Abstract
Copper sulfide (CuS) nanoparticles have attracted increasing attention from biomedical researchers across the globe, because of their intriguing properties which have been mainly explored for energy- and catalysis-related applications to date. This focused review article aims to summarize the recent progress made in the synthesis and biomedical applications of various CuS nanoparticles. After a brief introduction to CuS nanoparticles in the first section, we will provide a concise outline of the various synthetic routes to obtain different morphologies of CuS nanoparticles, which can influence their properties and potential applications. CuS nanoparticles have found broad applications in vitro, especially in the detection of biomolecules, chemicals, and pathogens which will be illustrated in detail. The in vivo uses of CuS nanoparticles have also been investigated in preclinical studies, including molecular imaging with various techniques, cancer therapy based on the photothermal properties of CuS, as well as drug delivery and theranostic applications. Research on CuS nanoparticles will continue to thrive over the next decade, and tremendous opportunities lie ahead for potential biomedical/clinical applications of CuS nanoparticles.
Keywords: Cupper sulfide (CuS), nanoparticle, molecular imaging, theranostics, cancer
1. Introduction
Nanotechnology, a vibrant research area over the last several decades, has had a remarkable impact on many aspects of the modern society. A wide variety of nanomaterials have attracted tremendous attention from researchers, because of their unique properties which can be quite different from those exhibited in the bulk state. The areas that have benefitted the most from advances in nanotechnology include electronics, energy, biomedical sciences, among others. In his paradigm shifting lecture entitled “there’s plenty of room at the bottom”,[1] Dr. Richard Feynman first spoke of nanosurgeons and nanomaterials which could enter the body and interact with the surrounding environment at the cellular level. Since then, the ever-evolving biomedical sciences have witnessed the investigation of many classes of novel and better nanomaterials, which could assist with disease diagnosis/therapy and improve patient management.
The major goals of nanotechnology in biomedical applications are to introduce new technologies, and improve the existing ones for more sensitive, accurate, efficient, and timely medical procedures. With the unprecedented initiatives such as the NCI Alliance for Nanotechnology in Cancer that encompasses the public and private sectors, designed to accelerate the applications of the best capabilities of nanotechnology to cancer,[2] it is expected that promising molecular discoveries will be efficiently translated into the clinic to benefit (cancer) patients. Tunable physicochemical properties, as well as the ability to be readily investigated/applied in biological systems upon appropriate functionalization, make nanoparticles among the most coveted systems for a range of applications including but not limited to biosensing,[3–4] imaging,[5–9] diagnosis,[10] drug delivery,[11] and therapy.[12–13]
Semiconducting nanoparticles have elicited a myriad of investigations into their unique properties such as charge transport, light emission, mechanics and thermal diffusion, etc., characteristic of the size scaling effects at nanometer dimensions. In addition, they are under active investigation in biomedical sciences, which represent a dynamic area of research in molecular and translational medicine. Their increasing importance in the detection and treatment of cancer and other diseases, drug delivery, and in vitro biosensing applications can be partly attributed to their favorable and easily tunable physical, chemical, magnetic, and/or optical properties.[14–15] Copper sulfide (CuS), a p type semiconductor with excellent optical and electrical properties, has been extensively studied for various applications.[16–21] However, reports on its biological applications had remained largely elusive until the last several years.
Recently, CuS nanoparticles are gradually emerging as a promising platform for sensing,[18, 22–29] molecular imaging,[30] photothermal therapy,[31–34] drug delivery,[35] as well as multifunctional agents that can integrate both imaging and therapy.[36] In this review article, we summarize the current status of CuS nanoparticles in biomedical research. A succinct discussion of the many forms of CuS nanoparticles and the synthesis procedures will be described first, and the potential applications of these nanoparticles are determined in part by their morphology, spatial orientation and arrangement, etc. The burgeoning role of CuS nanoparticles for in vitro and in vivo applications will then be illustrated in detail. Lastly, we discuss the progress that has been made to date, as well as the major challenges and future directions for these promising nanoparticles.
2. Controlled synthesis of CuS nanoparticles
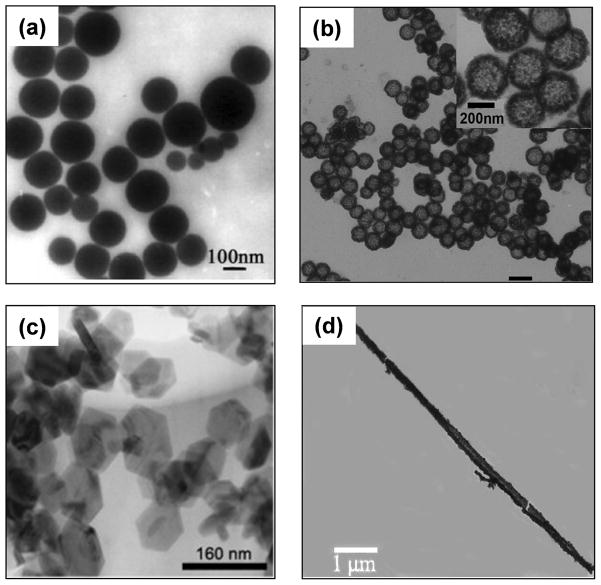
The early studies of CuS nanoparticles were mainly focused on the nanosphere morphology, whereas recent investigations involved a wider repertoire of nanostructures that span all three dimensions such as 3D hollow and solid nanospheres, core-shell particles and nanocages, 2D nanoplates, nanorods, and nanotubes/nanowires which are classified as 1D nanostructures (Figure 1). The methods of synthesis vary with the morphology, which in turn depends on the properties desired of the final product, as well as the applications it will be used for. For example, spherical CuS nanoparticles have found diverse applications in biomedicine, from photoacoustic imaging to therapeutic uses with photothermal ablation; hollow nanospheres and nanocages hold promising potential in drug delivery; CuS nanorods and nanowires have been successfully utilized for sensing of a variety of small molecules, food pathogens, and immunologically relevant moieties.
Figure 1.
Representative transmission electron micrographs of CuS with different morphologies: (a) nanospheres, (b) hollow nanospheres, (c) hexagonal nanoplates, (d) nanorods. Adapted with permission from references [50–51, 56, 68].
The synthesis of uniform and monodisperse nanoparticles is of utmost importance to their biomedical applications. Therefore, techniques for both physical and chemical characterization of the synthesized nanomaterials are indispensable. Characterization of CuS nanoparticles can be performed using a wide variety of techniques, including but not limited to X-Ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-Ray spectroscopy (EDS), transmission electron microscopy (TEM) and high resolution TEM (HRTEM), atomic force microscopy (AFM), fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), UV-visible and photoluminescence (PL) spectroscopy, etc. These techniques can provide important information on the elemental, structural (e.g. size and shape), and optical properties of CuS nanoparticles.
2.1. CuS nanospheres and nanocages
CuS nanospheres (Figure 1a) have been prepared by a variety of routes such as hydrothermal/solvothermal method,[37–40] microwave irradiation,[41–43] sonochemical synthesis,[44] etc. In the simplest process, reaction of Cu and S element was carried out in evacuated tubes. The drawbacks of this method include the large grain size of the product and high temperature required for the reaction. To overcome these drawbacks, hydrothermal route was commonly used, which has the advantages of easy fabrication and good yield of highly uniform and pure CuS nanoparticles, at comparatively lower temperature without the need of complex and toxic organometallic reactants. For example, precursors of Cu (CuO, CuCl2.2H2O etc.) and S (Na2S, Na2S2O3.5H2O, thiourea etc.) were autoclaved at 130–170 ºC for a few hours to synthesize CuS nanospheres of ~13 nm in diameter.[37, 39] In another report, ~10 nm sized CuS stabilized by citrate could be synthesized simply by mixing aqueous solutions of CuCl2, sodium citrate, and Na2S together at room temperature and subsequent reaction at 90 ºC for 15 min.[36] The size and shape of the nanoparticles could easily be tuned during the hydrothermal process by varying parameters such as the precursors used, temperature of the reaction, reaction time, etc.
Among the newly developed methods for the synthesis of CuS nanoparticles, microwave irradiation holds great promise because this process is simple, fast, and energy efficient.[41–43] With the same reactants as employed in the processes stated above, this method uses microwave irradiation (~180 W) in aqueous medium or ethylene glycol for ~20 minutes to carry forth the decomposition process. Other less frequently reported methods for the synthesis of CuS nanoparticles include the use of carboxylic acids as solvents for high nucleation rate and stabilization of nanoparticle dispersion,[45] enzymatic treatment of dextran stabilized CuS nanosuspensions in a green synthetic method to produce nanoparticles with good homogeneity,[46] and surfactant-based synthesis of CuS nanoparticles.[47]
Hollow nanospheres (Figure 1b) and nanocages have garnered much attention due to their capacity for chemical storage, drug delivery, catalysis, etc. An early report on hollow CuS nanostructures was based on the self-assembly of nanoflakes derived from Cu(II)-thiourea complex into hollow nanospheres, with the size of several microns in diameter.[48] Another study demonstrated the formation of hollow nanospheres as well as nanotubes from 5–10 nm CuS nanoparticles at room temperature.[49] It was suggested that such hollow structures may be formed as a result of decomposition of thiourea into H2S, which further reacts with the Cu precursor to produce CO2. The CO2 produced forms gaseous cavities which act as heterogeneous nucleation centers under hydrothermal conditions for the aggregation of CuS nanoflakes, ultimately giving rise to hollow nanostructures.
