Abstract
Background
Puerto Rican children share a disproportionate burden of prematurity and asthma in the United States. Little is known about prematurity and childhood asthma in Puerto Rican subjects.
Objective
We sought to examine whether prematurity is associated with asthma in Puerto Rican children.
Methods
We performed a case-control study of 678 children aged 6 to 14 years with (n = 351) and without (n = 327) asthma living in San Juan, Puerto Rico. Prematurity was defined by parental report for our primary analysis. In a secondary analysis, we only included children whose parents reported prematurity that required admission to the neonatal intensive care unit. Asthma was defined as physician-diagnosed asthma and wheeze in the prior year. We used logistic regression for analysis. All multivariate models were adjusted for age, sex, household income, atopy (≥1 positive IgE level to common allergens), maternal history of asthma, and early-life exposure to environmental tobacco smoke.
Results
In a multivariate analysis there was a significant interaction between prematurity and atopy on asthma (P = .006). In an analysis stratified by atopy, prematurity was associated with a nearly 5-fold increased odds of asthma in atopic children (adjusted odds ratio, 4.7; 95% CI, 1.5–14.3; P = .007). In contrast, there was no significant association between prematurity and asthma in nonatopic children. Similar results were obtained in our analysis of prematurity requiring admission to the neonatal intensive care unit and asthma.
Conclusions
Our results suggest that atopy modifies the estimated effect of prematurity on asthma in Puerto Rican children. Prematurity might explain, in part, the high prevalence of atopic asthma in this ethnic group.
Keywords: Childhood asthma, prematurity, Puerto Rican, atopy
Asthma is the most common chronic disease of childhood in the United States.1,2 In this country, the prevalence of childhood asthma is higher in Puerto Rican subjects (16.1%) and non-Hispanic black subjects (11.2%) than in non-Hispanic white subjects (7.7%) or Mexican American subjects (5.4%).2 Puerto Rican children living on the island of Puerto Rico have an even higher prevalence of asthma than those living on the US mainland.3 Poverty is a contributory factor but not the sole explanation for the high asthma prevalence in Puerto Rican children.4
Prematurity might explain, in part, the high prevalence of childhood asthma in Puerto Rican subjects. In the United States the preterm birth rate is higher among Puerto Rican (13.4%) or non-Hispanic black (17.1%) women than among non-Hispanic white (10.8%) orMexican American (11.3%) women.5 Furthermore, the proportion of children born prematurely is higher on the island of Puerto Rico (17.6%) than in any other state or territory of this country.6 Whereas prematurity has been consistently associated with an increased risk of asthma in preschool-age children,7–9 there is conflicting evidence of a link between prematurity and asthma in school-age children (in whom a diagnosis of asthma is more likely to be accurate). For example, a meta-analysis that included 19 observational studies (published between January 1966 and May 2005) showed that although prematurity was associated with a nearly 40% excess odds of asthma in children of all ages (pooled odds ratio [OR], 1.37; 95% CI, 1.30–1.43),7 this association became weaker and nonsignificant (pooled OR, 1.19; 95% CI, 0.94–1.51) when the analysis was restricted to studies of older participants (ie, children ≥10 years old). Whether atopy modifies the effects of prematurity on asthma at school age is unknown.
Even though Puerto Rican subjects share a disproportionate burden of prematurity and asthma in the United States, no study has examined the relation between these 2 conditions in this ethnic group.
We hypothesized that prematurity would be associated with asthma in Puerto Rican children but that this association would differ depending on atopic status. To test this hypothesis, we examined the relation between prematurity and asthma in a case-control study of 678 school-age Puerto Rican children living in San Juan, Puerto Rico.
METHODS
Subject recruitment
From March 2009 to June 2010, children in San Juan were chosen from randomly selected households. As previously described,10,11 households in the metropolitan area of San Juan were selected by using a multistage probability sampling design. Primary sampling units were randomly selected neighborhood clusters based on the 2000 US Census, and secondary sampling units were randomly selected households within each primary sampling unit. A household was eligible if 1 or more residents were children aged 6 to 14 years. In households with 1 or more eligible children, only 1 child was randomly selected for screening. On the basis of the sampling design, 7073 households were selected, and 6401 (approximately 91%) were contacted. Of these 6401 households, 1111 had 1 or more children within the age range of the study who met other inclusion criteria (see below). In an effort to reach a target sample size of approximately 700 children (which would give us ≥90% power to detect an OR of ≥2 for exposures with a prevalence of ≥25%), we attempted to enroll a random sample (n = 783) of these 1111 children. Parents of 105 of these 783 eligible households refused to participate or could not be reached. There were no significant differences in age, sex, or area of residence between eligible children who did (n = 678 [86.6%]) and did not (n = 105 [13.4%]) agree to participate. We selected as cases children who had physician-diagnosed asthma and wheeze in the previous year (n = 351). We selected as control subjects children who had neither physician-diagnosed asthma nor wheeze in the prior year (n = 327). All study participants had to have 4 Puerto Rican grandparents to ensure their Puerto Rican descent.
