Abstract
Biomaterials produced by nature have been honed through billions of years, evolving exquisitely precise structure-function relationships that scientists strive to emulate. Advances in genetic engineering have facilitated extensive investigations to determine how changes in even a single peptide within a protein sequence can produce biomaterials with unique thermal, mechanical and biological properties. Elastin, a naturally occurring protein polymer, serves as a model protein to determine the relationship between specific structural elements and desirable material characteristics. The modular, repetitive nature of the protein facilitates the formation of well-defined secondary structures with the ability to self-assemble into complex three-dimensional architectures on a variety of length scales. Furthermore, many opportunities exist to incorporate other protein-based motifs and inorganic materials into recombinant protein-based materials, extending the range and usefulness of these materials in potential biomedical applications. Elastin-like polypeptides can be assembled into 3D architectures with precise control over payload encapsulation, mechanical and thermal properties, as well as unique functionalization opportunities through both genetic and enzymatic means. An overview of current protein-based materials, their properties and uses in biomedicine will be provided, with a focus on the advantages of elastin-like polypeptides. Applications of these biomaterials as imaging and therapeutic delivery agents will be discussed. Finally, broader implications and future directions of these materials as diagnostic and therapeutic systems will be explored.
Keywords: Elastin-like peptides, biomimetic materials, recombinant polypeptides, nanotherapeutics, molecular imaging
1. Introduction
The evolution of biological systems has created a rich landscape of protein-based materials. Proteins possess a variety of genetically encoded structures and properties spanning many length scales, and an elegantly evolved biological system of production. Extensive studies over the last two decades have elucidated precise structure-function relationships for many proteins; an excellent example of this is elastin, a protein that imparts elasticity to a variety of tissues. Identifying the primary amino acid sequence and subsequent secondary structure of elastin has allowed an exquisite level of control over the thermal and mechanical properties of this material [1]. Utilizing nature’s machinery, recombinant polypeptides based on elastin and other proteins can be synthesized with a higher degree of specificity and control than is achievable by chemical methods. As a result, recombinant elastin-like polypeptides (ELPs) can be engineered to possess properties that are highly amenable to applications in biomedical diagnostic imaging and targeted therapeutic delivery [2]. Synthetic polymers, such as poly(caprolactone) (PCL), poly(D-,L-lactic acid) (PLA) and poly(lactic-co-glycolic acid) (PLGA) have been extensively employed [3], but lack the chemical flexibility, biodegradability, and thermal targeting and release mechanisms that can be exhibited by elastin-based materials. Triggered release mechanisms exhibited by poly(oxylalkylene) block copolymers [4] are similar to the lower critical solution temperature (LCST) phenomena seen in elastin-like materials, but lack the chemical diversity possible with elastin. The precise control over structure, general biocompatibility, and ease of functionalization make natural protein-based biomaterials, and elastin-based materials in particular, excellent candidates for biomedical applications.
This review will highlight recent work in the synthesis and purification of protein-based materials, with a focus on elastin-based materials. Methods for designing assembly of proteins into 3D architectures that would be useful for targeted imaging and therapeutic delivery are discussed. Special note is made of recent work to incorporate non-canonical amino acids and other biological motifs, as well as inorganic components, into elastin-like peptides and other biomaterials, extending the range and applicability of these unique biopolymers. Applications in bioimaging and therapeutic delivery are explored, with an emphasis on the use of elastin-like polypeptides in interrogating and treating pathologies such as cancer and cardiovascular disease. Finally, recent advances in imaging systems and targeting ligands will be briefly reviewed, and the potential for protein-based materials to be exploited in these applications will be discussed.
2. Elastin-Like Polypeptides and other Recombinant Proteins
Protein biopolymers have typically been modeled after structural proteins such as silk, collagen, and elastin. Elastin is a well-known extracellular matrix protein that provides elasticity to a variety of tissues such as blood vessels, ligaments, lungs, and skin. Originally derived from tropoelastin [5], elastin is composed of conserved pentapeptide repeat units with the classic form poly(Val-Pro-Gly-Val-Gly) [6]. The conserved peptide sequence found in mammalian elastins has been extensively studied to determine what essential components are useful for specific biomedical applications [7,8]. These polypeptides can interact to form fiber networks and other three-dimensional structures with controlled properties [9–13]. Synthesis of amphiphilic block polypeptide chains enables assembly mechanisms such as coacervation, a lower critical solution temperature (LCST) phenomenon. At an elevated transition temperature (Tt), interactions between hydrophobic domains enables self assembly of higher order structures, primarily determined by the peptide sequence [14,15]. The loss of entropy due to the formation of the ordered structure is counterbalanced by release of ordered water from the peptide backbone, particularly aliphatic residues [16,17]. The self-assembly process can be affected by other environmental factors, such as protein concentration [18], salt concentration, and pH [19–21]; these can be controlled, along with peptide sequence, to produce novel secondary structures and three dimensional architectures for a variety of biomedical applications.
2.1 Synthesis of Recombinant Polypeptides
The genetically encoded synthesis of polypeptide sequences provides a unique level of control over the molecular weight, sequence, and stereochemistry of the polypeptide [22], properties that can be difficult to control in chemical polymers [23]. These factors, combined with tunable mechanical properties, natural biocompatibility, and the established biodegradation profile [24] of peptide-based materials greatly enhances their potential. Many techniques exist for producing the desired amino acid sequence and molecular weight polypeptides for specific applications.
Initial methods for the production of ELPs focused on the simultaneous generation of a library of oligomeric genes by a concatemerization of a monomer gene [25–27]. Although this process is rapid, precise control over the oligomerization process is not maintained, and the yield contains an oligomer population with a statistical distribution of different lengths. While this may be acceptable in screening studies, more precise methods of generating repeat units for polypeptides of a certain chain length have been investigated.
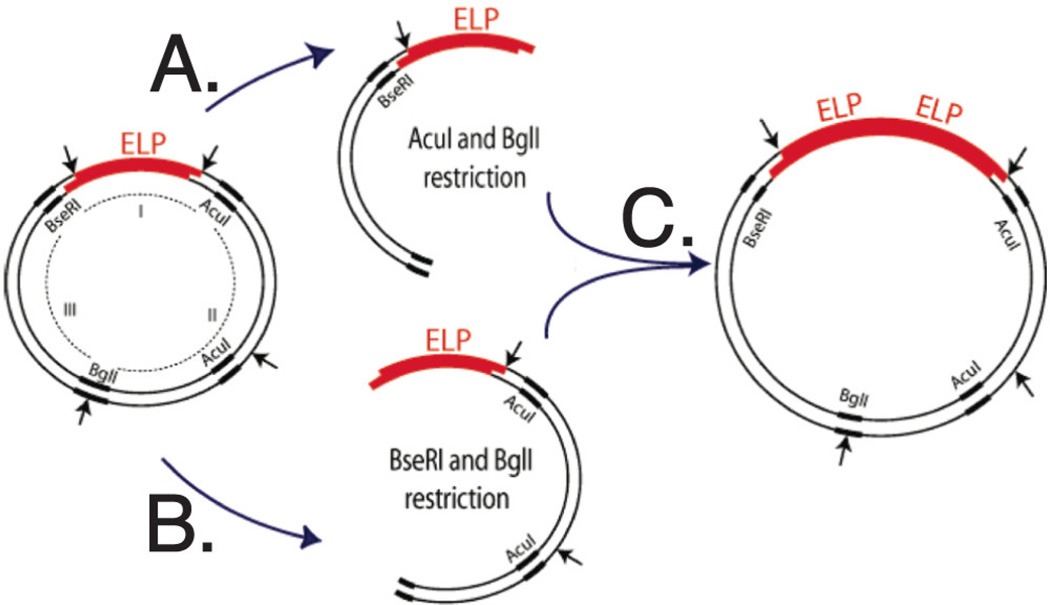
A more controlled method of producing repetitive gene sequences with a specific molecular weight was developed by Meyer et al. [22] and termed ‘recursive directional ligation’ (RDL). This method utilizes stepwise oligomerization with monomer DNA containing distinct recognition sequences at each end, cut by respective restriction endonucleases. This process produces complementary overhangs with no interruption of the repeat sequences; the two complementary ends are cohesive and ligated into a linearized vector cut by one of two restriction endonucleases, resulting in two repeats of monomer DNA in the vector. This procedure is performed recursively to grow the number of repeats of monomer DNA until the desired number of repetitive genes is achieved. However, this method is limited to specific biopolymer sequences, as the endonuclease restriction site overlaps the coding region. Furthermore, significant background can develop from clones lacking an insert due to self-ligation or incomplete digestion of a vector, reducing cloning efficiency. This method was optimized by McDaniel et al. [28] through recursive directional ligation by plasmid reconstruction (PRe-RDL), in which two halves of a parent plasmid are ligated together, resulting in a dimerized oligomer and reconstitution of a functional plasmid (Fig. 1). This method uses type II restriction endonucleases, which are applicable to any arbitrary oligonucleotide sequence, and produces a seamless junction between repeat peptides. A functional plasmid is only produced in the case of successful ligation, which decreases background from self-ligation and increases efficiency by preventing circularization of the insert.
Fig. 1.
Recursive directional ligation by plasmid reconstruction (Pre-RDL). In order to produce peptide oligomers with no extraneous peptides at the junction, two halves of a parent plasmid are ligated together. (A) The ELP-containing fragment is purified from the parent vector after digestion with AcuI and Bgll. (B) The parent ELP is also digested with BseRI and Bgll. (C) The two compatible halves are then reconstituted into the original vector, doubling the length of the insert. Reprinted with permission from [28]. Copyright (2010) American Chemical Society.
Another recently developed method, termed overlap extension rolling circle amplification (OERCA) overcomes some of the limitations of the above techniques. Developed by Amiram et al. [29], this rapid, robust and high-throughput method utilizes circular ssDNA and PCR methods to amplify repetitive sequences from a circular gene template. OERCA produces high yield and high fidelity repetitive gene libraries, ranging from 0.8 – 1.5 kb, with tunable distributions dependent upon the size range of the OERCA products before ligation. Synthesis of extensive gene libraries, has enabled investigation of previously inaccessible non-canonical elastin-like polypeptide polymers. However, the PRe-RDL method is often used to produce products with precise control over the final molecular weight of the ELP.
The completed expression vector is commonly transformed in E. coli, which is typically used for expressing recombinant proteins with tandem repeats due to its deficiency in homologous recombination [30]. For most applications, it is important to produce recombinant elastin-like peptides (ELPs) in large quantities and high purity in a cost effective manner. As ELPs and ELP-fusion proteins have disproportionate amounts of glycine, valine, proline and alanine compared to most cellular proteins, it was found that the synthesis could by optimized through the addition of supplemental amino acids. A higher concentration of proline and alanine during the expression process increased the final protein yield up to 3-fold over controls. This can result in yields of up to ~ 400 mg/L, a considerable improvement over unoptimized systems [31]. However, expression with E. coli systems still suffers from a variety of limitations, including the lack of eukaryotic post-translational systems, insolubility of the over-expressed mammalian proteins and subsequent sequestration into inclusion bodies, difficult purification from cellular contaminants, and endotoxin contamination. Endotoxin has been a specific concern for ELP expression, as it becomes associated with the protein product on cell lysis and is difficult to remove. Recently, yeast and plant [32] expression systems have been explored, with yeast offering the attractive advantage of ease of incorporation into industrial-scale fermentation systems. However, protein yields are often low when compared to E. coli, and Sallach et al. [33] has investigated a novel strategy to construct a gene with enhanced sequence diversity that encodes a highly repetitive elastin-like protein polymer for expression in Pischia pastoris. A modified concatemerization strategy was designed in which seven dissimilar monomer repeat units, encoding identical pentapeptide repeat sequences, served as a monomer library for the concatemerization reaction. This strategy would be used to create large, repetitive genes for a variety of expression systems with the potential to generate glycosylated ELPs.
Purification of ELPs has been investigated through a number of different methods, and affinity chromatography typically allows one-step purification. Though useful in laboratory-scale operations, this can represent a significant cost in scaled-up production of the final protein. Because ELPs undergo a reversible inverse temperature transition, it is possible to exploit this property to purify ELP products and even extend it to other fusion protein purification. It was noted by McPherson et al. [34] that by raising the temperature above the Tt, the protein-based polymer forms a visible aggregate that can be removed from the remaining soluble constituents by centrifugation. Several cycles can be used, in which centrifugation steps cycle above and below the Tt, preserving the ELP-fusion conjugate in each step while reducing endotoxin contamination from E. coli cell wall fragments.
2.2 Effect of Amino Acid Sequence
Genetic engineering techniques allow selection of the polypeptide amino acid sequence, precisely controlling features of the peptide polymer such as hydrophobicity [21,35–37], secondary structures [38,39], and presentation of biorecognizable motifs [40–42]. Extensive investigations into the role of the peptide sequence in the self-assembly of these materials has been carried out [35,43–45], facilitating the development of specific polypeptides for biomedical applications such as drug delivery and bioimaging.
Elastin-like materials are usually composed of pentapeptide repeats of the motif (V1P2G3Xaa4G5)n, where Xaa, the guest residue, is any amino acid other than proline and n indicates the desired number of repeats within the ELP. The peptide sequence has been shown through numerous investigations to directly impact the thermal and mechanical properties of the ELP. In particular, the polarity of Xaa has been shown to regulate the transition temperature of the ELP. Specifically, the addition of a more hydrophobic guest residue lowered the transition temperature, and increasing the polarity of the guest residue resulted in an increased transition temperature. This phenomenon, termed the “ΔTt effect,” scaled with the hydrophobic index of the amino acid at the guest residue position [8,46]. It was found that the formula poly-[fx(VPGXG)fv(VPGVG)] provided a series of polypentapeptides with tunable hydrophobicity, in which fx is the mole fraction of pentamers with a guest residue, Xaa, at position 4 and fv is the mole fraction of pentamers with valyl residues at position 4, with fx+fv=1. Using these parameters, the transition temperature and formation of a viscoelastic coacervate could be modified depending upon desired application.
