Highlights
-
•
Adolescence brings heightened risk for social anxiety disorder (SAD) onset.
-
•
Early-life temperament of behavioral inhibition is a documented risk for SAD.
-
•
Vulnerability to SAD reflects heightened salience of social and non-social rewards.
-
•
Avoidance-related (i.e., amygdala) and approach-motivational (i.e., striatum) systems are associated with adolescent SAD and behavioral inhibition.
Keywords: Social anxiety, Behavioral inhibition, Reward, Threat, Striatum, Amygdala
Abstract
Social anxiety disorder (SAD) markedly impairs daily functioning. For adolescents, SAD can constrain typical development precisely when social experiences broaden, peers’ opinions are highly salient, and social approval is actively sought. Individuals with extreme, impairing social anxiety fear evaluation from others, avoid social interactions, and interpret ambiguous social cues as threatening. Yet some degree of social anxiety can be normative and non-impairing. Furthermore, a temperament of behavioral inhibition increases risk for SAD for some, but not all adolescents with this temperament. One fruitful approach taken to understand the mechanisms of social anxiety has been to use neuroimaging to link affect and cognition with neural networks implicated in the neurodevelopmental social reorientation of adolescence. Although initial neuroimaging studies of adolescent SAD and risk for SAD underscored the role of fear-processing circuits (e.g., the amygdala and ventral prefrontal cortex), recent work has expanded these circuits to include reward-processing structures in the basal ganglia. A growing focus on reward-related neural circuitry holds promise for innovative translational research needed to differentiate impairing from normative social anxiety and for novel ways to treat adolescent SAD that focus on both social avoidance and social approach.
1. Introduction
Nearly everyone experiences normative, non-impairing levels of anxiety. Anxiety can be a motivator to overcome challenges, such as meeting an important deadline. It can also be adaptive in certain contexts, such as protecting one's social status until becoming familiar enough with new people to let down one's guard. For some individuals, however, anxiety becomes debilitating to the extent that daily functioning is markedly impaired. Furthermore, impairing levels of anxiety that onset early in life can interrupt the developmental progression of typical life experiences, such as socializing with peers at school.
One such anxiety disorder that commonly has an onset early in life is social anxiety disorder (SAD). SAD is indicated uniquely by an extreme, irrational and impairing fear of social situations, such as being criticized or negatively evaluated by other people (American Psychiatric Association, 2000). Although SAD can be diagnosed in early and middle childhood, a disproportionately high prevalence of cases emerges in late childhood and early adolescence (Beesdo et al., 2010, Stein et al., 2001). The age-of-onset distribution for SAD is unique from any other anxiety disorder. SAD onset rates increase considerably at age ten and plateau in the early twenties, whereas onset rates for generalized anxiety disorder (GAD), panic disorder, and specific phobias increase more steadily during this same period (Beesdo et al., 2010). About 50% of SAD cases onset by age 13, with 90% reaching onset by age 23 (Stein, 2006).
Given its age-of-onset patterns and characterization, SAD can constrain normative development in a life stage typically marked by expanding social experiences, a strong need for social approval and high social status, and an increased investment in friendships and romantic relationships. A multitude of expected, stage-dependent changes that facilitate social and emotional development renders adolescence as a period when fears of social evaluation or humiliation that characterize SAD can become especially pronounced. These changes in adolescence include but are not limited to brain maturation and puberty (Blakemore and Choudhury, 2006, Forbes and Dahl, 2010, Giedd, 2008, Nelson et al., 2005, Sisk and Zehr, 2005), broadened social opportunities and more exposure to unfamiliar peers (Graber and Brooks-Gunn, 1996, Smetana et al., 2006), and new motivations for peer and romantic relationships (Steinberg, 2008). Not only can these shifts contribute to vulnerability for SAD, they can exacerbate its consequences, as in the case of adolescents who withdraw from peers and social situations at a time when establishing healthy peer relationships is important for well-being.
A growing empirical base has shown that adolescents who are affected by SAD or at risk for SAD exhibit heightened neural activation in appetitive-motivational systems (Bar-Haim et al., 2009, Guyer et al., 2014, Guyer et al., 2012a, Guyer et al., 2006, Haber and Knutson, 2010, Hardin et al., 2006, Helfinstein et al., 2011, Helfinstein et al., 2012, Lahat et al., 2012, Perez-Edgar et al., 2013). This is an important consideration given that, historically, greater theoretical and research attention has been paid to the involvement of fear-avoidance neural systems, involving the amygdala for example, in SAD and risk for SAD (Davis, 1992, Kagan, 1996). The goal of this review paper is to highlight these recent findings from developmental cognitive neuroscience research that have deepened our understanding of the brain–behavior relationships in adolescent SAD and risk for SAD. This motivation stems from an understanding that the brain is a clear mediator of individual differences in behavior and that neurodevelopment in adolescence has distinct characteristics relative to other developmental periods. Although assessments based on other key physiological modalities (e.g., heart rate, heart rate variability) have provided critical insight into the development of and risk for SAD (Anderson and Hope, 2009, Beidel, 1988, Beidel et al., 1991, Porges, 2007) and relate to brain function (Thayer et al., 2012), the initial findings involving appetitive-motivational neural systems have come from studies using functional magnetic resonance imaging (fMRI). By mapping relationships among brain function, cognitive processes, and behavioral characteristics and phenotypes, we may eventually be able to use fMRI studies to test the effectiveness of treatments, such as cognitive behavioral therapy (CBT) on improving aberrant cognitive and affective processing in order to help alleviate anxiety (Pine et al., 2008).
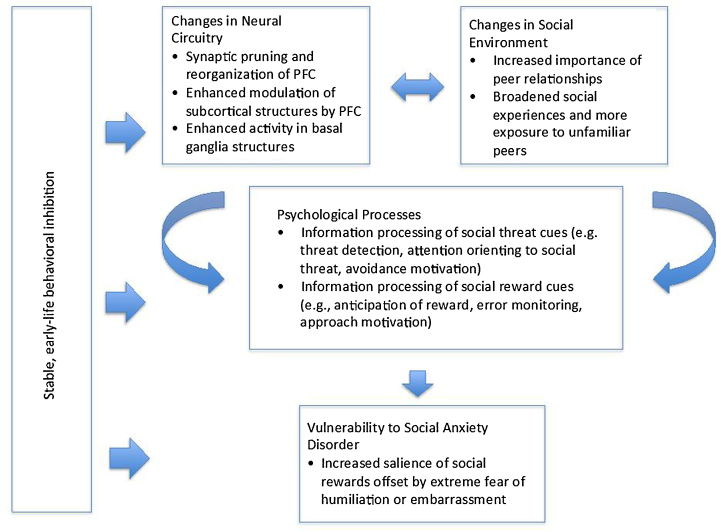
Our review paper proceeds as follows. First, we provide a characterization of adolescent SAD including its symptoms, prevalence, treatment strategies, and association with other disorders. Second, we describe how a better understanding of adolescent SAD has also emerged from studies of early-life behavioral inhibition (BI). BI is a type of temperament characterized by heightened fear reactions to unfamiliar stimuli and extreme social reticence that confers a degree of risk for later SAD. Third, we briefly review established findings involving avoidance-based neural systems that have associated elevated amygdala activation with SAD and BI. Fourth, we discuss recent studies that have focused attention on the neural circuitry underlying reward motivation as a system to consider, in conjunction with avoidance systems, in the study of adolescent SAD and risk for SAD (readers are referred to Bishop, 2007, Britton et al., 2011, LeDoux, 2007), for more detailed reviews on anxiety and fear-avoidance circuitry). This recent work primarily includes differential patterns of social-information processing and performance-based reward processing in appetitive-motivational circuitry specific to adolescence, BI, and SAD. Fifth, we consider adolescence as a vulnerable period for SAD and suggest that a fuller characterization of when and why adolescents face heightened risk for SAD can be accomplished by examining: early-life BI, the distributed network of brain structures implicated in both fear-avoidance and appetitive-motivational processes, and adolescents’ sensitivity to social evaluation and status (Fig. 1). We thus suggest a dimensional perspective of SAD that conceives of it as fueled by imbalances or conflicts between fear-avoidance and appetitive-motivational systems when responding to both social and non-social stimuli. We propose a conceptual model of adolescent vulnerability to SAD, central to which is the idea that socially anxious adolescents experience an approach-avoid conflict that arises from age-typical increased investment in peer evaluation coinciding with extreme fear of humiliation and embarrassment. Moreover, we stipulate that this conflict is mediated by aberrant functioning in a network of brain regions (i.e., amygdala, basal ganglia, and prefrontal cortex) that motivate cautious approach, and is moderated by individual differences in inhibited temperament that are rooted in childhood. Finally, we end with general conclusions based on the work reviewed.
