to an RGDS-targeting peptide to increase binding to integrins expressed on the tumor vasculature. In phase 1, SF1126 did not reach the maximum tolerated dose with a single dose limiting toxicity grade 3 diarrhea, reduced p-AKT and increased apoptosis90. Further studies are planned in combination with rituximab in CD20+ B-cell malignancies. Other compounds such as BEZ235, XL765 (also known as SAR245409), GDC-0980, PF-04691502 and PF-05212384 (also known as PKI-587) have completed phase 1 trials but data are not yet published.
Several new compounds have been designed to selectively inhibit PI3K with pan-PI3K targeting all class IA PI3Ks (BKM120, PX-866, XL147, GDC-0941, BAY80-6946, GSK2126458, CH5132799 and ATU027) or PI3K isoform-specific inhibitors (CAL101, BYL719, GSK2636771 and AZD6482). The phase 1 study with BKM12091 has shown hyperglycemia, skin rash and mood alteration as drug limiting toxicities. Once mood alterations were identified, selective serotonin reuptake inhibitors were prescribed, and no further mood alteration greater than grade 1 was seen. In the expansion study with the MTD (100 mg/d), most frequent grade 3/4 adverse effects (AEs) were transaminase increase (9.1%), asthenia (7.6%) and rash (6.1%). Anti-tumor activity is encouraging with 3 RECIST partial responses. A phase 1 study has also been completed for another compound (PX-86692) showing that dose limiting toxicities consisted of grade 3 diarrhea and elevated AST. No RECIST response was seen in this trial. New trials in association with other drugs are currently ongoing.
Finally, some compounds directly inhibit AKT with different mechanisms of action (plasma membrane disrupting agent, ATP-competitive and allosteric AKT inhibitors). Perifosine, a plasma membrane disrupting agent, has been extensively studied in phase I, II and even III trials93, 94. Despite promising first results with good tolerance and interesting anti-tumor activity, the latest phase III trial in metastatic refractory colorectal cancer was disappointing with no difference of overall survival or progression-free survival for perifosine plus capecitabine compared to capecitabine alone. In a small phase 1 trial with patient selection based on p-AKT positive tumor, triciribine phosphate monohydrate95 has shown no dose-limiting toxicities but a modest decrease of p-AKT after treatment. Response rates were not reported. Finally MK-2206, an allosteric AKT inhibitor, recently showed DLTs as mainly grade 3/4 skin rash96. In this trial no objective response was seen, however, tumor shrinkage was noted without reaching the level of the RECIST partial response. Of note, 9 patients have had paired tumor biopsy at baseline and at day 15. All of them demonstrated a decrease of p-AKT. Multiple drug combination and weekly schedule are now being tested to increase anti-tumor activity and minimize drug toxicity. Other compounds such as GSK690693, RX0201 (AKT antisense), PBI-05204, GSK2110183, GSK2141795, RG7440, GDC0068 and AZD5363 are under investigation or have completed phase 1 trials but the results are not yet published.
4. Perspective on mTOR inhibitors in HCC therapy
HCC is a very complex and heterogenous disease and progress in treatment of advanced HCC will likely occur slowly. One of the advantages of the first generation inhibitors (rapalogs) is the high specificity, the clinical approval and the comparatively few side-effects. Even if single agent therapies do not show the expected efficacy as has been revealed by the recent results from the EVOLVE study, rapalogs are still very attractive for use with other drugs. Moreover, sirolimus will most likely become first line treatment for HCC patients after liver transplantation.
With regard to current available second generation mTOR inhibitors we can anticipate limitations in their use in HCC patients, although they are more effective than first generation inhibitors in preclinical studies. First, for patients with impaired liver function, the increase in transaminase is troublesome, as observed in several clinical trials with the mTOR inhibitor AZD8055 but also with PI3K/mTOR dual inhibitor BGT226. Second, the performed phase 1 clinical trials demonstrated modest monotherapy efficacy, which was related to adverse events limiting dose escalation, but also related to a cytostatic effect more than a cytolytic effect of these drugs. Therefore, improved efficacy may mainly be reached in combination with other drugs. Third, new generation mTOR inhibitors may stimulate autophagy more than first generation inhibitors, and may consequently cause tumor cell protection against chemotherapy-induced death.