Though previous explorations of additional structural variations of the ELP motifs often failed to exhibit fully reversible phase transition behavior, recent work by Amiram et al. [29] has investigated the behavior of more complex hexapeptide motifs AVPGVG, VPAGVG, VPGAVG and VPGVAG, among others. It was found that these sequences exhibited fully reversible thermally triggered phase transitions and environmental sensitivity to both solution temperature and solution concentration. This exciting finding indicates the existence of a large and diverse set of motifs capable of exhibiting stimulus responsive behavior, further extending the potential range of responsive polypeptides.
Protein function has been extended beyond the limitations of natural amino acids through the site-specific placement and characterization of non-canonical amino acids into other protein-based biomaterials [47,48]. This has allowed further investigation of protein interactions in vivo [49,50], and has enabled the alteration of global protein properties, including raising the melting temperature of a model collagen peptide by more than 50°C [51]. Additional changes can be made to alter the hydrophobic content of proteins, changing aggregation properties [52,53], or even increasing fluorescence quantum yields and maxima [54,55].
2.3. Secondary and Higher Order Structures
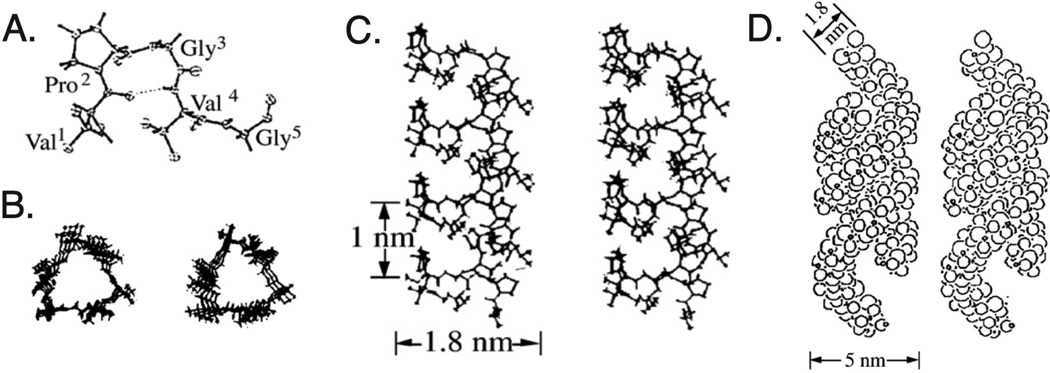
Polypeptide self-assembly into controlled, monodisperse structures is highly desirable for many biomedical applications. Assembly begins with formation of the protein secondary structure, which then leads to further tertiary and quaternary interactions based on both the protein properties and external stimuli. Biological molecules are able to form a variety of different structures with precise control, such as coiled-coils, α-helices, β-sheets, β-spirals, and other higher order structures [56]. These are guided by diverse interactions including electrostatic, hydrophobic, π-stacking, and hydrogen bonding, as well as covalent and steric contributions. The typical secondary structure associated with both elastin and elastin-like polypeptides is the Type II β-turn, a ten-atom hydrogen-bonded ring from the Val1 carboxyl to the Val4 amide (Fig. 2A). Upon an increase in temperature, this structure forms a helical configuration known as a β-spiral, connected through Val-Gly-Val segments, where the β-turns are spacers between the turns of the helix with a pitch of ~ 1 nm (Fig. 2B, C) [57]. These structures are present in both the solution and phase separated states of the ELP, indicating that they do not contribute to the energetics of the inverse transition temperature. Spirals can then assemble into filaments (Fig. 2D) or other 3D assemblies, controlled by the peptide sequence of the ELP.
Fig. 2.
Proposed structure of poly(GVGVP) based on extensive characterization. (A) The pentapeptide sequence Val-Pro-Gly-Val-Gly, with the (B) β-spiral formed though connections between the Val-Gly-Val segments. The side view of the spiral (C) reveals the pitch of the β-turns to be ~ 1 nm. These spirals can then assemble into filaments (D) or other 3D architectures. Adapted with permission from [199]. Copyright (1980) American Chemical Society.
Initial assembly mechanisms of elastin-like polypeptides focused primarily on hydrogels, cross-linked networks analogous to the elastomeric systems used as components of soft tissues and protein fibers [58]. Biological networks are usually post-translationally cross-linked via enzymatic modification, though many chemical strategies also exist for this purpose. The polypeptide sequence determines the position of the crosslinks, which can occur at well-defined intervals along the polypeptide chain [27]. This lends a structural uniformity to natural materials that is difficult to replicate in synthetic hydrogels. These structures are capable of forming lamellar crystallites [59], lyotrophic smetic phases [60] and thermoreversible gels [61] which are used in biomedical applications such as coatings, drug delivery, and scaffolding for tissue engineering [62,63]. The properties of these networks have been reviewed elsewhere [64–66].
Extensive work has been undertaken to understand how ELP primary and secondary structure affects the formation of 3D architectures. Thermally responsive polymeric nanoparticles have been extensively used for the encapsulation and release of small molecules [3], but elastin-like polypeptides present many additional advantages. Copolypeptide nanoparticle micelles were initially observed by Lee et al. [67], through the assembly of lipophilic and hydrophilic blocks. Observation by high resolution scanning electron microscopy and scanning transmission electron microscopy determined the presence of both spherical cylinders and micelles. Further investigations found that by varying the amino acid sequence, it was possible to generate thermally responsive elastin-like polypeptides in a linear AB diblock that would self-assemble into spherical micelles when heated slightly above body temperature. This arrangement was investigated extensively by Dreher et al. [68], in which a series of 10 ELP block copolymers with different molecular weights and hydrophilic-to-hydrophobic block ratios were genetically synthesized through recursive directional ligation. It was found that ELPs with hydrophilic-to-hydrophobic block ratios between 1:2 and 2:1 formed monodisperse spherical micelles, with the critical micelle temperature primarily affected by the length of the lower Tt block. The final structure was very stable, with the size of the micelle controlled by both the total ELP length and the hydrophilic-to-hydrophobic ratio. Sallach et al. [69] investigated protein tri-block structures, with a central hydrophilic block and two hydrophobic end blocks. These were found to form monodisperse micelles in a narrow range of RH ~ 100 nm; however, when the temperature was raised above Tt, a swift protein folding transition between α-helix and β-sheet structures caused an abrupt increase in micelle internal density, with a concomitant reduction in micelle size.
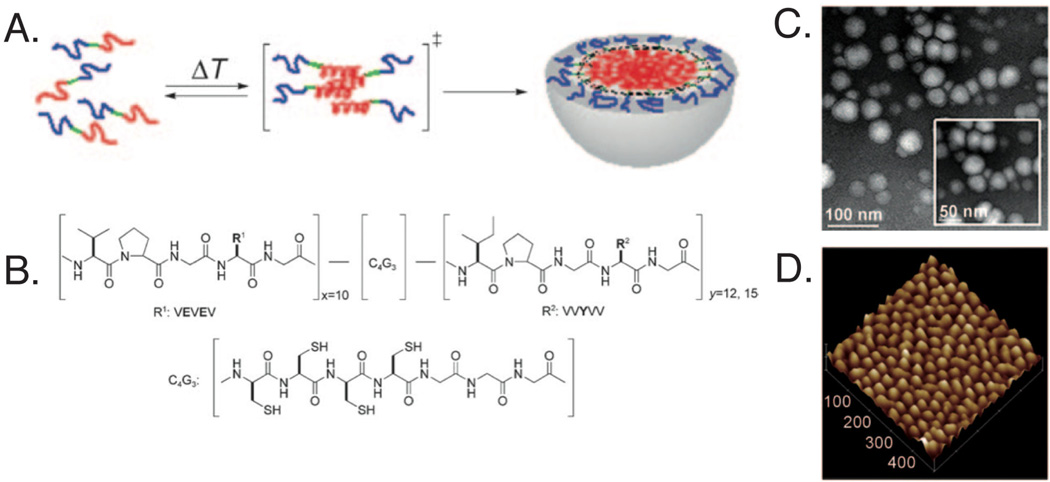
Though micelles present many advantages over synthetic polymers for delivery applications, they can be unstable in the complex biological environment. In order to stabilize elastin-like micellar structures, Kim et al. [70] synthesized amphiphilic diblock polypeptides with consecutive cysteine residues incorporated at the hydrophobic-hydrophilic core-shell interface (Fig. 3). It was found that above the inverse transition temperature of 25°C, well-defined micelles with ~ 20 nm RH were observed through transmission electron microscopy and atomic force microscopy. The formation of the disulfide bond at the amphiphilic interface stabilized the micelles over a range of temperatures and in the presence of bovine serum albumin, and only destabilized when exposed to a thiol-reducing microenvironment.
Fig. 3.
Developed elastin-like peptide for self-assembly into micelles. Upon heating, the (A) hydrophobic blocks (red) assemble into micelles with the hydrophilic blocks (blue) creating an outer shell and the cysteine containing regions (green) at the interface. (B) Chemical structure of synthesized amphiphilic diblock polypeptide, where ADP1 (x10y12) and ADP2 (x10y15). Micelles were characterized using (C) transmission electron microscopy and (D) atomic force microscopy, with ADP1 forming ~ 28 nm spheres with low polydispersity. Reprinted with permission from [70]. Copyright 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Elastin-like polypeptides can also form vesicles simply by changing the block arrangements and lengths [71]. It was found that a triblock ELP formed primarily micellar structures, based on dynamic and static light scattering measurements as well as electron microscopy interrogation. These and other genetically engineered proteins can be manipulated to form rod-like structures and compound micelles [72], electrospun nanofibers [73] and peptide-mineralized proteins utilizing lipid components to control the structure [74,75]. Factors affecting these morphologies included the size of the hydrophobic block, the presence of a purification tag or fusion proteins, and the solvent environment.
3. Synthesis of Hybrid Biomaterials
Though elastin is a convenient biomaterial platform, other natural structural proteins and inorganic materials display a wide variety of properties that are highly desirable for biomedical applications [76]. Nature has developed multifunctional composite materials, such as collagen-elastin matrices, that can provide a combination of strength and function required for specific tissues [77,78]. Genetic and protein engineering provide new tools for producing macromolecular polyamide copolymers with diversity and precision beyond the current capabilities of synthetic polymer chemistry. Proteins can be further modified to incorporate inorganic materials through strategies such as metal chelating protein motifs or direct chemical modification. These benefits allow the creation of truly engineered biomaterials that incorporate the both organic and inorganic components.
3.1 Protein Hybrid Materials
Silk-like polymers (SLPs) and silk-elastin-like polymers (SELPs) have been investigated for a number of biomedical applications, based on their ease of synthesis and high uniformity [79]. SLPs are based on repetitions of the silk fibroin amino acid motif GAGAGS and the mammalian elastin conserved motif VPGVG [80]. The proportions, number, and sequence of the repeated motifs govern the final properties of the polymer, with silk-like blocks imparting thermal and chemical stability through hydrogen-bonded β-sheet crystals. These biopolymers have been utilized in several biomedical applications, including controlled drug delivery [81,82] and gene therapy [83]. They can also be spun into microdiameter fibers that can withstand strains of up to 700%, and possess a tensile mechanical strength of up to 20 MPa [84]. Synthesis techniques have been developed to prepare silk nanospheres and microspheres for drug delivery [85,86]. Silk-like protein polymers typically exhibit extremely low solubility [87], which can frustrate their application in biomedicine; however, this can be alleviated through selfassembly or phosphorylation to inhibit hydrophobic interactions [88].
Collagen is another ubiquitous protein occurring primarily in the extracellular matrix. It mainly consists of fibrils formed through a primary structure of tandem (Gly-Xaa-Yaa) repeats, where glycine must be in every third position and arranged such that the Gly can form a hydrogen bond to an adjacent Pro, stabilizing the collagen structure [89]. The hydroxyproline residues at the Yaa position play an important role in the thermal stability of the collagen triple helix conformation [90]. The nanometer-sized triple helices self assemble to form higher-level supramolecular structures. Though it is difficult to produce a stable triple helix, synthetic collagen-like peptides derived from chemical synthesis have been investigated for their potential application as biomaterials, particularly in the areas of elastin-collagen based scaffolding [78,91], tissue engineering [92,93], and delivery [94]. The range of material properties of these hybrid materials has been extended through the synthesis of a resilin-elastin-collagen-like polypeptide (REC) [95]. The recombinant material incorporates both collagen and elastin as well as resilin, an elastomeric protein with is noted for high resilience and very high fatigue lifetime [96]. It was found that the hybrid polypeptide formed amyloid-like aligned fibers with an estimated Young’s modulus of 0.1–3 MPa.
Another potential area of interest is the conjugation of polypeptides with synthetic polymers. This has been accomplished with elastin by producing ABA triblock copolymers composed of PEG (segment B) and methacrylate-functionalized VPGVG (segment A) [97]. This work has been extended to the formation of PEGylated ELP micelles [98] and silk-based ordered β-sheets [99] for extended control over self-assembly and processing of the resulting copolypeptides.
3.2 Organic-Inorganic Hybrid Materials
Peptide materials can be utilized to direct the synthesis and self-assembly of inorganic materials. Extensive design rules have been developed, starting with the phage and cell surface display selection of peptide sequences that are capable of complexing with metallic ions [100–102]. Many of these peptides are capable of reducing metal ions and even templating crystal growth of metallic nanoparticles. Tan et al. [103] has developed a series of design rules to template nanoparticle growth, by a bottom-up approach of determining the reduction and binding abilities of the 20 natural α-amino acids, as well as rationally selected peptide sequences.