Fig. 1.
Conceptual model of adolescent vulnerability to social anxiety disorder (SAD). Aberrant processing of social threat and reward in SAD becomes pronounced in adolescence. Typically occurring changes in neural circuitry implicated in threat and reward processing coincide with changes in the social environment that afford greater opportunities for social reward from peers but also greater risk of humiliation and embarrassment in front of unfamiliar others. These changes are mediated by cognitive psychological processes that motivate approach and avoidance behaviors. Vulnerability to SAD in adolescence is viewed as a result of conflict between increased salience of social reward and extreme fear of humiliation or embarrassment. This vulnerability is moderated in part by a history of inhibited temperament.
2. Adolescent social anxiety disorder
SAD is defined by the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM) as a persistent and impairing fear of being in social or performance situations that involve potential evaluation or scrutiny by others. This fear often revolves around the potential for embarrassment and humiliation particularly within social contexts, such as public speaking, performing in class, starting conversations with peers, and social activities that involve interaction with others (e.g., dances, parties). The hypersensitivity to and fear of scrutiny from others that accompanies SAD heightens apprehension, arousal, and panic in these social and performance contexts (Heimberg et al., 2010, Kashdan and Herbert, 2001, Phan and Klumpp, 2010, Stein, 2006).
When exposed to the feared situation, the individual experiences anxiety despite the recognition of the excessive or unreasonable nature of this fear. The anxiety is often accompanied by physiological and behavioral reactions, such as elevated heart rate, sweating, shaking, crying, or refusal to speak. The individual will avoid the situations that put them at risk for public scrutiny, humiliation, or negative evaluation by others. Both adolescents and adults with SAD report extreme apprehension about appearing socially incompetent or boring when interacting with others (Stopa and Clark, 1993). Several cognitive factors also underlie the generation and maintenance of SAD, including negative interpretations of ambiguous or benign social cues, a negative mental representation of the self as seen by others, expectations for negative evaluation, and intense focus on one's own physical signs of anxiety (e.g., sweating, averted eye gaze) (Heimberg et al., 2010). In line with cognitive-behavioral models of SAD (Heimberg et al., 2010), cognitions about social fears could include thoughts, such as, “Kids at school will laugh at me if I start talking to them” or “No one wants to hang out with me after the bad presentation I gave in class.”
SAD is one of the most common mental disorders (Kessler, 2003). A national epidemiological study documented that 8.6% of 13–18 year olds meet lifetime criteria for SAD (Burstein et al., 2011). The risk for developing SAD increases dramatically in late childhood and early adolescence, but the risk for SAD onset then decreases dramatically by age 25 (Wittchen et al., 1999). Indeed, if SAD goes untreated in childhood or adolescence, then there is a high likelihood that it will persist into adulthood (Kessler, 2003). That said, as common as SAD is to emerge in adolescence, for many adolescents it can be transient and not persist into adulthood. Finally, SAD in adolescence also frequently co-occurs with other mental disorders. The most common disorders comorbid with SAD are other anxiety disorders and mood disorders (e.g., depression), and with more moderate frequency, Oppositional Defiant Disorder and later substance use (Lopes and Albano, 2013).
Treatments for pediatric SAD with the most empirical support include CBT and pharmacotherapy (e.g., selective serotonin reuptake inhibitors; SSRIs) (Lopes and Albano, 2013). CBT for SAD is typically given in a group setting to provide exposure to social situations and the ability to practice social skills in real time. In general, this type of exposure is expected to lessen anxiety. Goals of CBT are to provide social skills training (e.g., making eye contact, turn taking in conversations), create opportunities for role playing within a group, with a therapist, and at home, and target cognitions about social fears that are exaggerated, overgeneralized, and/or catastrophic in nature. Group CBT for adolescents with SAD has proven effective with methods, such as social skills training, cognitive reframing, and problem-solving (Albano et al., 1995). SSRIs also improve functioning in youth with SAD (Beidel et al., 2007), however, CBT increases social skills and social competence to a greater extent (Hayward et al., 2000).
3. Early-life behavioral inhibition
Much research has focused on a fearful temperament in studies of the etiology of anxiety disorders, with most evidence linking it to SAD. Different temperament profiles reflect an individual's early-life predisposition towards certain behavioral and physiological responses tied to affective reactions, such as fear, to novel stimuli (Fox et al., 2005, Kagan et al., 1988a, Kagan et al., 1988b, Rothbart and Derryberry, 1981). One well-studied classification of temperament linked to adolescent SAD is BI in infancy and early childhood. Found in about 15% of the population, BI is characterized by a heightened sensitivity to novelty and threat, fear of and wariness towards unfamiliar stimuli, and a tendency to withdraw from unfamiliar social situations (Fox et al., 2001, Perez-Edgar and Fox, 2005).
Although BI is not a psychiatric illness, a subset of children with early-life BI develops SAD, whereby the association persists over decades. Both retrospective and longitudinal characterizations of behaviorally inhibited temperament in early childhood have been linked to SAD in middle childhood and adolescence (Biederman et al., 2001, Chronis-Tuscano et al., 2009, Hirshfeld-Becker et al., 2007, Schwartz et al., 1999). For example, longitudinal maternal-reported and behavioral evidence suggests that early-life BI increases the odds of having SAD in adolescence by four-fold, and doubles the chance for any anxiety disorder (Chronis-Tuscano et al., 2009). The association between BI and later anxiety problems is especially strong when BI remains stable across development (Chronis-Tuscano et al., 2009, Hirshfeld et al., 1992). Thus, a benefit to examining adolescent SAD and early life BI is the identification of common behavioral and biological mechanisms that elicit similar reactions to feared social situations across development.
One possible mechanism linking BI and SAD stems from the extreme reactivity to and fear of novel and unknown stimuli and events, particularly of a social nature displayed in both conditions. The unfolding characterization of BI emerges as heightened reactivity to novelty and negative affect detected as early as 4 months (Fox et al., 2005, Fox et al., 2001), which relates to inhibited behavior with others in childhood (Calkins et al., 1996). Work has shown that BI in toddlerhood predicts social avoidance during free play with unfamiliar peers at age four (Rubin et al., 2002) and behaviorally inhibited children are quiet and socially avoidant in unfamiliar social situations at age seven (Kagan et al., 1988a, Kagan et al., 1988b). When infants with a BI temperament reach the school-age years they are socially reticent with peers (Coplan et al., 1994, Rubin et al., 2009). As behaviorally inhibited children's environments expand to include a broader range of social experiences, it is reasonable to expect, that for many of these youth, their heightened reactivity to unfamiliar or threatening stimuli will generalize to unfamiliar or threatening people. Indeed, later in childhood and adolescence, early-life BI relates to heightened attentional vigilance toward and greater difficulty disengaging from socially threatening stimuli (Bar-Haim et al., 2007, Perez-Edgar et al., 2011). Given the link between BI in early childhood and SAD in adolescence, consideration of key biological and social adolescent transitions that confer risk for SAD is important, as these contexts of change likely interact with stable temperament profiles rooted in early life. As such, early-life BI may act as a diathesis for SAD.
