Abstract
Pre-extinction administration of ∆9-tetrahydrocannibinol (THC) facilitates recall of extinction in healthy humans, and evidence from animal studies suggest that this likely involves via enhancement of the cannabinoid system within the ventromedial prefrontal cortex (vmPFC) and hippocampus (HIPP), brain structures critical to fear extinction. However, the effect of cannabinoids on the underlying neural circuitry of extinction memory recall in humans has not been demonstrated. We conducted a functional magnetic resonance imaging (fMRI) study using a randomized, double-blind, placebo-controlled, between-subjects design (N=14/group) coupled with a standard Pavlovian fear extinction paradigm and an acute pharmacological challenge with oral dronabinol (synthetic THC) in healthy adult volunteers. We examined the effects of THC on vmPFC and HIPP activation when tested for recall of extinction learning 24 hours after extinction learning. Compared to subjects who received placebo, participants who received THC showed increased vmPFC and HIPP activation to a previously extinguished conditioned stimulus (CS+E) during extinction memory recall. This study provides the first evidence that pre-extinction administration of THC modulates prefrontal-limbic circuits during fear extinction in humans and prompts future investigation to test if cannabinoid agonists can rescue or correct the impaired behavioral and neural function during extinction recall in patients with PTSD. Ultimately, the cannabinoid system may serve as a promising target for innovative intervention strategies (e.g. pharmacological enhancement of exposure-based therapy) in PTSD and other fear learning-related disorders.
Keywords: extinction, fMRI, ventromedial prefrontal cortex, hippocampus, amygdala, ∆9-tetrahydrocannabinol
1. Introduction
Anxiety disorders, such as post-traumatic stress disorder (PTSD), can be conceptualized by an inability to suppress inappropriate fear responses (Rauch, Shin, & Phelps, 2006; Rosen & Schulkin, 1998). A first-line and empirically-validated approach to treat this disorder is Prolonged Exposure Therapy (PE) (Foa, 2011), one component of which involves repeated exposure to fear-linked cues to produce “extinction” of fear and to prevent avoidance responses to these cues (Hofmann, 2008). PE is generally effective, but a significant number of patients have incomplete responses or fail to sustain improvements over time (Foa et al., 1999; Hembree et al., 2003; Rothbaum, Astin, & Marsteller, 2005). Limited efficacy and lack of sustainability could be due to the fact that extinction learning, which is the active ingredient of exposure-based therapy, is vulnerable to the return of fear (Bouton, 2004; Hermans, Craske, Mineka, & Lovibond, 2006; Myers & Davis, 2007; Robbins, 1990).
Convergent evidence from rat and human work have elucidated that discrete, yet anatomically and functionally interconnected, brain structures are critical for extinction learning and the retention of extinction memory (amygdala [AMYG], ventromedial prefrontal cortex [vmPFC], and hippocampus [HIPP]) (for a review see (Milad & Quirk, 2012)). For instance, AMYG activation has been correlated with fear responses during conditioning in human subjects based on functional magnetic resonance imaging (fMRI) studies (LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; Phelps, Delgado, Nearing, & LeDoux, 2004; Phelps et al., 2001). Prefrontal brain regions that interconnect with the AMYG, particularly the vmPFC, are important for consolidation and retrieval of extinction memories and consequent attenuation of conditioned fear responses perhaps via inhibiting AMYG output (Milad & Quirk, 2002; Phelps, et al., 2004; Quirk & Mueller, 2008). In humans, vmPFC activation during extinction recall and vmPFC thickness both correlate with magnitude of extinction retention (Milad et al., 2005; Milad et al., 2007; Phelps, et al., 2004). Similarly, the HIPP is associated with successful retrieval of extinction memory and is positively correlated with vmPFC activation during extinction recall in humans (Kalisch et al., 2006; Milad, et al., 2007). Interestingly, poor extinction retention and vmPFC-HIPP dysfunction have been implicated in anxiety disorders such as PTSD and could limit or undermine the maintenance of the therapeutic effects of exposure (Charney & Deutch, 1996; Milad et al., 2009; Pitman, Shin, & Rauch, 2001; van Minnen & Hagenaars, 2002). Despite having converging evidence of the critical neural mechanism underlying extinction recall and its retention, few strategies exist to augment the generalization and retention of extinction memory in the clinical setting in order to maximize treatment effects of exposure-based therapies.
Exciting new evidence have implicated several signaling pathways, such as the GABAergic, glutamatergic, noradrenergic, cholinergic, and cannabinoid systems, as potential pharmacological targets in the facilitation of extinction learning and the retention (for a review see (Kaplan & Moore, 2011)). Of particular interest, emerging studies in rodents suggest that activation of the cannabinoid (CB) system within brain structures important for extinction (e.g. AMYG, vmPFC, HIPP) may regulate extinction learning and retention. For instance, local infusions of AM251, a CB1 antagonist, into the dorsal HIPP (de Oliveira Alvares, Pasqualini Genro, Diehl, Molina, & Quillfeldt, 2008) or infralimbic cortex (IL), a homologous structure to the human vmPFC, (Lin, Mao, Su, & Gean, 2009), have been shown to block consolidation of contextual fear extinction and impair extinction of fear-potentiated startle, respectively. Several studies have corroborated and extended these findings by showing that CB1 antagonists lead to a profound disruption of extinction retention when given either prior to extinction learning or immediately following extinction learning, suggesting that CB1 receptor activation is an important mechanism for extinction learning and for consolidation of extinction memories in order to successfully retrieve these memories at a later time (Chhatwal, Davis, Maguschak, & Ressler, 2005; Pamplona, Bitencourt, & Takahashi, 2008; Pamplona, Prediger, Pandolfo, & Takahashi, 2006; Suzuki et al., 2004).
Based on these findings it is not surprising that activation of CB1 receptors, via agonists (e.g., WIN 55,212-2, HU210, Δ9-tetrahydrocannabinol [THC]), and pharmacological agents that enhance levels of released endogenous cannabinoids (eCBs) (e.g., AM404, an eCB reuptake inhibitor, and URB597, a fatty acid amide hydrolase (FAAH) inhibitor that blocks hydrolysis of anandamide) have also been shown to facilitate within-session extinction learning (Bitencourt, Pamplona, & Takahashi, 2008; Varvel, Wise, Niyuhire, Cravatt, & Lichtman, 2007) and enhance the retention of extinction (Bitencourt, et al., 2008; Chhatwal, et al., 2005; de Oliveira Alvares, et al., 2008; Lin, et al., 2009; Varvel, et al., 2007); but see (Chhatwal, et al., 2005)). For instance, local injections of WIN 55–212,2 (Lin, Mao, Chen, & Gean, 2008; Lin, et al., 2009) or AM404 (Lin, et al., 2009) into the IL cortex prior to extinction learning has been shown to facilitate the extinction of fear-potentiated startle and local injections of HUB210 and/or WIN 55-212,2 into the AMYG during fear extinction blocks spontaneous recovery of extinguished fear-potentiated startle in rats (Lin, Mao, & Gean, 2006). Similarly, extinction learning can be enhanced with local infusions of anandamide into the dorsal HIPP (de Oliveira Alvares, et al., 2008). Collectively, these animal studies suggest that the efficacy of extinction learning and retention can be enhanced via increasing activity of CB1 receptors acting specifically on fear extinction brain circuits (AMYG, HIPP, IL) and prompt us to translate these findings into human studies.