Hard-template assisted technique has also been used to synthesize CuS with a large hollow cavity (Figure 1b).[50] For this method, several different types of core supports have been used, including surfactant micelle microemulsions,[51] Cu2O nanoparticles,[50, 52] and poly-(styrene-acrylic) latex particles.[20] Cubic- and star-shaped, as well as octahedral nanoparticles, have been used as sacrificial templates to produce hollow CuS structures by solid-liquid reactions, with sizes ranging from 500 nm to several microns.[53–54] During this process, the templates are etched away in an appropriate solvent or serve as the reactants during the formation of the sulfide shell around them. Such template-based growth can be attributed either to the Kirkendall diffusion effect or the process of mass diffusion followed by Ostwald ripening.
2.2. CuS nanoplates
Aside from the 3D materials discussed above, 2D CuS nanostructures have also been prepared which are relatively rare and restricted mainly to nanoplates (Figure 1c) and thin films.[55] Since their biomedical applications are not widely explored, we will only briefly describe these nanostructures. The major routes for preparation of CuS nanoplates include hydrothermal/solvothermal methods,[39, 56–59] which yield nanoplates with edges ranging from 50 to 200 nm depending on the reaction conditions. Most research groups report the treatment of precursor-surfactant aqueous microemulsions at 130–180 ºC for a few hours. Variation of the process parameters can yield morphological and dimensional variants of the resulting CuS nanoplates. Through a surfactant-free approach using the sonochemical method, 20–40 nm single crystalline nanoplates were prepared under ambient conditions with in situ Cu(OH)2 nanoribbon templates.[44] Other representative procedures include single source method,[60] chemical vapor reaction,[61] and high-temperature precursor injection method to produce columnar arrays of CuS nanoplates.[62]
2.3. CuS nanotubes, nanorods, and nanowires
There has been an increasing interest in 1D nanostructures of semiconductor materials, such as CuS, for their applications in sensing and photocatalysis.[63–64] These elongated nanostructures have found widespread use in sensors, owing to their excellent electrochemical and catalytic properties, which will be briefly described below.
A wide range of procedures have been explored to synthesize these 1D nanostructures, where the parameters of the process vary with the morphology/dimension of the end product. For example, the diameters range from 30 to 80 nm for CuS nanowires[65–66] and 30 to 120 nm for CuS nanotubes,[38, 67] depending on the method of synthesis, conditions of growth, and the precursors used. Most of the procedures include hydrothermal processes and their variations, which can provide an easy and efficient way to produce good quality, uniform nanostructures with high aspect ratios (Figure 1d).[20, 38, 49, 65, 67–68] Meanwhile, thermolytic degradation of copper thiolate precursor without solvent,[69] the use of a paired cell at room temperature,[70] and amylose-directed synthesis,[71] have also been investigated for the preparation of these CuS nanostructures.
Several groups have utilized microwave irradiation techniques for the synthesis of CuS nanorods and tubular structures under different experimental conditions,[24, 41, 43, 66] whereas many other processes reported in the literature involved template or surfactant assisted routes.[72–75] In an early report, the fabrication of CuS nanorod arrays on arachidic acid monolayers was assembled on graphite with embedded copper ions.[74] This method provided controllable synthesis of nanowire arrays on a wide range of amphiphilic Langmuir-Blodgett films, which exhibited desirable characteristics for potential use in sensors.
3. Biomedical applications of CuS nanoparticles
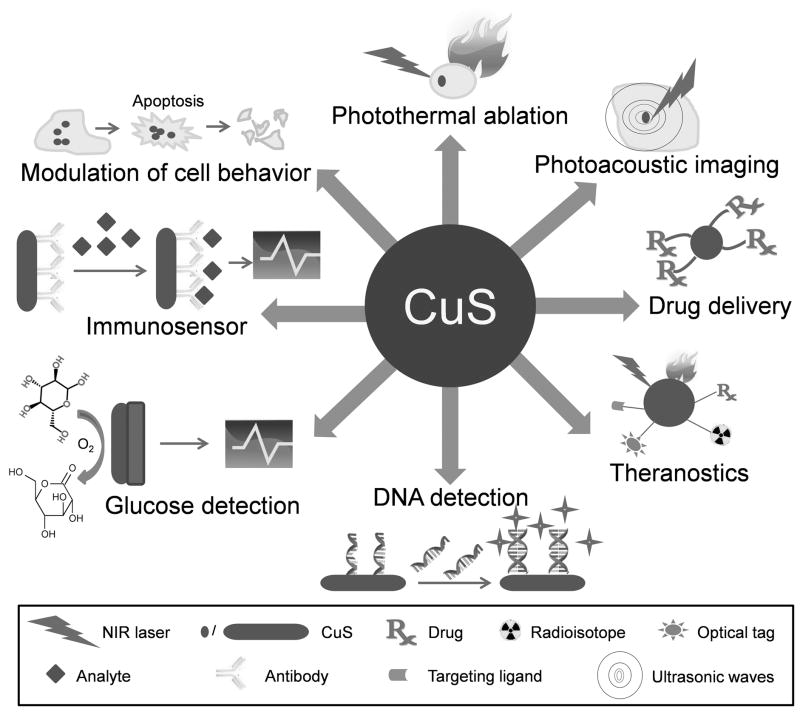
With the clear trend of increasing emphasis on interdisciplinary and translational research over the last decade,[76–79] advances in biomedical sciences rely heavily on the progress made in disciplines as varied as material sciences, engineering, mathematics, computer sciences, medical physics, among others. Novel biomaterials are actively been explored for superior properties over the current state-of-the-art. Nanomaterials that were previously considered only for uses in areas such as electronics, catalysis, and gas sensing etc. are gradually gaining importance in biomedical sciences and future health care.[80–82] The recent progress of CuS nanoparticles have spanned a wide variety of biomedical applications (Figure 2), which will be described in the following text of this review article.
Figure 2.
Representative biomedical applications of copper sulfide (CuS) nanoparticles.
3.1. In vitro applications of CuS nanoparticles
CuS nanoparticles and their conjugates have been widely used in the detection of biomolecules such as DNA, metabolites such as glucose (which can have important implications in diabetes and other diseases), food borne pathogens (which can be useful for prevention of food poisoning), hydrogen peroxide (involved in many biomedical processes and pathways), among others. The increasing popularity of CuS nanoparticles for use in sensing is based primarily on their metal-like electrical conductivity and the ability to promote electron transfer reactions with biomolecules.
3.1.1. DNA detection
Sequence specific detection of DNA is of utmost importance for many applications, such as various laboratory procedures (e.g. gene analysis), pathological tests for disease diagnosis, drug screening, forensic sciences, etc. A large number of strategies have been explored for detection of DNA hybridization,[83] among which nanomaterial-based chemiluminescence detection holds great promise. While the classical chemiluminescence assays depend on the luminescence of labels (usually enzymes) attached to the probe DNA upon hybridization to the target DNA,[84] newer versions of assays rely on the use of metal and semiconductor nanoparticles as the label. This strategy can bypass the inherent poor stability of enzymes, as well as the low detection sensitivity. Although the use of Au and Ag nanoparticle labels has been widely reported,[85–86] the instability of Ag+ and Au3+ in aqueous solutions remains a disadvantage. Cu2+, on the other hand, is highly soluble in water and much less expensive for DNA sequence detection.
A biosensor for short DNA sequences based on the flow injection chemiluminescence technique was reported.[22] Luminol-H2O2-Cu2+ CuS nanotags on probe DNA were used to generate the chemiluminescence signal, which was a result of the dissolution of Cu2+ ions upon hybridization of the target and probe DNA sequences. Enhanced signal intensity was obtained by electrochemical preconcentration with Cu2+ ions in an anodic stripping voltammetry (ASV) cell. In addition, the intensity of the signal was found to vary linearly with the concentration of the target sequence, with a detection limit of 5.5×10−13 M of the target DNA. When compared to Ag nanoparticle-based systems,[87] the luminol-H2O2-Cu2+ set up was reported to be simpler, faster, less expensive, and easier to fabricate. Modification of the DNA probe with CuS nanoparticles requires much shorter time than the Ag nanoparticles (13 h vs. 116 h). Subsequently, an improved sensor with lower detection limit and higher sensitivity was reported by the same group, in which the signal amplification ability of Au ions and Cu2+ preconcentration was exploited simultaneously.[23] The hybrid system of Au and CuS provided a detection limit as low as 4.8 fM (10−15 M) of target DNA with good specificity, as indicated by significantly weaker signal when there are two base-pair mismatch between the probe and the target DNA.