It is important to note that although for some children early tendencies associated with BI culminate in overt SAD, many pathways exist from early BI. Although the stability of BI has been established in several longitudinal studies (e.g., Gest, 1997, Rothbart, 1988), stability estimates are generally moderately sized. Some children with BI in infancy and early childhood grow out of their inhibited, socially reticent behavior and become non-inhibited in middle childhood and adolescence (Degnan and Fox, 2007, Fox et al., 2001). Another subset of children with BI does not develop impairing levels of anxiety. In either scenario, certain social experiences may direct the child's social development trajectory towards adaptive behavior and minimize impairing social avoidance. Indeed, evidence has demonstrated that behaviorally inhibited preschoolers who received non-maternal childcare were less likely to later exhibit anxious behaviors around peers (Almas et al., 2011). Similarly, just as SAD is common in adolescence, in many cases it does not persist into adulthood. Thus, examining individual differences in both the degree and stability of BI may be useful for understanding why some cases of SAD are adolescence-limited while others are stable into adulthood.
Nonetheless, as discussed earlier, the converse has been shown, as SAD has been reported in adolescents characterized in childhood as non-inhibited (Hirshfeld-Becker et al., 2007) and BI has been associated with anxiety disorders more generally, not just with SAD (Maack et al., 2012). Moreover, a number of individual difference and contextual factors have been found to moderate the association between early BI and later social anxiety problems. Stronger associations have been observed in females (Essex et al., 2010), children with insecure attachment styles (Muris et al., 2011, Nachmias et al., 1996), high parental trait anxiety (Muris et al., 2011), domineering parenting style and high maternal intrusive control (Rubin et al., 2002, Williams et al., 2009), and significant exposure to maternal stress (Essex et al., 2010).
It is also important to draw distinctions between BI and SAD. One perspective holds that BI reflects a core biologically-based system (the behavioral inhibition system, or BIS) that resolves conflicts among competing goals (e.g., approach-avoidance conflict) by inhibiting prepotent behavior, attending to and modulating emotional arousal toward threat-relevant information, and assessing risk (Corr, 2002). Whereas SAD is characterized by chronic impairment in functioning and atypical interpretation and response to specific social threats, BI is thought to represent a hyperactive end of an individual differences spectrum in general defensive approach that is rooted in temperament (McNaughton and Corr, 2004). High BIS scores, for example, may render individuals vulnerable to the cognitive biases experienced by individuals with SAD, but such biases are not a prerequisite for BI. However, typical BIS scores and normative social anxiety serve similar purposes in assessing the risk involved in approaching a reward.
Overall, a picture has emerged depicting the sensitivity of not only fear-avoidance neural circuitry but also reward neural circuitry underlying motivated action and learning in those with early-life BI and in adolescents with SAD. This picture also has particular relevance for the social domain in which BI and SAD have behavioral profiles in common. As children experience heightened sensitivity to social feedback during adolescence, behaviorally inhibited children may be at particular risk for experiencing hypersensitivity to aversive social outcomes, a profile that also characterizes SAD. Thus, in our conceptual model of risk for SAD, we stipulate that normative anxiety resulting from a multitude of changes in adolescence may become especially pronounced in a subset of individuals with stable, early-life BI (Fig. 1).
4. Fear-avoidance systems, SAD, and BI
Although our primary focus here is on the link between appetitive-motivational systems and SAD, it is important to consider that established, biologically based explanations for anxiety (Davis, 1992, LeDoux, 2007) and BI (Kagan, 1996) involve the neural networks implicated in “avoidance” responses to feared stimuli. Considerable research drawing on cross-species models delineates the amygdala, located in the medial temporal lobe, and regions of the ventral prefrontal cortex (vPFC), as key substrates underlying how the brain processes and responds to signals of danger in one's surroundings (Adolphs et al., 1995). The amygdala facilitates responses to salient stimuli either positive or negative in valence and mediates emotional responses (Kim et al., 2011). Within the vPFC, the lateral portion is involved in attention control and related processes and the medial portion is implicated in controlling flexible and adaptive motivational responses, such as acquisition of fearful responding, fear extinction, as well as approach and reward-driven behaviors (Dolan, 2007, Murray et al., 2007).
Evidence from fMRI research has pinpointed hyper-activation of the amygdala in association with adolescent SAD and early-life BI. The amygdala has been a key region of interest in this line of work because of its role in perceiving salience and attending to and disengaging from threat cues, including those of a social nature. Amygdala response has been probed primarily with the use of stimulus sets of faces depicting emotional expressions or as part of emotionally evocative social contexts. Studies of facial emotion encoding have found amygdala hyperactivation in adolescents with BI in early childhood (Perez-Edgar et al., 2007) and adolescents with GAD and/or SAD (Beesdo et al., 2009, McClure et al., 2007) versus control groups when they attend to fearful relative to neutral faces. A similar pattern of amygdala hyperactivation has been observed when adults who were behaviorally inhibited children viewed novel versus familiar faces (Schwartz et al., 2003). Another line of work has focused on attentional bias to social threat (e.g., looking longer and taking longer to disengage from angry versus neutral faces). Measured behaviorally, attentional bias to threat has been found in younger relative to older anxious individuals (Ladouceur et al., 2009) and in adolescents with a history of BI (Perez-Edgar et al., 2010). With regard to neural function, heightened amygdala response to rapid exposures of social threat cues has been observed in anxious relative to control adolescents (Monk et al., 2008) and stronger negative connectivity between amygdala and insula response to angry versus neutral faces has been found to moderate the association between early BI and later internalizing problems (Hardee et al., 2013). Finally, work that leveraged the very social context that elicits fear in SAD has shown amygdala hyperactivation in socially anxious versus healthy adolescents when anticipating being evaluated by unknown peers with whom participants expressed disinterest for a social interaction (Guyer et al., 2008).
Although anxious adolescents tend to fixate on rather than avoid threatening stimuli, biased allocation of attention toward threat is thought to influence downstream avoidance processes (e.g., initial hypervigilant threat detection, followed by withdrawal from the threat). Greater activation of the ventrolateral PFC (vlPFC) to angry compared to neutral faces has been found among anxious relative to non-anxious adolescents when threat cue presentations were prolonged (Monk et al., 2006). Although group differences in amygdala response to angry faces were not found during these longer threat cue durations, vlPFC activity was negatively associated with anxiety severity, such that greater vlPFC response was associated with lower symptom severity. Thus, vlPFC response to social threats after an initial orienting period may not be directly yoked to anxiety symptoms, but might modulate activity in fear-avoidance neural regions that generate anxiety. Interestingly, in the study of social evaluation anticipation, positive functional connectivity between the vlPFC and amygdala was found in socially anxious versus healthy adolescents when anticipating evaluation from undesirable peers, suggesting a possible role for the vlPFC in modulating avoidance of stimuli associated with the threat of social retaliation (Guyer et al., 2008). Thus, anxiety, and particularly SAD, in adolescence appears to involve heightened attention to social cues, both exogenous (e.g., threatening facial expressions) and endogenous (e.g., cues that indicate social rejection), via avoidance neural circuitry.
Overall, these findings implicate heightened amygdala response and vlPFC response to displays of social fear, social unfamiliarity, vigilance to social threat, and anticipation of social evaluation, underscoring the amygdala as a neurobiological mechanism underlying social-cognitive biases that may initiate or maintain SAD and BI. As depicted in Fig. 1, we propose that the cognitive mechanisms described in this section mediate avoidance behaviors in SAD. Hypervigilance and biased attentional allocation toward threat may lead socially anxious individuals to avoid situations in which they could potentially humiliate or embarrass themselves. Whether this hyperactive fear-avoidance circuitry precedes adolescence is as yet unknown and warrants further investigation.