Study procedures
Study participants completed a protocol that included administration of questionnaires, spirometry, and collection of blood samples (for measurement of serum total and allergen-specific IgE levels). One of the child’s parents (usually [for approximately 93% of subjects] the mother) completed a questionnaire that was slightly modified from one used in the Collaborative Study of the Genetics of Asthma.12 This questionnaire was used to obtain information about the child’s general and respiratory health; sociodemographic characteristics; family history of asthma, allergic rhinitis, or eczema; current exposure to environmental tobacco smoke (ETS); and early-life exposure to ETS (in utero or before 2 years of age).
Height and weight were measured to the nearest centimeter and pound, respectively. Spirometry was conducted with an EasyOne spirometer (NDD Medical Technologies, Andover, Mass). All participants had to be free of respiratory illnesses for 4 or more weeks, and they were also instructed to avoid (when possible) the use of inhaled short- and long-acting bronchodilators for 4 or more and 12 or more hours before testing, respectively. Forced expiratory maneuvers were judged to be acceptable if they met or exceeded American Thoracic Society criteria modified for children.13 The best FEV1 and forced vital capacity (FVC) values were selected for data analyses. Serum levels of total IgE and IgE specific to common allergens (dust mite [Der p 1], cockroach [Bla g 2], cat dander [Fel d 1], dog dander [Can f 1], and mouse urinary protein [Mus m 1]) were determined by using the UniCAP 100 system (Pharmacia & Upjohn, Kalamazoo, Mich). For each allergen, an IgE level of 0.35 IU/mL or greater was considered positive.
Written parental consent was obtained for participating children, from whom written assent was also obtained. The study was approved by the Institutional Review Boards of the University of Puerto Rico (San Juan, Puerto Rico), Brigham & Women’s Hospital (Boston, Mass), and the University of Pittsburgh (Pittsburgh, Pa).
Statistical analysis
For our primary analysis, prematurity was treated as a binary variable based on parental response to the following question: “Was your child born prematurely?” For our secondary analysis, prematurity requiring neonatal intensive care unit (NICU) admission was treated as a binary variable based on a positive response to the question on prematurity, as well as to the following question: “Was your child kept in a neonatal intensive care unit?” Our outcome of interest was asthma (defined as physician-diagnosed asthma and wheeze in the previous year).
For each continuous variable, we used 2-sample t tests to compare 2 groups. For the comparison of each binary variable between 2 groups, we used Fisher exact tests. For the multivariate analysis, we used a stepwise approach to build the logistic regression models. Because of their well-established association with prematurity, asthma, or both, all models included age,2 sex,14 household income (<$15,000/y vs ≥$15,000/y [near the median income for households in Puerto Rico in 2008–2009]),4,15,16 maternal history of asthma and early-life exposure to ETS.17 The following covariates were also included in the initial multivariate models if they were associated with asthma at a P value of .20 or less in bivariate analyses: body mass index as a z score (based on 2000 Centers for Disease Control and Prevention growth charts),18,19 low birth weight (<2500 g), mode of delivery (cesarean vs vaginal birth), total IgE level (transformed to a logarithmic [log10] scale), atopy (≥1 positive allergen-specific IgE), current exposure to ETS, parental education (≥1 parent completed high school vs none), type of health insurance (private or employer-based health insurance vs others), maternal history of 1 or more atopic diseases (asthma, allergic rhinitis, or eczema), and lung function measures (FEV1 and FEV1/FVC ratio). These additional covariates remained in the final models if they were associated with asthma at a P value of less than .05 or if they changed the parameter estimate (β) by 10% or greater. After the final models were built, we tested for first-order interactions between prematurity and the other covariates in the models. We assessed the overall goodness of fit of each model using the Hosmer-Lemeshow test.
As a confirmatory step, we conducted a conditional logistic regression analysis of prematurity and asthma after matching cases and control subjects through propensity scoring (see the Methods section in this article’s Online Repository at www.jacionline.org). Statistical significance was defined as a P value of less than .05. All statistical analyses were performed with SAS version 9.3 software (SAS Institute, Cary, NC).
RESULTS
Compared with control subjects, cases were significantly more likely to be younger, to be male, to be atopic (ie, to have ≥1 positive allergen-specific IgE), to be exposed to ETS (currently or in early life), and to have a history of prematurity or prematurity requiring NICU admission, a higher total IgE level, a maternal history of asthma or 1 or more atopic diseases, and a lower FEV1 and FEV1/FVC ratio (Table I).