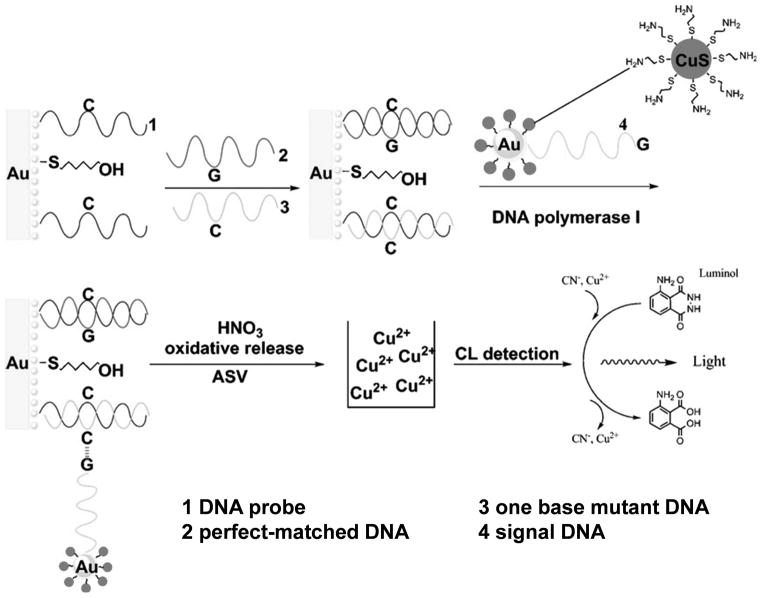
Using a similar setup, a more sensitive and accurate technique for quantification of single nucleotide polymorphisms (SNPs) down to the attomolar (10−18 M) concentration was developed (Figure 3).[27] This sensor was based on DNA polymerase-induced coupling of monobase (e.g. guanine in this study) functionalized nanoparticle probes to the corresponding sites on the mutated double-stranded DNA sequence. Higher sensitivity was achieved by incorporating Au nanoparticles, each of which was loaded with ~80 CuS nanoparticles for chemiluminescence detection of Cu2+ ions (generated by dissolution of CuS nanoparticles). It was suggested that a highly sensitive technique like this holds great promise for future genetic diagnostics and evolutionary studies.
Figure 3.
A chemiluminescent (CL) single nucleotide polymorphism detection assay based on Au and CuS nanoparticles. Adapted with permission from reference [27].
As we described above, a number of reports expounding the use of CuS nanoparticles for DNA detection exist in the literature. However, similar studies on other Cu-based nanoparticles (e.g. oxides) are largely non-existent. In one report, Cu2O hollow microspheres were employed for methylene blue-based DNA biosensing of Hepatitis B virus, which had a detection limit of ~10−10 M,[88] several orders of magnitude less sensitive than CuS-based DNA biosensors (typically lower than 10−13M).
3.1.2. Glucose Biosensors
Non-invasive detection of glucose levels has important implications in the monitoring of many diseases such as diabetes, which affect millions of people worldwide. The first biosensor for glucose was developed to monitor glucose oxidation about half a century ago, which was based on the enzyme glucose oxidase (GOX).[89] Big strides have been made since then and many glucose biosensors have been developed, based on nanoscale and mesoporous electrode surfaces incorporating metallic and/or semiconductor nanoparticles. These strategies allow direct transfer of electrons between the electrode and the enzyme that is trapped within the pore, thereby eliminating the need of mediators. CuS nanoparticles, with metal-like conductivity, provide low cost alternatives to noble metal-based detectors and have attracted significant interest in this field.
Glassy carbon electrodes coated with multi-walled carbon nanotubes (MWNTs), modified with single crystalline copper disulfide (Cu2S) nanocrystals ranging from spherical to triangular plate-like morphology, were used to generate glucose sensors.[90] This sensor design was found to be much more sensitive to H2O2 (released upon glucose oxidation by GOX) with a detection limit of 50 nM, as opposed to 10 μM for the conventional GOX-based sensor. A possible mechanism for such enhanced photocurrents was attributed to direct and efficient electron transfer from catalytic, photoexcited Cu2S nanocomposites to the MWNTs, forming an electrical network through direct contact.
The denaturation of enzymes during the immobilization process in GOX-based biosensors has severely impeded their widespread use. For example, the enzymatic activity can be influenced by temperature, pH, humidity, presence of chemicals, etc. In addition, there are also several other concerns associated with the mass production of these sensors, high dependency on oxygen, sensitivity to electroactive interferences present in the real blood samples, etc.[91] Therefore, enzyme-based biosensors have been gradually replaced by more stable and reliable non-enzymatic detectors.[92] Since these sensors rely on the direct oxidation of glucose at the electrode surface, which is kinetically a very slow process, the electrocatalytic properties of the electrode material play a pivotal role in the performance. For the development of enzyme-free biosensors, noble metals, metallic nanoparticles, and their alloys have been extensively investigated as electrode materials.[93–100] However, low selectivity and higher cost of Au- and Ag-based sensors have hindered their widespread utilization. In contrast, Cu-based nanomaterials, especially CuS nanoparticles, may provide a low cost, highly selective and reliable alternative.
While most applications of CuS nanoparticles are based on the nanosphere morphology, glucose biosensors mainly use nanotubes because of their excellent electrocatalytic properties. The CuS nanotubes employed as electrodes in biosensors have been generated through a number of routes: solvothermal oleic acid/water microemulsion system,[24] microwave assisted transformation of Cu complexes into CuS nanotubes,[28] and self-sacrificial template method.[29] In general, CuS nanotube-based biosensors have demonstrated good detection capability, sensitivity, anti-interference property, reproducibility, and stability. Similar results have also been reported for other sensors based on CuS nanoparticles complexed with mesoporous carbon,[26] nanocrystals of CuS, Pt and SnO2 grown on carbon nanotubes,[25] as well as other copper/copper oxide-based electrodes.[93, 95–99] However, a major hurdle for the use of CuO and Cu2O nanowires as electrode materials is that most of the synthetic procedures reported to date are grueling and time consuming.
3.1.3. Other applications of CuS nanoparticles
Besides sensing of various molecules such as DNA and glucose, several literature reports also focused on the in vitro uses of CuS nanoparticles for other applications. For example, a CuS thin film modified capacitive immunosenor was developed for the detection of human IgA antibody in serum samples, in which a goat anti-human IgA antibody was immobilized on the CuS thin film electrodes.[101] In another report, an electrochemical immunosensor based on CuS nanoparticle-MWNT composite electrodes was constructed for the detection of food borne pathogens (e.g. alpha-salmonella), which can have applications in mitigating food and water poisoning.[102] In both reports, the immunosensors were found to be reusable, sensitive, and specific to the desired analytes.[101–102] In an interesting study, bioactive nanocrystalline and amorphous CuS was found to specifically and significantly induce apoptosis of human cancer cells by entering the cells and localizing in specific organelles, thereby producing an anti-proliferative response.[103] Normal cells, however, were reported to be largely unaffected.
3.2. In vivo imaging and therapy with CuS nanoparticles
Molecular imaging is the visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans and/or other living systems.[104] Generally speaking, molecular imaging includes the use of optical techniques such as bioluminescence and fluorescence imaging,[105–107] molecular magnetic resonance imaging (MRI),[108], magnetic resonance spectroscopy, positron emission tomography (PET),[109–110] targeted ultrasound,[111–112] and single-photon emission computed tomography (SPECT).[113–114] Combination of molecular imaging and anatomical imaging (e.g. MRI and computed tomography [CT]) is now commonly used to provide complementary and more detailed information.[78, 115–116] In addition, many newly developed molecular imaging techniques are increasingly gaining popularity,[6, 8, 117–118] among which is photoacoustic imaging (also called optoacoustic imaging).
3.2.1. Photoacoustic imaging
Photoacoustic imaging relies on the absorption of short laser pulses by molecules in the body (e.g. hemoglobin and melanin), or exogenous contrast agents (e.g. Au and CuS nanoparticles), to generate heat which can lead to transient thermoelastic expansion and ultrasonic signals.[118] Hemoglobin absorbs light strongly at ~530 nm, making it a suitable endogenous contrast agent for photoacoustic imaging of the vasculature. Although photoacoustic tomography (PAT, i.e. tomographic photoacoustic imaging) can allow for imaging of deeper biological tissue (e.g. a few centimeters), its potential use with endogenous contrast is significantly hampered by tissue absorption and scattering of light at visible wavelengths.
Exogenous contrast agents that absorb in the near-infrared (NIR; > 700 nm) range can be used to address this issue, since the absorbance of biological molecules is at a minimum within this wavelength range thereby providing a relatively clear window for imaging with optical techniques.[113] Single-walled carbon nanotubes (SWNTs) and various Au nanoparticles remain the most widely used nanostructures for contrast enhancement in PAT.[119–120] Some major limitations of Au nanoparticles include their dependence of optical properties on complicated chemistries and environmental factors, relatively large size which can result in rapid clearance by the reticuloendothelial system (RES), etc.
In a recent study, CuS nanoparticles was reported as a novel class of contrast agents for PAT.[30] A NIR laser source of 1064 nm was chosen for its low absorption and scattering coefficient in normal tissue, which can be significantly absorbed by CuS nanoparticles. Of note, many of the other contrast agents used for PAT (e.g. organic dyes and other nanoparticles) have their absorption maxima between 560 and 840 nm. To tailor the CuS nanoparticles for optimal absorption at 1064 nm, the stoichiometric ratio between the Cu and S precursors were adjusted to synthesize CuS nanoparticles of 11 ± 3 nm in diameter. Successful imaging of the lymph nodes and brain was achieved in mouse models (Figure 4). Even when embedded at 5 cm depth into chicken breast, these CuS nanoparticles could be imaged with high in-plane resolution (~800 μm) and sensitivity (~0.7 nanomole per voxel).
Figure 4.