5. Appetitive-motivational systems, SAD, and BI
5.1. Approach-relevant neural circuitry
Recent data from several studies show that behaviorally inhibited and socially anxious adolescents also have elevated neural activation in networks that coordinate motivated behavior and reward processing (Bar-Haim et al., 2009, Guyer et al., 2014, Guyer et al., 2012a, Guyer et al., 2006, Haber and Knutson, 2010, Hardin et al., 2006, Helfinstein et al., 2011, Helfinstein et al., 2012, Lahat et al., 2012, Perez-Edgar et al., 2013). These networks include the basal ganglia and their projections from frontal cortical regions (e.g., the vPFC), the hippocampus, amygdala, and anterior cingulate cortex (ACC). Several structures comprise the basal ganglia including the striatum, globus pallidus, subthalamic nucleus, and substantia nigra. The striatum, in particular, has strong connections to cortical and limbic regions and its own functionally distinct structures. The dorsal striatum encompasses the caudate and putamen, which connect mainly to motor and cognitive areas of the PFC. The ventral striatum primarily contains the nucleus accumbens and projects to regions of the PFC implicated in emotion and motivation (Fareri et al., 2008).
The appetitive-motivational system involves several cortical-subcortical connections linked to reward-driven approach, reward valuation, and goal-directed cognitive control. The striatum and nucleus accumbens receive dopaminergic inputs from the substantia nigra and ventral tegmental area of the midbrain, respectively, and are involved in associating motivationally salient stimuli, such as rewards and punishments with anticipated outcomes to guide approach or avoidance behavioral responses (Haber and Knutson, 2010, Mattfeld et al., 2011). Connections between the striatum and orbitofrontal cortex have been implicated in continuous reward valuation and in the adjustment of behavioral responses based on errant predictions of aversive and appetitive events (Seymour et al., 2004). Furthermore, the ACC, vPFC, and dorsolateral PFC are involved in goal-directed cognitive control processes, such as goal planning, regulating motivation, and controlling flexible and adaptive motivational responses (Kim, 2013, Nelson and Guyer, 2011). As such, striatal structures have been main regions of interest in research on substance addiction (Hasler and Clark, 2013, Koob and Volkow, 2010), depression (Diener et al., 2012, Forbes et al., 2006), and basic drives for primary rewards, such as food, sex, and warmth (Sescousse et al., 2013). Numerous studies have identified the striatum as part of the appetitive-motivational neural system that responds during reward processing (Hardin et al., 2009). Specifically, fMRI studies implicate the striatum in generating approach responses during the anticipation of receiving monetary incentives, along with enhanced motor performance coordinating motivated behavior (Bjork et al., 2004, Knutson et al., 2001, Knutson et al., 2000). The degree of striatal activation has been found to increase with the salience of the reward cue (Zink et al., 2004) and in reinforcing action leading to rewards (Tricomi et al., 2004). A growing literature points to striatal circuitry in processing social cues and situations, a focus relevant to both BI and SAD. For example, studies have linked striatal response when attributing salience to social stimuli, such as human faces (Hare et al., 2005) and social motivations, such as romantic attachment (Bartels and Zeki, 2000) and peer acceptance (Guyer et al., 2012b).
5.2. Alterations in approach circuitry
Hyperactive striatal responses in BI and SAD may reflect anxiety during the anticipation of uncertain outcomes inclusive of both non-social incentives and social evaluation or interactions, and during social stress more generally. For example, adults with SAD show striatal hyperactivation during public speaking (Lorberbaum et al., 2004), and adolescents with BI show hyperactivation when anticipating social evaluation (Guyer et al., 2014). Anxiety symptoms and disorders in general, including social anxiety, have been linked with enhanced dopamine signaling in the striatum among individuals with the Met allele of the catechol O-methyltransferase (COMT) gene (Hariri, 2011, Olsson et al., 2005, Stein et al., 2006). Hyperactivation in the caudate has been observed in adolescents with BI during incentive anticipation, and this altered functioning is especially pronounced in adolescents with a genetic polymorphism (DRD4) that modulates dopamine uptake in striatal regions (Perez-Edgar et al., 2013). Thus, identifying specific patterns of striatal functional response during performance-based or social situations could reveal pathophysiological traits common to both adolescent SAD and BI. As such, consideration of approach-related neural circuitry could provide clues for understanding why adolescents experience heightened vulnerability to SAD and for developing treatments for adolescent SAD that leverage elicitation of this neural circuitry. As discussed below and in our model of adolescent risk for SAD (see Fig. 1), aberrant information processing of social reward cues may escalate the risk for SAD at a time when increased sensitivity to rewarding experiences such as peer acceptance typically occurs.
5.2.1. Reward anticipation
The groundwork for addressing reward processing and adolescent risk for SAD comes from several studies that used incentive-based performance-eliciting measures. One such measure, the Monetary Incentive Delay (MID) task (Knutson et al., 2000), assesses individual differences in behavioral and neural responses to performance-contingent rewards. In the task, subjects are shown visual cues that indicate potential monetary gain, loss, or no change. After a delay, a target is presented and subjects win money (gain trials) and avoid losing money (loss trials) by responding to the target within a set time. This task has been widely used and reliably elicits striatal activation during the anticipation of these outcomes (Knutson & Cooper, 2005).
Several neuroimaging studies paired fMRI with the MID task and similar incentive-based tasks to examine associations between approach-related neural circuitry and SAD or risk for SAD (focusing on BI) in adolescents (Bar-Haim et al., 2009, Guyer et al., 2012a, Guyer et al., 2006, Helfinstein et al., 2011). The first of these studies (Guyer et al., 2006) used the MID task to assess brain function during the anticipation of monetary gains and losses in three striatal sub-structures: the caudate, putamen, and nucleus accumbens. Key findings showed greater activation across these striatal structures in behaviorally inhibited versus non-inhibited adolescents, such that the more salient the outcome (i.e., the more money to gain or lose), the greater the striatal activation. Additionally, valence of the potential outcome did not modulate striatal activation, as both groups had similar activation levels to potential gains and losses. The group difference in striatal response demonstrated that adolescents with early-life BI process reward stimuli differently than non-inhibited adolescents. This may reflect an uncertainty of reinforcement or altered processing of reward-prediction error given the caudate and nucleus accumbens activation patterns documented. As such, behaviorally inhibited adolescents may experience not only heightened reward sensitivity, but also heightened vigilance toward evaluating their reward-contingent performance. Additionally, that the salience of reward magnitude but not reward valence modulated striatal activity suggests the potential receipt of rewards and avoidance of losing rewards may be equally salient events for behaviorally inhibited adolescents. Furthermore, the heightened motivation to respond to highly salient stimuli in BI may be fueled by a strong desire to avoid failure. This suggestion is supported by recent research that used a social evaluation task in which adolescents with and without BI anticipated receiving either rejection or acceptance feedback (Guyer et al., 2014). In this task, adolescents with but not without BI showed putamen hyperactivation during the anticipation of feedback, a result we view as analogous to the putamen hyperactivation observed for high-gain and high-loss trials in the MID task, and which we also interpret as suggesting a strong desire to avoid failure—in this case, social failure.
Demonstrating the same patterns of heightened striatal sensitivity to increasingly salient rewards in adolescents with SAD as seen in adolescents with early-life BI was an essential next step in confirming striatal function as a neurophysiological mechanism shared by clinically impairing SAD and risk for SAD. This gap was addressed in a study that used the MID task to compare striatal function in healthy adolescents, adolescents diagnosed with SAD, and adolescents diagnosed with GAD (Guyer et al., 2012a). As with the BI sample, increasing caudate and putamen response in adolescents with SAD, but not healthy adolescents or adolescents with GAD, was observed as a function of increasing incentive magnitude (for gains and losses) during anticipation. These results suggest that SAD involves heightened sensitivity to incentives relative to other forms of anxiety such as GAD, but similar to an early life risk factor, namely BI (Guyer et al., 2006). Given that BI is a risk factor both for GAD and for SAD, the finding of similar activity in the caudate and putamen during reward anticipation in the BI and SAD samples suggests that heightened striatal response may be a key neurobiological property for SAD but not for other anxiety disorders among youth with BI.