TABLE I.
| Covariate | Control subjects (n = 327) |
Cases (n = 351) |
|---|---|---|
| Age (y) | 10.5 (2.7) | 10.0 (2.6)‡ |
| Female sex | 168 (51.4%) | 150 (42.7%)‡ |
| Body mass index (z score) | 0.5 (1.1) | 0.7 (1.2) |
| Total IgE (IU/mL)§ | 151.4 (4.7) | 295.1 (4.8)‡ |
| Atopy (≥1 positive allergen-specific IgE) | 143 (49.8%) | 210 (68.9%)‡ |
| Exposure to ETS in utero or before age 2 y | 131 (40.2%) | 174 (49.6%)‡ |
| Current exposure to ETS | 113 (34.6%) | 155 (44.2%)‡ |
| Household income <$15,000/y | 196 (62.8%) | 225 (65.4%) |
| No parent graduated from high school | 64 (19.6%) | 63 (18.0%) |
| No private or employer-based health insurance | 205 (62.7%) | 239 (68.1%) |
| Maternal history of asthma | 67 (20.8%) | 172 (49.3%)‡ |
| Maternal history of asthma, allergic rhinitis, or eczema | 86 (26.7%) | 190 (54.8%)‡ |
| Low birth weight (<2500 g) | 14 (4.5%) | 18 (5.2%) |
| Birth by cesarean section | 108 (33.4%) | 131 (37.4%) |
| Prematurity | 15 (4.6%) | 31 (8.9%)‡ |
| Prematurity requiring NICU admission | 6 (1.9%) | 21 (6.0%)‡ |
| FEV1 (L)‖ | 2.0 (0.7) | 1.9 (0.7)‡ |
| FEV1/FVC ratio | 0.84 (0.1) | 0.81 (0.1)‡ |
Data are presented as numbers (percentages) for binary variables or means (SDs) for continuous variables.
Percentages were calculated for children with complete data. For example, 592 (287 control subjects and 305 cases) of the 678 participating children had allergen-specific IgE.
P < .05 for the comparisons between groups (performed by using 2-sample t tests or Fisher exact tests, as appropriate).
Total IgE transformed to a logarithmic (log10) scale. Results are shown as geometric means (SDs).
FEV1 values are presented as absolute values because of lack of predicted values for Puerto Rican subjects.
Table II shows a comparison of participating children with and without a history of prematurity (jointly and separately in cases and control subjects). In this analysis low birth weight was significantly associated with prematurity in cases, control subjects, and all subjects combined. Current exposure to ETS was significantly associated with prematurity in control subjects only. There was no significant association between any other variable (eg, indicators of socioeconomic status or atopy) and prematurity in cases, control subjects, or all subjects combined.
TABLE II.
Baseline characteristics of participating children according to case-control status and prematurity*†
| Covariate | Control subjects | Cases | All | |||
|---|---|---|---|---|---|---|
| Prematurity | Prematurity | Prematurity | ||||
| No (n = 308) | Yes (n = 15) | No (n = 318) | Yes (n = 31) | No (n = 626) | Yes (n = 46) | |
| Age (y) | 10.4 (2.7) | 10.3 (2.9) | 10.0 (2.6) | 10.0 (2.3) | 10.2 (2.7) | 10.1 (2.5) |
| Female sex | 157 (51.0%) | 9 (60.0%) | 137 (43.1%) | 13 (41.9%) | 294 (47.0%) | 22 (47.8%) |
| Body mass index (z score) | 0.5 (1.1) | 0.2 (1.5) | 0.7 (1.2) | 1.0 (1.1) | 0.6 (1.1) | 0.7 (1.3) |
| Total IgE (IU/mL)§ | 154.9 (4.7) | 102.3 (4.4) | 288.4 (4.9) | 288.4 (3.6) | 213.8 (5.0) | 199.5 (4.2) |
| Atopy (≥1 positive allergen-specific IgE) | 137 (51.1%) | 4 (26.7%) | 186 (67.4%) | 22 (81.5%) | 323 (59.4%) | 26 (61.9%) |
| Current exposure to ETS | 103 (33.4%) | 9 (60.0%)‡ | 139 (43.7%) | 15 (48.4%) | 242 (38.7%) | 24 (52.2%) |
| Exposure to ETS in utero or before age 2 y | 123 (39.9%) | 7 (46.7%) | 157 (49.4%) | 16 (51.6%) | 280 (44.7%) | 23 (50.0%) |
| Household income <$15,000/y | 184 (62.8%) | 10 (66.7%) | 205 (65.7%) | 19 (63.3%) | 389 (64.3%) | 29 (64.4%) |
| No parent graduated from high school | 57 (18.5%) | 6 (40.0%) | 55 (17.3%) | 8 (25.8%) | 112 (17.9%) | 14 (30.4%) |
| No private or employer-based health insurance | 191 (62.0%) | 11 (73.3%) | 216 (67.9%) | 22 (71.0%) | 407 (65.0%) | 33 (71.7%) |
| Maternal history of asthma | 64 (21.0%) | 3 (20.0%) | 157 (49.5%) | 13 (43.3%) | 221 (35.5%) | 16 (35.6%) |
| Maternal history of asthma, allergic rhinitis, or eczema | 82 (26.9%) | 4 (26.7%) | 174 (55.2%) | 14 (46.7%) | 256 (41.3%) | 18 (40.0%) |
| Low birth weight (<2500 g) | 8 (2.7%) | 6 (40.0%)‡ | 5 (1.6%) | 13 (41.9%)‡ | 13 (2.1%) | 19 (41.3%)‡ |
| Birth by cesarean section | 104 (34.0%) | 4 (26.7%) | 115 (36.3%) | 15 (48.4%) | 219 (35.2%) | 19 (41.3%) |
| FEV1 (L)‖ | 2.1 (0.8) | 1.8 (0.7) | 1.9 (0.7) | 2.1 (0.5) | 2.0 (0.7) | 2.0 (0.6) |
| FEV1/FVC ratio | 0.8 (0.1) | 0.9 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.1) |
Data are presented as numbers (percentage) for binary variables or means (SDs) for continuous variables.