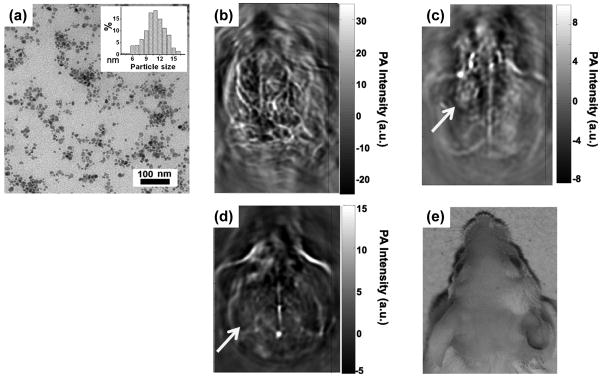
Photoacoustic tomography (PAT) imaging in mice with CuS nanoparticles. (a) A transmission electron microscopy image of CuS nanoparticles. Inset: particle size distribution. (b) A representative in vivo PAT image of a mouse brain, acquired using a 532 nm laser without contrast agent. (c) A PAT image of mouse brain acquired at 1064 nm, at 24 h after intracranial injection of CuS nanoparticles. (d) A PAT image of mouse brain acquired at 1064 nm, at 7 days after intracranial injection of CuS nanoparticles. (e) A photograph of the head of the mouse. Yellow arrow: injection site. Adapted with permission from reference [30].
These promising findings suggested that PAT imaging with CuS nanoparticles could be used for clinical applications, such as imaging breast lesions up to 4 cm deep, as well as other superficial lesions in the skin, limbs, head and neck, and lymph nodes. Traditional PAT imaging relies on the generation of contrast by differences in the blood perfusion between normal and tumor tissues, with the latter typically being under angiogenic and hypoxic conditions. However, such intrinsic contrast may be inadequate for early detection of cancer or imaging of deeper tumor tissues. If specific tumor targeting can be achieved in future investigations, these CuS nanoparticles can have enhanced specificity and sensitivity for potential clinical use, with the optimization of an efficient laser source. Besides CuS nanoparticles, phospholipid encapsulated Cu-neodecanoate nanoparticles (80–90 nm in diameter) have also been investigated for highly sensitive sentinel lymph node (SLN) imaging using PAT.[121] With the limited number of literature reports available, it is impossible to compare the in vivo performance of the “soft” Cu-polymer complexes with the “hard” CuS nanoparticles, which clearly warrant further investigation.
3.2.2. Photothermal ablation
The properties that make CuS nanoparticles suitable contrast agents for PAT imaging also render them good candidates for photothermal ablation applications. Although the use of CuS nanoparticles for imaging applications is rarely reported, their applications in cancer therapy are more extensively investigated. Hyperthermic ablation, especially photothermal ablation, is an active area of research where CuS nanoparticles are increasingly being employed. Generally speaking, hyperthermic ablation can kill tumor cells by heating them to 40–45 ºC, in a manner that the surrounding tissues are not significantly affected, since the severely hypoxic and low pH regions in tumor microenvironment make cancer cells more sensitive to heat than normal cells.[122] A variety of heat sources have been utilized, including microwave, magnetic field, radiofrequency, and laser stimulation.[123]
Photothermal ablation with a focused, skin penetrating NIR laser beam (typically in the range of 700–1065 nm) has been explored for the treatment of several tumor types. A severe limitation to the therapeutic window is posed by the non-specific absorption of heat by healthy tissues between the laser source and the tumor mass. This has motivated the search for novel photothermal agents with increased photothermal efficiency, thereby reducing the energy dose of the laser used and the damage to surrounding tissues. The use of CuS nanostructures and superstructures[33] as photothermal mediators offers several advantages over metal nanostructures such as Au nanoparticles, the most widely used photothermal agents. Besides the low cost of production for CuS nanoparticles compared to Au nanoparticles, the mechanism responsible for NIR absorption by CuS nanoparticles is also advantageous. While the NIR absorption of Au nanostructures stems from localized surface plasmon resonance (LSPR),[124] that of CuS nanoparticles rely on d-d transitions of Cu2+ ions. Such phenomenon of intra-band transition appears to be characteristic for CuS nanostructures, since deviations from this stoichiometry (e.g. Cu2–xS/Cu2-xSe where x = 1, 0.2, 0.03) have been shown to exhibit LSPR-based NIR absorption similar to metals.[125–126]
Such a difference has two important implications. First, the absorption wavelength for d-d transitions peaks at ~900 nm, which is in the NIR range and suitable for in vivo applications. This eliminates the need of specifically designed CuS nanoparticles which can require special and sometimes complicated procedures. On the other hand, the maximum absorption wavelength reported for Au counterparts does not exceed 850 nm. Furthermore, the absorbance at 900–980 nm is stronger than that at 808 nm, the wavelength commonly used for in vivo photothermal ablation.[31, 33] Second, LSPR absorption of Au nanoparticles is influenced by the dielectric constant of the surrounding medium, which may consequently have a shift in the absorption peak once they are delivered to the desired cells. Such complications are not applicable to CuS nanoparticles, since absorption wavelength due to intra-band transitions in CuS nanoparticles is not affected by the size and shape of the nanoparticles or the solvent.
Quantum confinement effects, however, can influence the absorption intensity of nanoparticles of different sizes.[31, 34] CuS nanoparticles typically have molar extinction coefficient on the order of 107–108 M−1cm−1, which is comparable to Cu2-xSe and much higher than that of organic dyes and quantum dots (typically 105–106 M−1cm−1).[126] Another advantage of photothermal ablation with CuS nanoparticles compared to Au nanoparticles is the small size, which can lead to better tumor targeting efficiency and potentially faster renal clearance. To date, the smallest Au nanostructures showing NIR absorption were reported to be ~30 nm in diameter,[127] whereas CuS nanoparticles as small as 3 nm could have NIR absorption.[31] However, one major drawback of using CuS nanoparticles for photothermal ablation is the poor photothermal conversion efficiency, which in turn requires very high concentration of CuS nanoparticles for practical applications.[31]
To overcome this limitation, several strategies have been explored to modify and optimize the physicochemical properties of CuS nanoparticles. While one strategy involves the use of local field enhancement from Au nanoparticle surface plasmon coupling,[34] another report suggested the use of core-shell structures with ZnS shells around the CuS cores in the future.[31] According to the theory of trapped excitons, excitons confined in the core of a small sized core-shell structure can exhibit greater absorbance and stability.
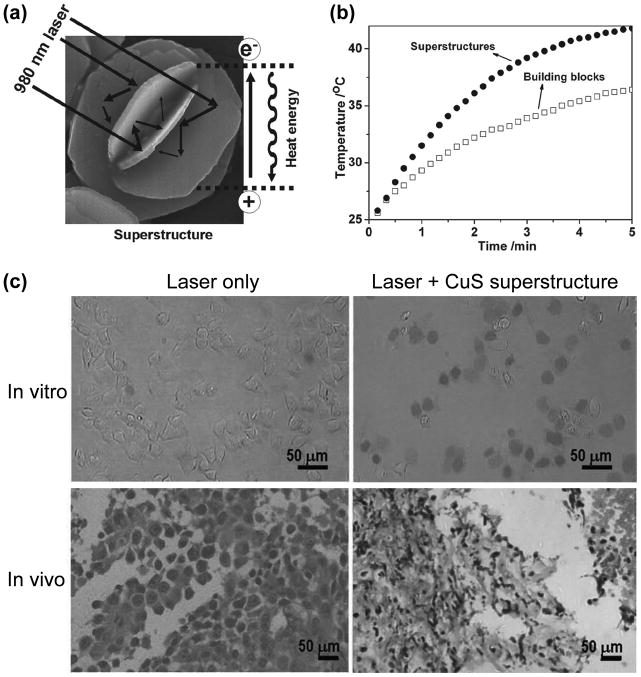
The use of flower-like hydrophilic superstructures of CuS was also proposed to enhance their absorbance of NIR light and photothermal conversion efficiency.[33] These uniform and monodispersed 3D superstructures were synthesized by the hydrothermal route, which were assembled from hexagonal plate-like building blocks (Figure 5a). An enhancement in absorption and ~50% increased photothermal conversion efficiency upon irradiation with 980 nm laser was observed for the CuS superstructures when compared to the nanoplates (Figure 5b). It was suggested that the faceted end planes of these crystalline superstructures could act as laser cavity mirrors for the 980 nm NIR light. The efficacy of these agents for photothermal ablation was evaluated both in vitro and in vivo, which showed that even at very low laser power density of < 1 W/cm2, the CuS nanostructures were capable of inducing cell death in vitro (Figure 5c). Furthermore, histological examination of tissues harvested from tumor-bearing mice revealed degenerative necrotic and karyolytic regions.
Figure 5.
Photothermal ablation with CuS superstructures. (a) Schematic representation of a CuS superstructure, which can serve as laser-cavity mirrors for 980 nm laser and its photothermal conversion. (b) The CuS superstructures exhibited superior photothermal properties when compared with the building blocks. (c) CuS superstructure can cause efficient photothermal ablation when excited with 980 nm laser with power density of < 1 W/cm2. In vitro, only dead Hela cells can be labeled with trypan blue. In PC-3 tumor-bearing mice, obvious tumor damage can be seen in H&E staining upon photothermal ablation. Adapted with permission from reference [33].