A recent study conducted in our laboratory showed that pre-extinction administration of THC facilitates recall of extinction in healthy humans (Rabinak et al., 2013). While participants that had received placebo during extinction learning exhibited spontaneous recovery of fear to an extinguished CS, THC attenuated spontaneous recovery of fear. Of note, THC did not affect within-session extinction learning, but only influenced the ability to successfully recall extinction memory, suggesting that THC affects are specific to maintaining and/or successfully retrieving extinction memory. In another study, consolidation of extinction learning was enhanced when cannabidiol (CBD), a non-psychotomimetic cannabinoid, was administered to healthy volunteers after extinction learning (Das et al., 2013). Together, these findings suggest that pharmacological enhancement of extinction recall is feasible in humans using cannabinoid system modulators; however, their effect on the underlying neural circuitry remains unknown.
In a randomized, double-blind, placebo-controlled, between-subjects design, we coupled a standard Pavlovian fear extinction paradigm in functional magnetic resonance imaging (fMRI) with an acute pharmacological challenge with oral dronabinol (synthetic THC) or placebo 2 hours prior to extinction learning in healthy adult volunteers and tested extinction retention 24 hours after extinction learning. Given the extant literature described above we had several hypotheses for each session of our study. We did not anticipate THC to have an effect on SCRs during extinction learning, consistent with what we have shown previously (Rabinak, et al., 2013). In addition, we hypothesized that during early extinction learning all participants would show increased AMYG activation to the CS+E compared to the CS-. However, because we previously found that THC did not affect the expression of conditioned fear responses during extinction learning (Rabinak, et al., 2013) we did not anticipate THC to have an effect on AMYG activity during early extinction learning. During extinction recall we hypothesized that relative to PBO, THC would decrease SCRs to a CS that was previously extinguished (CS+E) (Rabinak, et al., 2013). Moreover, we hypothesized that participants that had received PBO would engage vmPFC and HIPP to the CS+E (> CS+U) during extinction recall and that THC would further enhance regional activation in these regions to the CS+E relative to PBO.
2. Materials and Methods
2.1 Subjects
Twenty-eight healthy, right-handed volunteers (thirteen males; aged 21–45 years; Caucasian = 21; African American = 2; Asian = 3; More than one race = 2) participated in this study and were randomly assigned to the THC (n = 14) or placebo (n = 14) condition. Some participants (n = 15) had a minimal history of marijuana use (limited to < 10 lifetime exposures; mean: 1.61 ± 0.56); none had history or signs of neurological, psychiatric (including substance and alcohol abuse/dependence), or medical illness as confirmed by medical examination and a modified Structured Clinical Interview for DSM-IV (SCID-NP) (First, Spitzter, Gibbon, & Williams, 2002). All subjects had negative urine toxicology and alcohol breathalyzer screens at time of study.
All female subjects completed study sessions about 1 week prior to menses onset (based on self-reports of last period and cycle length), to ensure that they were studied while estrogen levels were low. This restriction was based on evidence that high estradiol levels can facilitate fear extinction (Milad et al., 2010; Zeidan et al., 2011). All participants gave written informed consent after explanation of the experimental protocol, as approved by the University of Michigan Institutional Review Board.
2.2 Experimental protocol and task
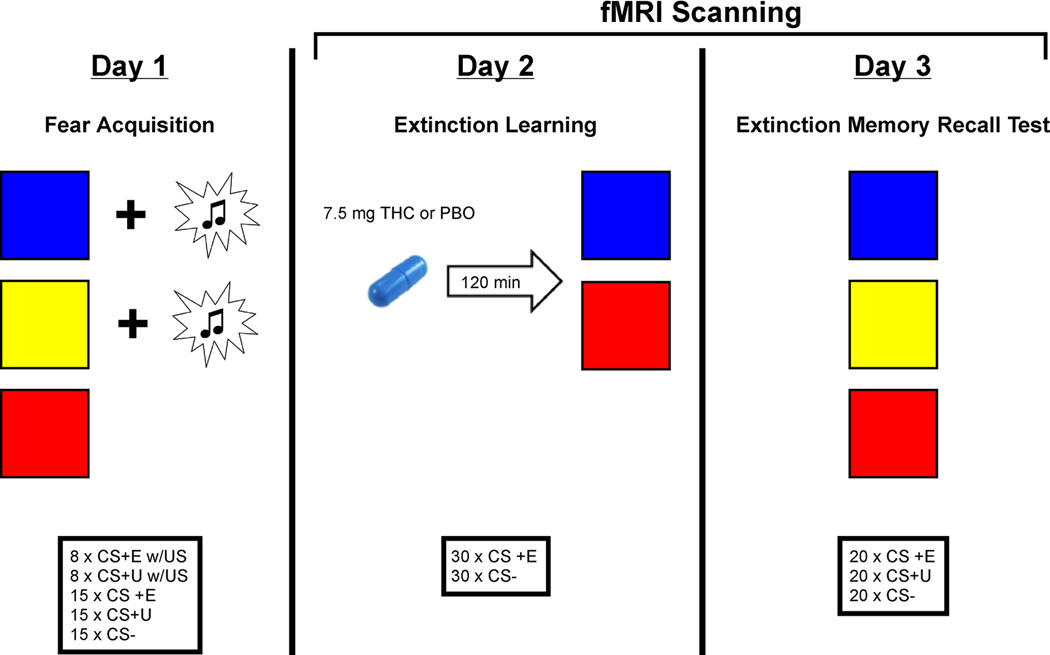
Participants were studied over 3 consecutive days as previously described (Rabinak, et al., 2013) (Figure 1). On Day 1, all participants underwent partial discrimination fear conditioning, in which they were presented with two neutral visual CSs on a computer screen (CS+s; e.g., blue and yellow squares) that co-terminated with an aversive white noise burst US through a pair of headphones (500 ms, 100dB) at a partial reinforcement rate of 35%. Fear acquisition consisted of 15 non-reinforced presentations of each of the CSs, intermixed with an additional 8 presentations of each CS+ that co-terminated with the US. A third CS (e.g. red square) was presented during fear conditioning but never paired with the US (CS-).
