Abstract
Objective
Despite being a common problem in Systemic Sclerosis (SSc), the extant literature on pain has primarily focused on biomedical correlates, or bivariate relationships with a few psychological characteristics. There is a need to investigate the more heuristic biopsychosocial model, which incorporates the simultaneous contributions of medical, psychological, and social variables in understanding pain.
Methods
Patients with SSc (N = 333) received clinical exams and completed self-report surveys at enrollment to the Genetics versus ENvironment In Scleroderma Outcome Study (GENISOS). Latent profile analysis was used to derive biopsychosocial profiles of patients using skin thickening, percent predicted forced vital lung capacity, perceived physical health, health worry, mental health, and social support. The profiles were examined in relation to pain and pain medication usage.
Results
A 3-profile solution provided the best fit to the data. Based on the biopsychosocial indicators, the profiles were characterized as Managing (n = 217), Resilient (n = 86), and Distressed (n = 30). Between-group differences for pain emerged, with the Distressed group, whose disease was less severe than the Resilient group, reporting the highest pain and the greatest utilization of pain medication.
Conclusion
Clinicians should consider biopsychosocial characteristics as contributing factors to the experience of pain in patients with SSc. Patients who are similar to those in the Distressed profile may be at an increased risk for pain and would likely benefit from a referral to a behavioral health or other ancillary service provider for pain management, rather than relying solely on pharmacological therapies.
Systemic Sclerosis (SSc) is a rheumatic disease characterized by skin thickening and fibrosis of internal organs due to a buildup of collagen and other extracellular matrix proteins [1]. There are two general classifications: limited cutaneous SSc (lcSSc), which has skin involvement only distal to the elbows and knees and is characterized by slow fibrosis and milder internal organ involvement [1-2], and diffuse cutaneous SSc (dcSSc), which has a worse prognosis, extensively affects the skin and internal organs, and is characterized by rapidly progressing fibrosis [1-2]. Clinical care for SSc is complicated by a lack of effective treatments for many manifestations of disease. Therefore, the primary goals of care are to preserve functioning, relieve symptoms, and improve quality of life.
Pain is a virtually ubiquitous problem in SSc. Indeed, 83% of patients in a large, recent sample reported significant pain [3], which is similar to previous rates [4-6]. Early in the disease process, patients report nonspecific muscle pain and stiffness [1], while other symptoms (e.g., difficulty swallowing, gastrointestinal discomfort) emerge as the disease progresses [7]. In SSc, pain has been typically conceptualized according to the biomedical model, which suggests that pain is a symptom secondary to disease activity and previously sustained tissue damage [8]. However, the level of pain one experiences is not always relative to disease severity [4, 9]. For example, although lcSSc patients typically report less pain than dcSSc patients, the differences are generally small and not clinically meaningful [3-4, 10-12]. Alternatively, a biopsychosocial framework for understanding pain, which has been widely accepted across disciplines and diseases, suggests that pain is not a purely physical phenomenon [13]. This model highlights interconnections among the disease, person, and environment, and postulates that none of these factors can independently explain pain. Instead, biological, psychological, and social factors work together in complex ways to shape pain perceptions [13].
At the broadest level, emotional health and pain share a significant connection, with up to half of chronic pain patients also reporting depression and/or anxiety [13]. Symptoms of depression [14] and anxiety [15] are common in SSc, and psychological health has been broadly linked with pain in this population [3-5, 10, 16-17]. For example, depressive [3] and anxious [17] symptomatology, and mental health-related quality of life [10] have demonstrated relationships with pain, even after accounting for other disease and psychosocial variables.
The way a person thinks about his/her health has also been linked with pain in clinical [13] and rheumatic [9, 18] populations. Illness cognitions can range from general concerns to more severe responses and preoccupation, with more extreme responses being of the greatest significance to pain. Research suggests that thinking about the serious consequences of SSc [12], castastrophizing thoughts [6], and maladaptive disease cognitions [19] are all associated with greater pain. Other variables have also been shown to influence the cognition-pain relationship; patients with less education and social support who engage in catastrophic cognitions report greater pain [6].
A great deal of research has supported a link between social support and pain both directly, and via mood [13]. Research in the rheumatic diseases has also largely supported this connection [9, 20]. For example, in a study of patients with rheumatoid arthritis, osteoarthritis, or ankylosing spondylitis, lower satisfaction with social support was correlated with greater pain [20]. To date, only one study of SSc patients has evaluated the social support-pain relationship. In this study, patients with poor social adjustment reported worse pain; although this relationship was accounted for by depression, suggesting that emotional health was the conduit for this association [4].