3.2.3. Drug delivery
Metastases are the cause of 90% of human cancer deaths.[128] Although chemotherapy remains the treatment modality of choice for most advanced cancers, it is rarely curative and has significant toxicity because of non-specific distribution of the cytotoxic drugs, which severely limits the maximum allowable dose.[129] On the other hand, rapid elimination and widespread distribution of the drugs into non-targeted organs/tissues mandates the administration of large doses to be therapeutically effective. This vicious cycle of large doses and concurrent toxicity is a major limitation of cancer chemotherapy. Therefore, development of biocompatible targeted drug delivery platforms will significantly improve metastatic cancer patient management. Because of the large surface area/loading capacity and versatile chemistry, nanomaterials are excellent carriers for targeted delivery of anti-cancer drugs.
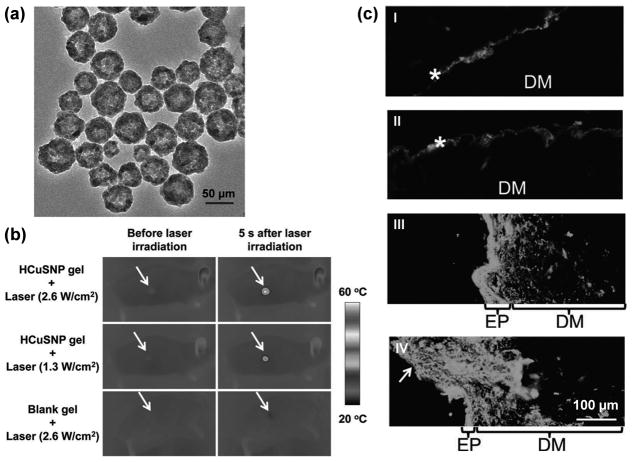
In an interesting report, CuS nanoparticles were investigated as drug delivery vehicles, where hollow CuS nanoparticles (~55 nm in diameter) were utilized for ablation assisted transdermal drug delivery (Figure 6).[35] Short (femto- to nanosecond) pulsed NIR irradiation (1.3–2.6 W/cm2) of the skin, mediated by CuS nanoparticles, led to focused thermal ablation of the stratum corneum. The use of short pulses resulted in rapid heating of the CuS nanoparticles to a high temperature, which was transmitted quickly to the tissues in contact, followed by an equally rapid cooling of the nanoparticles at the end of the pulse. This strategy ensured that the temperature of the skin never exceeds 40–50 ºC in the localized regions, which were coated with gel formulations of drug-bearing CuS nanoparticles. Although such elevated temperature did not produce any severe damage, it was sufficient to locally disrupt and decompose the keratin networks and cause disordering of the stratum corneum, which facilitated the uptake of hollow CuS nanospheres bearing a model hydrophilic “drug”, fluorescein isothiocynate (FITC)-labeled dextran. Thermographic and fluorescence microscopy studies confirmed localized heating of the epidermis by NIR irradiation, as well as subsequent enhanced penetration of FITC-dextran. Similar results were also obtained with a macromolecular drug, human growth hormone. Taken together, this technique holds the promise for efficient delivery of hydrophilic drugs, proteins, and vaccines, which may not be amenable to oral or intravenous administrations and can be obstructed by the hydrophobic stratum corneum barrier.
Figure 6.
Drug delivery with hollow CuS nanoparticles (demoted as HCuSNP). (a) A transmission electron microscopy image of the HCuSNPs. (b) Thermographic images of nude mice under NIR laser of various intensity. The mice were treated with 50 μL of gel with or without HCuSNPs. Arrows: area of skin treated with gel and laser. (c) Fluorescence microscopy images of diffusion of FITC-dextran in skin sections of nude mice, treated with (I) HCuSNP gel, (II) blank gel with pulsed laser (2.6 W/cm2), (III) HCuSNP gel with laser (1.3W/cm2), and (IV) HCuSNP gel with laser (2.6 W/cm2). Asterisk: stratum corneum; EP: epidermis; DM: dermis; Arrow: loss of epidermis. Adapted with permission from reference [35].
Extensive research effort has been devoted to the development of different families of drug delivery nanosystems such as polymers and organic/inorganic materials, each with their own set of advantages and disadvantages.[9, 119, 130–134] Because of the broad compositional and structural diversity, hybrid organic-inorganic nanosystems such as coordination polymer complexes are of substantial interest as an emerging class of drug nanocarriers.[135–136] Besides new formulations, innovative strategies for enhanced cargo delivery are also being developed. These strategies include specifically targeted delivery, extraordinary drug loading, controlled and environment/stimuli-responsive drug release, etc. CuS nanoparticles hold the potential as a novel class of drug delivery agents which can mediate drug release through their photothermal properties. Interestingly, no reports exist to date on the use of other Cu-based nanomaterials for drug/gene delivery applications to the best of our knowledge. CuSe nanocrystals, with their high photothermal conversion efficiency (~20%),[126] can be promising candidates for thermal-assisted drug delivery. In addition, porous CuO hollow nanostructures have been synthesized by thermal oxidation of CuS and Cu2S nanoparticles, which may also represent promising nanoplatforms for the delivery of therapeutic agents.[137]
3.2.4. Theranostics
Theranostics, which combines both therapy and diagnosis into a single platform,[138–139] is a highly dynamic research area over the last several years. Many nanomaterials are been actively explored for theranostic applications because of the enormous aspect ratio and/or surface area that they exhibit, which can allow for attachment of multiple copies of various theranostic moieties such as imaging labels (e.g. radioisotopes, fluorescent dyes, etc.), targeting ligands (e.g. peptides and antibodies), therapeutic agents (e.g. drugs, genes, etc.), as well as various polymers (e.g. PEG) to enhance their water solubility and biocompatibility. Ultimately, the combination of different targeting ligands, imaging labels, therapeutic drugs, and many other agents may allow for effective and controlled delivery of therapeutic agents in patients, which can be non-invasively monitored in real time.[5, 140] Because of the many intriguing properties discussed above, CuS nanoparticles have also been investigated for cancer theranostics.
Photothermal ablation has recently attracted significant attention as a locoregional, minimally invasive alternative to surgery. PET, on the other hand, is a widely used imaging modality in clinical oncology for cancer diagnosis, staging, and evaluation of therapeutic responses.[141–143] In one report, 64Cu-labeled hypericin was investigated to non-invasively assess the response to CuS nanoparticle-based photothermal ablation therapy in mouse tumor models.[144] Human mammary BT474 tumor-bearing mice were injected intratumorally with CuS nanoparticles, followed by NIR laser irradiation 24 h later. Uptake of 64Cu-labeled hypericin was found to be significantly higher in the treated mice compared to untreated control mice. Since 64Cu-labeled hypericin exhibited higher binding affinity to phosphatidylserine (PS) and phosphatidylethanolamine (PE) than to phosphatidylcholine, elevated tumor uptake upon photothermal ablation with CuS nanoparticles was attributed to the breakdown of the cell membrane and exposure of PS/PE to the PET tracer.
Recently, radiolabeling of nanoparticles with PET/SPECT isotopes have gained increasing interest for evaluating the pharmacokinetics and tumor targeting efficacy of various nanoparticles, since PET/SPECT is highly sensitive, quantitative, and clinical applicable.[140] However, one major concern is the potential detachment of radioisotopes from nanoparticles inside the animal body, which can cause misleading findings since PET/SPECT imaging detects the radioisotopes (whether they are on the nanoparticles or not) but not the nanoparticles themselves. Therefore, high in vivo stability of the radiolabeled nanoparticles is critical for more reliable experimental findings. In an intriguing study, multifunctional, chelator-free, PEG modified, 64Cu-labeled CuS nanoparticles (~11 nm in diameter) were constructed to serve as both a PET tracer and a photothermal ablation agent in live tumor-bearing mice (Figure 7).[36] Since CuCl2 was used as the precursor for the synthesis of CuS nanoparticles, 64CuCl2 was added during the procedure to prepare 64Cu-labeled CuS nanoparticles, in which 64Cu is an integral building block of CuS rather than being complexed through a chelator. Aside from the enhanced stability, this design also presents several desirable properties such as ease of synthesis, small size, higher tumor accumulation and hence better imaging results, as well as strong absorption in the NIR region (~930 nm). The PEG-[64Cu]CuS nanoparticles exhibited significant uptake and retention in U87 human glioblastoma xenografts in mice, based on the enhanced permeability and retention effect, which was successfully visualized by non-invasive PET imaging. Upon NIR laser irradiation, signs of thermonecrosis (e.g. loss of nucleus, cell shrinkage etc.) were observed in mice treated with the nanoparticles. Overall, this proof-of-principle study demonstrated the potential of CuS nanoparticles as a promising multifunctional platform for image guided photothermal ablation of cancer.
Figure 7.
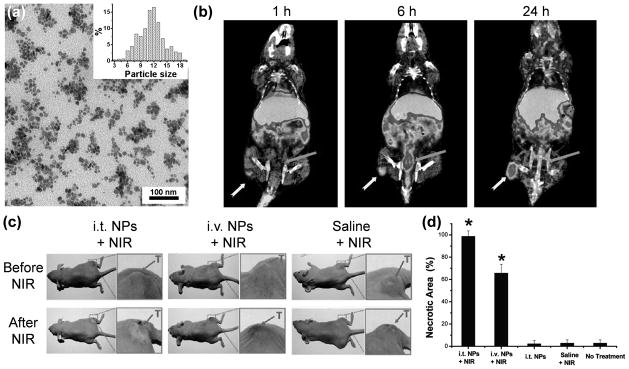
Cancer theranostics with 64Cu-labeled CuS nanoparticles. (a) A transmission electron microscopy image of PEGylated CuS nanoparticles. Inset shows the particle size distribution. (b) Coronal PET/CT images of U87 human glioblastoma xenografts in nude mice at 1, 6 and 24 h after intravenous injection of PEG-[64Cu]CuS nanoparticles. Yellow arrow: tumor; Orange arrow: bladder. (c) Photographs of tumor-bearing mice before and at 24 h after NIR laser irradiation (12 W/cm2 at 808 nm for 5 min). i.t.: intratumoral; i.v.: intravenous; NP: PEG-[64Cu]CuS nanoparticles. (d) Percentage of necrotic zone induced by various treatments based on H&E staining. *: statistically significant difference compared to the non-treated control mice. Adapted with permission from reference [36].