Percentages were calculated for children with complete data. For example, 592 (287 control subjects and 305 cases) of the 678 participating children had allergen-specific IgE.
P < .05 for the comparisons between groups (performed by using 2-sample t tests or Fisher exact tests, as appropriate).
Total IgE transformed to a logarithmic (log10) scale. Results are shown as geometric means (SDs).
FEV1 values are presented as absolute values because of lack of predicted values for Puerto Rican subjects.
A comparison of participating children with and without a history of prematurity requiring NICU admission (jointly and separately in cases and control subjects) is shown in Table E1 in this article’s Online Repository at www.jacionline.org. In this analysis, low birth weight was significantly associated with prematurity requiring NICU admission in cases, control subjects, and all subjects combined. There was no significant association between any other variable and prematurity requiring NICU admission in cases, control subjects, and all subjects combined.
After excluding subjects without data on allergen-specific IgE levels, 287 (approximately 88%) of the 327 control subjects and 305 (approximately 87%) of the 351 cases remained in the multivariate analysis of prematurity and asthma. Compared with those not included in this analysis, control subjects and cases were more likely to have a household income of less than $15,000/y, and control subjects were less likely to have private/employer-based health insurance. There were no other significant differences between control subjects or cases that were and were not included in the multivariate analysis (see Table E2 in this article’s Online Repository at www.jacionline.org).
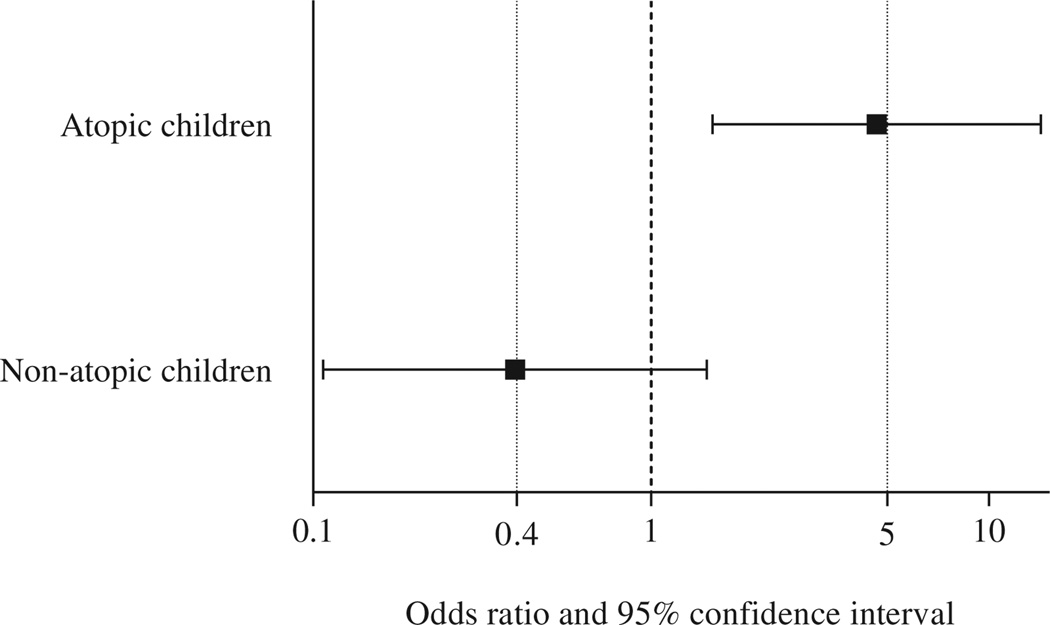
In our main multivariate analysis of prematurity and asthma, there was significant modification of the estimated effect of prematurity on asthma by atopy (P for interaction term = .006). Thus we conducted a multivariate analysis after stratification by atopy. In this stratified analysis prematurity was significantly associated with a nearly 5-fold increased odds of asthma in atopic children (OR, 4.7; 95% CI, 1.5–14.3; P=.007; Fig 1). In contrast, there was no significant association between prematurity and asthma in nonatopic children (OR, 0.4; 95% CI, 0.1–1.5; P =.2).