There is a growing appreciation for the biopsychosocial model of pain in SSc, however, a better understanding of how these factors interact is needed. A number of the reviewed studies included biopsychosocial variables, but these constructs were typically included in adjusted models, rather than considering heterogeneity among them. Alternatively, these variables may be modeled as multiplicative (combined) effects to understand general patterns at the level of the person. Although no analysis can capture all individual differences, the goal of this study was to determine whether general typologies that incorporate biological, psychological, and social characteristics could be identified to enhance understanding of SSc-related pain. The first aim was to evaluate the interrelationships of these factors by deriving homogeneous biopsychosocial trait profiles of SSc patients, and to interpret the response patterns that cluster together. The indicator variables (skin thickening, percent predicted forced vital lung capacity, perceived physical health, health worry, mental health, social support) were selected given the substantive reasoning that they may conjointly relate to pain. The second aim was to evaluate the predictive utility of each profile with respect to pain ratings and pain medication utilization. It was hypothesized that profiles characterized by poorer subjective ratings of perceived physical health, health worry, mental health, and social support would be related to pain and medication, whereas skin thickening and percent predicted forced vital lung capacity within the profiles would be less relevant.
Methods
Participants and Procedure
The sample (N = 333) was comprised of individuals who completed the baseline examination of the Genetics versus ENvironment In Scleroderma Outcome Study (GENISOS), an ongoing, prospective, early-disease (within 5 years of onset) cohort study aimed at understanding morbidity and mortality in SSc. Patients with SSc who lived within the geographic catchment area of one of the three centers (University of Texas Health Science Center at Houston, University of Texas Medical Branch at Galveston, University of Texas-Health Science Center at San Antonio) were recruited from the rheumatology faculty clinics, the county hospital, and chapters of the Scleroderma Foundation [21].
Baseline visits were conducted during outpatient appointments and inpatient services at facilities staffed by the clinician-investigators. During this visit, data from medical records were clarified. Patients received a standardized clinical exam which included an evaluation of skin thickening and pulmonary function and were administered a packet of psychosocial measures. All participants gave written informed consent. Institutional Review Board approval was obtained at all participating institutions.
Variables
Skin Thickening
The modified Rodnan Skin Score (mRSS [22]), an objective indicator of skin disease severity, is calculated by measuring the extent and severity of skin thickening on 17 body surfaces by palpation on a 4-point scale (0 = uninvolved to 3 = severe thickening). Scores range from 0-51.
Forced Vital Lung Capacity
Percent predicted forced vital lung capacity (%FVC) is an objective, validated measure for severity of SSc-related interstitial lung disease [23] indicating the ratio of the volume of air that the subject can forcibly exhale after a maximum inspiration to the same volume in age, gender, weight, height, and ethnicity matched unaffected controls. All pulmonary measurements met criteria outlined by the American Thoracic Society/European Respiratory Society, and were reviewed by a pulmonologist (R.M.E-Y-M.). Lower scores indicate greater severity of SSc-related interstitial lung disease.
Perceived Physical Health1
The Physical Functioning subscale from the Medical Outcomes Study Short-Form Health Survey (SF-36 [24]), was used to evaluate self-reported overall physical health. Scores are transformed into a 0-100 scale; lower scores indicate greater difficulties performing activities due to physical functioning. Internal consistency was α = .920.
Health Worry
Five items from the Illness Behavior Questionnaire (IBQ [25]) were used to generate the Health Worry scale for SSc [26]. An example item is, “Do you worry a lot about your health?” Scores range from 0-5; higher scores indicate greater worry and concern regarding one’s health. Internal consistency was α = .721.
Mental Health
The SF-36 [24] Mental Health Component Summary Score measures global emotional health and related functional impairment. It is comprised of four subscales (Mental Health, Role Limitations Due To Emotional Problems, Social Functioning, Vitality) which are transformed into a 0-100 scale; lower scores indicate greater psychological distress and more limitations due to emotional problems. Internal consistency was α = .807.
Social Support
The 40-item Interpersonal Support Evaluation List (ISEL [27]) was used to derive a measure of perceived social support. Respondents rate whether a statement is “probably true” or “probably false” based on their experience. An example item is, “There is at least one person I know whose advice I really trust.” The ISEL yields four subscales and an overall support score that is calculated by averaging the 4 subscales. Overall support scores range from 0-10; higher scores indicate better social support. Internal consistency was α = .870.
Pain
The Pain subscale from the SF-36 [24] was used to evaluate self-reported severity and impact of pain. Scores are transformed into a 0-100 scale; lower scores indicate greater pain severity and interference. Internal consistency was α = .884.
Pain Medication
Participants were asked whether they had taken acetominophen, non-steroidal anti-inflammatory drugs (NSAIDs), tramadol, and narcotics over the past month2. A variable with four categories (No medication, Acetaminophen/NSAIDs, Tramadol, Narcotics) was created to represent typical pain medication usage. Respondents taking multiple medications (11.1% of the sample; two medications: n = 34; three medications: n = 3) were coded with the strongest drug being taken (i.e., an individual taking both acetaminophen and tramadol was coded as Tramadol; an individual taking both tramadol and narcotics was coded as Narcotics).