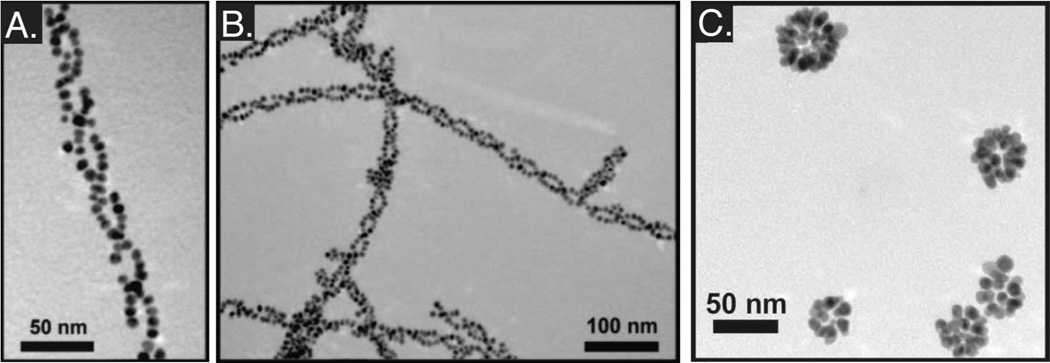
Combining these ideas and extensive knowledge of peptide assembly, Rosi et al. [104] has synthesized topographically complex assemblies of peptide-inorganic structures. Initial reports showed the development of complex helical structures mediated by the assembly of a peptide AYSSGAPPMPPF (PEPAu). This sequence was isolated via phage display and identified as possessing a high affinity for gold surfaces [105]. PEPAu assisted the formation of monodisperse spherical gold nanoparticles through both binding to tyrosine residues and mild reduction in HEPES buffer, with the [AYSS] residues facilitating the formation of β-sheet assemblies [106]. The addition of a small aliphatic carbon chain to generate [C11H23CO]-PEPAu allowed the formation of twisted extended nanoribbons with micrometer lengths (>4 µm) and ~ 6 nm widths, with AuNP decorating the outer surfaces of the ribbons. (Fig. 4A, 4B)
Fig. 4.
Synthesis and assembly of topographically complex hybrid materials. (A) Highly ordered double helices are formed using an organic molecule, PEPAu with an aliphatic carbon tail on the N-terminus. (B) By adding chloroauric acid (HAuCl4) to the solution, additional structures were formed in a precipitate. This work has been extended to spherical superstructures (C), including hollow spherical gold nanoparticles templated with structurally modified PEPAu. (A, B) Reprinted with permission from [108]. Copyright 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) Reprinted with permission from [104]. Copyright (2008) American Chemical Society.
These studies have been extended to the formation of spherical nanoparticle assemblies [107]. Changes to the length of the aliphatic chain attached to the PEPAu peptide, along with the inclusion of an alanine residue [C6-AA-PEPAu], resulted in the formation of hollow spherical nanoparticles ~140 nm in diameter upon the addition of HAuCl4 in HEPES buffer. Interestingly, it was hypothesized that the AuNP assisted in the assembly of the superstructures by facilitating the aggregation of the C6-AA-PEPAu peptide, indicating that the nanoparticle formation and self-assembly process may be coupled. Further adaptation of the peptide used in these studies, particularly the inclusion of a biphenyl group [BP-Ax-PEPAu], resulted in some control over assembly size, with resulting superstructures ranging from ~ 60–270 nm [108] (Fig. 4C).
It is intriguing to examine how the material properties of inorganic nanoparticles, gold nanoparticles in particular, can assist and complement the material properties of elastin-like polypeptides. Many colorimetric studies have used AuNP to assay the aggregation process of ELP upon increase in solution temperature, a completely reversible process [109]. Incorporation of gold nanorods into thermo responsive elastin-like polypeptide gels has also been investigated by Huang et al. [110] These nanoassemblies demonstrate a reversible optical response following exposure to NIR light. Incoming photons are converted to heat energy through absorption by the AuNR surface plasmon, resulting in localized heating above the polypeptide Tt and self-assembly of the ELP [110]. Studies such as these demonstrate opportunities for organic and inorganic materials with complementary properties to facilitate additional control over mechanisms of drug delivery, release profiles, and increased imaging contrast.
4. Targeting Strategies
Though there have been many advances in therapeutics for pathologies such as cancer and ischemic heart disease, many challenges remain. For example, though there are many kinds of anti-ischemic drugs, including calcium channel blockers and organic nitrates, antiplatelet and thrombolytic medications, many lack tissue specificity and have relatively short elimination half lives. Significantly reduced blood circulation in ischemic tissues results in low drug distribution in the desired areas, while relatively high systemic concentrations can cause adverse affects elsewhere. Biomaterials for drug delivery must enable the enhanced accumulation of therapeutics in targeted areas. This can be accomplished through a passive targeting system known as the ‘enhanced permeability and retention’ (EPR) effect, in which drugs and particles are accumulated in the target site due simply to changes in physiology associated with the pathology. Additional methods utilize a therapeutic or carrier is labeled with a targeting ligand, which over time enhances the local concentration of the drug through targeted biological interactions at the site and decreases the necessary dose.
4.1 Enhanced Permeability and Retention Effect
One of the earliest investigated drug delivery strategies is the enhanced permeability and retention (EPR) effect, a well known mechanism that describes the propensity of macromolecules and small particles to accumulate in regions surrounding tumor tissues [111]. If the half-life of a therapeutic can be extended, it has been shown that regions with greater vascular permeability and impaired filtration will contain an increased localized concentration of the therapeutic. PEGylation of therapeutics is considered the method of choice for improving the pharmacokinetics and stability of parenteral agents; however, encapsulation provides a protective environment and can improve the half-life and subsequent accumulation of the therapeutic in the desired area. For example, it was shown that (PEG-PE) nanoparticles (7–20 nm) were capable of accumulating in sites of myocardial infarction, similar to results seen in tumor necrotic areas [112].
EPR methods have been shown to act as a viable mode of imaging areas of vascular wall injury induced by angioplasty. It was found that elastin-like polypeptides conjugated with fluorescent dyes via an N-hydroxysuccinimide (NHS) ester linker generated nanoparticles with enhanced fluorescent intensity, which could then be tracked via fluorescent imaging [113]. It is also possible to exploit the thermal properties of ELP to enhance their accumulation in sites of injury. Fluorescently tagged ELP conjugates were injected and mild hyperthermia was applied to the tumor site, which was found to increase ELP concentration in tumor vasculature [114]. On cessation of heating, it was found that the ELP aggregates dispersed, demonstrating reversible accumulation of temperature-triggered aggregates in vivo. This method is assisted by the EPR effect and enhanced permeability of the heated tumor vasculature; however, thermal cycling was shown to increase the concentration ELP over the EPR effect alone, demonstrating the advantages of the combining the two approaches.
4.2 Targeting with Fusion Elastin-like Polypeptides
Elastin-like peptides offer additional opportunities to genetically encode targeting ligands directly onto the self-assembled nanoparticle surface. This type of targeting mechanism can enhance the accumulation of a delivery agent through specific interactions with biomarkers that are overexpressed in injured tissue as compared to healthy tissue. The natural assembly mechanisms of ELPs can be exploited to further enhance uptake into targeted regions. It has been shown that the incorporation of short peptide sequences (≤ 10 amino acids) can easily be tolerated at the hydrophilic end of elastin-like peptide block copolypeptides without disrupting the self-assembly of hydrophilic micelles [68]. Larger protein assemblies (>10 kDa) have also been incorporated into the ELP sequence for assembly into a micellar structure, facilitating active targeting and uptake.
4.2.1 Cell Penetrating Peptide Fusion Strategies
A class of molecules termed ‘cell penetrating peptides’ (CPP) are capable of facilitating internalization of a wide variety of cargo to diverse cell types, as unique cellular features are not required to enable cellular uptake [115,116]. A variety of CPPs have been identified from outside agents, such as HIV’s trans-activator of transcription (TAT) protein [117], penetratin from drosophila [118], and bactenecin from bovine neutrophils [119]. Initial study of the fusion of CPPs to unimer ELPs indicated that increased interaction with the cell membrane allowed greater internalization of the ELPs over time, though the rate of penetration was not dramatically affected. The non-specific nature of uptake appeared to vary with the CPP analyzed, though it occurred primarily through endocytotic mechanisms [120].
Current screening methods have also allowed identification of oligomers such as octa-arginine and other synthetic derivatives that allow cellular interaction and translocation across the cell membrane [41]. Instead, CPPs achieve internalization through a variety of mechanisms, most commonly macropinocytosis. Uptake is controlled through electrostatic interactions and hydrogen bonding between the peptide and the phospholipid and proteoglycan components of the cell membrane [121]. Studies of the uptake of arginine-rich CPP indicate that the charge density of the peptides may trigger actin organization on the cell membrane and subsequent macropinocytosis [122]. The opportunity to create controlled multivalent structures using the thermally activated assembly of ELP micelles was elegantly demonstrated by MacEwan et al. [123] to create an external trigger for localization and delivery. Oligoarginine CPPs are promiscuous and robust ligands that can efficiently delivery a wide variety of cargo to many different cell types; however, the lack of cellular specificity can be problematic. It has been demonstrated that oligoarginine demonstrates a strong cut-off effect: fewer than six consecutive arginines (Arg) in the CPP results in dramatically decreased cellular uptake. Exploiting the thermal assembly properties of ELPs, five Arg – below the necessary threshold for cellular uptake – were recombinantly incorporated into ELPBC. When the targeted region area was heated, there was a greater than 8-fold increase in cellular uptake over controls. This was attributed to the increased surface density of the Arg residues upon assembly of the ELPBC into micellar structures.
4.2.2 Targeted Peptide and Protein Fusion Strategies
Incorporation of short peptides was demonstrated by Dreher et al. [68], with an RGD and NGR tripeptide, both known for targeting of angiogenic tumor vasculature. Formation of the multivalent micelles at clinically relevant temperatures (37–42°C) was theorized to allow enhancement of the nanoparticle avidity 103–108 fold over the monovalent state of the ELPBC. This work was expanded by Simnick et al. [124] with a GRGDS peptide incorporated onto the N-terminus of the linear unimer elastin-like peptide block copolymer (ELPBC), which could then be incorporated into micelles at different concentrations of ELPBC to create thermally triggered micelles with different ligand densities. This property was exploited to create a dynamic affinity modulation (DAM) targeting system, in which the ELPBC morphs from a low affinity unimer form to a high-avidity micellar state in response to an external stimulus, such as heat. Once ELPBC assembled into multivalent micelles in targeted areas, it was found that increased cellular interaction was observed over the GRGDS-ELPBC peptide in its monovalent state. This work was further extended to incorporation of an NGR tripeptide ligand targeting the CD13 receptor, which is up-regulated in tumor vasculature and perivascular cells [125].
Antibody-based targeting strategies have been demonstrated in the use of ELPs in antithrombotic therapies. Topcic et al. [126] has shown that an activation-specific glycoprotein (GP) IIb/IIIa blocking single chain antibody can successfully be fused to an elastin-like peptide (ELP-scFv). The β-spiral of the polypeptide created steric hindrance that prevented the action of the scFv at temperatures above 37°C. However, at hypothermic body temperatures (≤ 32°C), the linearized form of the ELP-scFV bound to activated platelets, demonstrating a novel technique to produce hypothermia-induced antiplatelet therapy.
Recognizing the value of incorporating targeting proteins into the corona of the ELPBC, it was shown possible to incorporate even larger intact fusion proteins (95–110 residues). Two model proteins, thioredoxin (Trx) and fibronectin type III domain (Fn3), were genetically encoded onto the ELPBC construct, abrogating the need for additional covalent attachment chemistry [42]. These protein-ELPBC micelles showed enhanced receptor-mediated cell uptake compared to that of the protein-ELPBC in its unimer state and ligand-negative states. Additional systems have been developed utilizing the knob domain of adenovirus fiber protein, which exhibits selective internalization into tissues expressing coxsackievirus and adenovirus receptor (CAR) -dependent endocytosis. Knob proteins fused with ELP formed nanoparticles at a slightly lower critical temperature than the ELP alone [127], a phenomenon that has been reported for fusion proteins with a significant number of hydrophobic amino acids. The knob-ELP fusion protein exhibited greater internalization and proof-of-principal that larger fusion proteins can be assembled by diblock ELPs without the need for significant bioconjugate chemistry. This system shows promise for further exploitation of targeting proteins (such as engineered antibody domains, antibody mimics, and larger protein domains) fused to monodisperse and biocompatible ELP micelles.
5. Applications in Imaging
The biocompatibility and targeting capabilities of ELP-based nanomaterials makes them attractive as contrast agents in developing theranostic systems. For example, fluorescent imaging yields excellent spatial and temporal resolution both in vitro and in vivo, using appropriate fluorophores, optics, and photon sensors. Though this technique is limited by the attenuation of fluorescent light by tissue, it has been successfully adopted for intraoperative fluorescent imaging systems, fluorescent endoscopes [128], intravascular fluorescent catheters [129], and in vivo confocal microscopy. Current clinical usage of fluorescent dyes is limited to indocyanine green (ICG) and methylene blue (MB) [130]. ICG was first synthesized in the 1950s, and binds tightly to plasma proteins, typically confining its use to intraoperative assessment of vascular flow in cardiovascular surgery [131]. These fluorescent probes and others under development are typically untargeted and hydrophobic, which presents challenges including poor aqueous solubility, nonspecific uptake in normal tissues and organs, and exclusion by the blood-nerve-barrier and the blood-brain-barrier. Many of these issues could be addressed by conjugation or encapsulation of the fluorophore within a targeted nanoparticle.
Current work by Kim et al. [113] has demonstrated the potential for elastin-based amphiphilic diblock polypeptides (ADP) as a platform for fluorescent imaging. ADP micelles were functionalized with fluorescent dyes via an N-hydroxysuccinimide (NHS) ester linkage, generating ELP nanoparticles with enhanced in vivo fluorescent intensity (Fig. 5). This platform was used to characterize vascular wall injury in a rat aortic balloon injury model via an EPR effect, and demonstrated the feasibility of multivalent attachment of fluorophores to the EMP micelle with minimal fluorescent quenching. This area of investigation indicates the promise of ELPs for encapsulation and delivery of a variety of fluorescent and NIR dyes [132], capitalizing on the ELPs biocompatibility.
Fig. 5.