FIG 1.
Results of the multivariate logistic regression analysis of prematurity and asthma after stratification by atopy (defined as ≥1 positive allergen-specific IgE). Multivariate models were adjusted for age, sex, household income, maternal history of asthma, and early-life exposure to ETS. Goodness of fit for each of the multivariate models was assessed by using the Hosmer-Lemeshow test (P = .9 for the model in nonatopic children and P = .9 for the model in atopic children).
We then conducted our secondary multivariate analysis of prematurity requiring NICU admission and asthma. In this analysis, we also found a significant modification of the effect of prematurity requiring NICU admission on asthma by atopy (P for interaction term = .04). Thus, we conducted a multivariate analysis after stratification for atopy, obtaining similar results to those for prematurity and asthma (ie, significant results in atopic children but nonsignificant results in nonatopic children; Table III).
TABLE III.
Analysis of prematurity requiring NICU admission and asthma in participating children after stratification by atopy (≥1 positive allergen-specific IgE)*†
| Covariate | OR (95% CI) | |
|---|---|---|
| Nonatopic children (n = 239) |
Atopic children (n = 353) |
|
| Unadjusted | ||
| Prematurity requiring NICU admission | 1.1 (0.2–5.1), P = .9 | 5.0 (1.1–22.4), P = .03 |
| Adjustedठ| ||
| Prematurity requiring NICU admission | 0.4 (0.1–3.0), P = .4 | 6.3 (1.4–28.9), P = .02 |
| Maternal history of asthma | 4.7 (2.5–8.7), P < .001 | 3.6 (2.2–5.9), P < .001 |
| Exposure to ETS in utero or before age 2 y | 1.6 (0.9–3.0), P = .1 | 1.5 (0.9–2.4), P = .1 |
Allergen-specific IgE levels were available for 592 (287 control subjects and 305 cases) of the 678 participating children.
Asthma was defined as physician-diagnosed asthma and wheeze in the previous year.
Multivariate logistic regression models were adjusted for age, sex, and household income in addition to the covariates listed in the first column.
Goodness of fit for each of the multivariate models was assessed by using the Hosmer-Lemeshow test (P =.9 for the model in nonatopic children and P =.8 for the model in atopic children).
Because of high collinearity between the various indicators of socioeconomic status (household income, type of health insurance, and parental education), we did not include all of these variables in the same models. Replacing household income with type of health insurance or parental education did not significantly change our findings (see Table E3 in this article’s Online Repository at www.jacionline.org). Likewise, we obtained similar results after replacing maternal history of asthma with maternal history of 1 or more atopic disease in the multivariate models (see Table E4 in this article’s Online Repository at www.jacionline.org). To attempt to further exclude potential confounding, we also repeated the stratified multivariate analyses of prematurity and asthma after additional adjustment for low birth weight and lung function measures, obtaining similar results (see Table E5 in this article’s Online Repository at www.jacionline.org).
As an additional confirmatory step, we first conducted propensity score matching, in which 297 (approximately 85%) of the 351 cases were paired to control subjects with regard to age, sex, household income, and early-life exposure to ETS (see the Methods section in this article’s Online Repository). As expected, this matching led to no significant differences in any of these covariates between cases and control subjects. In spite of a decreased sample size in the matched data set, we obtained similar results for the conditional logistic regression analysis of prematurity and asthma stratified by atopy (see Table E6 in this article’s Online Repository at www.jacionline.org). In particular, the direction of the association in either atopic or nonatopic children was the same as in our primary analysis, although it was of borderline statistical significance (P = .06) in atopic children (likely because of reduced statistical power).
DISCUSSION
We found a significant and strong association between prematurity and atopic asthma in Puerto Rican children. In contrast, we found no significant association between prematurity and nonatopic asthma in Puerto Rican children.
To our knowledge, this is the first study to present the separate effects of prematurity on atopic and nonatopic asthma in childhood. In contrast to our findings, prematurity was reportedly associated with nonatopic asthma in a study of 741 German children (age, 5–7 years).20 The discrepant findings between that study and ours could be explained by differences in sample size (only 64 children ever given a diagnosis of asthma [44 nonatopic] were included in the German study), age, environment and ethnicity, and definition of asthma. Of note, small sample size precluded an analysis of prematurity and atopic asthma in the German study. In another study of 137 school-age Finnish children, prematurity was not associated with asthma or current wheeze, but premature children with current wheeze at age 10 years were more likely to be atopic.21 Our findings further underscore the need to identify subgroups of premature infants who might be at a higher risk for asthma because interventions targeted to prevent preterm deliveries22,23 might help decrease the burden of asthma in high-risk populations.