Data Analysis
Latent Profile Analysis (LPA [28]), an empirically driven statistical technique that defines taxonomies (classes) of people based on common characteristics, was used to derive categorical latent variables representing classes of SSc patients with similar biopsychosocial profiles. Because it is difficult to interpret interactions with more than three variables, and because traditional analytic methods are at the level of the variable, not the person, LPA is a preferred technique for making inferences about individuals. This method summarizes complicated relationships among variables, similar to the way in which symptom clusters are categorized in medical settings to help inform diagnosis and treatment, and to make predictions about an individual. LPA uses all observations of the continuous indicator variables to define these classes via maximum likelihood estimation [29]. The probability that an individual was properly classified, which enables each person to be categorized into the best-fitting class, is estimated simultaneously with the overall model [30]. Models are estimated with classes added iteratively to determine which model is the best fit. It is recommended that the sample size for LPA be large because class solutions produced from smaller samples may be unstable [31]. Recommendations mirror that of Structural Equation Modeling, with sample sizes of 200 being adequate [32].
To achieve the first aim, LPA was conducted using MPlus 6.1 [33]. Models were evaluated using the Lo-Mendell-Rubin Adjusted Likelihood Ratio Test (LMRT [34]), the Bootstrapped Likelihood Ratio Test (BLRT [35]), Akaike information criteria (AIC [36]), sample size-adjusted Bayesian information criteria (sBIC [37]), and Entropy [38] to determine the optimal number of classes. The LMRT and the BLRT compare the fit of a target model (e.g., 2-class model) to a comparison model specifying one less class (e.g., 1-class model). The p-value generated for the LMRT and BLRT indicates whether the solution with more (p < .05) or fewer (p > .05) classes fits better. The AIC and sBIC are descriptive fit indices wherein smaller values indicate better model fit. Entropy describes the accuracy of classification of individuals into a class; bigger values (i.e., closer to 1) indicate greater accuracy. Models were also evaluated on interpretability to determine whether the classes truly represented different categories, rather than being an artifact of a nonnormal distribution [39]. Given that small classes (i.e., those with less than 5% of the sample) are typically considered spurious, a condition often associated with extracting too many classes/profiles [40], the number of patients categorized into each class was also considered. The overall sample means (and SDs) and conditional response means (and SDs) of each indicator variable from the best-fitting solution were compared for interpretation. The classes were then related to disease and demographic characteristics. For the second aim, ANOVA and chi-square tests were conducted to evaluate potential differences in pain and pain medication usage as a function of class. To identify between-class differences, Bonferroni post-hoc tests were conducted and adjusted standardized residuals were examined using a familywise error rate of .05.
Results
Sample characteristics are described in Table 1. Most participants were women, married, and had at least a high school diploma or General Education Development certificate. Ages ranged from 16 to 86. Age of disease onset ranged from 14 to 84. Disease duration ranged from 0 to 5 years. Individuals with dcSSc had greater skin thickening (t [330] = -15.22, p < .001; dcSSc = 21.98 ± 10.95; lcSSc = 6.82 ± 5.10) and lower forced vital lung capacity (t [309] = 2.54, p = .011; dcSSc = 80.07 ± 20.74; lcSSc = 86.33 ± 22.37).
Table 1.
Sample characteristics
| Variable | M ± SD or n (%) | |
|---|---|---|
| Age | 48.00 ± 13.04 | |
| Sex | Women | 278 (83.5%) |
| Men | 55 (16.5%) | |
| Race/Ethnicity | White | 157 (47.2%) |
| Hispanic | 97 (29.1%) | |
| Black | 68 (20.4%) | |
| Asian | 10 (3.0%) | |
| American Indian | 1 (0.3%) | |
| Marital status | Married/Partnered | 180 (56.6%) |
| Never married | 42 (13.2%) | |
| Divorced/Separated | 80 (25.2%) | |
| Widowed | 16 (5.03%) | |
| Education | Less than high school | 49 (15.3%) |
| High school diploma/GED | 166 (51.7%) | |
| Associate’s degree | 32 (10.0%) | |
| Bachelor’s degree | 47 (14.6%) | |
| Post-graduate | 27 (8.4%) | |
| Family income | < $14,999 | 78 (25.0%) |
| $15,000 - $29,999 | 71 (22.8%) | |
| $30,000-$49,999 | 64 (20.5%) | |
| $50,000-$99,999 | 60 (19.2%) | |
| ≥ $100,000 | 39 (12.5%) | |
| Disease subtype | Diffuse cutaneous | 192 (57.8%) |
| Limited cutaneous | 140 (42.2%) | |
| Age of disease onset | 46.02 ± 13.20 | |
| Disease duration (years) | 1.20 ± 1.40 | |
| Skin thickening (MRSS) | 15.62 ± 11.67 | |
| Forced Vital Lung Capacity | 82.70 ± 21.63 | |
| History of digital ulcers | 200 (60.1%) | |
| Arthritis | 101 (30.3%) | |
| IBQ | Health Worry Scale | 2.19 ± 1.63 |
| ISEL | Total Social Support Scale | 8.20 ± 1.63 |
| SF-36 | Mental Health Composite Score | 45.51 ± 12.91 |
| Physical Functioning Scale | 43.32 ± 28.66 | |
| Pain Scale | 48.81 ± 27.18 | |
| Pain medication use | Not taking pain medication | 190 (57.1%) |
| Acetaminophen/NSAIDs | 66 (19.8%) | |
| Tramadol | 18 (5.41%) | |
| Narcotics | 59 (17.7%) |
Development of Biopsychosocial Classes Using Latent Profile Analysis
Intercorrelations among the indicator variables were nonsignificant or small/moderate in size which allowed for more differentiation between classes3. Latent profile models containing 1-4 classes were fit to the data. Fit indices for each LPA are presented in Table 2. The LMRT and BLRT indicated that the 2-class solution fit better than the 1-class solution (p = .005). The 3-class solution was superior to the 2-class solution according to the LMRT (p = .05) and BLRT values (p < .0001), and lower AIC and sBIC values. Although the 4-class solution revealed slightly lower AIC and sBIC values, and a statistically significant BLRT value (p < .0001), Entropy was lower, and the LMRT indicated that it was not statistically different from the 3-class solution (p = .32). Therefore, the 3-class solution was considered the best fit to the data.