4. Summary and future perspectives
It’s been more than 4 decades since the declaration of the “War on Cancer”. Tremendous investment has been devoted to cancer research, and it is clear that personalized medicine is the key for improving clinical cancer patient management. Nanotechnology is one of the most promising tools for both ex vivo sensing applications and in vivo imaging/therapy applications, as illustrated in this review article for CuS nanoparticles, an emerging class of promising nanoparticles with many desirable features for biomedical applications. Not limited to cancer, these CuS nanoparticles can also play multiple roles in other diseases because of their versatility and multifunctionality. Targeting ligands (none has been successfully used for CuS nanoparticle-based tumor targeting, to the best of our knowledge), diagnostic labels, and therapeutic agents may all be accommodated within the nanoparticle because of its small size and resultant large surface area. Ex vivo nanosensors and in vivo imaging are both critical for future optimization of cancer patient management, and a combination of the two can offer synergistic advantages.
In this review article, we have discussed in detail the methods to synthesize CuS nanoparticles with various morphologies, as well as how they have been investigated in multiple disciplines of biomedical research such as in vitro sensing, in vivo imaging, photothermal ablation, drug delivery, and theranostics. Although they are much less extensively studied compared to many other nanoparticles (e.g. those that are magnetic and/or fluorescent), CuS nanoparticles have proven themselves to be highly versatile and readily tunable for various biomedical applications. Many critical proof-of-principle experiments have been reported in live animal models (e.g. PAT, photothermal ablation, drug delivery, and theranostics), it is expected that many more studies will emerge in the near future for CuS nanoparticles which exhibit a variety of desirable properties.
Research in the near future should focus on developing better targeted CuS nanoparticles for greater specificity in vivo. To date, the investigation of CuS nanoparticles in cancer research are almost exclusively based on non-specific accumulation of these nanoparticles in the tumor, taking advantage of the leaky tumor vasculature. Improved tumor targeting efficiency is needed to reduce the side effects of anti-cancer drugs and therapies on normal tissues, which is one of the major barriers for successful advancement of various nanomaterials beyond small animal studies. Another aspect of CuS nanoparticles that needs improvement is their photoconversion efficiency. One major application of CuS nanoparticles in biomedicine is dependent on their ability to convert NIR light into thermal energy, which can subsequently be used for ablation of cancer cells or heat-assisted drug delivery. The greater the conversion efficiency, the lower will be the dose needed for hyperthermic procedures and the greater will be the possibility of future clinical translation. The favorable properties and biocompatibility of CuS nanoparticles merit further research to develop them into more sophisticated and multifunctional systems. The many different morphologies and surface chemistries of CuS nanoparticles can be explored to enhance their drug delivery capabilities. Various combinations of hollow/porous/core-shell architectures, polymeric coating, and functional moieties (e.g. imaging/therapeutic agents, genes, targeting ligands etc.) can offer numerous new avenues for future research.
Research on the biomedical applications of CuS nanoparticles is still in its infancy. A few major challenges that need to be overcome in future research include: 1) The prohibitively high power of laser that is needed for CuS nanoparticle activation, especially under in vivo conditions, severely limits their therapeutic and drug delivery applications; 2) Proper surface modifications and precise control in shape/size distribution are imperative for successful future applications. Simple, clever, reproducible, and scalable techniques need to be developed to manufacture more uniform, appropriately sized, and bioinert CuS nanoparticles with reduced RES sequestration, optimal pharmacokinetics, and potential renal clearance; 3) The in vivo interaction of CuS nanoparticles with the body is unknown and difficult to predict, casting the greatest hurdle to successful clinical and commercial translation of these systems. More research effort on elucidating the pharmacokinetics and potential toxicity of CuS nanoparticles in mammalian systems are required before any commercial applications of these nanosystems can be envisaged.
Many other hurdles also need to be overcome in terms of clinical applications of novel nanomaterials including CuS nanoparticles, from the idea conception of a novel nanomedicine to its eventual approval for clinical use.[140, 145] For example, there are many commercial and regulatory challenges to be tackled with the emerging generation of more complex nanoparticles, in part owing to their multicomponent nature. However, on a positive note, some highly complex nanoparticles have reached clinical trials.[146] Although these and potentially other challenges exist for the translation of nanoparticles that are currently research tools into approved products for patients, their tremendous potential should drive the development and continuing emergence of novel nanoparticles for cancer imaging and therapy. The integration of diagnostic imaging capability with therapeutic interventions (i.e. theranostics) is critical for addressing the challenges of cancer heterogeneity and adaptation. Much remains to be done before this can be a clinical reality and continuous multidisciplinary efforts on the use/optimization of various nanoplatforms (including those based on CuS nanoparticles) will shed new light on molecular diagnostics and molecular therapy. It has been several decades since applications of nanomaterials in healthcare were first conceived. The field remains largely untapped and offers ample opportunities.
Acknowledgments
This work is supported, in part, by the University of Wisconsin - Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365), the Department of Defense (W81XWH-11-1-0644), and the American Cancer Society (125246-RSG-13-099-01-CCE).
Biographies
Shreya Goel is a graduate student in the Materials Science Program at the University of Wisconsin - Madison. She received her Master in Technology (M.Tech) degree in Nanotechnology from Indian Institute of Technology Roorkee, India in 2012 and is now pursuing her PhD degree under the supervision of Prof. Cai. Her research interests involve the development of novel nanomaterials as platforms for multimodality molecular imaging, cancer diagnosis and therapy, and other biomedical applications.
Feng Chen received his PhD degree in Materials Physics and Chemistry from Shanghai Institute of Ceramics, Chinese Academy of Sciences (P.R. China) in 2012. He is currently a Research Associate under the supervision of Prof. Weibo Cai in the Department of Radiology, University of Wisconsin - Madison. Dr. Chen has published > 30 peer-reviewed articles and his research interests involve the design and synthesis of multifunctional nanosystems for cancer targeted imaging and therapy.
Weibo Cai is an Assistant Professor in the Department of Radiology at the University of Wisconsin - Madison. He received a PhD degree in Chemistry from UCSD in 2004. After post-doctoral training at Stanford University, he launched his career at UW - Madison in early 2008. Prof. Cai has authored > 120 peer-reviewed publications and 13 book chapters, served on the Editorial Board of ~20 scientific journals, and won many awards including the Society of Nuclear Medicine Young Professionals Committee Best Basic Science Award (2007), European Association of Nuclear Medicine Springer Prize (2011 & 2013), American Cancer Society Research Scholar (2013–2017), among many others.