Figure 1.
Diagram of the experimental design.
The next day (Day 2) participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (Marinol; 7.5 mg; Solvay Pharmaceuticals, Marietta, GA) or dextrose alone (placebo; PBO). The THC dose used is the lowest effective dose found to produce behavioral and subjective effects (Kirk & de Wit, 1999; Phan et al., 2008; Rabinak, Sripada, Angstadt, de Wit, & Phan, 2012; Wachtel, ElSohly, Ross, Ambre, & de Wit, 2002). Approximately 120 minutes after drug administration [PBO, 128.93 ± 2.43 min; THC, 120.33 ± 2.87 min] all participants underwent an extinction session. The timing ensured presence of peak subjective effects and plasma levels of THC (Wachtel, et al., 2002) during the extinction training. During extinction learning one of the CS+s (e.g., blue square) was extinguished (CS+E), by presenting it in the absence of the US, whereas the other CS+ (e.g., yellow square) was not presented (CS+U). There were 30 CS+E and 30 CS− trials over two runs (15 of each stimulus type per run).
To assess extinction retention, we conducted an extinction memory recall test approximately 24 hours after the extinction learning session (Day 3). This consisted of 20 non-reinforced presentations of each of the CSs (CS+E, CS+U, and CS-) over two runs (10 of each stimulus type per run). In each experimental session (fear acquisition, extinction learning, and extinction recall test) all CSs were presented for 4 sec each with an inter-trial interval (ITI) from 6 to 18 sec. The designation of colored squares (blue, yellow, or red) as CS+E, CS+U, or CS-was counterbalanced across the participants and the order of trials was pseudo-randomized, such that no more than 2 presentations of the same colored square (red, yellow, or blue) occurred in a row.
At the beginning of each session participants were told that they may or may not hear a loud noise burst and were instructed to pay attention to the computer screen and try to figure out the relationship between the colored squares and the noise burst. During each presentation of the colored square stimuli, participants were asked to rate their expectancy that the US would occur on a 5-point scale (“Will you hear a loud noise burst?”: 1 = Definitely not; 3 = Unsure; 5 = Definitely). US expectancy was scored as the first response within 3 sec of CS onset. Conditioned fear was indexed by changes in skin conductance responses (SCRs) and US expectancy ratings for each CS trial. Electrodes and headphones remained in place during all sessions (removed only during breaks).
2.3 Functional imaging: acquisition and analysis
FMRI scanning was conducted during the extinction learning and extinction recall test sessions and was performed on a 3T GE Signa System (General Electric; Milwaukee, WI) using a standard radiofrequency coil at the University of Michigan Functional MRI Laboratory. Whole-brain functional images (i.e., blood oxygenated level-dependent [BOLD]) were collected from 43 axial, 3-mm-thick slices using a T2*-sensitive gradient echo reverse spiral acquisition sequence (repetition time, 2000 ms; echo time, 30 ms; 64 × 64 matrix; 220 mm field of view; flip angle, 90°), optimized to minimize susceptibility artifacts (signal loss) at the medial temporal lobe (including the AMYG) (Stenger, Boada, & Noll, 2000). A T1-weighted anatomical image was collected in the same planes as the functional data, but with higher in-plane resolution (1mm2, T1-overlay) to aid in later co-registration. A high resolution, T1-weighted volumetric anatomical scan (T1-SPGR; three-dimensional spoiled gradient echo) was also acquired for precise anatomical localization and normalization.
Data from all participants met criteria for high quality and scan stability with minimum motion correction and were subsequently included in fMRI analyses (< 2 mm displacement in any one direction). The first four volumes were discarded to allow for T1 equilibration effects. Functional data were processed and analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm) (Friston et al., 1995). Images were temporally corrected to account for slice time acquisition differences and spatially realigned to correct for head movement. Each participant’s T1-overlay was co-registered to the time-series data and the T1-SPGR was then co-registered to the co-registered T1-overlay image. The co-registered T1-SPGR was then segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using the VBM8 toolbox of SPM8 and normalized to Montreal Neurological Institute (MNI) space using DARTEL (Ashburner, 2007) and the resulting deformation field was applied to the time-series data. These normalized time-series data were subsequently re-sampled to 2 mm3 voxels and smoothed with a 6 mm Gaussian kernel to minimize noise and effects due to residual differences in functional and gyral anatomy during inter-subject averaging.
Estimates of the stimulus effects at each voxel for each subject were made with an event-related design and by convolving the functional signal for each event with a canonical hemodynamic response function and with a 128 s high-pass filter. Individual statistical parametric maps (SPMs) were calculated according to the general linear model for the contrasts of interest for each participant. In addition, the six movement parameters obtained during realignment were included in the model as regressors to account for motion-related effects in BOLD. Individual SPMs were then analyzed at the second level in a random-effects statistical model. The contrast of interest during the extinction learning session was CS+E versus CS-. In order to examine the effects of THC on changes in neural activation over time we divided the extinction session into early (first 15 trials of each stimulus type) and late (last 15 trials of each stimulus type) time windows (Phelps, et al., 2004).
Based on prior evidence, vmPFC and HIPP activation is most robust and signals extinction recall during the early phase of extinction recall (Milad & Quirk, 2002; Milad, et al., 2007); therefore our primary contrast of interest during extinction recall was the first 10 CS+E versus the first 10 CS+U trials during the extinction recall session. We had a priori hypotheses that extinction recall would engage vmPFC-HIPP circuitry, and that THC would have CS+E− specific and localized effects on these regions. We searched for significant activations that fell within our regions of interest (ROI), defined a priori as the vmPFC and HIPP. Anatomical localization of these activations within ROIs were defined by anatomical landmarks using MARINA software (Walter et al., 2003) based on masks from the atlas of Tzourio-Mazoyer and colleagues (Tzourio-Mazoyer et al., 2002). Of note, the vmPFC mask was comprised of the following bilateral, anatomically-defined brain regions: the orbital part of the inferior, middle, and superior frontal gyri, the gyrus rectus, and the olfactory cortex. Within our ROIs, activations surviving small volume correction (within the anatomical mask) at p < 0.05 family-wise error (FWE) were considered significant.