Table 2.
Model fit indices for skin thickening, forced vital lung capacity, perceived physical health, health worry, mental health, total social support
| Solution | LMRT (p) | BLRT (p) | AIC | sBIC | Entropy |
|---|---|---|---|---|---|
| 1 class | 12802.40 | 12810.04 | |||
| 2 class | 118.590 (.005) | 121.507 (<.0001) | 12694.90 | 12706.98 | .806 |
| 3 class | 95.834 (.05) | 98.191 (<.0001) | 12610.71 | 12627.24 | .799 |
| 4 class | 35.530 (.32) | 35.530 (<.0001) | 12589.18 | 12610.17 | .695 |
Note. LMRT = Lo-Mendell-Rubin Test, BLRT = Bootstrapped Lo-Mendell Rubin Test, AIC = Akaike Information Criterion, sBIC = sample size-adjusted Bayesian Information Criterion
The overall sample means and conditional response means used to substantively interpret each class are available in Table 3. Figure 1 presents the z-transformed conditional response means (Ms = 0, SDs = 1) for the purposes of illustration. To facilitate interpretation of the profiles, the z scores for the figure were set such that higher scores represented better functioning. Class 1 is comprised of 65.2% of the sample and represents individuals with relatively less severe skin thickening and forced vital lung capacity, better perceived physical health, fewer health worries, better mental health, and more social support. Accordingly, this profile was referred to as Managing. Class 2 is comprised of 25.8% of the sample and was termed Resilient because it represents individuals with relatively more severe skin thickening and forced vital lung capacity and poorer perceived physical health, but fewer health worries, better mental health, and more social support. Class 3, labeled Distressed, is comprised of 9.0% of the sample and is characterized by individuals with relatively less severe skin thickening and forced vital lung capacity, but poorer perceived physical health, more health worries, poorer mental health, and lower social support.
Table 3.
Overall sample means ± SD and Biopsychosocial profile conditional response means ±SD
| n | Skin Thickening‡ | Forced Vital Lung Capacity† | Perceived Physical Health† | Health Worry‡ | Mental Health† | Social Support† | |
|---|---|---|---|---|---|---|---|
| Sample | 333 | 15.62 ± 11.67 | 82.70 ± 21. 63 | 43.32 ± 28.66 | 2.19 ± 1.63 | 45.51 ± 12.91 | 8.20 ± 1.63 |
| 3-class solution | |||||||
| Class 1 (Managing) | 217 | 10.17 ± 20.08 | 86.38 ± 23.63 | 53.22 ± 31.54 | 1.94 ± 1.97 | 46.72 ± 15.36 | 8.70 ± 1.92 |
| Class 2 (Resilient) | 86 | 30.96 ± 13.20 | 73.19 ± 33.22 | 26.95 ± 51.30 | 2.38 ± 2.64 | 48.10 ± 15.17 | 8.40 ± 2.32 |
| Class 3 (Distressed) | 30 | 10.53 ± 12.25 | 84.17 ± 28.11 | 26.94 ± 35.78 | 3.10 ± 1.81 | 32.78 ± 16.60 | 4.92 ± 1.75 |
Note.
higher scores indicate better functioning;
lower scores indicate better functioning
Figure 1.