References
- 1.Feynman R. Caltech Engineering and Science. 1960;23:22. [Google Scholar]
- 2.Farrell D, Alper J, Ptak K, Panaro NJ, Grodzinski P, Barker AD. ACS Nano. 2010;4:589. doi: 10.1021/nn100073g. [DOI] [PubMed] [Google Scholar]
- 3.Doria G, Conde J, Veigas B, Giestas L, Almeida C, Assunção M, Rosa J, Baptista PV. Sensors. 2012;12:1657. doi: 10.3390/s120201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haun JB, Yoon TJ, Lee H, Weissleder R. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2010;2:291. doi: 10.1002/wnan.84. [DOI] [PubMed] [Google Scholar]
- 5.Cai W, Chen X. Small. 2007;3:1840. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Hong H, Myklejord DV, Cai W. Small. 2011;7:3261. doi: 10.1002/smll.201100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai W, Hong H. Am J Nucl Med Mol Imaging. 2012;2:136. [PMC free article] [PubMed] [Google Scholar]
- 8.Yigit MV, Medarova Z. Am J Nucl Med Mol Imaging. 2012;2:232. [PMC free article] [PubMed] [Google Scholar]
- 9.Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. ACS Nano. 2008;2:889. doi: 10.1021/nn800072t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigger I, Dubernet C, Couvreur P. Adv Drug Deliv Rev. 2002;54:631. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 11.Farokhzad OC, Langer R. ACS Nano. 2009;3:16. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 12.Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Adv Mater. 2011;23:H18. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 13.West JL, Halas NJ. Annu Rev Biomed Eng. 2003;5:285. doi: 10.1146/annurev.bioeng.5.011303.120723. [DOI] [PubMed] [Google Scholar]
- 14.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Anal Bioanal Chem. 2008;391:2469. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 15.Walkey C, Sykes EA, Chan WCW. ASH Education Program Book. 2009;2009:701. doi: 10.1182/asheducation-2009.1.701. [DOI] [PubMed] [Google Scholar]
- 16.Chung JS, Sohn HJ. J Power Sources. 2002;108:226. [Google Scholar]
- 17.Raevskaya AE, Stroyuk AL, Kuchmii SY, Kryukov AI. J Mol Catal A Chem. 2004;212:259. [Google Scholar]
- 18.Sagade AA, Sharma R. Sens Actuators B Chem. 2008;133:135. [Google Scholar]
- 19.Wu Y, Wadia C, Ma W, Sadtler B, Alivisatos AP. Nano Lett. 2008;8:2551. doi: 10.1021/nl801817d. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, Xiao H, Chen S, Wang C. Ceramics International. 2009;35:905. [Google Scholar]
- 21.Wang X-y, Fang Z, Lin X. J Nanopart Res. 2009;11:731. [Google Scholar]
- 22.Ding C, Zhong H, Zhang S. Biosens Bioelectron. 2008;23:1314. doi: 10.1016/j.bios.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Zhong H, Ding C. Anal Chem. 2008;80:7206. doi: 10.1021/ac800847r. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Wang G, Gu A, Wei Y, Fang B. Chem Commun (Camb) 2008:5945. doi: 10.1039/b814725f. [DOI] [PubMed] [Google Scholar]
- 25.Myung Y, Jang DM, Cho YJ, Kim HS, Park J, Kim JU, Choi Y, Lee CJ. J Phys Chem C. 2009;113:1251. [Google Scholar]
- 26.Bo X, Bai J, Wang L, Guo L. Talanta. 2010;81:339. doi: 10.1016/j.talanta.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Ding C, Wang Z, Zhong H, Zhang S. Biosens Bioelectron. 2010;25:1082. doi: 10.1016/j.bios.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Xue D. J Mater Chem. 2011;21:223. [Google Scholar]
- 29.Qian L, Mao J, Tian X, Yuan H, Xiao D. Sens Actuators B Chem. 2013;176:952. [Google Scholar]
- 30.Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. ACS Nano. 2012;6:7489. doi: 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Nanomedicine (Lond) 2010;5:1161. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 32.Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J. ACS Nano. 2011;5:9761. doi: 10.1021/nn203293t. [DOI] [PubMed] [Google Scholar]
- 33.Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J. Adv Mater. 2011;23:3542. doi: 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- 34.Lakshmanan SB, Zou X, Hossu M, Ma L, Yang C, Chen W. J Biomed Nanotechnol. 2012;8:883. doi: 10.1166/jbn.2012.1486. [DOI] [PubMed] [Google Scholar]
- 35.Ramadan S, Guo L, Li Y, Yan B, Lu W. Small. 2012;8:3143. doi: 10.1002/smll.201200783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. J Am Chem Soc. 2010;132:15351. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghezelbash A, Korgel BA. Langmuir. 2005;21:9451. doi: 10.1021/la051196p. [DOI] [PubMed] [Google Scholar]
- 38.Lu Q, Gao F, Zhao D. Nano Lett. 2002;2:725. [Google Scholar]
- 39.Zhang YC, Qiao T, Ya Hu X. J Cryst Growth. 2004;268:64. [Google Scholar]
- 40.Gorai S, Ganguli D, Chaudhuri S. Cryst Growth Des. 2005;5:875. [Google Scholar]
- 41.Ni Y, Liu H, Wang F, Yin G, Hong J, Ma X, Xu Z. Appl Phys A. 2004;79:2007. [Google Scholar]
- 42.Tadjarodi AKD. Proc 14th Int Electron Conf Synth Org Chem. 2010;c007:1. [Google Scholar]
- 43.Thongtem T, Phuruangrat A, Thongtem S. Mater Lett. 2010;64:136. [Google Scholar]
- 44.Xu H, Wang W, Zhu W. Mater Lett. 2006;60:2203. [Google Scholar]
- 45.Armelao L, Camozzo D, Gross S, Tondello E. J Nanosci Nanotechnol. 2006;6:401. doi: 10.1166/jnn.2006.916. [DOI] [PubMed] [Google Scholar]
- 46.Kim YY, Walsh D. Nanoscale. 2010;2:240. doi: 10.1039/b9nr00194h. [DOI] [PubMed] [Google Scholar]
- 47.Khiew PS, Radiman S, Huang NM, Ahamd MS. J Cryst Growth. 2004;268:227. [Google Scholar]
- 48.Chen X, Wang Z, Wang X, Zhang R, Liu X, Lin W, Qian Y. J Cryst Growth. 2004;263:570. [Google Scholar]
- 49.Liu X, Xi G, Liu Y, Xiong S, Chai L, Qian Y. J Nanosci Nanotechnol. 2007;7:4501. doi: 10.1166/jnn.2007.899. [DOI] [PubMed] [Google Scholar]
- 50.Zhu H, Wang J, Wu D. Inorg Chem. 2009;48:7099. doi: 10.1021/ic900201p. [DOI] [PubMed] [Google Scholar]
- 51.Yu XL, Cao CB, Zhu HS, Li QS, Liu CL, Gong QH. Adv Funct Mater. 2007;17:1397. [Google Scholar]
- 52.Yang Z-h, Zhang D-p, Zhang W-x, Chen M. J Phys Chem Solids. 2009;70:840. [Google Scholar]
- 53.Jiao S, Xu L, Jiang K, Xu D. Adv Mater. 2006;18:1174. [Google Scholar]
- 54.Xu H, Wang W, Zhu W, Zhou L. Nanotechnology. 2006;17:3649. [Google Scholar]
- 55.Rao CN, Kalyanikutty KP. Acc Chem Res. 2008;41:489. doi: 10.1021/ar700192d. [DOI] [PubMed] [Google Scholar]
- 56.Chu L, Zhou B, Mu H, Sun Y, Xu P. J Cryst Growth. 2008;310:5437. [Google Scholar]
- 57.Li F, Kong T, Bi W, Li D, Li Z, Huang X. Appl Surf Sci. 2009;255:6285. [Google Scholar]
- 58.Zhang J, Zhang Z. Mater Lett. 2008;62:2279. [Google Scholar]
- 59.Zhang P, Gao L. J Mater Chem. 2003;13:2007. [Google Scholar]
- 60.Lou W, Chen M, Wang X, Liu W. J Phys Chem C. 2007;111:9658. [Google Scholar]
- 61.Wang KJ, Li GD, Li JX, Wang Q, Chen JS. Cryst Growth Des. 2007;7:2265. [Google Scholar]
- 62.Wu H, Chen W. Nanoscale. 2011;3:5096. [Google Scholar]
- 63.Shifu C, Mingsong J, Yunguang Y. J Nanosci Nanotechnol. 2012;12:4898. doi: 10.1166/jnn.2012.4886. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Tian J, Li H, Wang L, Qin X, Asiri AM, Al-Youbi AO, Sun X. Langmuir. 2012;28:12893. doi: 10.1021/la303049w. [DOI] [PubMed] [Google Scholar]
- 65.Roy P, Srivastava SK. Cryst Growth Des. 2006;6:1921. [Google Scholar]
- 66.Liao XH, Chen NY, Xu S, Yang SB, Zhu JJ. J Cryst Growth. 2003;252:593. [Google Scholar]
- 67.Wu C, Yu SH, Chen S, Liu G, Liu B. J Mater Chem. 2006;16:3326. [Google Scholar]
- 68.Tan C, Zhu Y, Lu R, Xue P, Bao C, Liu X, Fei Z, Zhao Y. Mater Chem Phys. 2005;91:44. [Google Scholar]
- 69.Larsen TH, Sigman M, Ghezelbash A, Doty RC, Korgel BA. J Am Chem Soc. 2003;125:5638. doi: 10.1021/ja0342087. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Lu W, Hou J, Li X, Han S. J Nanosci Nanotechnol. 2011;11:9818. doi: 10.1166/jnn.2011.5316. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Hu J, Liu G, Zhang G, Zou H, Shi J. Carbohydr Polym. 2013;92:555. doi: 10.1016/j.carbpol.2012.08.102. [DOI] [PubMed] [Google Scholar]
- 72.Cao G, Liu D. Adv Colloid Interface Sci. 2008;136:45. doi: 10.1016/j.cis.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Gao L, Wang E, Lian S, Kang Z, Lan Y, Wu D. Solid State Commun. 2004;130:309. [Google Scholar]
- 74.Mao G, Dong W, Kurth DG, Möhwald H. Nano Lett. 2004;4:249. [Google Scholar]
- 75.Singh KV, Martinez-Morales AA, Bozhilov KN, Ozkan M. Chem Mater. 2007;19:2446. [Google Scholar]
- 76.Weissleder R, Pittet MJ. Nature. 2008;452:580. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Dongen GA, Ussi AE, de Man FH, Migliaccio G. Am J Nucl Med Mol Imaging. 2013;3:166. [PMC free article] [PubMed] [Google Scholar]