To clarify the signal direction, variance and specificity of differences in activation between the PBO and THC groups in each CS condition (Extinction Learning: CS+E, CS-; Extinction Recall: CS+E, CS+U), we extracted BOLD signal responses (parameter estimates, β-weights in arbitrary units [a.u.] of activation) averaged across all voxels within 5-mm radius sphere surrounding the peak activation within these a priori regions. Of note, we did not conduct statistical test on the extracted BOLD signal responses, as they were defined from significant activations within our ROI masks. We calculated Cohen’s d, an index of effect size, on drug effects based on the following: Cohen’s d = mean βPBO - mean βTHC/σ pooled, where σpooled = √(σPBO2 + σTHC2)/2; (σ = SD).
2.4 Psychophysiological and behavioral measures: acquisition and analysis
SCRs were measured by two disposable carbon fiber electrodes attached between the first and second phalanges of the second and third digits of the left hand (EL509, BIOPAC Systems, Inc., Goleta, CA). The electrodes were connected to a BIOPAC Systems skin conductance module (GSR100C) and skin conductance was continuously sampled at a rate of 1000 samples per second, amplified, and stored on a Dell laptop computer for offline analysis using AcqKnowledge 4.2 software (BIOPAC Systems, Inc.). The recorded waveforms were low pass filtered using a Blackman window (cutoff frequency = 125 Hz) and mean value smoothed over 100 adjacent data points prior to scoring.
SCR for each CS trial was calculated by subtracting the mean skin conductance level during the 2 sec before stimulus onset from the highest skin conductance level that occurred in the 0.5 to 4.5 sec latency window after stimulus onset. SCRs below 0.02 µS were scored as zero (LaBar, et al., 1998; Schiller et al., 2010). Raw SCRs from each session were square root transformed to normalize the distributions and scaled according to each subject’s mean square root transformed US response during the acquisition session (LaBar, et al., 1998; Milad, Orr, Pitman, & Rauch, 2005; Orr et al., 2000; Schiller, et al., 2010). To assess the level of conditioned responding in anticipation of the aversive US separate from unconditioned responses to the noise bursts, themselves, we included only non-reinforced trials of the CS+s in the analysis. Extinction learning success was calculated with an Extinction Learning Index: 100 – ([the average SCR during the last four trials of the Extinction Learning session divided by the largest SCR during the Acquisition session] × 100) and extinction memory recall success was calculated with an Extinction Retention Index with the same formula as the Extinction Learning Index except that SCRs for the first two trials during the Extinction Recall session were used (Holt et al., 2009; Milad, et al., 2007).
SCRs and US expectancy ratings were analyzed using analysis of variance (ANOVA). Post-hoc comparisons between and within drug groups, using independent and paired t tests, respectively, were performed after a significant F ratio was obtained. We used a significance threshold of p < 0.05 (two-tailed), corrected for multiple comparisons using Bonferroni correction. Unless otherwise stated all data are presented as means ± SEM.
3. Results
3.1 Fear Acquisition
3.1.1 SCR
All participants acquired differential fear conditioning as evidenced by greater SCR responses to the CS+ [THC = 0.40 ± 0.09; PBO = 0.30 ± 0.05] than to the CS− [THC = 0.22 ± 0.07; PBO = 0.21 ± 0.06; main effect of stimulus: F(1,26) = 10.91, p = 0.003]. There were no significant differences in SCR during fear acquisition between participants assigned to the THC and PBO groups [absence of main effect of drug: F(1,26) = 0.40, p = 0.53; and drug by stimulus interaction: F(1,26) = 1.38, p = 0.25]. The two drug groups responded similarly to the US and displayed high levels of unconditioned SCRs [THC = 0.85 ± 0.13; PBO = 1.05 ± 0.10; t(26) = −1.19, p = 0.24].
3.1.2 US Expectancy
Participants were asked to rate their expectancy that the US would occur during each presentation of the CSs on a 5-point scale (“Will you hear a loud noise burst?”: 1 = Definitely not; 3 = Unsure; 5 = Definitely). Consistent with the SCR results, participants rated the US as more likely to occur during the CS+ [THC = 3.57 ± 0.21; PBO = 3.43 ± 0.18] than to the CS-[THC = 2.07 ± 0.32; PBO = 2.25 ± 0.26; main effect of stimulus: F(1,26) = 22.21, p < 0.001]. There were no significant differences in US expectancy ratings during fear acquisition between participants in the THC and PBO groups [absence of main effect of drug: F(1,26) = 0.01, p = 0.93; and drug by stimulus interaction: F(1,26) = 0.32, p = 0.58].
3.2 Extinction Learning
3.2.1 SCR
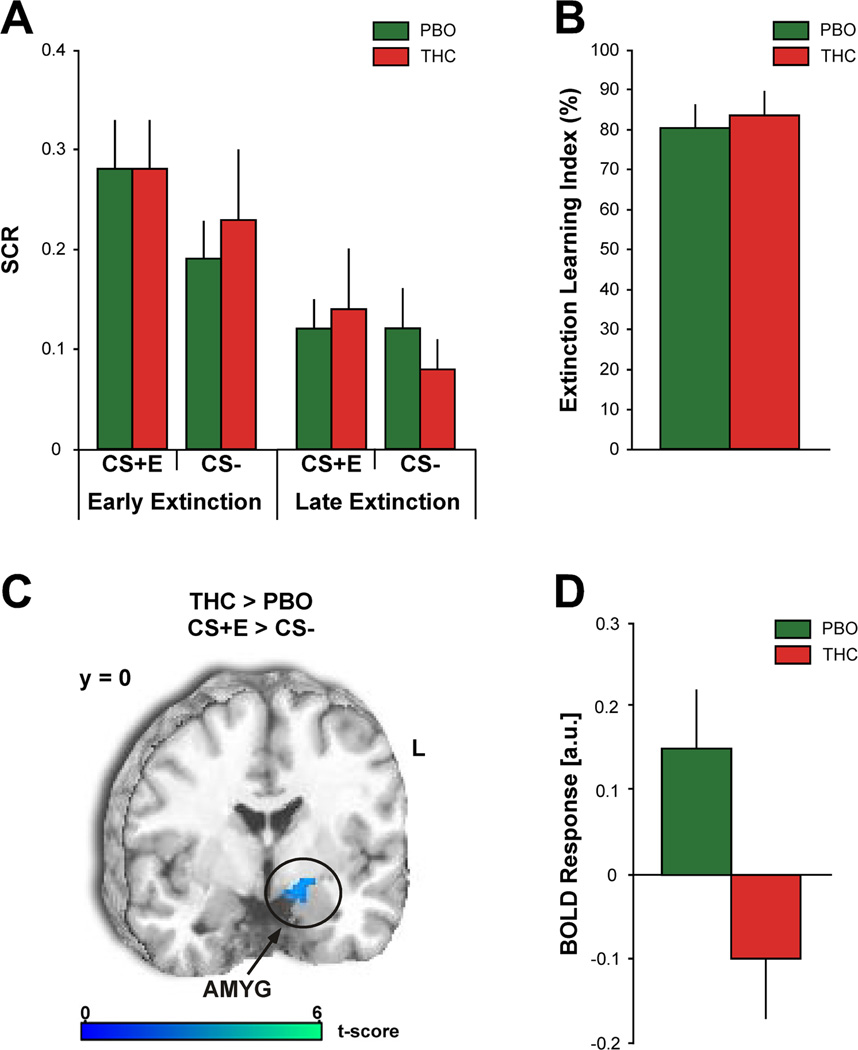
An ANOVA of SCR with three, two-level factors – drug (THC, PBO), stimulus (CS+E, CS-), and time (early extinction, late extinction) – revealed a significant main effect of stimulus [F(1,26) = 6.00, p = 0.02] and a significant main effect of time [F(1,26) = 18.34, p < 0.001]. Both drug groups displayed significantly greater SCRs to the CS+E during early extinction [THC = 0.28 ± 0.05; PBO = 0.28 ± 0.05] compared to late extinction [THC = 0.14 ± 0.06; t (13) = 2.41, p = 0.03; PBO = 0.12 ± 0.03; t (13) = 3.00, p = 0.01] (Figure 2A). Of note, elevated SCRs to the CS+E during early extinction learning were comparable to SCRs to the CS+E during fear acquisition in both drug groups [THC: t(13) = 1.38, p = 0.19; PBO: t(13) = 0.27, p = 0.79], supporting successful acquisition and next day retention of conditioned fear. Moreover, both groups demonstrated equivalent levels of extinction learning, as evidenced by their mean Extinction Learning Index [THC = 83.45 ± 6.30%; PBO = 80.35 ± 6.01%; t(24) = 0.36, p = 0.73 (Figure 2B)].