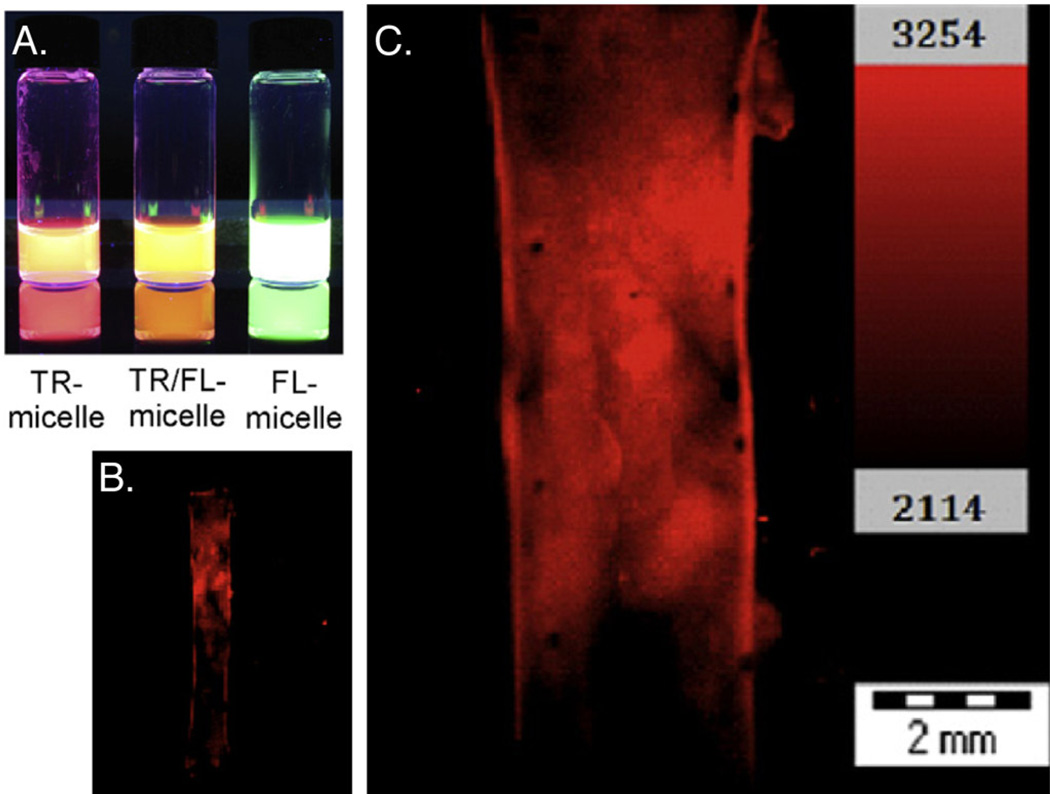
Development of fluorescent-dye labeled micelles for in vivo imaging. (A) UV illumination of aqueous dispersions of Texas Red (TR)-micelle, Texas Red/fluorescein micelle, and a fluorescein-micelle. (B) It was found that in the rat aortic balloon injury model, TR-micelles accumulated only in the injury zone (defined by Evans Blue), with (C) significant penetration correlated to areas of greater injury (6× magnification). Reprinted from [113]. Copyright (2012), with permission from Elsevier.
As demonstrated by Rosi et al. [133], the incorporation of inorganic particles into ELP nanoparticles is entirely feasible and this work has been extended to other organic nanoparticulates, such as liposomes [134]. Metallic [135] and semiconductor nanoparticles [136] possess qualities that are highly desirable in fluorescent probes, including increased brightness, greater photostability, narrow and symmetric emission spectra, and a wide range of tunability. Quantum dots (QDs) exhibit excellent characteristics for fluorescent studies [137]; however, they have also been shown to exhibit cytotoxicity over longer term and extreme conditions, generating concern about their use [136]. Development of a new class of QDs using less toxic elements is underway [138,139]. Several studies have indicated that recombinant proteins can be engineered to improve the solubility and direct self-assembly in solution. Mattoussi et al. [140] demonstrated that a recombinant maltose binding protein (MBP) with a highly positively charged leucine zipper successfully binds QDs through electrostatic interactions. This allowed the formation of self-assembled QDs while maintaining the functionality of the MBP, presenting a strategy for targeting strong fluorescent agents.
Molecular imaging is a powerful tool for characterizing biological processes at the cellular and sub-cellular level, as well as non-invasive diagnostic imaging of pathologies. Imaging techniques that can capture high levels of detail and spatial resolution have been reviewed elsewhere [141–144], yet have in common the requirements of strong signal generation, low attenuation, and precise targeting to regions and markers of interest. Positron emission tomography (PET) is a quantitative technique used to track molecular events with appropriate radionucleotides, and can be combined with other techniques, such as computed tomography, to increase the resolution of the image. However, many PET contrast agents are low molecular weight compounds with rapid clearance profiles, which limits clinical applications. Janib et al. [145] demonstrated that the metallic radionuclide 64Cu could be conjugated to a suitable chelating agent, AmBaSar [146], and attached to the N-terminus of an ELP block copolymer for thermally triggered assembly. Using microPET, it was possible to track the biodistribution and pharmacokinetics of four different ELP constructs over several days. The predominant finding from the study was that the lowest molecular weight ELP, was cleared via the kidneys while the higher molecular weight ELPs exhibited improved circulation half-life. This tracking facilitated the development of a model for better understanding the biodistribution and pharmacokinetics of ELP nanoparticles that accumulated through the EPR effect, and could be widely applicable to other targeted systems.
6. Applications in Therapeutic Delivery
Recombinant protein-based materials have been extensively developed for applications in drug delivery over the past decade. Elastin-like peptides in particular are versatile biopolymers with many routes of accumulation, and the added benefit of precise control over ELP architecture. Ideal drug carriers increase the therapeutic index of the drug by targeting the site of injury, thereby decreasing the necessary dosage and nonspecific interactions with healthy tissues. ELPs retain the biologically friendly properties of polymeric drug delivery systems, such as extending the circulation half-life of the drug by increasing its apparent molecular weight, consequentially decreasing the clearance rate and nonspecific absorption into system tissues. Furthermore, the precise nature of the genetic engineering techniques used to produce ELP micelles allows control over polypeptide parameters such as molecular weight and composition, which can further impact tissue distribution and sub-cellular uptake. In particular, amphiphilic diblock copolypeptides, with spatially separated hydrophobic and hydrophilic segments, can self assemble into micelles with a hydrophilic corona and a hydrophobic core. This is an attractive modality for the encapsulation of small, hydrophobic therapeutics that would otherwise not be soluble in the biological environment. The hydrophilic corona can prevent uptake by the reticuloendothelial system, and the size can be tuned for targeting via the EPR effect or conjugated targeting ligands.
6.1 Delivery of Chemical Small Molecule Therapeutics
There are many strategies to drive therapeutic encapsulation within a polypeptide micelle. Chemotherapeutics can be conjugated to the C-terminus of a hydrophilic ELP, where the amphiphilic nature of the conjugate drives self-assembly into nanoparticles. Early investigations to optimize the design of ELP-small molecule conjugates focused on synthesizing conjugation chemistries and linkers that would minimize perturbation of the transition behavior of the ELP while providing efficient, quantitative release of the therapeutic [147,148]. It was demonstrated that short, aliphatic spacers minimize changes to ELP transition temperatures and biophysical characteristics, and the effect of the linker on the drug release profile is modest. Additional studies to create macromolecular drug carriers focused on controlling circulation and the drug release profile. For example, ELP [VA8G7]-160 was shown to solubilize conjugated molecules in aqueous plasma, display favorable pharmacokinetics, and posses an extended half-life in vivo [149]. Addition of a short peptide consisting of Cys-(Gly-Gly-Cys)7 to the C-terminus of the ELP provided multiple, unique sites for drug attachment. Doxorubicin (Dox), chosen for its promise as a chemotherapeutic agent with dose-limiting side effects, was conjugated to n-β-maleimidopropionic acid hydrazide trifluoroacetic acid, an acid-labile hydrazine based linker containing a terminal maleimide, and then conjugated to the ELP via a maleimide-thiol reaction. It was found that the ELP-conjugated Dox showed a 3.5-fold increase in tumor concentration after 24 hours over controls, and a 2.6-fold decrease in heart concentration (Fig. 6) [150]. Decreased cardiac exposure is critical for clinical use, as Dox suffers from dose-limiting cardiomyopathy.
Fig. 6.
Use of self-assembling chimeric polypeptide-doxorubicin (CP-Dox nanoparticles for tumor therapy. (A) Concentration of free Dox and CP-Dox in the tumor at 2 and 24 hours after systemic injection. (B) Concentration of Dox and CP-Dox in heart tissues at 2 and 24 hours. (C) Tumor volume after administration of Dox and CP-Dox 15 days after implantation. A substantial increase in animal survival was correlated with administration of CP-Dox. Laser scanning confocal microscopy images of C26 cells (blue) showing cellular uptake of CP-Dox (red) at (D) 5 min, (E) 30 min and (F) 24 hours. Scale bar: 20 µM. Reprinted by permission from Macmillan Publishers Ltd: Nature Materials [150], copyright 2009.
Several ELP formulations have been developed specifically for drug delivery applications. A novel ELP explored the use of self-assembled poly(VPAVG) micro- and nanoparticles as a vehicle for the controlled release of the model drug dexamethasone phosphate (DMP) [151,152]. Significantly, poly(VPAVG) forms stable particles in water or phosphate buffer saline when warmed above its transition temperature of ~30°C, but exhibits asymmetric hysteresis behavior. The particles do not re-dissolve until a strong undercooling of 12–15°C is achieved, resulting in particles that are stable either at room or body temperature. Further investigation found that the presence of the alanine in the 3rd position contains less water bound to the carbonyl of amide groups than typical poly(VPGVG), resulting in a more rigid structure and the formation of a more stable particle suspension [153]. It was found that poly(VPAVG) efficiently encapsulated DMP, though the kinetics of drug release showed a burst-release profile and required further improvement.
In order to improve the targeted delivery of therapeutics such as Dox, an ELP formulation was developed with the cell penetrating peptide SynB1 on the N-terminus of the ELP by Moktan et al. [154]. The cell penetrating, thermally responsive fusion protein was conjugated to a thiol-reactive doxorubicin prodrug, through attachment to cysteine resides at the C-terminus of the ELP. The acid-sensitive hydrazone linker ensured effective cleavage of the drug in the acidic tumor microenvironments or after uptake into the acidic compartments of the endosomes/lysosomes within targeted cells. This strategy resulted in improved pharmacokinetics, enhanced tumor uptake and an increase in the maximum tolerated dose (MTD) over free Dox. Thermal targeting resulted in complete tumor growth inhibition and increased therapeutic benefit, demonstrating the potential advantages of fusion protein-ELP systems. This system was extended to test the efficacy of other chemotherapeutics, such as paclitaxel. Although it is a potent anti-tumor therapeutic, paclitaxel is practically insoluble and is generally administered in a formulation containing Cremophor EL® and ethanol, which can have undesirable side effects [155]. In vitro studies [156] indicated that conjugation of paclitaxel to the CPP-ELP delivery system did increase the solubility and internalization of the drug, though the overall potency of the conjugate was reduced when compared to free paclitaxel; this may be explained by the different modes of cellular entry adopted by the two formulations [157].
Amphiphilic block copolypeptides (ADP) have also been shown as effective drug delivery agents for dipyridamole (DIP) as a model hydrophobic, anti-inflammatory compound. The ADP, synthesized by Kim et al. [158], consisted of a hydrophilic block of 10 pentapeptide repeats and a hydrophobic block of 15 repeat units, which contain 20 glutamic acids ([Glu2])10 and 15 tyrosine residues ([Tyr]15), respectively. Above the transition temperature (Tt ≈ 10°C), the diblock polypeptide self-assembles into micellar nanoparticles with disulfide cross-links at the hydrophobic core-hydrophilic shell interface. It was found that the micelles were stable in solution at room temperature under constant agitation for several weeks, as assessed by DLS. Though DIP is known to have poor solubility, upon addition of the ADP followed micellarization at 25°C, the precipitate disappeared to yield a translucent yellow solution, consistent with solubilized DIP. The average diameters of DIP-loaded micelles were 57 and 55 nm at 25 and 37°C respectively, and displayed uniform morphology as assessed by TEM. Drug encapsulation was monitored via UV spectroscopy, and it was found that drug loading was approximately 11–12% w/w, corresponding to a 60- to 70-fold increase in drug solubility. Drug release studies conducted by determining the amount of DIP remaining within the micelles indicated that the drug release was most rapid during the first 8 hours and slowed over time, indicating the remaining compound was stably associated within protein micelles. Additional studies were conducted with the DIP-loaded micelles under dialysis to characterize release rates under a variety of conditions. It was found that the addition of bovine serum albumin and dithiothreitol, which cleaves the disulfide cross-links increased the DIP release rate. It was also found that protein micelles with a longer hydrophobic block release drugs more slowly than micelles with shorter hydrophobic blocks, potentially due to a more densely packed micelle core that increases hydrophobic interactions with the DIP. Initial in vivo studies indicated that the DIP-micelles significantly reduced the total cell number and neutrophil infiltration in a zymosaninduced murine model of peritonitis when compared to controls.
Elastin-like polypeptides have also been evaluated as a drug delivery system for localized cancer radiotherapy. In this system [159], a thermally sensitive ELP was synthesized and radiolabeled with either 125I or 131I, as well as fluorescent labels. Upon injection, thermally activable ELP conjugates were found to accumulate more significantly in region of the tumor, due to enhanced accumulation of the ELP aggregates. This work has also been extended to liposomes [160], which exhibit thermally triggered intravascular release.