Some,24–28 but not all,20,21,29–31 studies of school-age children have found an association between prematurity and asthma. In a registry-based study of 150,204 children age 5 to 18 years in the United States, both a gestational age of 32 weeks or less and a gestational age of between 33 and 36 weeks were associated with asthma.24 In contrast, neither a gestational age of 32 weeks or less nor a gestational age of between 33 and 36 weeks was associated with asthma at age 16 years in a population-based study of 3,968 Finnish twins.31 The discrepant results across studies could be explained by differences in study populations (including age and prevalence of atopic asthma) or asthma definitions (eg, based on use of health care services for asthma vs parental report of physician-diagnosed asthma).
Puerto Rican subjects have increased rates of preterm birth,5,6 as well as increased risks of atopy32 and atopic asthma. For example, 466 (72.1%) of 646 children and adults with asthma were found to be atopic in a previous study in Ponce, Puerto Rico.33 In our study, approximately 60% of children with asthma were atopic. Although this rate is lower than that reported for Latin American countries, such as Costa Rica,34 it is higher than those reported for other areas of the world.35 Although limited evidence in non–Puerto Rican subjects suggests that prematurity might decrease the risk of atopy,36,37 we found no significant association between prematurity and atopy in Puerto Rican children.
Whether prematurity partly explains racial or ethnic disparities in asthma has been insufficiently studied. In a previous study24 black children had a higher unadjusted prevalence of asthma than white children. After adjustment for gestational age, there was no longer a significant difference in asthma prevalence between the 2 groups, suggesting that prematurity underlies certain race-specific differences in asthma. In contrast to ours, that prior study24 used registry-based records and lacked information on Hispanic ethnicity, atopy, and other relevant covariates.
Early-life changes in lung structure, function, or immune responses (secondary to a short gestational age) can have substantial implications for the subsequent occurrence of respiratory diseases.7,27,38 Our results suggest that a child with prematurity-related lung abnormalities who also develops atopic airway inflammation might be at a greater risk of asthma (ie, a “2-hit” hypothesis) than a child with prematurity-related lung abnormalities but no atopy. Given our results in premature children requiring NICU admission, this risk seems to be further increased in children with more severe prematurity. This is consistent with several studies that have found an inverse dose-response relationship between gestational age and asthma.24–26
Our study has considerable strengths, including a multistage probability sampling design for subject recruitment and an analytic approach accounting for potential confounders and effect modifiers, such as atopy. We also recognize several limitations to our findings.
First, either selection bias or recall bias are possible in any cross-sectional study. However, recall bias is an improbable explanation for our results because we obtained similar findings in an analysis of more severe prematurity (ie, prematurity requiring NICU admission, which is less likely to be affected by parental recall). Major selection bias is also unlikely because there were no significant differences in prematurity or indicators of asthma severity/control (eg, lung function) between children who were and were not included in the current analysis. Thus neither selection bias nor recall bias is likely to fully account for the marked differences in the magnitude and significance of the observed associations for atopic versus nonatopic asthma.
Second, we cannot separate the effects of prematurity on asthma by gestational age because we lack that information.
Third, there could be residual confounding by variables not measured in our study (eg, perinatal or postnatal infections,39,40 neonatal anthropometrics other than birth weight,41,42 bronchopulmonary dysplasia,43 or mechanical ventilation44). However, such confounding is unlikely to fully account for the difference in the estimated effects of prematurity on atopic versus nonatopic asthma.
Fourth, there is controversy regarding a diagnosis of asthma in children born preterm because recurrent wheezing in premature children (particularly those with bronchopulmonary dysplasia) might represent a separate clinical entity.43 However, prematurity and asthma are not mutually exclusive, and a diagnosis of asthma is more likely to be accurate in a premature child with atopy and/ or a family history of atopic diseases (approximately 82% and approximately 50%of premature cases in our study, respectively).45
Fifth, a premature child with wheezing and atopy might receive a diagnosis of asthma more often than a premature child with wheezing but no atopy. Although we cannot exclude this because of our study design, most physicians caring for our participants did not know the participants’ IgE levels when they gave them asthma diagnoses (because of limited resources in Puerto Rico). In addition, we found no significant difference in the lifetime prevalence of physician-diagnosed atopic diseases other than asthma (allergic rhinitis, eczema, or both) between premature and nonpremature cases (data not shown).
Finally, our results might not be generalizable to Puerto Rican children in the US mainland or non–Puerto Rican children.