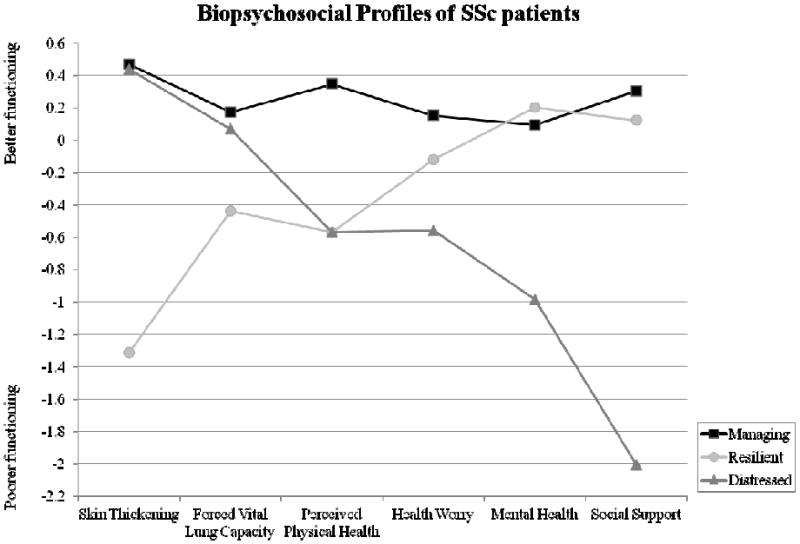
z transformed conditional response means of the 3-class solution
Note. For illustrative purposes, z scores were set so that higher scores represented better functioning
In sum, the Managing and Distressed classes were similar with regard to skin thickening and percent predicted forced vital lung capacity; however, they differed on their perceived physical health and their psychosocial characteristics. Specifically, the Distressed class had the poorest psychosocial functioning of the three groups. Resilient patients had more severe skin thickening and percent predicted forced vital lung capacity than the other groups, and accordingly, their perceived physical health was poorer. However, the Resilient class reported better psychosocial functioning, with scores equivalent to the healthier Managing patients. The profiles differed somewhat on how much they worried about their health: Distressed patients reported the most worry, Managing patients reported the least worry, and the Resilient class, which was the sickest class, reported moderate worry.
Disease Characteristic and Sociodemographic Group Differences
Follow-up analyses suggested that the classes differed by disease type, χ2 (2) = 75.77, p < .0001. The proportions of lcSSc and dcSSc patients were similar to the overall sample for the Distressed class (60.0% lcSSc, 40.0% dcSSc) and the Managing class (55.6% lcSSc, 44.4% dcSSc). However, the Resilient class had more dcSSc (97.7%) than lcSSc (2.3%) patients than would be expected due to chance. There was a significant difference for income (χ2 [8] = 21.44, p =.006), with Distressed patients reporting a higher proportion of lower income than would be expected by chance (51.9% reported an annual income lower than $14,999). The classes did not differ on history of digital ulcers, arthritis, disease duration, age, gender, race/ethnicity, or education (ps > .05).
Association of Biopsychosocial Profiles with Pain and Pain Medication
Figure 2 provides a graphic depiction of pain and medication use between the classes. ANOVA results suggested overall group differences in pain, F (2, 290) = 16.47, p < .001, partial η2 = .102. Post-hoc comparisons revealed a large difference (d = 1.00, p < .001) between the Managing (54.91 ± 26.26) and Distressed classes (29.48 ± 24.38), such that Distressed patients reported greater pain4. A moderate significant difference (d = .52, p < .001) suggested that Resilient patients (41.44 ± 25.36) reported more pain than Managing patients. Although the difference between the Distressed and Resilient classes was not statistically significant, there was a trend and medium-sized effect suggesting that Distressed patients had greater pain than Resilient patients (d = .48, p = .103).
Figure 2.
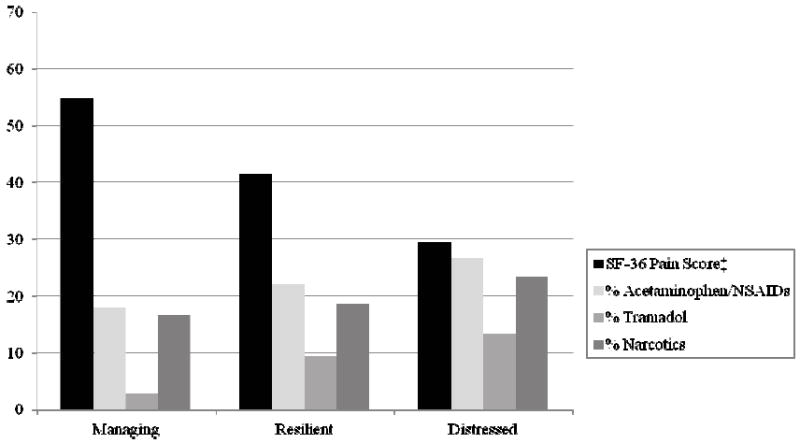
Self-reported pain (SF-36) and pain medication usage for each biopsychosocial class
Note. ‡higher scores on the SF-36 indicate better functioning (i.e., less pain); Significant between-group differences were observed for pain, F (2, 290) = 16.47, p < .001, and pain medication usage, χ2 (6) = 14.88, p = .021.
Chi-square test results suggested that pain medication usage was not equal among the classes, χ2 (6) = 14.88, p = .021. These relationships are described in Table 4. Inspection of the standardized residuals for each class by pain medication category revealed that the Managing class (62.67%) was significantly more likely to not be taking pain medication whereas the Distressed class (36.67%) was significantly less likely to not be taking pain medication than would be expected based on the total sample. Additionally, the Managing class (2.76%) was significantly less likely to be taking tramadol, whereas the Distressed class (13.33%) was significantly more likely to be taking tramadol.
Table 4.