5.2.2. Role of agency in reward anticipation
An important question remained regarding the role of the striatum during reward anticipation, as it was unclear whether adolescents with SAD or risk for SAD were simply more sensitive to anticipated rewards in the absence of active decision-making or whether this heightened striatal sensitivity would emerge when exerting agency over their actions. The latter proposition aligns with the heightened performance monitoring hypothesis referred to earlier. To address this question, a study of adolescents with early life BI used an fMRI task that manipulated performance contingency during incentive processing (Bar-Haim et al., 2009), separating reward-contingent performance monitoring and anticipation of reward to test for dissociation between these processes as indexed by striatal response. As expected, adolescents with BI relative to adolescents without BI history showed increased nucleus accumbens activation when the reward outcome was contingent on their action (Bar-Haim et al., 2009). No group differences were observed, however, for non-contingent or motor trials. These results highlight an association between early childhood BI and increased striatal activity in adolescence when agency is involved in anticipating rewards. Taken together, the findings from Guyer et al. (2006) and Bar-Haim et al. (2009) underscore the heightened concerns about performance experienced by socially inhibited individuals, and an approach-avoid conflict related to generating the approach behaviors necessary for performing well and the avoidance behaviors related to a fear of performing poorly.
5.2.3. Reward receipt
Given that heightened striatal activity during reward anticipation is associated with motivated action in avoiding failure in BI (Bar-Haim et al., 2009), then learning that one actually failed may be more salient in BI versus non-BI adolescents and feature prominently in neural response during feedback processing. Recent work thus probed beyond reward anticipation to examine BI-related differences in striatal response to rewarding and punishing outcomes that followed performance (Helfinstein et al., 2011). It was hypothesized that, although BI moderated the relationship between reward salience and striatal activation during the anticipation of reward (Guyer et al., 2006), BI would moderate the valence of the subsequent outcome (gain or loss) and striatal response to feedback related to one's actions. As expected, adolescents with versus without BI history showed heightened caudate activation to punishment but not reward outcomes (Helfinstein et al., 2011). Furthermore, group differences emerged in the ventromedial prefrontal cortex (vmPFC), a region implicated in appraising a stimulus's reward value (Kringelbach, 2005). Specifically, non-BI adolescents showed greater vmPFC activation to reward versus punishment outcomes, whereas for BI adolescents, vmPFC activity did not distinguish between these outcomes.
The caudate and vmPFC findings reported in Helfinstein et al. (2011) have two key implications for understanding risk for SAD as a function of neurally based reward processing. First, adolescents with BI history may experience the motivation to avoid an aversive outcome to a greater extent than their non-inhibited peers. For example, because the heightened response to negative outcomes in behaviorally inhibited adolescents occurred in the caudate, a region known for hyper-responsiveness to appetitive stimuli in typical individuals (Knutson et al., 2001), it may be that the reward circuitry usually recruited to generate appetitive-motivational behavior is perturbed in youth with BI by eliciting avoidance rather than approach representations of feedback upon learning the outcomes of their motivated actions. Results from the social evaluative task discussed in Section 5.2.1 lend further support to this suggestion. In this task, after anticipating social feedback, participants received actual bids of acceptance and rejection from peers. Here, caudate hypoactivation after the receipt of rewarding feedback (peer acceptance) was observed in behaviorally inhibited relative to non-inhibited adolescents, further implicating a shift in the caudate's role away from reward responsiveness (Guyer et al., 2014). A second key implication of Helfinstein et al. (2011) is that the diminished vmPFC response to reward and punishment feedback in adolescents with BI may signal a lowered capacity for distinguishing between reward cue values. Because the vmPFC modulates striatal responses (Haber et al., 1995), adolescents with BI may code aversive outcomes via reduced striatal response that contributes to the muted discrimination between positive and negative outcomes in the vmPFC. Nevertheless, it remains unknown whether muted response to reward in the vmPFC was a cause or a consequence of enhanced responding in the caudate, given the bidirectional functional connections between the vmPFC and caudate.
6. Adolescence-specific vulnerability to SAD
6.1. Changes in appetitive-motivational processes
As depicted in Fig. 1, we propose that vulnerability to SAD in adolescence is partially a function of age-expectant changes in a distributed network of brain regions that modulate reward motivation in normal adolescents and become altered in anxious youth. Adolescence signals a time of increased exploration and risk-taking, behaviors that possibly evolved as adaptive strategies for achieving autonomy and survival without parental protection, as well as for finding mates for sexual reproduction. Longitudinal evidence points to increased reward sensitivity from early to late adolescence (Urosevic et al., 2012). Some studies have reported evidence of decreased striatal recruitment in adolescents relative to children and/or adults during reward tasks (Bjork et al., 2004, Blum et al., 1996, Geier et al., 2010). Increased risk-taking and approach behavior is interpreted through these data as reflecting a deficiency in reward processing in the adolescent brain, thus ascribing a greater salience of rewards to adolescents relative to adults. Other studies, in contrast, have reported evidence of increased recruitment of reward-related brain regions in adolescents relative to children and/or adults when anticipating or viewing reward cues (Ernst et al., 2005, Galvan et al., 2006, Geier et al., 2010, Van Leijenhorst et al., 2010a, Van Leijenhorst et al., 2010b). These data suggest that increased risk-taking and approach behavior in adolescence is attributable to enhanced, rather than deficient reward processing in the brain. One study reported both striatal hypoactivity during response preparation in a reward task, followed by striatal hyperactivity when anticipating feedback about the reward in adolescents versus adults (Geier et al., 2010). Other work has reported a U-shaped pattern of striatal activity across childhood, adolescence, and adulthood suggesting that adolescence marks a phase of development that is highly sensitive to reward cues relative to earlier and later developmental periods (Van Leijenhorst et al., 2010b). Notably, a U-shaped trajectory also characterizes hormonal levels in striatal regions, with an adolescent peak in the transmission of dopaminergic signals that facilitate incentive motivation (Luciana et al., 2012).
There is general agreement that the preponderance of data support striatal hyperactivity versus hypoactivity in adolescence (Ernst et al., 2006, Galvan, 2010), and that these neurobiological changes underlie behavioral change in reward valuation, goal-oriented performance monitoring, and cognitive regulation of motivation. These insights into the neural signature of reward processing among typical adolescents have provided some impetus for examining appetitive-motivational systems among adolescents with or at risk for SAD. Although these shifts are age-expectant and adaptive, in a subset of adolescents at high risk for anxiety the changes in reward processing that accompany adolescence may elicit hyperarousal and emotion regulation difficulties in the presence of performance-contingent rewards.
Age-expectant changes in reward processing coincide with a number of other neurobiological changes that may escalate risk for SAD. In our model of adolescent vulnerability to SAD, we stipulate that neurobiological risk factors include both structural and functional changes in cortical regions (including the PFC) and in subcortical regions (including the striatum). Changes in striatal activity occur at a time when significant reorganization takes place in a distributed neural network. Structural changes include synaptic pruning, increased white matter in prefrontal regions, and increased synaptic connectivity and integration (Giedd, 2008). Functional changes are marked by shifts from more diffuse to more focal task-based activity and enhanced modulation of subcortical structures by the PFC (Blakemore and Choudhury, 2006, Giedd, 2008). Though this reorganization ultimately functions to enhance efficiency and flexibility in cognitive tasks, it occurs over a relatively protracted period that may continue into adulthood. In contrast, subcortical structures that control motivation and emotion mature relatively early in development (Lenroot and Giedd, 2006). A potential imbalance between the time courses of cortical and subcortical neural circuitry development is one hypothesized mechanism of adolescent vulnerability to disorders such as SAD (Paus et al., 2008). Risk avoidance, emotion dysregulation, and impulsivity related to hyper-arousal are all indicators of SAD, and these processes may become exaggerated at a time when heightened reward responsiveness overrides the still-maturing regulatory mechanisms of the PFC.