Figure 2.
A, Mean SCRs to the CS+E and CS− during early and late extinction learning. B, Mean Extinction Learning Index values. C, Between-group voxel-wise statistical t map overlaid on a canonical brain rendering (MNI coronal, y-plane = 0) showing attenuated AMYG reactivity to the CS+E (> CS−) during early extinction learning in the THC group compared to the PBO group. Image is masked to show only the activation in this hypothesized brain region. Threshold for displaying the image is set a p = 0.05; color bar represents statistical t scores. D, Mean BOLD response (β weights ± SEM) from the left AMYG (5 mm radius sphere from all voxels around the peak MNI coordinate [−26, −8, −12]) showing activation to CS+E (> CS−) in the PBO group and deactivation in the THC group. PBO (green bars) and THC (red bars).
In the THC group SCRs to the CS− were greater during early extinction [0.23 ± 0.07] compared to late extinction [0.08 ± 0.03; t (13) = 2.20, p = 0.05]; there was no difference in the PBO group [Early = 0.19 ± 0.04; Late = 0.12 ± 0.04; t (13) = 1.50, p = 0.16]. In addition, there were no significant differences in SCRs to the CS+E and CS− during early extinction and late extinction [absence of a stimulus by time interaction: F(1,26) = 0.37, p = 0.55], as well as no significant differences in SCRs between the THC and PBO groups [absence of a significant main effect of drug: F(1,26) = 0.02, p = 0.90; drug by stimulus interaction: F(1,26) = 0.25, p = 0.63; drug by time interaction: F(1,26) = 0.21, p = 0.65; and a drug by stimulus by time interaction: F(1,26) = 0.87, p = 0.36].
3.2.2 US Expectancy
An ANOVA of US expectancy ratings with three, two-level factors – drug (THC, PBO), stimulus (CS+E, CS-), and time (early extinction, late extinction) – revealed a significant main effect of stimulus [F(1,26) = 32.52, p < 0.001], a significant main effect of time [F(1,26) = 25.99, p < 0.001], and a significant stimulus by time interaction [F(1,26) = 16.90, p < 0.001]. Both drug groups displayed significantly greater US expectancy ratings to the CS+E during early extinction [THC = 2.67 ± 0.24; PBO = 3.24 ± 0.20] compared to late extinction [THC = 1.64 ± 0.32; t(13) = 2.46, p = 0.03; PBO = 1.43 ± 0.25; t (13) = 6.25, p < 0.001]. There were no significant differences in US expectancy ratings for the CS− within drug groups between early [THC = 1.71 ± 0.24; PBO = 1.57 ± 0.22] and late extinction [THC = 1.36 ± 0.20; t (13) = 1.26, p = 0.23; PBO = 1.21 ± 0.15; t (13) = 1.82, p = 0.09]. Additionally, both drug groups displayed significantly greater US expectancy ratings to the CS+E compared to the CS− [THC = t (13) = 3.55, p = 0.004; PBO = t (13) = 5.44, p < 0.001] during early extinction, but during late extinction US expectancy ratings to the CS+E and CS− were not significantly different [THC = t (13) = 0.94, p = 0.37; PBO = t (13) = 1.39, p = 0.19]. There were no significant differences in US expectancy ratings between the THC and PBO groups, as evidence by the absence of a significant main effect of drug [F(1,26) = 0.01, p = 0.93], drug by stimulus interaction [F(1,26) = 1.39, p = 0.25], drug by time interaction [F(1,26) = 1.28, p = 0.27], and a drug by stimulus by time interaction [F(1,26) = 2.32, p = 0.14].
3.2.3 Brain Activation
During early extinction learning the PBO group displayed increased activation in the left AMYG (peak MNI coordinate [−26, −8, −12]; volume = 272mm3; Z = 2.84; p < 0.05 FWE) to the CS+E (> CS-) compared to the THC group (Figure 2C). Follow-up ROI analyses on the extracted BOLD signals (β weights) from the left AMYG clarified the direction of THC effects and confirmed left AMYG activation to CS+E in the PBO group, which was attenuated in the THC group (Figure 2D; mean β ± SEM: PBO, 0.15 ± 0.07 vs. THC, −0.10 ± 0.07; Cohen’s d =1.01). During late extinction there were no significant differences in brain activation within our a priori ROIs between the two groups.