6.2 Delivery of Therapeutic Peptides and Proteins
In addition to encapsulating hydrophobic drugs, elastin-like polymers are also capable of acting as delivery agents for therapeutic peptide and protein delivery. Therapeutic peptides can modulate important protein/protein interactions and elicit a therapeutic response with great specificity. However, poor stability and inability to penetrate biological membranes limits their potential applications. ELP delivery systems present an opportunity to overcome these limitations by stabilizing the peptide in circulation, targeting the peptide to the desired site and facilitating penetration and intracellular uptake. For example, peptide therapeutics can be potent inhibitors of the cell cycle, making them attractive for use in cancer therapy. Several cell cycle inhibitors [161,162] and proapoptic agents [163,164] have been identified and incorporated into ELP delivery platforms. Initial studies used Bac-7, a transduction domain from the bactenecin family of antimicrobial peptides, to deliver ELP modified at its C-terminus with a derivative of the p21 peptide, which has been shown to interfere with PCNA function and inhibit cyclin-dependent kinase activity [165]. The conjugate was shown to localize in the nucleus of SKOV-3 cells, where it arrests the cell cycle, induces caspase activation and inhibits Rb phosphorylation. These effects were enhanced through the application of hyperthermia, indicating thermal cycling of the targeted region was an effective way to increase intracellular uptake. Similar systems have been developed to deliver other peptide therapeutics, such as GRG motifs to induce cell cycle arrest through the accumulation of cyclin dependent kinase inhibitors [166]; the helix 1 motif from of c-My, which controls cells growth, proliferation, apoptosis and tumorigenesis [167–170]; the S100B inhibitory peptide, which inhibits oxidative stress-mediated injury to neurons involved in Alzheimer’s disease [171]; and the cationic α-helical KLAKLAKKLAKLAK peptide, which induces apoptosis through mitochondrial membrane disruption [172].
In addition to delivery of therapeutic peptides, ELP have also been used to deliver therapeutic proteins. Poly(VPAVG) was used to form micelles encapsulating bone morphogenetic proteins (BMP), therapeutic proteins that have demonstrated a successful regenerative potential in the areas of spinal disk regeneration, healing of long bone fractures, and periodontal repair [173]. The uptake of both BMP-2 and BMP-14 was investigated, as well as the release profile and biological activity of the growth factors in C2C12 cells [151]. The particles exhibited a high encapsulation efficiency of BMP-2 and BMP-14 (94.5% and 99.2% per total amount of growth factor added during preparation, 20 µg/mL). It was found that both growth factors exhibited a burst release of approximately 20% in the first 24 hours, with additional sustained release of approximately 30% of the growth factor released over the next two weeks. Delivery of therapeutic growth factors has been extended to keratinocyte growth factor (KGF) for applications in wound healing. In many cases, chronic wounds associated with ulcerations, pressure sores, and diabetic foot ulcers are treated with topically delivered KGF, which enhances reepithelialization, accelerated wound closure and reduced inflammation [174–176]. However, in order for keratinocyte treatments to be effective, repeated applications are required. Koria et al. [177] have fabricated a fusion protein composed of recombinant human KGF and ELPs, which forms into a nanoparticulate carrier that can penetrate into the wound. These particles were applied to full-thickness wounds in genetically diabetic mice and improved wound healing by enhancing reepithlialization (2-fold) and granulation (3-fold) when compared to controls.
6.3 Delivery of Genetic Material
There are many obstacles to the delivery, internalization, and translation of genetic material. The extracellular environment contains many biomolecules designed to trap and degrade DNA, and upon entry to the cell, the enzymatic environment promotes degradation. Many delivery systems are unable to deliver sufficient amounts of intact coding sequence entering the nucleus. Viral vectors are usually favored for delivery of therapeutic genes; however, there are concerns related to the immunogenicity and safety of viruses.
There have been numerous strategies incorporating viruses into biomaterials to overcome delivery inefficiencies, nonspecific delivery, and immunogenesis [178]. Recombinant silk-elastin like protein polymers (SELPs) combine silk-like and elastin-like blocks in various ratios in order to control solubility, gelation, stimuli-sensitivity, material strength, biorecognition, and biodegradation. These copolymers undergo an irreversible sol-to-gel transition at body temperature, forming robust hydrogels that can act as an excellent scaffold for the encapsulation and release of adenovirus materials. Initial studies investigated the release of plasmid DNA and adenoviral particles post injection in xenograft tumors. Gene delivery and transfection efficiency can by influenced through a variety of parameters, including shear modulus, gel formation, degree of swelling, and sensitivity to environmental stimuli such as pH, temperature, and ionic strength [179,180]. Studies with optimized SELPs ladened with Ad.GFP indicated that the hydrogel system could release encapsulated adenovirus particles over a period of four weeks, with persistent GFP expression up to 15 days after the initial SELP+virus injection. Transduction and expression of GFP was localized to the injection site [181]. A direct comparison between SELPs for gene delivery and a commercially available synthetic copolymer, Poloxomer 407, found that SELP injection was characterized with mild injection site inflammation, less pronounced local toxicity, and retention at the injection site for 12 weeks [182]. Virus encapsulated in the SELP exhibited greater efficacy of tumor suppression, with increased levels and duration of viral gene expression over both Polaxamer 407 and direct virus controls. These materials show great promise for gene delivery; however, long-term retention of the encapsulated hydrogel mass within the tissue must be addressed.
Additionally, ELP-micelles have been investigated as biocompatible and biodegradable gene therapy vehicles. Cationic elastin diblock copolymers were synthesized for multimodal gene therapy [183]. Oligolysine blocks (VGK8G)n were incorporated into the ELP in order to condense and protect the plasmid DNA (pDNA) during transport. The ELP was synthesized in low yield and polyplexed with the pDNA in a nanoparticulate form. The polyplexes were stable in the presence of anionic proteins at levels corresponding to physiological conditions, but at higher heparin concentrations (0.47 USP unites heparin/ug DNA) it was possible to displace the DNA from the polyplexes. Further studies indicated that transfection of MCF-7 cells in vitro was possible using the polyplexes. Low exhibited toxicity indicates that K8-ELP(1–60)/pDNA polyplexes may safely deliver and release DNA cargo. However, transfection efficiency was only qualitatively measured and appeared to be relatively low when compared with positive controls.
This work was extended through the synthesis of a new ELP-based cationic diblock copolymer incorporating diethylenetriamine (DET), p[Asp(DET)]53ELP(1–90) [184]. Cationic polymers possessing DET have been shown to exhibit minimal cytotoxicity and high gene transfection efficiency, overcoming the limitations of bacterial expression of diblock copolymers with longer cationic blocks [185]. The new ELP-based cationic diblock copolymer is biodegradable, less toxic that previous biosynthetic biopolymers, and exhibits the desirable thermal phase transition behavior. Though the transfection efficiency was one order of magnitude lower than the positive control utilizing 25kDa branched polyethyleneimine (BPEI) as the delivery vehicle, cell viability remained high (80%) even at mM amine concentrations of p[Asp(DET)]53ELP(1–90), in contrast to BPEI’s much higher cytotoxicity.
The potential for targeted delivery of therapeutics to treat a variety of disorders continues to expand, but is limited by safety, efficiency and specificity. ELP biomaterials provide a modular and versatile tool to address some of these challenges through precise targeting and novel therapeutic release strategies.
7. Discussion and Future Directions
Development of protein-based biomaterials has hugely advanced the success of medical devices and drug delivery systems. These versatile biopolymers can be engineered with a high degree of chemical and structural specificity, making them a flexible platform for a variety of biomedical applications. Self-assembly of the proteins can be controlled, leading to unique biomaterials with morphologies that facilitate therapeutic or contrast agent encapsulation, protein purification, and fusion proteins for targeted delivery. Directed evolution experiments through phage and cell surface display have identified amino acids particularly suited to chelating specific metallic ions, which allows for incorporation of inorganic elements into protein motifs. The addition of non-natural amino acids has also extended the applicability of these materials, indicating that the transition temperatures, nanoparticulate morphology, and final thermal and mechanical properties can be precisely engineered. These techniques have been extended to biomaterials for applications such as bioadhesives [186], another important class of genetically encoded biomaterials that has benefited from the incorporation and investigation of non-canonical amino acids [187].
Protein-based biomaterials are uniquely poised to assist in the development of new diagnostic imaging systems. Recent efforts in ultrasound, optical and magnetic resonance imaging have generated significant interest in the use of advanced techniques to improve the resolution and stage at which pathology can be diagnosed. Studies in this area have already identified increased inflammation-regulated cysteine protease activity in atheromata and stent-induced arterial injury. Jaffer et al. [188] utilized an activable near infrared fluorescent (NIRF) imaging agent to verify the activity of cathepsin B eight weeks after balloon injury (Fig. 7). An intravascular ultrasound (IVUS) catheter was used to obtain arterial structure information, which was then correlated with NIRF reporters and used to identify mildly stenotic plaques in vivo. These techniques would benefit from the development of contrast agents with optimized half-lives, increased specificity, and provided significant signal-to-noise enhancement.
Fig. 7.
In vivo investigation of plaque distribution and protease activity in the iliac artery. An activable near infra-red fluorescent (NIRF) imaging agent was utilized to provide contrast for a pull-back scan on a monorail NIRF catheter (A) with areas of intensity shown. (B) An intravascular ultrasound (IVUS) of the same region was overlaid and provided both structural and molecular information for arterial information in atheroma and coronary stent-induced vascular injury. Reprinted from [188], Copyright (2011) with permission from Elsevier.
The incorporation of inorganic elements such as gold, iron, and silver into biomaterials facilitates broadens the general applicability of protein-based biomaterials. Many inorganic particles possess properties that cannot be matched by their biological counterparts, including strong absorptive cross-sections, magnetic properties, photostability, and chemical stability. Conversely, protein-based materials possess general biocompatibility, biodegradability and well-understood clearance profiles, ease of functionality, and a more controlled interaction with the biological environment. By creating organic-inorganic hybrids, it is possible to meld the best properties of both materials while ameliorating some of the potential downsides. An elegant example of this is light-mediated release from biodegradable liposomes investigated by Troutman et al. [134], in which hybrid Au-liposomes are synthesized with an optical response in the NIR region. Upon exposure to wavelengths in the NIR, the liposome breaks apart and the small (4–7 nm) Au nanoparticles are easily cleared through the renal system. Incorporation of inorganic particles into protein-based biomaterials affords opportunities for precise engineering of assembled structures that could be used in localized hyperthermia, taking advantage of the thermal responsiveness of ELP to develop remotely triggered delivery of therapeutics.
Protein-based materials have been more widely applied to applications in therapeutic delivery, and have been shown to provide significant benefits. Many useful therapeutics are frustrated by (i) low therapeutic index (ii) significant nonspecific interactions, with many undesirable side effects; and (iii) poor stability or solubility in vivo. Clinical formulations have addressed this through the use of polymeric carriers and liposomes. However, ELP and other protein-based materials provide significant potential opportunities for additional control through the inclusion of cell-adhesion or penetrating peptides, enzymatic degradation sequences, ligand-binding sites, self-assembly triggers and chemical modification sites. Controlled assembly of protein-based materials offers many advantages for therapeutic encapsulation and multivalent ligand presentation, which has been shown to significantly increase the targeting potential of nanocarriers. The unique potential of ELP-therapeutics to accumulate in regions of acute hyperthermia have been amply demonstrated [125,149,189–191]. In vivo studies by Dreher et al. [192] utilize the application of hyperthermic conditions while imaging through a dorsal window to demonstrate accumulation in tumors. These experiments are an excellent proof of principle, but the technique could be extended with additional methods of hyperthermia application [193,194]. Namely, external radio frequency devices [195], hyperthermic perfusion [196], and microwave energy applied using waveguides [197] would expand the potential targeting area, allowing accumulation of ELPs in deep tissues. Additionally, hyperthermic techniques utilizing nanomaterials have been explored for cancer treatments, with the nanomaterials collected in the tumor reaching temperatures of 45°C [194,198]. Engineering of ELP properties could be undertaken, tuning the transition temperature to respond to specific hyperthermia techniques and gradients.
Studies over the past several decades have yielded methods to precisely engineered protein polymers based on natural proteins. Though polypeptides modeled after structural proteins such as elastin, collagen and silk have dominated this area, significant interest has developed in de novo sequences which can be designed or evolved for specific applications. It is now possible to rationally design peptides that respond to specific environmental stimuli over a wide range of temperatures, incorporate non-natural amino acids, or even peptides which facilitate the assembly of inorganic components such as metallic nanoparticles into the protein structure. These materials can then be assembled into a variety of 3D architectures, such as multivalent protein aggregates and micelles, hybrid organic-inorganic structures, and hydrogels, to benefit a variety of biomedical applications (Fig. 8). The scientific community is in a unique position to leverage techniques perfected by nature to create precisely engineered nanostructures. Advances in diagnostic testing and therapeutic development have clearly outlined the required features of delivery systems; the necessary information is in place to design protein-based materials to meet these challenges.
Fig. 8.