6.2. Changes in the social environment
We also propose that changes in the social environment make an independent contribution to SAD risk in adolescence (Fig. 1). Altered avoidance and appetitive-motivational processes may contribute to SAD onset within the context of a changing social landscape in adolescence. Relative to their peer interactions earlier in development, adolescents spend significantly more time with peers than with family (Steinberg and Morris, 2001). Adolescence is also a period when interactions with peers are highly rewarding and influential, which increases the salience and impact of both positive and negative aspects of interacting with one's peers (Rubin et al., 2006). As children move into middle and high school, opportunities to create friendships and romantic relationships expand and youth are exposed to more unfamiliar peers than at younger ages. This increased social exposure can lead to timidity and fear of embarrassment and humiliation in the face of uncertain social status. For some adolescents, the anxiety that comes with broadened social opportunities may be extreme enough to lead to SAD, particularly for those with a temperamentally based bias towards reticence in social situations.
The conflict between approach and avoidance systems observed in youth with early-life BI and youth with SAD in a monetary incentive context may also underlie motivational conflicts in peer relations. For example, whereas typically developing adolescents have enhanced sensitivity in reward-related neural networks that motivate approaching unfamiliar peers with a bid for social affiliation and acceptance, socially anxious or at-risk adolescents may experience approach-avoid conflict in this situation, as they anticipate rejection but are also highly invested in what their peers think of them (Lucock & Salkovskis, 1988). Such conflict is likely driven by enhanced sensitivity in both approach and withdrawal motivation systems. Furthermore, immaturity of the brain's cognitive control networks could contribute to negative attentional and interpretational biases among socially anxious adolescents, making it difficult for them to process positive and negative social information (Weeks and Howell, 2012). Resolving motivational conflicts in peer-driven environments can be especially difficult when social cues are ambiguous (e.g., one cannot tell whether another person thinks he or she is boring). In these instances, socially anxious adolescents are likely to interpret social information as overly threatening and to generate negative self-evaluations, and thus, avoid social bids with uncertain outcomes (Heimberg et al., 2010). As such, heightened social anxiety in adolescence may not only be a function of age-typical increases in social motivation, but it may also reflect difficulty in encoding the reward value of social cues that arises from cognitive biases typical of SAD.
6.3. Conceptual model of adolescent vulnerability to SAD
Adolescence brings distinct contextual and biological changes that motivate social behavior, but it also brings increased vulnerability for SAD. Thus, our model for understanding adolescent vulnerability to SAD highlights the relationships among individual differences in temperament (namely BI), changes in cortical and subcortical neural circuitry during adolescence underlying social and non-social information processing, and changes in the social environment (Fig. 1). The model stipulates that heightened risk for SAD in adolescence is associated with approach-avoidance conflicts whereby the increased salience of social reward is offset by extreme fear of humiliation, embarrassment, or lowered social status. These conflicts are generated by normative changes in several, interacting processes at multiple levels. At the biological level, changes in neural circuitry occur in threat-related (i.e., amygdala and vlPFC) and reward-related (i.e., basal ganglia and vmPFC) systems. These neurobiological mechanisms are associated with psychological processes that differ between non-anxious adolescents and those with SAD or at risk for SAD, specifically processing of threats and rewards of a social and non-social nature. The central motivational conflict between increased desire in adolescence for peer acceptance and fear of embarrassment or humiliation is driven by a number of cognitive factors described earlier, such as anticipation of punishment (i.e., rejection), enhanced performance monitoring to avoid failure, and enhanced processing of negative outcomes. Such a view is consistent with behavioral evidence indicating that socially anxious individuals tend to expect rejection, show heightened concern for their own appearance of anxiety, and display overly negative interpretations of social outcomes (Heimberg et al., 2010).
Adolescent changes in neural and motivation systems occur within a changing social context: more time is spent with peers, opportunities for social interactions expand, and exposure to unfamiliar peers increases. The relationships among the changes occurring at biological, psychological, and environmental levels may be moderated by individual differences such as a behaviorally inhibited temperament in predicting SAD. Given the vast research linking SAD with a multitude of contributing influences, our model is not comprehensive. For example, many children who are not behaviorally inhibited develop SAD. Although temperament is a strong factor, other developmental influences exist; yet the model focuses on temperament because many of the findings on adolescent SAD in the developmental cognitive neuroscience literature are linked with BI. Furthermore, pubertal status is likely to modulate social fears and motivations, due to changing hormones and changing physical appearance (Forbes et al., 2010, Sisk and Zehr, 2005). In spite of these constraints, the model highlights key relationships of interest in developmental cognitive neuroscience and its application to psychopathology. Furthermore, it generates hypotheses for differentiating normative and atypical levels of social anxiety in adolescence.
The functional neuroimaging studies linking adolescent SAD and early life BI to approach-related neural circuitry have made strides in characterizing hypersensitivity to rewards and fear of failure related to performance (Bar-Haim et al., 2009, Guyer et al., 2014, Guyer et al., 2012a, Guyer et al., 2006, Helfinstein et al., 2011). To more fully establish a clinically relevant relationship between striatal circuitry and SAD, tasks designed to probe constructs linked specifically with social anxiety symptoms, such as extreme fear of social evaluation and humiliation, ought to be used in conjunction with neuroimaging. Progress towards understanding adolescent vulnerability for SAD has been initiated by pairing neuroscience methodologies with social evaluation tasks and by increasing the ecological validity of our assessments of the processes that are tightly linked with risk for SAD, such as social evaluation and social avoidance (Gunther Moor et al., 2010, Guyer et al., 2014, Guyer et al., 2012a, Guyer et al., 2008, Guyer et al., 2009, Lau et al., 2011).
7. Conclusions
Why is adolescence a period of heightened risk for a diagnosis of clinical social anxiety? The preponderance of research points to a complicated and dynamic cluster of changes that may confer this vulnerability: a history of behaviorally inhibited temperament that interacts with changes in fear- and reward-related neural circuits, social information processing, and changes in the social environment. The present paper reviewed recent data to introduce novel conceptual and methodological approaches to addressing this question: moving beyond a focus solely on fear processing and incorporating constructs of reward processing, integrating social tasks into established neuroimaging paradigms, and assessing neural and behavioral change over time, or at least between multiple age groups, among those affected by and at risk for SAD. The social world is an extremely complex environment. The more that developmental cognitive neuroscience research can account for that complexity, the more fully and effectively we can understand adolescent SAD and differentiate it from typical anxieties that can be expected in adolescence.
Conflict of interest
The authors report no financial relationships with commercial interests.
Acknowledgements
This work was supported by National Institute of Health grants to A.E.G. (R00-MH080076) and to J.D.C. (T32-MH020006).The authors wish to thank Dr. Daniel S. Pine and Dr. Ross Thompson for their helpful comments on initial drafts.