3.3 Extinction Recall Test
3.3.1 SCR
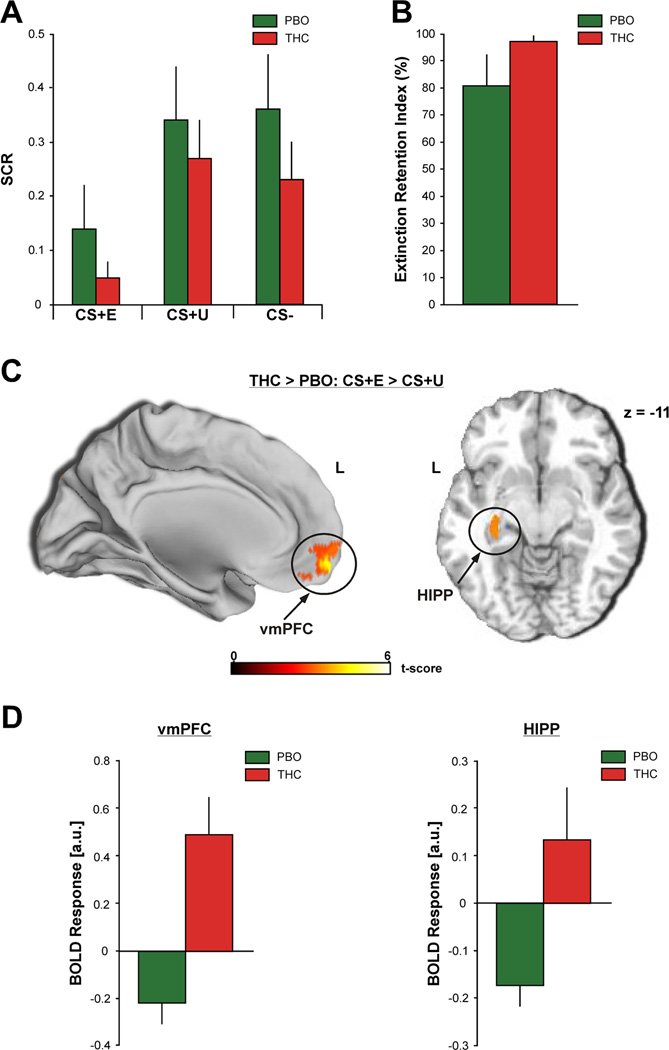
An ANOVA of SCR with two factors – drug (THC, PBO) and stimulus (CS+E, CS+U, CS-) – revealed a significant main effect of stimulus [F(2,52) = 7.47, p < 0.001]. Both drug groups displayed significantly smaller SCRs to the CS+E [THC = 0.05 ± 0.03; PBO = 0.14 ± 0.08] compared to the CS+U [THC = 0.27 ± 0.07; t (13) = −3.00, p = 0.01; PBO = 0.34 ± 0.10; t (13) = − 3.81, p = 0.002] and the CS− [THC = 0.23 ± 0.07; t (13) = −2.24, p = 0.04; PBO = 0.36 ± 0.10; t (13) = −2.31, p = 0.04] during extinction recall (Figure 3A). In addition, responses to the CS+E were not significantly different from those displayed during late extinction learning [THC: t (13) = −1.30, p = 0.22; PBO: t (13) = 0.27, p = 0.79], further suggesting good retention of extinction memory. Contrary to our hypothesis, SCR magnitude to the CS+E was not significantly different between the THC and PBO groups [absence of main effect of drug: F(1,26) = 1.16, p = 0.29; and drug by stimulus interaction: F(2,52) = 0.08, p = 0.92]. Interestingly, the THC group had a high Extinction Retention Index [97.25 ± 1.85%] with very little within group variation (Figure 3B). In contrast, the PBO group had a lower Extinction Retention Index [80.44 ± 11.51%], albeit not significantly lower [t(24) = 1.34, p = 0.19], and greater within group variability (Figure 3B). Of note, there was no significant difference in SCRs to the CS+U and CS− [THC = t (13) = 0.37, p = 0.72; PBO = t (13) = −0.12, p = 0.90].
Figure 3.
A, Mean SCRs to the CS+E (left), CS+U (middle), and CS− (right) during the extinction recall test. B, Mean Extinction Retention Index values. C, Between-group voxel-wise statistical t map overlaid on a canonical brain rendering showing increased vmPFC (MNI sagittal) (left) and HIPP (MNI horizontal, z-plane = −11) (right) reactivity to the CS+E (> CS+U) during the extinction recall test in the THC group compared to the PBO group. Images are masked to show only the activations in these a priori brain regions. Threshold for displaying the images are set at p = 0.05; color bar represents statistical t scores. D, Mean BOLD response (β weights ± SEM) from the vmPFC (5 mm radius sphere from all voxels around the peak MNI coordinate [−4, 60, −12]) (left) and the left HIPP (peak MNI coordinate [−30, −6, −16]) (right) showing activation to CS+E (> CS+U) in the THC group compared to the PBO group. PBO (green bars) and THC (red bars).
3.3.2 US Expectancy
An ANOVA of US expectancy with two factors – drug (THC, PBO) and stimulus (CS+E, CS+U, CS-) – revealed a significant main effect of stimulus [F(2,48) = 13.92, p < 0.001]. Both drug groups displayed significantly lower US expectancy ratings to the CS− [THC = 1.67 ± 0.26; PBO = 1.57 ± 0.25] compared to the CS+E [THC = 2.62 ± 0.33; t (11) = 2.42, p = 0.03; PBO = 2.50 ± 0.25; t (13) = 3.24, p = 0.006] and CS+U [THC = 2.62 ± 0.29; t (11) = 2.57, p = 0.03; PBO = 3.21 ± 0.24; t (13) = 4.14, p = 0.001] during extinction recall. In the PBO group US expectancy ratings to the CS+E were lower compared to the CS+U [t (13) = −2.50, p = 0.03]; there was no difference between the CS+E and CS+U in the THC group [t (11) = −0.18, p = 0.86]. There were no significant differences between the drug groups on US expectancy rating, as evidenced by the absence of a significant main effect of drug [F(1,24) = 0.23, p = 0.64] and drug by stimulus interaction [F(248) = 1.03, p = 0.37].
3.3.3 Brain Activation
Within our a priori regions participants who received THC during extinction learning showed significantly more activation in the vmPFC (peak MNI coordinate [−4, 60, −12]; volume = 5344mm3; Z = 3.96; p = 0.03 FWE; Figure 3C, left) and the left HIPP (peak MNI coordinate [−30, −6, −16]; volume = 184mm3; Z = 3.57; p = 0.04 FWE; Figure 3C, right) to the CS+E (> CS+U) during extinction recall compared to participants who had received PBO during extinction learning. Follow-up ROI analyses on the extracted BOLD signals from these regions revealed relative deactivation within these regions in the PBO group, which was reversed in the THC group (mean β ± SEM: vmPFC: PBO, −0.22 ± 0.09 vs. THC, 0.49 ± 0.16; Cohen’s d = 1.63; Figure 3D, left; HIPP: PBO, −0.18 ± 0.04 vs. THC, 0.13 ± 0.11; Cohen’s d = 1.14; Figure 3D, right). Of note, there was no significance difference in vmPFC or HIPP activation to the CS+U between the THC and PBO groups.