In summary, our results suggest that prematurity is strongly associated with atopic asthma in Puerto Rican children. Birth cohort studies are needed to further examine prematurity and atopic asthma in Puerto Rican children and children of other ethnicities.
Supplementary Material
Key messages.
Atopy modifies the estimated effect of prematurity on asthma in Puerto Rican children.
Prematurity is strongly associated with atopic asthma but not significantly associated with nonatopic asthma in Puerto Rican children.
Acknowledgments
Supported by grant HL079966 from the National Institutes of Health (NIH). J.M.B.’s contribution was supported by grant HD052892 from the NIH.
J. C. Celedón has received grants from the National Institutes of Health (NIH) and the Heinz Foundation, has received fees for a one-time consultant arrangement with Genentech, and receives royalties from UpTo-Date. J. M. Brehm has received grants from the NIH.
We thank all the participating children and their families for their invaluable contribution to the study.
Abbreviations used
- ETS
Environmental tobacco smoke
- FVC
Forced vital capacity
- NICU
Neonatal intensive care unit
- OR
Odds ratio
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Malveaux FJ. The state of childhood asthma: introduction. Pediatrics. 2009;123(Suppl 3):S129–S130. doi: 10.1542/peds.2008-2233B. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 3.Cohen RT, Canino GJ, Bird HR, Shen S, Rosner BA, Celedon JC. Area of residence, birthplace, and asthma in Puerto Rican children. Chest. 2007;131:1331–1338. doi: 10.1378/chest.06-1917. [DOI] [PubMed] [Google Scholar]
- 4.Forno E, Celedon JC. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9:154–160. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Wilson EC, Mathews TJ. Births: final data for 2010. Natl Vital Stat Rep. 2012;61:1–100. [PubMed] [Google Scholar]
- 6.2012 Premature Birth Report Card. [Accessed March 5, 2013];March of Dimes. 2012 Available at: http://www.marchofdimes.com/mapflashfilespad/reportcards/2012/english/US.pdf.
- 7.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, et al. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ. 2012;344:e896. doi: 10.1136/bmj.e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal NK, Fiks AG, Lorch SA. Association of late-preterm birth with asthma in young children: practice-based study. Pediatrics. 2011;128:e830–e838. doi: 10.1542/peds.2011-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bird HR, Canino GJ, Davies M, Duarte CS, Febo V, Ramirez R, et al. A study of disruptive behavior disorders in Puerto Rican youth: I. Background, design, and survey methods. J Am Acad Child Adolesc Psychiatry. 2006;45:1032–1041. doi: 10.1097/01.chi.0000227878.58027.3d. [DOI] [PubMed] [Google Scholar]
- 11.Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada MM, Boutaoui N, et al. African ancestry and lung function in Puerto Rican children. J Allergy Clin Immunol. 2012;129:1484–1490. doi: 10.1016/j.jaci.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C. Collaborative studies on the genetics of asthma—National Heart, Lung and Blood Institute. Clin Exp Allergy. 1995;25:29–32. doi: 10.1111/j.1365-2222.1995.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.Weiss ST, Gold DR. Gender differences in asthma. Pediatr Pulmonol. 1995;19:153–155. doi: 10.1002/ppul.1950190302. [DOI] [PubMed] [Google Scholar]
- 15.Smith LK, Manktelow BN, Draper ES, Springett A, Field DJ. Nature of socioeconomic inequalities in neonatal mortality: population based study. BMJ. 2010;341:c6654. doi: 10.1136/bmj.c6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Census Bureau. Household income for states: 2008 and 2009. [Accessed March 18, 2013]; Available at: http://www.census.gov/prod/2010pubs/acsbr09-2.pdf.