A strong focus should also be directed towards the discovery and validation of biomarkers which predict tumor-response after therapy. Biomarkers for mTOR inhibitor efficacy have been evaluated in preclinical, but also in clinical studies97, 98. Among others, these biomarkers include inactivation of PTEN, activating mutations of PI3KCA, expression levels of pS6, pS6K, S6K, and pAkt. Interestingly, a study demonstrated that human cancer cell lines carrying PI3KCA mutations were responsive to everolimus, except when KRAS mutations occurred concomitantly99. Similar results were obtained in a study with colon cancer cell lines100, and mTOR resistance was also shown in ovarian cancer cell lines, which overexpressed the apoptosis-inhibitory protein Bcl2101. Novel technologies such as next-generation sequencing may further path the way for the identification of new biomarkers, as has been shown in a recent study with bladder cancer patients, in which response to everolimus was clearly more effective in patients with a somatic mutation in the TSC1 complex102. However, none of these markers have been confirmed in clinical trials. It is thus currently difficult to recommend any of these biomarkers for patient selection in clinical trials. In addition, it has to be emphasized, that substantial progress in the identification of biomarkers for HCC treatment is unlikely to occur, if tumor tissue is not taken before and after chemo-therapy by means of a liver biopsy. Acquiring tumor tissue should therefore be mandatory for any phase II or phase III clinical study and will further help to develop novel therapies.
In spite of these drawbacks, mTOR inhibitors together with AKT and PI3K inhibitors still remain an attractive and promising therapeutic option for the treatment of HCC and ongoing as well as future clinical studies will reveal if they can be used for the therapy of this devastating disease.
Key point box.
-
-
The mTOR pathway is upregulated in 40-50% of HCC cases, is related to bad prognosis and contributes to sorafenib resistance in HCC. Preclinical in vitro and in vivo studies showed a reduction of HCC tumor growth by mTOR inhibitors.
-
-
Rapamycin and first generation mTOR inhibitors (rapalogs) are used in clinical trials for advanced HCC but also for adjuvant therapy in HCC patients after transplantation and TACE.
-
-
Preclinical studies in HCC have shown that second generation mTOR inhibitors are more efficacious than first generation mTOR inhibitors and could synergize with them.
-
-
Phase 1 clinical trials with second generation mTOR inhibitors in various solid cancers revealed dose limiting toxicities such as diarrhea, skin rash and increase of transaminase
-
-
Predictive biomarkers for efficacy of mTOR inhibitors have been identified in preclinical studies but not yet confirmed in clinic studies.
Acknowledgments
Financial support
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. M.S.M. is supported by “Schweizerische Stifung für Medizinisch-Biologische Stipendien” (PASMP-3_140071). T.D. is supported by the “Fondation Monahan” and “Prix Amgen pour la Recherche et l’Innovation en oncologie digestive 2011”.
Footnotes
Conflict of interest
The authors have no competing financial interests to declare.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin D, Colombi M, Moroni C, et al. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 3.Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tee AR, Fingar DC, Manning BD, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 6.DeYoung MP, Horak P, Sofer A, et al. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22:239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinzalla V, Stracka D, Oppliger W, et al. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Sieghart W, Fuereder T, Schmid K, et al. Mammalian target of rapamycin pathway activity in hepatocellular carcinomas of patients undergoing liver transplantation. Transplantation. 2007;83:425–432. doi: 10.1097/01.tp.0000252780.42104.95. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. 1983, e1–e11. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahin F, Kannangai R, Adegbola O, et al. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 11.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–S51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baba HA, Wohlschlaeger J, Cicinnati VR, et al. Phosphorylation of p70S6 kinase predicts overall survival in patients with clear margin-resected hepatocellular carcinoma. Liver Int. 2009;29:399–405. doi: 10.1111/j.1478-3231.2008.01798.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Huang Y, Li J, et al. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27:255–261. doi: 10.1007/s12032-009-9201-4. [DOI] [PubMed] [Google Scholar]