Since the Extinction Retention Index was more variable across participants in the PBO group than the THC group, a Pearson’s correlation coefficient was computed to assess the relationship between the Extinction Retention Index and the individual BOLD signals from the vmPFC and HIPP during extinction recall (CS+E > CS+U) in the PBO group. We did not detect a significant correlation between the Extinction Retention Index and vmPFC (r(11)= −0.24, p = 0.43) or HIPP (r(11) = −0.28; p = 0.36 ) activation in the PBO group.
4. Discussion
In the present study we found that THC attenuated AMYG reactivity to a CS that was previously paired with an aversive US (CS+E > CS-) during early extinction learning compared to PBO. In addition, participants who received THC during extinction learning showed significantly more activation in the vmPFC and the HIPP to the CS+E (> CS+U) during extinction recall compared to participants who had previously received PBO during extinction learning. Together, these findings provide the first evidence that pre-extinction administration of THC modulates the underlying neural circuits involved in fear extinction in humans.
During extinction learning we expected the PBO and THC groups to successfully extinguish conditioned fear responses (e.g. SCRs and US expectancy ratings) to the CS+E and did not expect THC to have an effect on within-session extinction learning, consistent with our previous findings (Rabinak, et al., 2013). In addition, we hypothesized that during early extinction learning presentations of the CS+E would elicit increased AMYG activation (compared to the CS-), consistent with prior fMRI studies in healthy humans that have shown that AMYG activation is associated with the expression of conditioned fear responses (e.g. SCR) (LaBar, et al., 1998; Milad, et al., 2007; Phelps, et al., 2004). However, since we did not expect THC to have an effect on the expression of behavioral fear responses during extinction learning we did not anticipate THC to have an effect on AMYG activity during extinction learning. As expected, both drug groups displayed significantly greater conditioned fear responses to the CS+E during early extinction compared to late extinction (Figure 2A) and there was no effect of THC on behavioral indices of extinction learning. Moreover, both groups demonstrated equivalent levels of extinction learning, as evidenced by their mean Extinction Learning Index (Figure 2B). During early extinction learning presentations of the CS+E (> CS-) elicited increased activation in the left AMYG; however THC attenuated AMYG responding to the CS+E (Figures 2C and 2D).
Based on previous research using rodent models of fear, it has been hypothesized that during extinction learning eCB release in the AMYG may promote the extinction of fear responses via two pathways: 1) activation of CB1 receptors on GABAergic interneurons within the AMYG, which decrease GABAergic transmission, and thus lead to the potentiation of glutamatergic “extinction” pathways; and/or 2) activation of CB1 receptors on glutamatergic neurons within the AMYG, which decrease glutamate transmission, and thus lead to a de-potentiation of “fear” pathways (Lafenetre, Chaouloff, & Marsicano, 2007; Marsicano et al., 2002). Although THC’s effect on AMYG reactivity to the CS+E during early extinction learning was unanticipated it is consistent with previous fMRI studies in humans using a social-threat paradigm. For instance, a study conducted in our lab found that oral THC (vs. PBO) attenuates AMYG reactivity to aversive/fear stimuli (fearful and angry faces) (Phan, et al., 2008) and others have shown that the level of AMYG reactivity is inversely related to the level of cannabis use (Cornelius, Aizenstein, & Hariri, 2010). Besides THC, CBD has also been shown to attenuate AMYG to fearful faces (Bhattacharyya et al., 2010; Fusar-Poli et al., 2009). Studies using imaging genetics (coupling functional brain imaging with genotyping) in humans have shown that genetic variation in FAAH inhibition (FAAH 385A), which would alter the extent of hydrolysis of anandamide and thereby increase endocannabinoid signaling, is associated with decreased AMYG reactivity to threatening faces (Gunduz-Cinar et al., 2012; Hariri et al., 2009), further supporting a role for the cannabinoid system in fear regulation. Collectively, these data demonstrate that modulation of the cannabinoid system would have down-stream effects on the neural substrates involved in processing signals of fear (e.g., AMYG reactivity to fearful faces/CS+s).
In a previous study, we have demonstrated that pre-extinction administration of THC facilitates extinction of conditioned fear in humans (Rabinak, et al., 2013). Specifically, compared to subjects that received PBO, participants that received THC prior to extinction learning displayed low SCR to a previously extinguished CS during extinction memory recall 24 hours after extinction learning, suggesting that THC prevented the recovery of fear. In the present study we expected to find similar results on behavioral indices of fear within and between the PBO and THC groups during extinction memory recall. We found that the PBO and THC groups displayed significantly smaller SCRs to the CS+E compared to the CS+U during extinction recall (Figure 3A); however, there was no significant difference between the THC and PBO groups. This finding is consistent with findings from another group that also did not detect an effect of THC on fear extinction (Klumpers et al., 2012). It is not entirely clear what factors may have contributed to the conflicting results of the present study with those from our previous study (Rabinak, et al., 2013), given that the study designs were similar, with the exception that in the present study extinction learning and recall were conducted in the scanner. One possibility may be potential context-specificity of the effects of THC. For instance, a previous study using d-cycloserine (DCS), a N-methyl-d-aspartic acid (NMDA) receptor partial agonist, as a potential “cognitive enhancer,” suggested that DCS may exert its effects through some form of context learning rather than on the conditioned association between the CS and US (Guastella, Lovibond, Dadds, Mitchell, & Richardson, 2007). Another possibility may be differential sensitivity to the effects of THC between individuals (Bhattacharyya et al., 2012; Henquet, Di Forti, Morrison, Kuepper, & Murray, 2008) contributing to larger variability and smaller effect size. In addition, in the present study SCR levels were very low in the PBO group; this may have created a “floor effect,” leaving little opportunity for THC to further reduce SCRs. A more sensitive approach to test THC’s potential to enhance extinction recall may be to use a population that exhibits extinction recall deficits, such as those seen in patients with PTSD, (Bailey, Cordell, Sobin, & Neumeister, 2013; Milad et al., 2008; Milad, et al., 2009; Neumeister et al., 2013), which are currently underway. Moreover, THC may not be effective in a non-clinical population. In fact, studies using dcs as a potential “cognitive enhancer,” have only shown enhancement of fear extinction in anxiety populations and no effect in healthy participants (Davis, Ressler, Rothbaum, & Richardson, 2006; Guastella, Dadds, Lovibond, Mitchell, & Richardson, 2007; Guastella, Lovibond, et al., 2007; Guastella et al., 2008; Hofmann, 2007, 2008; Ledgerwood, Richardson, & Cranney, 2003, 2004, 2005; Norberg, Krystal, & Tolin, 2008; Ressler et al., 2004; Walker, Ressler, Lu, & Davis, 2002). Therefore, additional studies are needed to reconcile some of these findings and investigate the whole range of THC effects on extinction of fear responses.