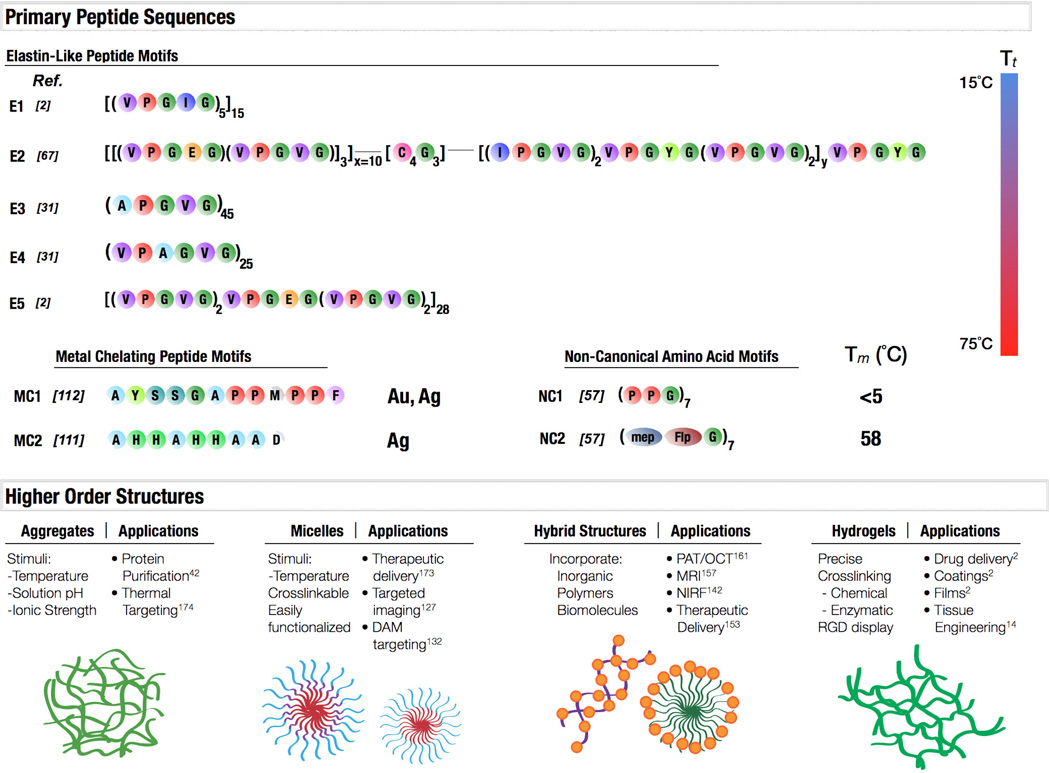
A schematic depicting primary peptide sequences, associated properties, and potential applications. Elastin-like polypeptides E1-E5 exhibit transition temperatures between 15°C and 75°C. E1 and E5 are elastomeric-like. E2 is an amphiphilic diblock copolymer with an intermediate crosslinking domain. E3 and E4 represent potential for hexapeptide elastin-like motifs, shown to have a wide range of temperature and structural variability. Polypeptides have also been identified which chelate metallic ions, such as MC1, which is capable of capturing both gold and silver. MC2 has been shown to efficiently bind silver. Further expansion of the basic collagen motif, NC1, with modified prolines (2S,4R)-4-methylproline (mep) and (2S,4R)-4-fluoroproline (Flp) showed how side chain modification could stabilize the collagen triple helix, concepts that could be applied to many protein motifs. These protein motifs can then organize into higher order structures in response to environmental stimuli or composition, such as protein aggregates, micelles, hybrid organic-inorganic structures, and hydrogels. All of these protein materials can be utilized in a variety of applications.
8. Conclusions
The extensive literature on genetically engineered biomaterials, in particular elastin-based materials, presents a wealth of knowledge on the synthesis of biomaterials with precisely tuned properties. Incorporation of non-natural elements and inorganic materials further extends the range of these versatile materials and improves their capabilities in the realms of diagnostic imaging and therapeutic delivery. Furthermore, new techniques for leveraging high contrast nanocarriers in areas of diagnostics and imaging may allow for quicker diagnoses and better identification of risk factors, facilitating personalized treatment. Molecular imaging in particular may yield important information about underlying fundamental mechanisms in pathologies such as cancer and cardiovascular disease. Development of novel targeting ligands will further benefit therapeutic delivery, enabling the use of powerful therapeutics otherwise limited by dosage or stability. Further investigation is required to develop methods of controlling the release profile of therapeutics after the target has been reached, and this can be facilitated by clever exploitation of the ELPs engineered properties. A wealth of knowledge exists in each fundamental area: protein engineering, nanoparticle synthesis, targeting methods, and therapeutic development. In combination with advances in diagnostic tools, this knowledge is already being leveraged in an exciting new era for targeted and personalized medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urry DW, Urry KD, Szaflarski W, Nowicki M. Elastic-contractile model proteins: physical chemistry, protein function and drug design and delivery. Adv Drug Deliv Rev. 2010;62:1404–1455. doi: 10.1016/j.addr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Kim W, Chaikof EL. Recombinant elastin-mimetic biomaterials: Emerging applications in medicine. Adv Drug Deliv Rev. 2010;62:1468–1478. doi: 10.1016/j.addr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C-Y, Kim TH, Wu W-C, Huang C-M, Wei H, Mount CW, et al. pH-dependent, thermosensitive polymeric nanocarriers for drug delivery to solid tumors. Biomaterials. 2013;34:4501–4509. doi: 10.1016/j.biomaterials.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatham AS, Shewry PR. Elastomeric proteins: biological roles, structures and mechanisms. Trends Biochem Sci. 2000;25:567–571. doi: 10.1016/s0968-0004(00)01670-4. [DOI] [PubMed] [Google Scholar]
- 6.Urry DW, Shaw RG, Prasad KU. Polypentapeptide of elastin: temperature dependence of ellipticity and correlation with elastomeric force. Biochem Biophys Res Commun. 1985;130:50–57. doi: 10.1016/0006-291x(85)90380-8. [DOI] [PubMed] [Google Scholar]
- 7.Urry DW, Parker TM. Mechanics of elastin: molecular mechanism of biological elasticity and its relationship to contraction. J Muscle Res Cell Motil. 2002;23:543–591. [PubMed] [Google Scholar]
- 8.Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, et al. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J Am Chem Soc. 1991;113:4346–4348. [Google Scholar]
- 9.D'Souza A, Hart DS, Middaugh CR, Gehrke SH. Characterization of the changes in secondary structure and architecture of elastin–mimetic triblock polypeptides during thermal gelation. Macromolecules. 2006;39:7084–7091. [Google Scholar]
- 10.Sallach RE, Cui W, Wen J, Martinez A, Conticello VP, Chaikof EL. Elastin-mimetic protein polymers capable of physical and chemical crosslinking. Biomaterials. 2009;30:409–422. doi: 10.1016/j.biomaterials.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Hest J, Tirrell DA. Protein-based materials, toward a new level of structural control. Chem Commun (Camb) 2001:1897–1904. doi: 10.1039/b105185g. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta D, Heilshorn SC. Protein-engineered biomaterials: highly tunable tissue engineering scaffolds. Tissue Eng Part B Rev. 2010;16:285–293. doi: 10.1089/ten.teb.2009.0591. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Macosko CW, Urry DW. Elastomeric polypentapeptides cross-linked into matrixes and fibers. Biomacromolecules. 2001;2:170–179. doi: 10.1021/bm0000900. [DOI] [PubMed] [Google Scholar]
- 14.Kakivaya SR, Hoeve CA. The glass point of elastin. Proc Natl Acad Sci USA. 1975;72:3505–3507. doi: 10.1073/pnas.72.9.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lillie MA, Gosline JM. The effects of hydration on the dynamic mechanical properties of elastin. Biopolymers. 1990;29:1147–1160. doi: 10.1002/bip.360290805. [DOI] [PubMed] [Google Scholar]
- 16.Urry DW. Entropic elastic processes in protein mechanisms. I. Elastic structure due to an inverse temperature transition and elasticity due to internal chain dynamics. J Protein Chem. 1988;7:1–34. doi: 10.1007/BF01025411. [DOI] [PubMed] [Google Scholar]
- 17.Urry DW, Hugel T, Seitz M, Gaub HE, Sheiba L, Dea J, et al. Elastin: a representative ideal protein elastomer. Philos Trans R Soc Lond B Biol Sci. 2002;357:169–184. doi: 10.1098/rstb.2001.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaoka T, Tamura T, Seto Y, Tada T, Kunugi S, Tirrell DA. Mechanism for the phase transition of a genetically engineered elastin model peptide (VPGIG)40 in aqueous solution. Biomacromolecules. 2003;4:1680–1685. doi: 10.1021/bm034120l. [DOI] [PubMed] [Google Scholar]
- 19.Urry DW. Molecular machines: how motion and other functions of living organisms can result from reversible chemical changes. Angew Chem Int Ed Engl. 1993;32:819–841. [Google Scholar]
- 20.Li B, Daggett V. The molecular basis of the temperature- and pH-induced conformational transitions in elastin-based peptides. Biopolymers. 2002;68:121–129. doi: 10.1002/bip.10204. [DOI] [PubMed] [Google Scholar]
- 21.Luan CH, Parker TM, Gowda DC, Urry DW. Hydrophobicity of amino acid residues: differential scanning calorimetry and synthesis of the aromatic analogues of the polypentapeptide of elastin. Biopolymers. 1992;32:1251–1261. doi: 10.1002/bip.360320914. [DOI] [PubMed] [Google Scholar]
- 22.Meyer DE, Chilkoti A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: examples from the elastin-like polypeptide system. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 23.McGrath KP, Tirrell DA, Kawai M, Mason TL, Fournier MJ. Chemical and biosynthetic approaches to the production of novel polypeptide materials. Biotechnol Prog. 1990;6:188–192. doi: 10.1021/bp00003a004. [DOI] [PubMed] [Google Scholar]
- 24.Shah M, Hsueh P-Y, Sun G, Chang HY, Janib SM, MacKay JA. Biodegradation of elastin-like polypeptide nanoparticles. Protein Sci. 2012;21:743–750. doi: 10.1002/pro.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, et al. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 26.Tirrell DA, Fournier MJ, Mason TL. Protein engineering for materials applications. Curr Opin Struct Biol. 1991;1:638–641. [Google Scholar]
- 27.McMillan RA, Conticello VP. Synthesis and characterization of elastin-mimetic protein gels derived from a well-defined polypeptide precursor. Macromolecules. 2000;33:4809–4821. [Google Scholar]
- 28.McDaniel JR, MacKay JA, Quiroz FG, Chilkoti A. Recursive directional ligation by plasmid reconstruction allows rapid and seamless cloning of oligomeric genes. Biomacromolecules. 2010;11:944–952. doi: 10.1021/bm901387t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amiram M, Quiroz FG, Callahan DJ, Chilkoti A. A highly parallel method for synthesizing DNA repeats enables the discovery of “smart” protein polymers. Nat Mater. 2011;10:141–148. doi: 10.1038/nmat2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 31.Chow DC, Dreher MR, Trabbic-Carlson K, Chilkoti A. Ultra-high expression of a thermally responsive recombinant fusion protein in E. coli. Biotechnol Prog. 2006;22:638–646. doi: 10.1021/bp0503742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conley AJ, Joensuu JJ, Jevnikar AM, Menassa R, Brandle JE. Optimization of elastinlike polypeptide fusions for expression and purification of recombinant proteins in plants. Biotechnol Bioeng. 2009;103:562–573. doi: 10.1002/bit.22278. [DOI] [PubMed] [Google Scholar]
- 33.Sallach RE, Conticello VP, Chaikof EL. Expression of a recombinant elastin-like protein in Pichia pastoris. Biotechnol Prog. 2009;25:1810–1818. doi: 10.1002/btpr.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPherson DT, Xu J, Urry DW. Product purification by reversible phase transition following Escherichia coli expression of genes encoding up to 251 repeats of the elastomeric pentapeptide GVGVP. Protein Expr Purif. 1996;7:51–57. doi: 10.1006/prep.1996.0008. [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Macosko CW, Urry DW. Phase transition and elasticity of protein-based hydrogels. J Biomater Sci Polym Ed. 2001;12:229–242. doi: 10.1163/156856201750180942. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Flynn NT, Langer R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv Mater. 2004;16:1074–1079. [Google Scholar]
- 37.Dublin S, Zimenkov Y, Conticello VP. Engineering responsive mechanisms to control the assembly of peptide-based nanostructures. Biochem Soc Trans. 2009;37:653. doi: 10.1042/BST0370653. [DOI] [PubMed] [Google Scholar]
- 38.Kim W, McMillan A, Snyder JP, Conticello VP. A stereoelectronic effect on turn formation due to proline substitution in elastin-mimetic polypeptides. J Am Chem Soc. 2005;127:18121–18132. doi: 10.1021/ja054105j. [DOI] [PubMed] [Google Scholar]
- 39.Kim W, Conticello VP. Protein engineering methods for investigation of structure function relationships in protein-based elastomeric materials. Polym Rev (Phila Pa) 2007;47:93–119. [Google Scholar]
- 40.Walker L, Perkins E, Kratz F, Raucher D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative. Int J Pharm. 2012;436:825–832. doi: 10.1016/j.ijpharm.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacEwan SR, Chilkoti A. Harnessing the power of cell-penetrating peptides: activatable carriers for targeting systemic delivery of cancer therapeutics and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;5:31–48. doi: 10.1002/wnan.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassouneh W, Fischer K, MacEwan SR, Branscheid R, Fu CL, Liu R, et al. Unexpected multivalent display of proteins by temperature triggered self-assembly of elastin-like polypeptide block copolymers. Biomacromolecules. 2012;13:1598–1605. doi: 10.1021/bm300321n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urry DW, Chang DK, Krishna NR, Huang DH, Trapane TL, Prasad KU. Twodimensional proton NMR studies on poly(VPGVG) and its cyclic conformational correlate, cyclo(VPGVG)3. Biopolymers. 1989;28:819–833. doi: 10.1002/bip.360280404. [DOI] [PubMed] [Google Scholar]
- 44.Sciortino F, Urry DW, Palma MU, Prasad KU. Self-assembly of a bioelastomeric structure: solution dynamics and the spinodal and coacervation lines. Biopolymers. 1990;29:1401–1407. doi: 10.1002/bip.360291007. [DOI] [PubMed] [Google Scholar]
- 45.Nagapudi K, Brinkman WT, Leisen J, Thomas BS, Wright ER, Haller C, et al. Proteinbased thermoplastic elastomers. Macromolecules. 2005;38:345–354. [Google Scholar]
- 46.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 47.Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Curr Opin Chem Biol. 2010;14:774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoesl MG, Budisa N. In vivo incorporation of multiple noncanonical amino acids into proteins. Angew Chem Int Ed Engl. 2011;50:2896–2902. doi: 10.1002/anie.201005680. [DOI] [PubMed] [Google Scholar]
- 49.Tcherkezian J, Brittis PA, Thomas F, Roux PP, JG F. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–644. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang MM, Tsou LK, Charron G, Raghavan AS, Hang HC. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci U S A. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shoulders MD, Satyshur KA, Forest KT, Raines RT. Stereoelectronic and steric effects in side chains preorganize a protein main chain. Proc Natl Acad Sci U S A. 2010;107:559–564. doi: 10.1073/pnas.0909592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steiner T, Hess P, Bae JH, Wiltschi B, Moroder L, Budisa N. Synthetic biology of proteins: tuning GFPs folding and stability with fluoroproline. PLoS One. 2008;3:e1680. doi: 10.1371/journal.pone.0001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolschner C, Giese A, Kretzschmar HA, Huber R, Moroder L, Budisa N. Design of anti- and pro-aggregation variants to assess the effects of methionine oxidation in human prion protein. Proc Natl Acad Sci USA. 2009;106:7756–7761. doi: 10.1073/pnas.0902688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goulding A, Shrestha S, Dria K, Hunt E, Deo SK. Red fluorescent protein variants with incorporated non-natural amino acid analogues. Protein Eng Des Sel. 2008;21:101–106. doi: 10.1093/protein/gzm075. [DOI] [PubMed] [Google Scholar]