- 15.Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guichard C, Amaddeo G, Imbeaud S, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleary SP, Jeck WR, Zhao X, et al. Identification of driver genes in hepatocellular carcinoma by exome sequencing. Hepatology. 2013 doi: 10.1002/hep.26540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kan Z, Zheng H, Liu X, et al. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23:1422–1433. doi: 10.1101/gr.154492.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyault S, Rickman DS, de Reynies A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 20.Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JS, Chu IS, Heo J, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Thorgeirsson SS. Genome-scale profiling of gene expression in hepatocellular carcinoma: classification, survival prediction, and identification of therapeutic targets. Gastroenterology. 2004;127:S51–S55. doi: 10.1053/j.gastro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Nault JC, De Reynies A, Villanueva A, et al. A Hepatocellular Carcinoma 5-Gene Score Associated With Survival of Patients After Liver Resection. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Woo HG, Park ES, Lee JS, et al. Identification of potential driver genes in human liver carcinoma by genomewide screening. Cancer Res. 2009;69:4059–4066. doi: 10.1158/0008-5472.CAN-09-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 29.Sezgin Goksu S, Bilal S, Coskun HS. Hepatitis B reactivation related to everolimus. World J Hepatol. 2013;5:43–45. doi: 10.4254/wjh.v5.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno S, Yamagishi Y, Ebinuma H, et al. Progressive liver failure induced by everolimus for renal cell carcinoma in a 58-year-old male hepatitis B virus carrier. Clin J Gastroenterol. 2013;6:188–192. doi: 10.1007/s12328-013-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Parent R, Kolippakkam D, Booth G, et al. Mammalian target of rapamycin activation impairs hepatocytic differentiation and targets genes moderating lipid homeostasis and hepatocellular growth. Cancer Res. 2007;67:4337–4345. doi: 10.1158/0008-5472.CAN-06-3640. [DOI] [PubMed] [Google Scholar]
- 33.Buitrago-Molina LE, Pothiraju D, Lamle J, et al. Rapamycin delays tumor development in murine livers by inhibiting proliferation of hepatocytes with DNA damage. Hepatology. 2009;50:500–509. doi: 10.1002/hep.23014. [DOI] [PubMed] [Google Scholar]
- 34.Menon S, Yecies JL, Zhang HH, et al. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5:ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenerson HL, Yeh MM, Kazami M, et al. Akt and mTORC1 Have Different Roles During Liver Tumorigenesis in Mice. Gastroenterology. 2013;144:1055–1065. doi: 10.1053/j.gastro.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horie Y, Suzuki A, Kataoka E, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neef M, Ledermann M, Saegesser H, et al. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786–796. doi: 10.1016/j.jhep.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 38.Patsenker E, Schneider V, Ledermann M, et al. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55:388–398. doi: 10.1016/j.jhep.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 39.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 41.Larsson O, Morita M, Topisirovic I, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A. 2012;109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 43.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ZJ, Zheng ZJ, Shi R, et al. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 45.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 46.Zheng L, Yang W, Wu F, et al. Prognostic Significance of AMPK Activation and Therapeutic Effects of Metformin in Hepatocellular Carcinoma. Clin Cancer Res. 2013;19:5372–5380. doi: 10.1158/1078-0432.CCR-13-0203. [DOI] [PubMed] [Google Scholar]
- 47.Phoenix KN, Vumbaca F, Claffey KP. Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERalpha negative MDA-MB-435 breast cancer model. Breast Cancer Res Treat. 2009;113:101–111. doi: 10.1007/s10549-008-9916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin MJ, Hayward R, Viros A, et al. Metformin accelerates the growth of BRAF V600E-driven melanoma by upregulating VEGF-A. Cancer Discov. 2012;2:344–355. doi: 10.1158/2159-8290.CD-11-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu N, Gu HJ, Li Q. Effects of antidiabetic drug metformin on the migration and invasion abilities of human pulmonary adenocarcinoma A549 cell line in vitro. J Thorac Dis. 2010;2:76–80. [PMC free article] [PubMed] [Google Scholar]