- 17.Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94:136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 19.Perez-Perdomo R, Perez-Cardona C, Disdier-Flores O, Cintron Y. Prevalence and correlates of asthma in the Puerto Rican population: Behavioral Risk Factor Surveillance System, 2000. J Asthma. 2003;40:465–474. doi: 10.1081/jas-120018713. [DOI] [PubMed] [Google Scholar]
- 20.Bolte G, Schmidt M, Maziak W, Keil U, Nasca P, von Mutius E, et al. The relation of markers of fetal growth with asthma, allergies and serum immunoglobulin E levels in children at age 5–7 years. Clin Exp Allergy. 2004;34:381–388. doi: 10.1111/j.1365-2222.2004.01890.x. [DOI] [PubMed] [Google Scholar]
- 21.Siltanen M, Savilahti E, Pohjavuori M, Kajosaari M. Respiratory symptoms and lung function in relation to atopy in children born preterm. Pediatr Pulmonol. 2004;37:43–49. doi: 10.1002/ppul.10402. [DOI] [PubMed] [Google Scholar]
- 22.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–175. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 23.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381:223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dombkowski KJ, Leung SW, Gurney JG. Prematurity as a predictor of childhood asthma among low-income children. Ann Epidemiol. 2008;18:290–297. doi: 10.1016/j.annepidem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Gessner BD, Chimonas MA. Asthma is associated with preterm birth but not with small for gestational age status among a population-based cohort of Medicaid-enrolled children <10 years of age. Thorax. 2007;62:231–236. doi: 10.1136/thx.2005.053363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogt H, Lindstrom K, Braback L, Hjern A. Preterm birth and inhaled corticosteroid use in 6- to 19-year-olds: a Swedish national cohort study. Pediatrics. 2011;127:1052–1059. doi: 10.1542/peds.2010-3083. [DOI] [PubMed] [Google Scholar]
- 27.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly YJ, Brabin BJ, Milligan P, Heaf DP, Reid J, Pearson MG. Maternal asthma, premature birth, and the risk of respiratory morbidity in schoolchildren in Mersey-side. Thorax. 1995;50:525–530. doi: 10.1136/thx.50.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ. 1993;306:817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz KA, Pocock SJ, Strachan DP. Neonatal head circumference, neonatal weight, and risk of hayfever, asthma and eczema in a large cohort of adolescents from Sheffield, England. Clin Exp Allergy. 2003;33:737–745. doi: 10.1046/j.1365-2222.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 31.Rasanen M, Kaprio J, Laitinen T, Winter T, Koskenvuo M, Laitinen LA. Perinatal risk factors for asthma in Finnish adolescent twins. Thorax. 2000;55:25–31. doi: 10.1136/thorax.55.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celedon JC, Sredl D, Weiss ST, Pisarski M, Wakefield D, Cloutier M. Ethnicity and skin test reactivity to aeroallergens among asthmatic children in Connecticut. Chest. 2004;125:85–92. doi: 10.1378/chest.125.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Montealegre F, Meyer B, Chardon D, Vargas W, Zavala D, Hart B, et al. Comparative prevalence of sensitization to common animal, plant and mould allergens in subjects with asthma, or atopic dermatitis and/or allergic rhinitis living in a tropical environment. Clin Exp Allergy. 2004;34:51–58. doi: 10.1111/j.1365-2222.2004.01855.x. [DOI] [PubMed] [Google Scholar]
- 34.Celedon JC, Soto-Quiros ME, Hanson LA, Weiss ST. The relationship among markers of allergy, asthma, allergic rhinitis, and eczema in Costa Rica. Pediatr Allergy Immunol. 2002;13:91–97. doi: 10.1034/j.1399-3038.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 35.Weinmayr G, Weiland SK, Bjorksten B, Brunekreef B, Buchele G, Cookson WO, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007;176:565–574. doi: 10.1164/rccm.200607-994OC. [DOI] [PubMed] [Google Scholar]
- 36.Siltanen M, Kajosaari M, Pohjavuori M, Savilahti E. Prematurity at birth reduces the long-term risk of atopy. J Allergy Clin Immunol. 2001;107:229–234. doi: 10.1067/mai.2001.112128. [DOI] [PubMed] [Google Scholar]
- 37.Siltanen M, Wehkalampi K, Hovi P, Eriksson JG, Strang-Karlsson S, Jarvenpaa AL, et al. Preterm birth reduces the incidence of atopy in adulthood. J Allergy Clin Immunol. 2011;127:935–942. doi: 10.1016/j.jaci.2010.12.1107. [DOI] [PubMed] [Google Scholar]
- 38.Maritz GS, Morley CJ, Harding R. Early developmental origins of impaired lung structure and function. Early Hum Dev. 2005;81:763–771. doi: 10.1016/j.earlhumdev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164:187–192. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey CD, Gold DR, Litonjua AA, Sredl DL, Ryan L, Celedon JC. Respiratory illnesses in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;119:150–156. doi: 10.1016/j.jaci.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 41.Kindlund K, Thomsen SF, Stensballe LG, Skytthe A, Kyvik KO, Backer V, et al. Birth weight and risk of asthma in 3–9-year-old twins: exploring the fetal origins hypothesis. Thorax. 2010;65:146–149. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 42.Sevelsted A, Bisgaard H. Neonatal size in term children is associated with asthma at age 7, but not with atopic dermatitis or allergic sensitization. Allergy. 2012;67:670–675. doi: 10.1111/j.1398-9995.2012.02805.x. [DOI] [PubMed] [Google Scholar]
- 43.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 44.Grischkan J, Storfer-Isser A, Rosen CL, Larkin EK, Kirchner HL, South A, et al. Variation in childhood asthma among former preterm infants. J Pediatr. 2004;144:321–326. doi: 10.1016/j.jpeds.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 45.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med. 2008;358:743–745. doi: 10.1056/NEJMc073362. author reply 5–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.