- 55.Merkel L, Hoesl MG, Albrecht M, Schmidt A, Budisa N. Blue fluorescent amino acids as in vivo building blocks for proteins. ChemBioChem. 2010;11:305–314. doi: 10.1002/cbic.200900651. [DOI] [PubMed] [Google Scholar]
- 56.Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822. doi: 10.1039/b607706d. [DOI] [PubMed] [Google Scholar]
- 57.Chang DK, Urry DW. The molecular dynamics of the β-spiral of the polypentapeptide of elastin in “state III” with 2.9 pentamers per turn. Theochem. 1989;199:303–312. [Google Scholar]
- 58.Cirulis JT, Keeley FW. Kinetics and morphology of self-assembly of an elastin-like polypeptide based on the alternating domain arrangement of human tropoelastin. Biochemistry. 2010;49:5726–5733. doi: 10.1021/bi100468v. [DOI] [PubMed] [Google Scholar]
- 59.Krejchi MT, Atkins ED, Waddon AJ, Fournier MJ, Mason TL, Tirrell DA. Chemical sequence control of beta-sheet assembly in macromolecular crystals of periodic polypeptides. Science. 1994;265:1427–1432. doi: 10.1126/science.8073284. [DOI] [PubMed] [Google Scholar]
- 60.Tirrell DA, Yu SM, Conticello VP, Zhang G, Kayser C, Fournier MJ, et al. Smectic ordering in solutions and films of a rod-like polymer owing to monodispersity of chain length. Nature. 1997;389:167–170. doi: 10.1038/38254. [DOI] [PubMed] [Google Scholar]
- 61.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 62.Annabi N, Fathi A, Mithieux SM, Martens P, Weiss AS. The effect of elastin on chondrocyte adhesion and proliferation on poly (ε-caprolactone)/elastin composites. Biomaterials. 2011:1517–1525. doi: 10.1016/j.biomaterials.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 63.Martín L, Alonso M, Girotti A, Arias FJ, Rodríguez-Cabello JC. Synthesis and characterization of macroporous thermosensitive hydrogels from recombinant elastinlike polymers. Biomacromolecules. 2009;10:3015–3022. doi: 10.1021/bm900560a. [DOI] [PubMed] [Google Scholar]
- 64.Wu X, Levenston ME, Chaikof EL. A constitutive model for protein-based materials. Biomaterials. 2006;27:5315–5325. doi: 10.1016/j.biomaterials.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Wu X, Sallach R, Haller CA, Caves JA, Nagapudi K, Conticello VP, et al. Alterations in physical cross-linking modulate mechanical properties of two-phase protein polymer networks. Biomacromolecules. 2005;6:3037–3044. doi: 10.1021/bm0503468. [DOI] [PubMed] [Google Scholar]
- 66.Wu X, Sallach RE, Caves JM, Conticello VP, Chaikof EL. Deformation responses of a physically cross-linked high molecular weight elastin-like protein polymer. Biomacromolecules. 2008;9:1787–1794. doi: 10.1021/bm800012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee T, Cooper A, Apkarian RP, Conticello VP. Thermo-reversible self-assembly of nanoparticles derived from elastin-mimetic polypeptides. Adv Mater. 2000;12:1105–1110. [Google Scholar]
- 68.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, et al. Temperature triggered self-assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sallach RE, Leisen J, Caves JM, Fotovich E, Apkarian RP, Conticello VP, et al. A permanent change in protein mechanical responses can be produced by thermallyinduced microdomain mixing. J Biomater Sci Polym Ed. 2009;20:1629–1644. doi: 10.1163/156856208X386228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim W, Thévenot J, Ibarboure E, Lecommandoux S, Chaikof EL. Self-assembly of thermally responsive amphiphilic diblock copolypeptides into spherical micellar nanoparticles. Angew Chem Int Ed Engl. 2010;49:4257–4260. doi: 10.1002/anie.201001356. [DOI] [PubMed] [Google Scholar]
- 71.Martín L, Castro E, Ribeiro A, Alonso M, Rodríguez-Cabello JC. Temperaturetriggered self-assembly of elastin-like block co-recombinamers: the controlled formation of micelles and vesicles in an aqueous medium. Biomacromolecules. 2012;13:293–298. doi: 10.1021/bm201436y. [DOI] [PubMed] [Google Scholar]
- 72.Rabotyagova OS, Cebe P, Kaplan DL. Self-assembly of genetically engineered spider silk block copolymers. Biomacromolecules. 2009;10:229–236. doi: 10.1021/bm800930x. [DOI] [PubMed] [Google Scholar]
- 73.Benitez PL, Sweet JA, Fink H, Chennazhi KP, Nair SV, Enejder A, et al. Sequencespecific crosslinking of electrospun, elastin-like protein preserves bioactivity and native-like mechanics. Adv Healthc Mater. 2012;2:114–118. doi: 10.1002/adhm.201200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptideamphiphile nanofibers. Science. 2001;294:1684–1687. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 75.Aluri S, Pastuszka MK, Moses AS, MacKay JA. Elastin-like peptide amphiphiles form nanofibers with tunable length. Biomacromolecules. 2012;13:2645–2654. doi: 10.1021/bm300472y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu X, Cebe P, Weiss AS, Omenetto F, Kaplan DL. Protein-based composite materials. Mater Today (Kidlington) 2012;15:208–215. [Google Scholar]
- 77.Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH. Elastin as a biomaterial for tissue engineering. Biomaterials. 2007;28:4378–4398. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 78.Jansen JA, Daamen WF, van Kuppevelt TH. Controlled fabrication of triple layered and molecularly defined collagen/elastin vascular grafts resembling the native blood vessel. Acta Biomater. 2010;6:4666–4674. doi: 10.1016/j.actbio.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 79.Anumolu R, Gustafson JA, Magda JJ, Cappello J, Ghandehari H, Pease LF., III Fabrication of highly uniform nanoparticles from recombinant silk-elastin-like protein polymers for therapeutic agent delivery. ACS Nano. 2011;5:5374–5382. doi: 10.1021/nn103585f. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Machado R, Azevedo-Silva J, Correia C, Collins T. High level expression and facile purification of recombinant silk-elastin-like polymers in auto induction shake flask cultures. AMB Express. 2013;3:1–15. doi: 10.1186/2191-0855-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Deliv Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 82.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev. 2010;62:1497–1508. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Adv Drug Deliv Rev. 2010;62:1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 84.Qiu W, Teng W, Cappello J, Wu X. Wet-spinning of recombinant silk-elastin-like protein polymer fibers with high tensile strength and high deformability. Biomacromolecules. 2009;10:602–608. doi: 10.1021/bm801296r. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010;31:1025–1035. doi: 10.1016/j.biomaterials.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen A, Li Y, Hu J, Liu X, Li J, Zhang Y, et al. Fabrication of silk fibroin nanoparticles for controlled drug delivery. J Nanopart Res. 2012;14:736–746. [Google Scholar]
- 87.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 88.Winkler S, Wilson D, Kaplan DL. Controlling β-sheet assembly in genetically engineered silk by enzymatic phosphorylation/dephosphorylation. Biochemistry. 2000;39:12739–12746. doi: 10.1021/bi001335w. [DOI] [PubMed] [Google Scholar]
- 89.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. A hyperstable collagen mimic. Chem Biol. 1999;6:63–64. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 90.Hobza P, Hurych J, Zahradník R. Quantum chemical study of the mechanism of collagen proline hydroxylation. Biochim Biophys Acta. 1973;304:466–472. doi: 10.1016/0304-4165(73)90266-3. [DOI] [PubMed] [Google Scholar]
- 91.Faraj KA, van Kuppevelt TH, Daamen WF. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007;13:2387–2394. doi: 10.1089/ten.2006.0320. [DOI] [PubMed] [Google Scholar]
- 92.Shamloo A, Xu H, Heilshorn S. Mechanisms of vascular endothelial growth factorinduced pathfinding by endothelial sprouts in biomaterials. Tissue Eng Part A. 2012;18:320–330. doi: 10.1089/ten.tea.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cen L, Liu W, Cui L, Zhang W, Cao Y. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res. 2008;63:492–496. doi: 10.1203/PDR.0b013e31816c5bc3. [DOI] [PubMed] [Google Scholar]
- 94.Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, et al. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater. 2012;24:749–754. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 95.Bracalello A, Santopietro V, Vassalli M, Marletta G, Del Gaudio R, Bochicchio B, et al. Design and production of a chimeric resilin-, elastin-, and collagen-like engineered polypeptide. Biomacromolecules. 2011;12:2957–2965. doi: 10.1021/bm2005388. [DOI] [PubMed] [Google Scholar]
- 96.Li L, Tong Z, Jia X, Kiick KL. Resilin-like polypeptide hydrogels engineered for versatile biological function. Soft Matter. 2012;9:665. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ayres L, Vos MRJ, Adams PJHM, Shklyarevskiy IO, van Hest JCM. Elastin-based side-chain polymers synthesized by ATRP. Macromolecules. 2003;36:5967–5973. [Google Scholar]
- 98.Pechar M, Brus J, Kostka L, Konák C, Urbanová M, Šlouf M. Thermoresponsive selfassembly of short elastin-like polypentapeptides and their poly(ethylene glycol) derivatives. Macromol Biosci. 2007;7:56–69. doi: 10.1002/mabi.200600196. [DOI] [PubMed] [Google Scholar]
- 99.Rathore O, Sogah DY. Nanostructure formation through β-sheet self-assembly in silk-based materials. Macromolecules. 2001;34:1477–1486. [Google Scholar]
- 100.Huang Y, Chiang C-Y, Lee SK, Gao Y, Hu EL, De Yoreo J, et al. Programmable assembly of nanoarchitectures using genetically engineered viruses. Nano Lett. 2005;5:1429–1434. doi: 10.1021/nl050795d. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y, Kim J, Yun DS, Nam YS, Shao-Horn Y, Belcher AM. Virus-templated Au and Au-Pt core-shell nanowires and their electrocatalytic activities for fuel cell applications. Energy Environ Sci. 2012;5:8328. doi: 10.1039/C2EE21156D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Djalali R, Chen Y-F, Matsui H. Au nanowire fabrication from sequenced histidine-rich peptide. J Am Chem Soc. 2002;124:13660–13661. doi: 10.1021/ja028261r. [DOI] [PubMed] [Google Scholar]
- 103.Tan YN, Lee JY, Wang DIC. Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc. 2010;132:5677–5686. doi: 10.1021/ja907454f. [DOI] [PubMed] [Google Scholar]
- 104.Chen C-L, Zhang P, Rosi NL. A new peptide-based method for the design and synthesis of nanoparticle superstructures: construction of highly ordered gold nanoparticle double helices. J Am Chem Soc. 2008;130:13555–13557. doi: 10.1021/ja805683r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slocik JM, Stone MO, Naik RR. Synthesis of gold nanoparticles using multifunctional peptides. Small. 2005;1:1048–1052. doi: 10.1002/smll.200500172. [DOI] [PubMed] [Google Scholar]
- 106.Minor DL, Jr, Kim PS. Measurement of the β-sheet-forming propensities of amino acids. Nature. 1994;367:660–663. doi: 10.1038/367660a0. [DOI] [PubMed] [Google Scholar]
- 107.Song C, Zhao G, Zhang P, Rosi NL. Expeditious synthesis and assembly of sub-100 nm hollow spherical gold nanoparticle superstructures. J Am Chem Soc. 2010;132:14033–14035. doi: 10.1021/ja106833g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hwang L, Zhao G, Zhang P, Rosi NL. Size-controlled peptide-directed synthesis of hollow spherical gold nanoparticle superstructures. Small. 2011;7:1939–1942. doi: 10.1002/smll.201100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nath N, Chilkoti A. Interfacial phase transition of an environmentally responsive elastin biopolymer adsorbed on functionalized gold nanoparticles studied by colloidal surface plasmon resonance. J Am Chem Soc. 2001;123:8197–8202. doi: 10.1021/ja015585r. [DOI] [PubMed] [Google Scholar]
- 110.Huang H-C, Koria P, Parker SM, Selby L, Megeed Z, Rege K. Optically Responsive Gold Nanorod–Polypeptide Assemblies. Langmuir : the ACS Journal of Surfaces and Colloids. 2008;24:14139–14144. doi: 10.1021/la802842k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 112.Lukyanov AN, Hartner WC, Torchilin VP. Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release. 2004;94:187–193. doi: 10.1016/j.jconrel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Kim W, Brady C, Chaikof EL. Amphiphilic protein micelles for targeted in vivo imaging. Acta Biomater. 2012;8:2476–2482. doi: 10.1016/j.actbio.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 115.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 117.Frankel AD, Pabo CO. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 118.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptorindependent. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 119.Sadler K, Eom KD, Yang J-L, Dimitrova Y, Tam JP. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry. 2002;41:14150–14157. doi: 10.1021/bi026661l. [DOI] [PubMed] [Google Scholar]
- 120.Massodi I, Bidwell GL, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. J Control Release. 2005;108:396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 122.Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 123.MacEwan SR, Chilkoti A. Digital switching of local arginine density in a genetically encoded self-assembled polypeptide nanoparticle controls cellular uptake. Nano Lett. 2012;12:3322–3328. doi: 10.1021/nl301529p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simnick AJ, Valencia CA, Liu R, Chilkoti A. Morphing low-affinity ligands into highavidity nanoparticles by thermally triggered self-assembly of a genetically encoded polymer. ACS Nano. 2010;4:2217–2227. doi: 10.1021/nn901732h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simnick AJ, Amiram M, Liu W, Hanna G, Dewhirst MW, Kontos CD, et al. In vivo tumor targeting by a NGR-decorated micelle of a recombinant diblock copolypeptide. J Control Release. 2011;155:144–151. doi: 10.1016/j.jconrel.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Topcic D, Kim W, Holien JK, Jia F, Armstrong PC, Hohmann JD, et al. An activationspecific platelet inhibitor that can be turned on/fff by medically used hypothermia. Arterioscler Thromb Vasc Biol. 2011;31:2015–2023. doi: 10.1161/ATVBAHA.111.226241. [DOI] [PubMed] [Google Scholar]