- 50.Janku F, McConkey DJ, Hong DS, et al. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 51.Cui J, Gong Z, Shen HM. The role of autophagy in liver cancer: Molecular mechanisms and potential therapeutic targets. Biochim Biophys Acta. 2013;1836:15–26. doi: 10.1016/j.bbcan.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Colombo M, Raoul JL, Lencioni R, et al. Multidisciplinary strategies to improve treatment outcomes in hepatocellular carcinoma: a European perspective. Eur J Gastroenterol Hepatol. 2013;25:639–651. doi: 10.1097/MEG.0b013e32835e33bb. [DOI] [PubMed] [Google Scholar]
- 53.Freise CE, Ferrell L, Liu T, et al. Effect of systemic cyclosporine on tumor recurrence after liver transplantation in a model of hepatocellular carcinoma. Transplantation. 1999;67:510–513. doi: 10.1097/00007890-199902270-00003. [DOI] [PubMed] [Google Scholar]
- 54.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 55.Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 56.Semela D, Piguet AC, Kolev M, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 58.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 59.Buitrago-Molina LE, Vogel A. mTor as a potential target for the prevention and treatment of hepatocellular carcinoma. Curr Cancer Drug Targets. 2012;12:1045–1061. doi: 10.2174/156800912803988011. [DOI] [PubMed] [Google Scholar]
- 60.Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–5102. doi: 10.1002/cncr.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiah HS, Chen CY, Dai CY, et al. Randomised clinical trial: comparison of two everolimus dosing schedules in patients with advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:62–73. doi: 10.1111/apt.12132. [DOI] [PubMed] [Google Scholar]
- 62.Schoniger-Hekele M, Muller C. Pilot study: rapamycin in advanced hepatocellular carcinoma. Aliment Pharmacol Ther. 2010;32:763–768. doi: 10.1111/j.1365-2036.2010.04404.x. [DOI] [PubMed] [Google Scholar]
- 63.Rizell M, Andersson M, Cahlin C, et al. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol. 2008;13:66–70. doi: 10.1007/s10147-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 64.Decaens T, Luciani A, Itti E, et al. Phase II study of sirolimus in treatment-naive patients with advanced hepatocellular carcinoma. Dig Liver Dis. 2012;44:610–616. doi: 10.1016/j.dld.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piguet AC, Saar B, Hlushchuk R, et al. Everolimus augments the effects of sorafenib in a syngeneic orthotopic model of hepatocellular carcinoma. Mol Cancer Ther. 2011;10:1007–1017. doi: 10.1158/1535-7163.MCT-10-0666. [DOI] [PubMed] [Google Scholar]
- 67.Newell P, Toffanin S, Villanueva A, et al. Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo. J Hepatol. 2009;51:725–733. doi: 10.1016/j.jhep.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Zhou J, Fan J, et al. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 69.Choo SP, Chowbay B, Ng QS, et al. A Phase 1 dose-finding and pharmacodynamic study of rapamycin in combination with bevacizumab in patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2013;49:999–1008. doi: 10.1016/j.ejca.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Kelley RK, Nimeiri HS, Munster PN, et al. Temsirolimus combined with sorafenib in hepatocellular carcinoma: a phase I dose-finding trial with pharmacokinetic and biomarker correlates. Ann Oncol. 2013;24:1900–1907. doi: 10.1093/annonc/mdt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choo AY, Yoon SO, Kim SG, et al. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jimenez RH, Boylan JM, Lee JS, et al. Rapamycin response in tumorigenic and non-tumorigenic hepatic cell lines. PLoS One. 2009;4:e7373. doi: 10.1371/journal.pone.0007373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez RH, Lee JS, Francesconi M, et al. Regulation of gene expression in hepatic cells by the mammalian Target of Rapamycin (mTOR) PLoS One. 2010;5:e9084. doi: 10.1371/journal.pone.0009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrich AM, Leshchenko V, Kuo PY, et al. Akt inhibitors MK-2206 and nelfinavir overcome mTOR inhibitor resistance in diffuse large B-cell lymphoma. Clin Cancer Res. 2012;18:2534–2544. doi: 10.1158/1078-0432.CCR-11-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Totary-Jain H, Sanoudou D, Ben-Dov IZ, et al. Reprogramming of the microRNA transcriptome mediates resistance to rapamycin. J Biol Chem. 2013;288:6034–6044. doi: 10.1074/jbc.M112.416446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013 doi: 10.1002/hep.26369. [DOI] [PubMed] [Google Scholar]