- 78.Nolting DD, Nickels ML, Guo N, Pham W. Am J Nucl Med Mol Imaging. 2012;2:273. [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L, Chang RC, Chu LW, Mak HK. Am J Nucl Med Mol Imaging. 2012;2:386. [PMC free article] [PubMed] [Google Scholar]
- 80.Hong H, Gao T, Cai W. Nano Today. 2009;4:252. doi: 10.1016/j.nantod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang K, Feng L, Shi X, Liu Z. Chem Soc Rev. 2013;42:530. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Nayak TR, Hong H, Cai W. Nanoscale. 2012;4:3833. doi: 10.1039/c2nr31040f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J. Nucleic Acids Res. 2000;28:3011. doi: 10.1093/nar/28.16.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X, Zhang XE, Chai YQ, Hu WP, Zhang ZP, Zhang XM, Cass AE. Biosens Bioelectron. 1998;13:451. doi: 10.1016/s0956-5663(97)00095-x. [DOI] [PubMed] [Google Scholar]
- 85.Liu YM, Mei L, Liu LJ, Peng LF, Chen YH, Ren SW. Anal Chem. 2011;83:1137. doi: 10.1021/ac103166n. [DOI] [PubMed] [Google Scholar]
- 86.Taton TA, Mirkin CA, Letsinger RL. Science. 2000;289:1757. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- 87.Liu CH, Li ZP, Du BA, Duan XR, Wang YC. Anal Chem. 2006;78:3738. doi: 10.1021/ac0522409. [DOI] [PubMed] [Google Scholar]
- 88.Zhu H, Wang J, Xu G. Cryst Growth Des. 2008;9:633. [Google Scholar]
- 89.Clark LC, Lyons C. Ann N Y Acad Sci. 1962;102:29. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 90.Lee H, Yoon SW, Kim EJ, Park J. Nano Lett. 2007;7:778. doi: 10.1021/nl0630539. [DOI] [PubMed] [Google Scholar]
- 91.Toghill CRG, KE Int J Electrochem Sci. 2010;5:1246. [Google Scholar]
- 92.Mayorga-Martinez CC, Guix M, Madrid RE, Merkoci A. Chem Commun (Camb) 2012;48:1686. doi: 10.1039/c2cc16601a. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y, Su L, Manuzzi D, de los Monteros HV, Jia W, Huo D, Hou C, Lei Y. Biosens Bioelectron. 2012;31:426. doi: 10.1016/j.bios.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Niu X, Lan M, Chen C, Zhao H. Talanta. 2012;99:1062. doi: 10.1016/j.talanta.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 95.Xu Q, Zhao Y, Xu JZ, Zhu JJ. Sens Actuators B Chem. 2006;114:379. [Google Scholar]
- 96.Cao F, Guo S, Ma H, Yang G, Yang S, Gong J. Talanta. 2011;86:214. doi: 10.1016/j.talanta.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Jiang LC, Zhang WD. Biosens Bioelectron. 2010;25:1402. doi: 10.1016/j.bios.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 98.Li C, Su Y, Zhang S, Lv X, Xia H, Wang Y. Biosens Bioelectron. 2010;26:903. doi: 10.1016/j.bios.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Wang AJ, Feng JJ, Li ZH, Liao QC, Wang ZZ, Chen JR. Cryst Eng Comm. 2012;14:1289. [Google Scholar]
- 100.Wang W, Zhang L, Tong S, Li X, Song W. Biosens Bioelectron. 2009;25:708. doi: 10.1016/j.bios.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Wu Z, Cao Z, Zeng JL, Zhang L, Chu X, Shen GL, Yu RQ. Anal Sci. 2010;26:1001. doi: 10.2116/analsci.26.1001. [DOI] [PubMed] [Google Scholar]
- 102.Viswanathan S, Rani C, Ho JA. Talanta. 2012;94:315. doi: 10.1016/j.talanta.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 103.Guo Y, Zhang J, Yang L, Wang H, Wang F, Zheng Z. Chem Commun (Camb) 2010;46:3493. doi: 10.1039/c001714k. [DOI] [PubMed] [Google Scholar]
- 104.Mankoff DA. J Nucl Med. 2007;48:18N. [PubMed] [Google Scholar]
- 105.Huang X, Lee S, Chen X. Am J Nucl Med Mol Imaging. 2011;1:3. [PMC free article] [PubMed] [Google Scholar]
- 106.Wu Y, Zhang W, Li J, Zhang Y. Am J Nucl Med Mol Imaging. 2013;3:1. [PMC free article] [PubMed] [Google Scholar]
- 107.Contag CH, Bachmann MH. Annu Rev Biomed Eng. 2002;4:235. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 108.Sosnovik DE, Weissleder R. Curr Opin Biotechnol. 2007;18:4. doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 109.Gambhir SS. Nat Rev Cancer. 2002;2:683. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 110.Alauddin MM. Am J Nucl Med Mol Imaging. 2012;2:55. [PMC free article] [PubMed] [Google Scholar]
- 111.Liang HD, Blomley MJ. Br J Radiol. 2003;76(Spec No 2):S140. doi: 10.1259/bjr/57063872. [DOI] [PubMed] [Google Scholar]
- 112.Dayton PA, Rychak JJ. Front Biosci. 2007;12:5124. doi: 10.2741/2553. [DOI] [PubMed] [Google Scholar]
- 113.James ML, Gambhir SS. Physiol Rev. 2012;92:897. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 114.Bhargava P, He G, Samarghandi A, Delpassand ES. Am J Nucl Med Mol Imaging. 2012;2:221. [PMC free article] [PubMed] [Google Scholar]
- 115.Balyasnikova S, Lofgren J, de Nijs R, Zamogilnaya Y, Hojgaard L, Fischer BM. Am J Nucl Med Mol Imaging. 2012;2:458. [PMC free article] [PubMed] [Google Scholar]
- 116.Fakhri GE. Am J Nucl Med Mol Imaging. 2012;2:415. [PMC free article] [PubMed] [Google Scholar]
- 117.Thorek D, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, Grimm J. Am J Nucl Med Mol Imaging. 2012;2:163. [PMC free article] [PubMed] [Google Scholar]
- 118.Wang LV, Hu S. Science. 2012;335:1458. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boisselier E, Astruc D. Chem Soc Rev. 2009;38:1759. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 120.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Nat Nanotechnol. 2008;3:557. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pan D, Cai X, Yalaz C, Senpan A, Omanakuttan K, Wickline SA, Wang LV, Lanza GM. ACS Nano. 2012;6:1260. doi: 10.1021/nn203895n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Zee J. Ann Oncol. 2002;13:1173. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 123.Atkinson WJ, Brezovich IA, Chakraborty DP. IEEE Trans Biomed Eng. 1984;BME-31:70. doi: 10.1109/TBME.1984.325372. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J, Noguez C. Plasmonics. 2008;3:127. [Google Scholar]
- 125.Zhao Y, Pan H, Lou Y, Qiu X, Zhu J, Burda C. J Am Chem Soc. 2009;131:4253. doi: 10.1021/ja805655b. [DOI] [PubMed] [Google Scholar]
- 126.Hessel CM, Pattani VP, Rasch M, Panthani MG, Koo B, Tunnell JW, Korgel BA. Nano Lett. 2011;11:2560. doi: 10.1021/nl201400z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Clin Cancer Res. 2009;15:876. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehlen P, Puisieux A. Nat Rev Cancer. 2006;6:449. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 129.Chabner BA, Roberts TG., Jr Nat Rev Cancer. 2005;5:65. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 130.Xu ZP, Zeng QH, Lu GQ, Yu AB. Chem Eng Sci. 2006;61:1027. [Google Scholar]
- 131.Murakami T, Tsuchida K. Mini Rev Med Chem. 2008;8:175. doi: 10.2174/138955708783498078. [DOI] [PubMed] [Google Scholar]
- 132.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. J Control Release. 2001;70:1. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 133.Fréchet JMJ. J Polym Sci, Part A: Polym Chem. 2003;41:3713. [Google Scholar]
- 134.Svenson S, Tomalia DA. Adv Drug Deliv Rev. 2005;57:2106. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 135.Imaz I, Hernando J, Ruiz-Molina D, Maspoch D. Angew Chem Int Ed Engl. 2009;48:2325. doi: 10.1002/anie.200804255. [DOI] [PubMed] [Google Scholar]
- 136.Imaz I, Rubio-Martinez M, Garcia-Fernandez L, Garcia F, Ruiz-Molina D, Hernando J, Puntes V, Maspoch D. Chem Commun (Camb) 2010;46:4737. doi: 10.1039/c003084h. [DOI] [PubMed] [Google Scholar]
- 137.Liu J, Xue D. Adv Mater. 2008;20:2622. [Google Scholar]
- 138.Kelkar SS, Reineke TM. Bioconjug Chem. 2011;22:1879. doi: 10.1021/bc200151q. [DOI] [PubMed] [Google Scholar]
- 139.Melancon MP, Zhou M, Li C. Acc Chem Res. 2011;44:947. doi: 10.1021/ar200022e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hong H, Zhang Y, Sun J, Cai W. Nano Today. 2009;4:399. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. J Nucl Med. 2001;42:1S. [PubMed] [Google Scholar]
- 142.Iagaru A. Am J Nucl Med Mol Imaging. 2011;1:63. [PMC free article] [PubMed] [Google Scholar]
- 143.Grassi I, Nanni C, Allegri V, Morigi JJ, Montini GC, Castellucci P, Fanti S. Am J Nucl Med Mol Imaging. 2012;2:33. [PMC free article] [PubMed] [Google Scholar]
- 144.Song S, Xiong C, Zhou M, Lu W, Huang Q, Ku G, Zhao J, Flores LG, Jr, Ni Y, Li C. J Nucl Med. 2011;52:792. doi: 10.2967/jnumed.110.086116. [DOI] [PubMed] [Google Scholar]
- 145.Venditto VJ, Szoka FC., Jr Adv Drug Deliv Rev. 2013;65:80. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Davis ME. Mol Pharm. 2009;6:659. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]