- 127.Sun G, Hsueh P-Y, Janib SM, Hamm-Alvarez S, Andrew MacKay J. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J Control Release. 2011;155:218–226. doi: 10.1016/j.jconrel.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soltesz EG, Laurence RG, De Grand AM, Cohn LH, Mihaljevic T, Frangioni JV. Image-guided quantification of cardioplegia delivery during cardiac surgery. Health Sociol Rev. 2007;10:E381–E386. doi: 10.1532/HSF98.20071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Funovics MA, Alencar H, Su HS, Khazaie K, Weissleder R, Mahmood UU. Miniaturized multichannel near infrared endoscope for mouse imaging. Mol Imaging. 2003;2:350–357. doi: 10.1162/15353500200303166. [DOI] [PubMed] [Google Scholar]
- 130.Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 131.Chang K, Jaffer F. Advances in fluorescence imaging of the cardiovascular system. J Nucl Cardiol. 2008;15:417–428. doi: 10.1016/j.nuclcard.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 132.Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen C-L, Rosi NL. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew Chem Int Ed Engl. 2010;49:1924–1942. doi: 10.1002/anie.200903572. [DOI] [PubMed] [Google Scholar]
- 134.Troutman TS, Barton JK, Romanowski M. Biodegradable plasmon resonant nanoshells. Adv Mater. 2008;20:2604–2608. doi: 10.1002/adma.200703026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin C-AJ, Lee C-H, Hsieh J-T, Yu W-C, Yang H-Z, Li JK, et al. Synthesis and surface modification of highly fluorescent gold nanoclusters and their exploitation for cellular labeling. Proc SPIE, vol. 7575, International Society for Optics and Photonics. 2010:757506–757508. [Google Scholar]
- 136.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zrazhevskiy P, Gao X. Quantum dot imaging platform for single-cell molecular profiling. Nat Commun. 2013;4 doi: 10.1038/ncomms2635. 1619-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pons T, Pic E, Lequeux N, Cassette E, Bezdetnaya L, Guillemin F, et al. Cadmiumfree CuInS2/ZnS quantum dots for sentinel lymph node imaging with reduced toxicity. ACS Nano. 2010;4:2531–2538. doi: 10.1021/nn901421v. [DOI] [PubMed] [Google Scholar]
- 139.Park J, Dvoracek C, Lee KH, Galloway JF, Bhang H-EC, Pomper MG, et al. Imaging: CuInSe/ZnS core/shell NIR quantum dots for biomedical imaging. Small. 2011;7:3106–3106. doi: 10.1002/smll.201101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, et al. Self-assembly of CdSe–ZnS quantum dot bioconjugates using an engineered recombinant orotein. J Am Chem Soc. 2000;122:12142–12150. [Google Scholar]
- 141.Janib SM, Moses AS, MacKay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62:1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sevick-Muraca EM, Houston JP, Gurfinkel M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr Opin Chem Biol. 2002;6:642–650. doi: 10.1016/s1367-5931(02)00356-3. [DOI] [PubMed] [Google Scholar]
- 143.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011;29:213–221. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bayer CL, Chen Y-S, Kim S, Mallidi S, Sokolov KV, Emelianov S. Multiplex photoacoustic molecular imaging using targeted silica-coated gold nanorods. Biomedical Optics Express. 2011;2:1828–1835. doi: 10.1364/BOE.2.001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Janib SM, Liu S, Park R, Pastuszka MK, Shi P, Moses AS, et al. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integr Biol. 2012;5:183. doi: 10.1039/c2ib20169k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cai H, Fissekis J, Conti PS. Synthesis of a novel bifunctional chelator AmBaSar based on sarcophagine for peptide conjugation and (64)Cu radiolabelling. Dalton Trans. 2009:5395–5400. doi: 10.1039/b902210d. [DOI] [PubMed] [Google Scholar]
- 147.Furgeson DY, Dreher MR, Chilkoti A. Structural optimization of a “smart” doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors. J Control Release. 2006;110:362–369. doi: 10.1016/j.jconrel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 148.Dreher MR, Raucher D, Balu N, Colvin OM, Ludeman SM, Chilkoti AA. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 149.Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release. 2001;74:213–224. doi: 10.1016/s0168-3659(01)00319-4. [DOI] [PubMed] [Google Scholar]
- 150.Andrew MacKay J, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Selfassembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bessa PC, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha AM, et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J Control Release. 2010;142:312–318. doi: 10.1016/j.jconrel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 152.Herrero-Vanrell R, Rincón AC, Alonso M, Reboto V, Molina-Martinez IT, Rodríguez-Cabello JC. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J Control Release. 2005;102:113–122. doi: 10.1016/j.jconrel.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt P, Dybal J, Rodríguez-Cabello JC, Reboto V. Role of water in structural changes of poly(AVGVP) and poly(GVGVP) studied by FTIR and Raman spectroscopy and ab initio calculations. Biomacromolecules. 2005;6:697–706. doi: 10.1021/bm049461t. [DOI] [PubMed] [Google Scholar]
- 154.Moktan S, Perkins E, Kratz F, Raucher D. Thermal Targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo. Molecular Cancer Therapeutics. 2012;11:1547–1556. doi: 10.1158/1535-7163.MCT-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 156.Moktan S, Ryppa C, Kratz F, Raucher D. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Invest New Drugs. 2010;30:236–248. doi: 10.1007/s10637-010-9560-x. [DOI] [PubMed] [Google Scholar]
- 157.Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 158.Kim W, Xiao J, Chaikof EL. Recombinant amphiphilic protein micelles for drug delivery. Langmuir. 2011;27:14329–14334. doi: 10.1021/la202864x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu W, MacKay JA, Dreher MR, Chen M, McDaniel JR, Simnick AJ, et al. Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model. J Control Release. 2010;144:2–9. doi: 10.1016/j.jconrel.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72:5566–5575. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gius DR, Ezhevsky SA, Becker-Hapak M, Nagahara H, Wei MC, Dowdy SF. Transduced p16INK4a peptides inhibit hypophosphorylation of the retinoblastoma protein and cell cycle progression prior to activation of Cdk2 complexes in late G1. Cancer Res. 1999;59:2577–2580. [PubMed] [Google Scholar]
- 162.Miyamoto Y, Fujimoto KK, Doi R, Otaka A, Fujii N, Imamura M. Trojan p16 peptide suppresses pancreatic cancer growth and prolongs survival in mice. Clin Cancer Res. 2002;8:1271–1276. [PubMed] [Google Scholar]
- 163.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 164.Zhang X-X, Eden HS, Chen XX. Peptides in cancer nanomedicine: drug carriers, targeting ligands and protease substrates. J Control Release. 2012;159:2–13. doi: 10.1016/j.jconrel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ball KL, Lain S, Fâhraeus R, Smythe C, Lane DP. Cell-cycle arrest and inhibition of Cdk4 activity by small peptides based on the carboxy-terminal domain of p21WAF1. Curr Biol. 1997;7 doi: 10.1016/s0960-9822(06)00029-7. 10-0. [DOI] [PubMed] [Google Scholar]
- 166.Bidwell GL, Whittom AA, Thomas E, Lyons D, Hebert MD, Raucher D. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides. 2010;31:8–8. doi: 10.1016/j.peptides.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bidwell GL, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4:1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 168.Bidwell GL, Davis AN, Raucher D. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135:2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 169.Bidwell GL, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012;319:136–143. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bidwell GL, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055104. e55104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hearst SM, Walker LR, Shao Q, Lopez M, Raucher D, Vig PJS. The design and delivery of a thermally responsive peptide to inhibit S100B-mediated neurodegeneration. Neuroscience. 2011;197:369–380. doi: 10.1016/j.neuroscience.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moktan S, Raucher D. Anticancer activity of proapoptotic peptides is highly improved by thermal targeting using elastin-like polypeptides. Int J Pept Res Ther. 2012;18:227–237. doi: 10.1007/s10989-012-9295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thamm OC, Koenen P, Bader N, Schneider A, Wutzler S, Neugebauer EA, et al. Acute and chronic wound fluids influence keratinocyte function differently. Int Wound J. 2013 doi: 10.1111/iwj.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell ALM, Kyriakides TR, Saltzman WMW. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials. 2013;34:3891–3901. doi: 10.1016/j.biomaterials.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xia Y-P, Zhao Y, Marcus J, Jimenez PA, Ruben SM, Moore PA, et al. Effects of keratinocyte growth factor-2 (KGF-2) on wound healing in an ischaemia-impaired rabbit ear model and on scar formation. J Pathol. 1999;188:431–438. doi: 10.1002/(SICI)1096-9896(199908)188:4<431::AID-PATH362>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 177.Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, et al. Selfassembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc Natl Acad Sci USA. 2011;108:1034–1039. doi: 10.1073/pnas.1009881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jang J-H, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Haider M, Megeed Z, Ghandehari H. Genetically engineered polymers: status and prospects for controlled release. J Control Release. 2004;95:1–26. doi: 10.1016/j.jconrel.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 180.Wald Cresce von A, Dandu R, Burger A, Cappello J, Ghandehari H. Characterization and real-time imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels. Mol Pharm. 2008;5:891–897. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hatefi A, Cappello J, Ghandehari HH. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24:773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 182.Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. Int J Pharm. 2012;427:97–104. doi: 10.1016/j.ijpharm.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen T-HH, Bae Y, Furgeson DY. Intelligent biosynthetic nanobiomaterials (IBNs) for hyperthermic gene delivery. Pharm Res. 2007;25:683–691. doi: 10.1007/s11095-007-9382-5. [DOI] [PubMed] [Google Scholar]
- 184.Chen T-HH, Bae Y, Furgeson DY, Kwon GS. Biodegradable hybrid recombinant block copolymers for non-viral gene transfection. Int J Pharm. 2012;427:105–112. doi: 10.1016/j.ijpharm.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 185.Itaka K, Ishii T, Hasegawa Y, Kataoka K. Biodegradable polyamino acid-based polycations as safe and effective gene carrier minimizing cumulative toxicity. Biomaterials. 2010;31:8–8. doi: 10.1016/j.biomaterials.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 186.Mehdizadeh M, Yang J. Design Strategies and Applications of Tissue Bioadhesives. Macromol Biosci. 2012;13:271–288. doi: 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Larregola M, Moore S, Budisa N. Congeneric bio-adhesive mussel foot proteins designed by modified prolines revealed a chiral bias in unnatural translation. Biochem Biophys Res Commun. 2012;421:646–650. doi: 10.1016/j.bbrc.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 188.Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, et al. Twodimensional intravascular near-infrared fluorescence molecular maging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dreher M, Chilkoti A. Intravital laser scanning confocal microscopy for drug carrier imaging in solid tumors. Mam. 2007;13 [Google Scholar]
- 190.McDaniel JR, Callahan DJ, Chilkoti A. Drug delivery to solid tumors by elastin-like polypeptides. Adv Drug Deliv Rev. 2010;62:1456–1467. doi: 10.1016/j.addr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McDaniel JR, Bhattacharyya J, Vargo KB, Hassouneh W, Hammer DA, Chilkoti A. Self-Assembly of thermally responsive nanoparticles of a genetically encoded peptide polymer by drug conjugation. Angew Chemie Int Ed. 2012;52:1683–1687. doi: 10.1002/anie.201200899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Chilkoti A. Thermal cycling enhances the accumulation of a temperature-sensitive biopolymer in solid tumors. Cancer Res. 2007;67:4418–4424. doi: 10.1158/0008-5472.CAN-06-4444. [DOI] [PubMed] [Google Scholar]
- 193.Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17:1–18. doi: 10.1080/02656730150201552. [DOI] [PubMed] [Google Scholar]
- 194.IP Soares P, MM Ferreira I, AGBN Igreja R, MM Novo C, PMR Borges J. Application of Hyperthermia for Cancer Treatment: Recent Patents Review. Recent Pat Anticancer Drug Discov. 2012;7:64–73. doi: 10.2174/157489212798358038. [DOI] [PubMed] [Google Scholar]
- 195.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070417. e70417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cameron RB, Hou D. Intraoperative hyperthermic chemotherapy perfusion for malignant pleural mesothelioma: an in vitro evaluation. J Thorac Cardiovasc Surg. 2013;145:496–504. doi: 10.1016/j.jtcvs.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 197.Vogl TJ, Farshid P, Naguib NNN, Zangos S. Thermal ablation therapies in patients with breast cancer liver metastases: a review. Eur Radiol. 2013;23:797–804. doi: 10.1007/s00330-012-2662-4. [DOI] [PubMed] [Google Scholar]
- 198.Wust P, Gneveckow U, Wust P, Gneveckow U, Johannsen M, Böhmer D, et al. Magnetic nanoparticles for interstitial thermotherapy - feasibility, tolerance and achieved temperatures. IInt J Hyperthermia. 2006;22:673–685. doi: 10.1080/02656730601106037. [DOI] [PubMed] [Google Scholar]
- 199.Cook WJ, Einspahr H, Trapane TL, Urry DW, Bugg CE. Crystal structure and conformation of the cyclic trimer of a repeat pentapeptide of elastin, cyclo-(L-valyl-Lprolylglycyl-L-valylglycyl)3. J Am Chem Soc. 1980;102:5502–5505. [Google Scholar]