- 77.Kirstein MM, Boukouris AE, Pothiraju D, et al. Activity of the mTOR inhibitor RAD001, the dual mTOR and PI3-kinase inhibitor BEZ235 and the PI3-kinase inhibitor BKM120 in hepatocellular carcinoma. Liver Int. 2013;33:780–793. doi: 10.1111/liv.12126. [DOI] [PubMed] [Google Scholar]
- 78.Grabinski N, Ewald F, Hofmann BT, et al. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer. 2012;11:85. doi: 10.1186/1476-4598-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda M, Shimomura M, Kobayashi K, et al. Growth inhibition by NVP-BEZ235, a dual PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol Rep. 2011;26:1273–1279. doi: 10.3892/or.2011.1370. [DOI] [PubMed] [Google Scholar]
- 80.Thomas HE, Mercer CA, Carnevalli LS, et al. mTOR inhibitors synergize on regression, reversal of gene expression, and autophagy in hepatocellular carcinoma. Sci Transl Med. 2012;4:139ra84. doi: 10.1126/scitranslmed.3003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evert M, Calvisi DF, Evert K, et al. V-AKT murine thymoma viral oncogene homolog/mammalian target of rapamycin activation induces a module of metabolic changes contributing to growth in insulin-induced hepatocarcinogenesis. Hepatology. 2012;55:1473–1484. doi: 10.1002/hep.25600. [DOI] [PubMed] [Google Scholar]
- 82.Xin HW, Ambe CM, Hari DM, et al. Label-retaining liver cancer cells are relatively resistant to sorafenib. Gut. 2013 doi: 10.1136/gutjnl-2012-303261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen KF, Chen HL, Tai WT, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 84.Gedaly R, Angulo P, Chen C, et al. The role of PI3K/mTOR inhibition in combination with sorafenib in hepatocellular carcinoma treatment. Anticancer Res. 2012;32:2531–2536. [PubMed] [Google Scholar]
- 85.Gedaly R, Angulo P, Hundley J, et al. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176:542–548. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- 86.Serova M, de Gramont A, Tijeras-Raballand A, et al. Benchmarking effects of mTOR, PI3K, and dual PI3K/mTOR inhibitors in hepatocellular and renal cell carcinoma models developing resistance to sunitinib and sorafenib. Cancer Chemother Pharmacol. 2013;71:1297–1307. doi: 10.1007/s00280-013-2129-6. [DOI] [PubMed] [Google Scholar]
- 87.Shao H, Gao C, Tang H, et al. Dual targeting of mTORC1/C2 complexes enhances histone deacetylase inhibitor-mediated anti-tumor efficacy in primary HCC cancer in vitro and in vivo. J Hepatol. 2012;56:176–183. doi: 10.1016/j.jhep.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 88.Naing A, Aghajanian C, Raymond E, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer. 2012;107:1093–1099. doi: 10.1038/bjc.2012.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol. 2012;23:2399–2408. doi: 10.1093/annonc/mds011. [DOI] [PubMed] [Google Scholar]
- 90.Mahadevan D, Chiorean EG, Harris WB, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012;48:3319–3327. doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 92.Hong DS, Bowles DW, Falchook GS, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 93.Van Ummersen L, Binger K, Volkman J, et al. A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 94.Unger C, Berdel W, Hanauske AR, et al. First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer. 2010;46:920–925. doi: 10.1016/j.ejca.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 95.Garrett CR, Coppola D, Wenham RM, et al. Phase I pharmacokinetic and pharmacodynamic study of triciribine phosphate monohydrate, a small-molecule inhibitor of AKT phosphorylation, in adult subjects with solid tumors containing activated AKT. Invest New Drugs. 2011;29:1381–1389. doi: 10.1007/s10637-010-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 97.Delbaldo C, Albert S, Dreyer C, et al. Predictive biomarkers for the activity of mammalian target of rapamycin (mTOR) inhibitors. Target Oncol. 2011;6:119–124. doi: 10.1007/s11523-011-0177-6. [DOI] [PubMed] [Google Scholar]
- 98.Liao YM, Sy A, Yen Y. Markers for efficacy of mammalian target of rapamycin inhibitor. Anticancer Res. 2012;32:4235–4244. [PubMed] [Google Scholar]
- 99.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ducker GS, Atreya CE, Simko JP, et al. Incomplete inhibition of phosphorylation of 4E-BP1 as a mechanism of primary resistance to ATP-competitive mTOR inhibitors. Oncogene. 2013 doi: 10.1038/onc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aguirre D, Boya P, Bellet D, et al. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis. 2004;9:797–805. doi: 10.1023/B:APPT.0000045781.46314.e2. [DOI] [PubMed] [Google Scholar]
- 102.Iyer G, Hanrahan AJ, Milowsky MI, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]