References
- Adolphs R., Tranel D., Damasio H., Damasio A.R. Fear and the human amygdala. J. Neurosci. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano A.M., Marten P.A., Holt C.S., Heimberg R.G., Barlow D.H. Cognitive-behavioral group treatment for social phobia in adolescents. A preliminary study. J. Nerv. Ment. Dis. 1995;183(10):649–656. doi: 10.1097/00005053-199510000-00006. [DOI] [PubMed] [Google Scholar]
- Almas A.N., Phillips D.A., Henderson H.A., Hane A.A., Degnan K.A., Moas O.L., Fox N.A. The relations between infant negative reactivity, non-maternal childcare, and children's interactions with familiar and unfamiliar peers. Soc. Dev. 2011;20(4):718–740. doi: 10.1111/j.1467-9507.2011.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic criteria from DSM-IV-TR. [Google Scholar]
- Anderson E.R., Hope D.A. The relationship among social phobia, objective and perceived physiological reactivity, and anxiety sensitivity in an adolescent population. J. Anxiety Disord. 2009;23(1):18–26. doi: 10.1016/j.janxdis.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Fox N.A., Benson B., Guyer A.E., Williams A., Nelson E.E., Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol. Sci. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van I.M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. The neural basis of romantic love. Neuroreport. 2000;11(17):3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Beesdo K., Lau J.Y., Guyer A.E., McClure-Tone E.B., Monk C.S., Nelson E.E., Pine D.S. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch. Gen. Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K., Pine D.S., Lieb R., Wittchen H.U. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch. Gen. Psychiatry. 2010;67(1):47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- Beidel D.C. Psychophysiological assessment of anxious emotional states in children. J. Abnorm. Psychol. 1988;97(1):80–82. doi: 10.1037//0021-843x.97.1.80. [DOI] [PubMed] [Google Scholar]
- Beidel D.C., Christ M.G., Long P.J. Somatic complaints in anxious children. J. Abnorm. Child Psychol. 1991;19(6):659–670. doi: 10.1007/BF00918905. [DOI] [PubMed] [Google Scholar]
- Beidel D.C., Turner S.M., Sallee F.R., Ammerman R.T., Crosby L.A., Pathak S. SET-C versus fluoxetine in the treatment of childhood social phobia. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(12):1622–1632. doi: 10.1097/chi.0b013e318154bb57. [DOI] [PubMed] [Google Scholar]
- Biederman J., Hirshfeld-Becker D.R., Rosenbaum J.F., Herot C., Friedman D., Snidman N., Faraone S.V. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry. 2001;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Bishop S.J. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn. Sci. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J., Choudhury S. Brain development during puberty: state of the science. Dev. Sci. 2006;9(1):11–14. doi: 10.1111/j.1467-7687.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- Blum K., Sheridan P.J., Wood R.C., Braverman E.R., Chen T.J., Cull J.G., Comings D.E. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med. 1996;89(7):396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Lissek S., Grillon C., Norcross M.A., Pine D.S. Development of anxiety: the role of threat appraisal and fear learning. Depress. Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein M., He J.P., Kattan G., Albano A.M., Avenevoli S., Merikangas K.R. Social phobia and subtypes in the national comorbidity survey-adolescent supplement: prevalence, correlates, and comorbidity. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(9):870–880. doi: 10.1016/j.jaac.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S.D., Fox N.A., Marshall T.R. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67(2):523–540. [PubMed] [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., Perez-Edgar K., Henderson H.A., Diaz Y., Fox N.A. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan R.J., Rubin K.H., Fox N.A., Calkins S.D., Stewart S.L. Being alone, playing alone, and acting alone: distinguishing among reticence and passive- and active-solitude among children. Child Dev. 1994;65(1):129–137. [PubMed] [Google Scholar]
- Corr P.J., Gray's J.A. Reinforcement sensitivity theory: tests of the joint subsystems hypothesis of anxiety and impulsivity. Pers. Indiv. Diff. 2002;33:511–532. [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Degnan K.A., Fox N.A. Behavioral inhibition and anxiety disorders: multiple levels of a resilience process. Dev. Psychopathol. 2007;19(3):729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Diener C., Kuehner C., Brusniak W., Ubl B., Wessa M., Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61(3):677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Dolan R.J. Keynote address: revaluing the orbital prefrontal cortex. Ann. N. Y. Acad. Sci. 2007;1121:1–9. doi: 10.1196/annals.1401.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E., Pine D.S. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex M.J., Klein M.H., Slattery M.J., Goldsmith H.H., Kalin N.H. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am. J. Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Martin L.N., Delgado M.R. Reward-related processing in the human brain: developmental considerations. Dev. Psychopathol. 2008;20(4):1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Christopher May J., Siegle G.J., Ladouceur C.D., Ryan N.D., Carter C.S., Dahl R.E. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J. Child Psychol. Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L., Dahl R.E. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(2):162. doi: 10.1097/00004583-201002000-00010. e161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Marshall P.J., Nichols K.E., Ghera M.M. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Rubin K.H., Calkins S.D., Schmidt L.A. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4(6) doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb. Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gest S.D. Behavioral inhibition: stability and associations with adaptation from childhood to early adulthood. J. Person. Soc. Psychol. 1997;72(2):467–475. doi: 10.1037//0022-3514.72.2.467. [DOI] [PubMed] [Google Scholar]
- Giedd J.N. The teen brain: insights from neuroimaging. J. Adolesc. Health. 2008;42(4):335–343. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Graber J.A., Brooks-Gunn J. Transitions and turning points: navigating the passage from childhood through adolescence. Dev. Psychol. 1996;32(4):768–776. [Google Scholar]
- Gunther Moor B., van Leijenhorst L., Rombouts S.A., Crone E.A., Van der Molen M.W. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc. Neurosci. 2010;5(5-6):461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Benson B., Choate V.R., Bar-Haim Y., Perez-Edgar K., Jarcho J.M., Nelson E.E. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev. Psychopathol. 2014 doi: 10.1017/S0954579413000941. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Detloff A., Benson B., Nelson E.E., Perez-Edgar K., Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am. J. Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cogn. Affect. Neurosci. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Lau J.Y., McClure-Tone E.B., Parrish J., Shiffrin N.D., Reynolds R.C., Nelson E.E. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., McClure-Tone E.B., Shiffrin N.D., Pine D.S., Nelson E.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., Hardin M.G., Roberson-Nay R., Monk C.S., Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J. Neurosci. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Kunishio K., Mizobuchi M., Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J. Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee J.E., Benson B.E., Bar-Haim Y., Mogg K., Bradley B.P., Chen G., Perez-Edgar K. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin M.G., Perez-Edgar K., Guyer A.E., Pine D.S., Fox N.A., Ernst M. Reward and punishment sensitivity in shy and non-shy adults: relations between social and motivated behavior. Pers. Indiv. Diff. 2006;40(4):699–711. doi: 10.1016/j.paid.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin M.G., Pine D.S., Ernst M. The influence of context valence in the neural coding of monetary outcomes. Neuroimage. 2009;48(1):249–257. doi: 10.1016/j.neuroimage.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Davidson M.C., Glover G.H., Casey B.J. Contributions of amygdala and striatal activity in emotion regulation. Biol. Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hariri A.R. The what, where, and when of catechol-O-methyltransferase. Biol. Psychiatry. 2011;70(3):214–215. doi: 10.1016/j.biopsych.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Hasler B.P., Clark D.B. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcohol Clin. Exp. Res. 2013;37(4):558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C., Varady S., Albano A.M., Thienemann M., Henderson L., Schatzberg A.F. Cognitive-behavioral group therapy for social phobia in female adolescents: results of a pilot study. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(6):721–726. doi: 10.1097/00004583-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Brozovich F.A., Rapee R.M. A cognitive behavioral model of social anxiety disorder: update and extension. In: Heimberg R.G., Brozovich F.A., Rapee R.M., editors. Social Anxiety: Clinical, Developmental, and Social Perspectives. (2nd ed. Elsevier Academic Press; San Diego, CA, USA: 2010. pp. 395–422. [Google Scholar]