Percentage of patients within each biopsychosocial group regularly taking pain medication
| Not taking pain medication | Acetaminophen/NSAIDS | Tramadol | Narcotics | |
|---|---|---|---|---|
| Managing (n = 217) | 62.67%* | 17.97% | 2.76%** | 16.59% |
| Resilient (n = 86) | 50.00% | 22.09% | 9.30% | 18.60% |
| Distressed (n = 30) | 36.67%* | 26.67% | 13.33%* | 23.33% |
Note. Overall model, χ2 (6) = 14.88, p = .021;
p < .05;
p < .01
Discussion
Skin thickening, percent predicted forced vital lung capacity, perceived physical health, health worry, mental health, and social support were used to identify biopsychosocial profiles of patients with SSc. Three classes emerged and were termed Managing, Resilient, and Distressed. One remarkable finding was that while the Managing and Distressed groups were similar with regard to skin thickening and percent predicted forced vital lung capacity, they differed on perceived physical health, mental health, and social support. Specifically, the Managing group was functioning well psychosocially; the Distressed group was not. The Resilient group had a much more severe disease manifestation; however, Resilient patients mirrored the Managing group psychosocially.
When the groups were evaluated in relation to other clinical variables that cause persistent pain (i.e., digital ulcers, arthritis), there were no differences. Moreover, it is significant that the proportion of lcSSc and dcSSc patients in the Managing and Distressed typologies was roughly equivalent to the overall sample, but that the Resilient typology was predominantly comprised of dcSSc patients. This suggests that disease severity is not the key factor for differentiating between patients who are at risk for decreased quality of life, consistent with previous findings [5, 11]. Indeed, when the classes were evaluated in relation to pain and medication, the Distressed group, which was less severely affected, reported greater pain and medication usage.
One interesting finding from this study was that social support in the Distressed group was approximately two standard deviations lower than the other profiles. The health benefits of social support from family, friends and other informal groups (as opposed to health professional or therapeutic support), have been recognized across disease populations [13, 41], including rheumatic diseases [9, 42]. The current findings suggest that social support is of great interest in understanding the experience of SSc patients, particularly given that SSc patients may avoid socializing due to appearance concerns [43], and that over half of patients with rheumatic disorders report moderate to high levels of loneliness [44]. It is also worth mentioning that, social support is not characterized by the number of relationships one has, but rather the perceived availability and quality of support [27]. A person may have many social contacts but not feel supported by them, or, conversely, a person may derive adequate support from just one relationship.
Effective pain management is a primary goal of patient care, although it has not been well investigated in SSc [45]. Because not all patients respond well to pharmacological pain management [46], other methods that target modifiable psychosocial factors (i.e., emotional health, cognitions, social support) should be considered. Approaches such as cognitive-behavioral therapy (which involves skill building in areas such as mindfulness, relaxation, coping, social support, changing maladaptive beliefs), have already been identified and used in other pain populations [9, 13, 47-48]. While outcomes to these treatments are less straightforward (e.g., successful treatment may mean that pain is partially ameliorated, a patient is experiencing their pain differently, healthcare costs have decreased), they may be promising as an adjunct for patients who are not benefiting from pharmacological therapy.
However, prior to implementing and evaluating interventions for individuals with characteristics similar to the Distressed profile, it is important to determine whether such patients can be feasibly identified within clinical settings. To this end, researchers and clinicians are encouraged to assess for perceived physical health, health worry, mental health, and social support in addition to disease severity of the skin and lungs, which are routinely evaluated.
Limitations of this study include limited generalizability to late-stage patients, who typically experience greater pain. Also, because the data were cross sectional, it is not possible to know whether Distressed patients were functioning poorly prior to their diagnosis, or these characteristics emerged during the disease process. Because the medication question did not specify that the medication must be for SSc discomfort, it is possible that acetaminophen/NSAIDs taken for other pain (e.g., headaches) may have been erroneously captured in that response category. It is also important to note that other variables likely relate to SSc pain, and the six indicators selected for the current study are not exhaustive. Rather, the choices were guided by theoretical rationale, as the goal of the current study was not to investigate all potential corollaries of pain, but to evaluate whether biopsychosocial variables could be modeled synergistically. This study was the first to use a person-centered approach to model biopsychosocial traits in relation to pain in SSc. The results suggest that psychosocial functioning is fundamental to understanding pain in this population. Clinicians are encouraged to take a holistic approach in assessments and to make referrals for ancillary pain management services when indicated.
Significance and Innovations.
Researchers and clinicians have rarely considered how medical, psychological, and social traits are synergistically linked with pain in SSc patients.
Although disease severity is a risk factor for increased pain, psychological and social characteristics are important corollaries of pain, particularly in those with less severe disease.
The current findings facilitate better identification of patients who may benefit from referrals to ancillary services for alternative treatments of pain.
Acknowledgments
We thank Scott C. Roesch for statistical guidance.
Grant support: This study was funded by the National Institute of Health (NIH/NIAMS) Center of Research Translation (CORT) in Scleroderma P50AR054144 (Mayes); NIH-KL2RR024149 and K23AR061436 (Assassi); Department of Defense PR1206877 (Mayes).