Of note, in the present study all participants in the THC group had a near perfect recall of the extinction memory, as evinced by their high Extinction Retention Index, whereas the PBO group had a relatively lower percentage of successful extinction retention with greater within group variability (Figure 3B). This finding may suggest that perhaps THC was able to facilitate extinction recall in those individuals who may have otherwise performed worse had they not received THC prior to extinction learning. The between-subject nature of our drug condition does not allow us to explore this possibility and future studies are needed to further test this. Like with the previous question, our study might have been underpowered to test some of the THC effects and these might be further clarified with a larger sample size.
Within our a priori regions participants who received THC during extinction learning showed significantly more activation in the vmPFC and the left HIPP to the CS+E (> CS+U) during extinction recall compared to participants who had previously received PBO during extinction learning. These results are in line with previous rodent studies that have suggested that during extinction activation of vmPFC CB1 receptors induces neuronal plasticity, which subsequently increases top-down inhibition on fear-output neurons in the AMYG (Lin, et al., 2009) and activation of HIPP CB1 receptors may support long-term extinction memory formation via enhanced glutamatergic neurotransmission (de Oliveira Alvares, et al., 2008). Surprisingly, follow-up ROI analyses on the extracted BOLD signals from these regions revealed deactivation within these regions in the PBO group, which was reversed in the THC group. In our study, vmPFC and HIPP activation did not track extinction retention (e.g. SCR) to the CS+E in the PBO group during extinction recall, which raises the question whether this specific experimental design optimal for recruiting the fear extinction recall network. One factor contributing to the lack of vmPFC-HIPP activation in the PBO group may be the large within group variability of extinction recall success (Extinction Retention Index), which may have masked any vmPFC and/or HIPP activation in those participants that had good recall.
This study provides the first evidence that pre-extinction administration of THC modulates prefrontal-limbic circuits during fear extinction in humans. Together with previous rodent and human findings, these results prompt future investigation to test if cannabinoid agonists can rescue or correct the impaired behavioral and neural function during extinction recall in patients with PTSD (Inslicht et al., 2013; Milad, et al., 2008; Milad, et al., 2009; Rougemont-Bucking et al., 2011). Ultimately, the cannabinoid system may serve as a promising target for innovative intervention strategies (e.g. pharmacological enhancement of exposure-based therapy) in PTSD and other fear learning-related disorders (Bailey, et al., 2013; Rabinak & Phan, 2013).
Acknowledgements
This work was supported by a grant from the National Center for Research Resources (UL1RR024986) and from the National Institute of Mental Health (1R21MH093917-01A1) to KLP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bailey CR, Cordell E, Sobin SM, Neumeister A. Recent progress in understanding the pathophysiology of post-traumatic stress disorder: implications for targeted pharmacological treatment. CNS Drugs. 2013;27(3):221–232. doi: 10.1007/s40263-013-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D, McGuire PK. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of [delta]-9-tetrahydrocannabinol on midbrain and striatal function. Molecular Psychiatry. 2012;17(12):1152–1155. doi: 10.1038/mp.2011.187. doi: http://www.nature.com/mp/journal/v17/n12/suppinfo/mp2011187s1.html. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. European Neuropsychopharmacology. 2008;18(12):849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Critical Reviews in Neurobiology. 1996;10(3–4):419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30(3):516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35(6):644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RK, Kamboj SK, Ramadas M, Yogan K, Gupta V, Redman E, Morgan CJ. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60(4):369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience. 2008;154(4):1648–1655. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzter RL, Gibbon M, Williams JB. Structured clinical interivew for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Reserach Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foa EB. Prolonged exposure therapy: past, present, and future. Depress Anxiety. 2011;28(12):1043–1047. doi: 10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol. 1999;67(2):194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2(4):189–210. [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, McGuire PK. Distinct effects of {delta }9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41(6):466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45(4):663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63(6):544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Macpherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Manuck SB. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biological Psychiatry. 2009;66(1):9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J Trauma Stress. 2003;16(6):555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- Henquet C, Di Forti M, Morrison P, Kuepper R, Murray RM. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34(6):1111–1121. doi: 10.1093/schbul/sbn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Craske MG, Mineka S, Lovibond PF. Extinction in human fear conditioning. Biol Psychiatry. 2006;60(4):361–368. doi: 10.1016/j.biopsych.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Enhancing exposure-based therapy from a translational research perspective. Behav Res Ther. 2007;45(9):1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clinical Psychology Review. 2008;28(2):199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Goff DC. Extinction memory is impaired in schizophrenia. Biological Psychiatry. 2009;65(6):455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, Neylan TC. Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res. 2013;47(1):64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99(2):217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63(1):137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Delta9-THC and D-cycloserine on extinction of conditioned fear in humans. J Psychopharmacol. 2012;26(4):471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56(5):367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117(2):341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118(3):505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57(8):841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem. 2008;15(12):876–884. doi: 10.1101/lm.1081908. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13(3):316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19(1):165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Huang Y. Elevated brain cannabinoid CB receptor availability in post-traumatic stress disorder: a positron emission tomography study. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90(1):290–293. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188(4):641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28(10):2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry. 2001;62(Suppl 17):47–54. [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33(1):56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Phan KL. Cannabinoid Modulation of Fear Extinction Brain Circuits: A Novel Target to Advance Anxiety Treatment. Curr Pharm Des. 2013 doi: 10.2174/13816128113199990437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Sripada CS, Angstadt M, de Wit H, Phan KL. Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. J Neural Transm. 2012;119(6):701–707. doi: 10.1007/s00702-011-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robbins S. Mechanisms underlying spontaneous recovery in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16(3):235–249. [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105(2):325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Astin MC, Marsteller F. Prolonged Exposure versus Eye Movement Desensitization and Reprocessing (EMDR) for PTSD rape victims. J Trauma Stress. 2005;18(6):607–616. doi: 10.1002/jts.20069. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T(*)(2)-weighted functional MRI. Magn Reson \Med. 2000;44(4):525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24(20):4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Minnen A, Hagenaars M. Fear activation and habituation patterns as early process predictors of response to prolonged exposure treatment in PTSD. Journal of Traumatic Stress. 2002;15(5):359–367. doi: 10.1023/A:1020177023209. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32(5):1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161(4):331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D. MARINA: An easy tool for the creation for MAsks for Region of Interest Analyses; Paper presented at the 9th International Conference on Functional Mapping of the Human Brain; New York, N.Y.. 2003. Jun 19–22, 2003. [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry. 2011;70(10):920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]