- Helfinstein S.M., Benson B., Perez-Edgar K., Bar-Haim Y., Detloff A., Pine D.S., Ernst M. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein S.M., Fox N.A., Pine D.S. Approach-withdrawal and the role of the striatum in the temperament of behavioral inhibition. Dev. Psychol. 2012;48(3):815–826. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- Hirshfeld D.R., Rosenbaum J.F., Biederman J., Bolduc E.A., Faraone S.V., Snidman N., Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker D.R., Biederman J., Henin A., Faraone S.V., Davis S., Harrington K., Rosenbaum J.F. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. J. Dev. Behav. Pediatr. 2007;28(3):225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- Kagan J. Temperament in Human Nature. Boulder; Westview: 1996. Galen's prophecy. [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Snidman N., Gibbons J., Johnson M.O. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988;59(6):1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kashdan T.B., Herbert J.D. Social anxiety disorder in childhood and adolescence: current status and future directions. Clin. Child Fam. Psychol. Rev. 2001;4(1):37–61. doi: 10.1023/a:1009576610507. [DOI] [PubMed] [Google Scholar]
- Kessler R.C. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr. Scand. Suppl. 2003;417:19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Loucks R.A., Palmer A.L., Brown A.C., Solomon K.M., Marchante A.N., Whalen P.J. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.I. Neuroscientific model of motivational process. Front. Psychol. 2013;4:98. doi: 10.3389/fpsyg.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Cooper J.C. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Silk J.S., Dahl R.E., Ostapenko L., Kronhaus D.M., Phillips M.L. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9(6):855–864. doi: 10.1037/a0017747. [DOI] [PubMed] [Google Scholar]
- Lahat A., Perez-Edgar K., Degnan K.A., Guyer A.E., Lejuez C.W., Ernst M., Fox N.A. Early childhood temperament predicts substance use in young adults. Transl. Psychiatry. 2012;2 doi: 10.1038/tp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J.Y.F., Guyer A.E., Tone E.B., Jenness J., Parrish J.M., Pine D.S., Nelson E.E. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal complex. Int. J. Behav. Dev. 2011;36(1):36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr. Biol. 2007;17(20):R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lopes V.M., Albano A.M. Pediatric social phobia. In: Vasa R.A., Roy A.K., editors. Pediatric Anxiety Disorders: A Clinical Guide. Springer; NY: 2013. [Google Scholar]
- Lorberbaum J.P., Kose S., Johnson M.R., Arana G.W., Sullivan L.K., Hamner M.B., George M.S. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15(18):2701–2705. [PubMed] [Google Scholar]
- Luciana M., Wahlstrom D., Porter J.N., Collins P.F. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev. Psychol. 2012;48(3):844–861. doi: 10.1037/a0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucock M.P., Salkovskis P.M. Cognitive factors in social anxiety and its treatment. Behav. Res. Ther. 1988;26:297–302. doi: 10.1016/0005-7967(88)90081-2. [DOI] [PubMed] [Google Scholar]
- Maack D.J., Tull M.T., Gratz K.L. Examining the incremental contribution of behavioral inhibition to generalized anxiety disorder relative to other Axis I disorders and cognitive-emotional vulnerabilities. J. Anxiety Disord. 2012;26:689–695. doi: 10.1016/j.janxdis.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Mattfeld A.T., Gluck M.A., Stark C.E. Functional specialization within the striatum along both the dorsal/ventral and anterior/posterior axes during associative learning via reward and punishment. Learn. Mem. 2011;18(11):703–711. doi: 10.1101/lm.022889.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Parrish J.M., Adler A., Blair R.J., Pine D.S. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch. Gen. Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McNaughton N., Corr P.J. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Nelson E.E., McClure E.B., Mogg K., Bradley B.P., Leibenluft E., Pine D.S. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am. J. Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., Bradley B.P., Mai X., Louro H.M., Pine D.S. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., van Brakel A.M., Arntz A., Schouten E. Behavioral Inhibition as a risk factor for the development of childhood anxiety disorders: a longitudinal study. J. Child Fam. Stud. 2011;20(2):157–170. doi: 10.1007/s10826-010-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., O’Doherty J.P., Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J. Neurosci. 2007;27(31):8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M., Gunnar M., Mangelsdorf S., Parritz R.H., Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. The development of the ventral prefrontal cortex and social flexibility. Dev. Cogn. Neurosci. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Olsson C.A., Anney R.J., Lotfi-Miri M., Byrnes G.B., Williamson R., Patton G.C. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatry Genet. 2005;15(2):109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K., Fox N.A. Temperament and anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2005;14(4):681–706. doi: 10.1016/j.chc.2005.05.008. viii. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K., Hardee J., Guyer A.E., Benson B., Nelson E.E., Gorodetsky E., Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K., Reeb-Sutherland B.C., McDermott J.M., White L.K., Henderson H.A., Degnan K.A., Fox N.A. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J. Abnorm. Child Psychol. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K., Roberson-Nay R., Hardin M.G., Poeth K., Guyer A.E., Nelson E.E., Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Klumpp H. Neuroendocrinology and neuroimaging studies of social anxiety disorder. In: Phan K.L., Klumpp H., editors. Social Anxiety: Clinical, Developmental, and Social Perspectives. 2nd ed. Elsevier Academic Press; San Diego, CA, USA: 2010. pp. 273–312. [Google Scholar]
- Pine D.S., Guyer A.E., Leibenluft E. Functional magnetic resonance imaging and pediatric anxiety. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(11):1217–1221. doi: 10.1097/CHI.0b013e318185dad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: a commentary on contemporary heart rate variability research. Biol. Psychol. 2007;74(2):301–307. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K. Temperament and the development of inhibited approach. Child Dev. 1988;59(5):1241–1250. [PubMed] [Google Scholar]
- Rothbart M.K., Derryberry D. Development of individual differences in temperament. In: Lamb A.B.M., editor. vol. 1. Erlbaum; Hillsdale, NJ: 1981. (Advances in Developmental Psychology). [Google Scholar]
- Rubin K.H., Bukowski W.M., Parker J.G. Peer interactions, relationships, and groups. In: Eisenberg N.E., Damon W.E., Lerner R.M.E., editors. Handbook of Child Psychology. 6th ed. John Wiley & Sons Inc; Hoboken, N.J., U.S: 2006. [Google Scholar]
- Rubin K.H., Burgess K.B., Hastings P.D. Stability and social-behavioral consequences of toddlers’ inhibited temperament and parenting behaviors. Child Dev. 2002;73(2):483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- Rubin K.H., Coplan R.J., Bowker J.C. Social withdrawal in childhood. Annu. Rev. Psychol. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Snidman N., Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz C.E., Wright C.I., Shin L.M., Kagan J., Rauch S.L. Inhibited and uninhibited infants grown up: adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldu X., Segura B., Dreher J.C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37(4):681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Seymour B., O’Doherty J.P., Dayan P., Koltzenburg M., Jones A.K., Dolan R.J., Frackowiak R.S. Temporal difference models describe higher-order learning in humans. Nature. 2004;429(6992):664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- Sisk C.L., Zehr J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smetana J.G., Campione-Barr N., Metzger A. Adolescent development in interpersonal and societal contexts. Annu. Rev. Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Stein D.J., Newman T.K., Savitz J., Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11(10):745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- Stein M.B. An epidemiologic perspective on social anxiety disorder. J. Clin. Psychiatry. 2006;67(Suppl. 12):3–8. [PubMed] [Google Scholar]
- Stein M.B., Fuetsch M., Muller N., Hofler M., Lieb R., Wittchen H.U. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch. Gen. Psychiatry. 2001;58(3):251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. Adolescent development. Annu. Rev. Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stopa L., Clark D.M. Cognitive processes in social phobia. Behav. Res. Ther. 1993;31(3):255–267. doi: 10.1016/0005-7967(93)90024-o. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Ahs F., Fredrikson M., Sollers J.J., 3rd., Wager T.D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tricomi E.M., Delgado M.R., Fiez J.A. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Urosevic S., Collins P., Muetzel R., Lim K., Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev. Psychol. 2012;48(5):1488–1500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Weeks J.W., Howell A.N. The bivalent fear of evaluation model of social anxiety: further integrating findings on fears of positive and negative evaluation. Cogn. Behav. Ther. 2012;41(2):83–95. doi: 10.1080/16506073.2012.661452. [DOI] [PubMed] [Google Scholar]
- Williams L.R., Degnan K.A., Perez-Edgar K., Henderson H.A., Rubin K.H., Pine D.S., Fox N.A. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. J. Abnorm. Child Psychol. 2009;37(8):1063–1075. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U., Stein M.B., Kessler R.C. Social fears and social phobia in a community sample of adolescents and young adults: prevalence, risk factors and co-morbidity. Psychol. Med. 1999;29(2):309–323. doi: 10.1017/s0033291798008174. [DOI] [PubMed] [Google Scholar]
- Zink C.F., Pagnoni G., Martin-Skurski M.E., Chappelow J.C., Berns G.S. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]