Footnotes
The Physical Functioning scale score was used instead of the Physical Component score because the Component score includes an indicator of pain.
Participants were also asked about aspirin and muscle relaxers, but these were not included in the pain medication variable given that aspirin is usually taken as an anti-platelet agent, and muscle relaxers (n = 4) are typically taken for fibromyalgia and are not considered pain medication. The 4 individuals taking muscle relaxers were also taking narcotics and were coded as such.
A table of these relationships is available from the study authors upon request.
Note that on the SF-36, lower scores indicate greater pain severity and interference, whereas higher scores indicate less pain severity and interference.
References
- 1.Medsger TA. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29:255–73. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 3.Schieir O, Thombs BD, Hudson M, Boivin JF, Steele R, Bernatsky S, et al. Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care Res. 2010;62:409–17. doi: 10.1002/acr.20108. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson LM, Haythornwaite JA, Heinberg LJ, Boling C, Reed J, White B, et al. The impact of pain and symptoms of depression in scleroderma. Pain. 2002;95:267–75. doi: 10.1016/S0304-3959(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 5.Richards HL, Herrick AL, Griffin K, Gwilliam PD, Fortune DG. Psychological adjustment to systemic sclerosis-exploring the association of disease factors, functional ability, body related attitudes and fear of negative evaluation. Psychol Health Med. 2004;9:29–39. [Google Scholar]
- 6.Edwards RR, Goble L, Kwan A, Kudel I, McGuire L, Heinberg L, et al. Catastrophizing, pain, and social adjustment in scleroderma: Relationships with educational level. Clin J Pain. 2006;22:639–46. doi: 10.1097/01.ajp.0000210918.26159.94. [DOI] [PubMed] [Google Scholar]
- 7.Franck-Larsson K, Graf W, Rönnblom A. Lower gastrointestinal symptoms and quality of life in patients with systemic sclerosis: A population-based study. Eur J Gastroenterol Hepatol. 2009;21:176–82. doi: 10.1097/MEG.0b013e32831dac75. [DOI] [PubMed] [Google Scholar]
- 8.Carreira PE. “Quality of pain” in systemic sclerosis. Rheumatology. 2006;45:1185–6. doi: 10.1093/rheumatology/kel247. [DOI] [PubMed] [Google Scholar]
- 9.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–24. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 10.Georges C, Chassany O, Toledano C, Mouthon L, Tiev K, Meyer O, et al. Impact of pain in health related quality of life of patients with systemic sclerosis. Rheumatology. 2006;45:1298–302. doi: 10.1093/rheumatology/kel189. [DOI] [PubMed] [Google Scholar]
- 11.Malcarne VL, Hansdottir I, McKinney A, Upchurch R, Greenbergs HL, Henstorf GH, et al. Medical signs and symptoms associated with disability, pain, and psychosocial adjustment in systemic sclerosis. J Rheumatol. 2007;34:359–67. [PubMed] [Google Scholar]
- 12.Richards HL, Herrick AL, Griffin K, Gwilliam PD, Loukes J, Fortune DG. Systemic sclerosis: patients’ perceptions of their condition. Arthritis Rheum. 2003;49:689–96. doi: 10.1002/art.11385. [DOI] [PubMed] [Google Scholar]
- 13.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 14.Thombs BD, Taillefer SS, Hudson M, Baron M. Depression in patients with systemic sclerosis: A systematic review of the evidence. Arthritis Rheum. 2007;57:1089–97. doi: 10.1002/art.22910. [DOI] [PubMed] [Google Scholar]
- 15.Legendre C, Allanore Y, Ferrand I, Kahan A. Evaluation of depression and anxiety in patients with systemic sclerosis. Joint Bone Spine. 2005;72:408–11. doi: 10.1016/j.jbspin.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Kwakkenbos L, van Lankveld WG, Vonk MC, Becker ES, van den Hoogen FH, van den Ende CH. Disease-related and psychosocial factors associated with depressive symptoms in patients with systemic sclerosis, including fear of progression and appearance self-esteem. J Psychosom Res. 2012;72:199–204. doi: 10.1016/j.jpsychores.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Hyphantis TN, Tsifetaki N, Pappa C, Voulgari PV, Siafaka V, Bai M, Alamanos Y, Drosos AA, Mavreas V. Clinical features and personality traits associated with psychological distress in systemic sclerosis patients. J Psychosom Res. 2007;62:47–56. doi: 10.1016/j.jpsychores.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55:325–32. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 19.van Lankveld WG, Teunissen H, Näring G, Vonk MC, van den Hoogen FH. Social support, disease-related cognitions and coping as predictors of depressed mood in systemic sclerosis. Cognit Ther Res. 2008;32:434–47. [Google Scholar]
- 20.Savelkoul M, Post MW, de Witte LP, van den Borne HB. Social support, coping, and subjective well-being in patients with rheumatic diseases. Patient Educ Couns. 2000;39:205–18. doi: 10.1016/s0738-3991(99)00033-6. [DOI] [PubMed] [Google Scholar]
- 21.Reveille JD, Fischbach M, McNearney T, Friedman AW, Aguilar MB, Lisse J, et al. Systemic sclerosis in 3 US ethnic groups: A comparison of clinical, sociodemographic, serologic, and immunogenetic determinants. Semin Arthritis Rheum. 2001;30:332–46. doi: 10.1053/sarh.2001.20268. [DOI] [PubMed] [Google Scholar]
- 22.Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clin Exp Rheumatol. 1986;4:367–9. [PubMed] [Google Scholar]
- 23.Furst D, Khanna D, Matucci-Cerinic M, Clements P, Steen V, Pope J, et al. Systemic sclerosis - continuing progress in developing clinical measures of response. J Rheumatol. 2007;34:1194–200. [PubMed] [Google Scholar]
- 24.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 25.Pilowsky I, Spence ND. Patterns of illness behaviour in patients with intractable pain. J Psychosom Res. 1975;19:279–87. doi: 10.1016/0022-3999(75)90026-4. [DOI] [PubMed] [Google Scholar]
- 26.Merz EL, Malcarne VL, Roesch SC, Sharif R, Harper BE, Draeger HT, et al. Measuring illness behavior in patients with systemic sclerosis. Arthritis Care Res. 2013;65:585–93. doi: 10.1002/acr.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the Functional Components of Social Support. In: Sarason I, Sarason B, editors. Social support: theory, research, and applications. Boston, MA: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- 28.Lanza ST, Flaherty BP, Collins LM. Latent class and latent transition analysis. In: Schinka JA, Velicer WA, editors. Handbook of psychology: Research methods in psychology. New York, NY: Wiley; 2003. pp. 663–85. [Google Scholar]
- 29.Little RJ, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- 30.Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: The roles of emotion regulation and inattention. Dev Psychol. 2006;42:913–28. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- 31.Roesch SC, Villodas M, Villodas F. Latent class/profile analysis in maltreatment research: A commentary on Nooner et al., Pears et al., and looking beyond. Child Abuse Negl. 2010;34:155–60. doi: 10.1016/j.chiabu.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Schumacker RE, Lomax RG. A beginner’s guide to structural equation modeling. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2004. [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus Users’ Guide. 6. Los Angeles, CA: Muthén & Muthén; 1998-2010. [Google Scholar]
- 34.Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–78. [Google Scholar]
- 35.McLachlan GJ, Peel D. Finite mixture models. New York, NY: John Wiley; 2000. [Google Scholar]
- 36.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–23. [Google Scholar]
- 37.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–64. [Google Scholar]
- 38.Ramaswamy V, Desarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Market Sci. 1993;12:103–24. [Google Scholar]
- 39.Muthén B. Should substance use disorders be considered as categorical or dimensional? Addiction. 2006;101(Suppl 1):6–16. doi: 10.1111/j.1360-0443.2006.01583.x. [DOI] [PubMed] [Google Scholar]
- 40.Hipp JR, Bauer DJ. Local solutions in the estimation of growth mixture models. Psychol Methods. 2006;11:36–53. doi: 10.1037/1082-989X.11.1.36. [DOI] [PubMed] [Google Scholar]
- 41.Barth J, Schneider S, von Känel R. Lack of social support in the etiology and prognosis of coronary heart disease: A systematic review and meta-analysis. Psychosom Med. 2010;72:229–38. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- 42.Mazzoni D, Cicognani E. Social support and health in patients with systemic lupus erythematosus: A literature review. Lupus. 2011;20:1117–25. doi: 10.1177/0961203311412994. [DOI] [PubMed] [Google Scholar]
- 43.Haythornthwaite JA, Heinberg LJ, McGuire L. Psychologic factors in scleroderma. Rheum Dis Clin North Am. 2003;29:427–39. doi: 10.1016/s0889-857x(03)00020-6. [DOI] [PubMed] [Google Scholar]
- 44.Kool MB, Geenen R. Loneliness in patients with rheumatic diseases: The significance of invalidation and lack of social support. J Psychol. 2012;146:229–41. doi: 10.1080/00223980.2011.606434. [DOI] [PubMed] [Google Scholar]
- 45.Giuggioli D, Manfredi A, Colaci M, Ferri C. Oxycodone in the Long-Term Treatment of Chronic Pain Related to Scleroderma Skin Ulcers. Pain Med. 2010;11:1500–3. doi: 10.1111/j.1526-4637.2010.00849.x. [DOI] [PubMed] [Google Scholar]
- 46.Lundborg CN, Nitescu PV, Appelgren LK, Curelaru ID. Progressive systemic sclerosis: Intrathecal pain management. Reg Anesth Pain Med. 1999;24:89–93. doi: 10.1016/s1098-7339(99)90171-2. [DOI] [PubMed] [Google Scholar]
- 47.Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;24:299–309. doi: 10.1016/j.berh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Kerns RD, Sellinger J, Goodin BR. Psychological Treatment of Chronic Pain. Annu Rev Clin Psychol. 2011;7:411–34. doi: 10.1146/annurev-clinpsy-090310-120430. [DOI] [PubMed] [Google Scholar]


