Abstract
Background
Use and effectiveness of tobacco quitlines by weight is unknown.
Purpose
Determine if baseline weight is associated with treatment engagement, cessation or weight gain following quitline treatment.
Methods
Quitline participants (n=595) were surveyed at baseline, three and six months.
Results
Baseline weight was not associated with treatment engagement. In unadjusted analyses, overweight smokers reported higher quit rates and were more likely to gain weight after quitting than obese or normal weight smokers. At three months, 40% of overweight vs. 25% of normal weight or obese smokers quit smoking (p=0.01); 42% of overweight, 32% of normal weight, 33% of obese quitters gained weight (p=0.05). After adjusting for covariates, weight was not significantly related to cessation (approaching significance at six months, p=.06) or weight gain.
Conclusions
In the first quitline study of this kind, we found no consistent patterns of association between baseline weight and treatment engagement, cessation or weight gain.
Keywords: obesity, smoking, weight gain, quitline
Background
The comorbidity of obesity and smoking is responsible for a substantial burden of personal suffering, functional impairment, and disease.[1–3] While smoking cessation can drastically reduce disease risks,[4] weight gain that commonly accompanies quitting can be problematic for this population. Research indicates that 80% of smokers will gain weight after quitting with an average weight gain of 8–16 pounds [5–16] and some smokers gain more than 20 pounds.[5–9,11–12,15,17] Importantly, evidence suggests that obese smokers have lower quit rates, [18–19] tend to gain more weight after quitting, [12,20] and are more concerned about cessation-related weight gain than normal weight and overweight smokers. [21–23]
Concerns about gaining weight after quitting are important because of their association with cessation treatment adherence, cessation outcomes [15,24–26] and weight gain. [11,27] Although research on the most efficacious treatment for smoking related weight concerns has been inconclusive [28], recent reviews suggest that combining weight-focused and smoking cessation interventions together does not necessarily have a negative impact on quit rates. [29,30] Since these prior studies involved intensive, in-person group interventions and often only enrolled women, there remains uncertainty in whether such treatments can be disseminated on a population level (e.g. through a state quitline). In fact, the lack of a definitive treatment approach for smokers with weight concerns can lead to confusion among cessation treatment specialists who provide telephone counseling through state quitlines. [31] Tobacco quitlines are the most widely used and effective forms of cessation treatment: they are available in all states and territories in the United States; and, are recommended by the Tobacco Clinical Guidelines. [28, 32–33] Quitlines provide a potential avenue for integration of weight-based content to smoking cessation treatment that may be feasible and acceptable to obese smokers. However, little is known of the needs of overweight and obese smokers who use quitlines.
Given that the sparse literature that exists suggests obese smokers who try to quit smoking are less successful and are more likely to gain weight after quitting, there remains an urgent need to develop and test population based cessation treatments that can improve quit rates and prevent cessation related weight gain in obese smokers. However, prior to such treatment development, it is first necessary to determine if standard quitline interventions need to be changed or if they are equally effective as is, across all weight groups. In fact there are no data on the use and effectiveness of state quitlines across the body mass index spectrum for both smoking cessation efficacy and weight gain following abstinence. Two previous quitline studies that included weight focused interventions (addressing weight concerns or offering weight loss treatment for those who successfully complete cessation treatment) [34,35] only recruited smokers with significant weight concerns, only those calling a single quitline and data on weight gain and abstinence were not reported separately by baseline weight. To address these limitations, the present observational study was designed to determine among quitline participants if baseline weight is associated with: 1) engagement in quitline services (number of counseling calls completed and use of nicotine replacement therapy); 2) the effectiveness of quitlines (abstinence at three and six months); and, 3) weight gain after quitting. Given the limited data available regarding these associations in a quitline setting, we had no apriori hypotheses about the impact baseline weight would have on treatment engagement and outcomes.
Methods
This observational study was conducted by Alere Wellbeing, the largest service provider for state quitlines in the U.S. Adult smokers who called one of the five state quitlines (Georgia, Louisiana, Maryland, South Carolina, and Texas) between August 10, 2010 and December 29, 2010 were asked questions about their height, weight, and concerns about gaining weight after quitting. Study questions were added to the standard questions asked when a person calls the quitline and registers for services. Individuals who met eligibility requirements (18 years or older, not pregnant, English speakers, smoked at least five cigarettes per day, provided a phone number and address, and were ready to quit within 30 days) were invited to participate in the study. Recruitment goals were to enroll participants of all body weights but with a proportional grouping following standard cut-offs for normal weight, overweight, and obese. Eligibility questions and consent scripts were built into the quitline’s service delivery application. An automated algorithm calculated body mass index from self-reported height and weight using standard methods and classified individuals as normal weight (body mass index = 18.5 to 24.99 kilogram/meter2), overweight (body mass index = 25 to 29.99 kilogram/meter2), or obese (body mass index ≥ 30 kg/m2). [36] Eligible and interested individuals were transferred to a specially trained Quit Coach® who obtained informed verbal consent and baseline data, and completed the first comprehensive counseling call per the standard quitline protocol. There were no changes to quitline services provided. An external independent survey company administered telephone surveys at three and six months, and mailed gift cards upon completion of each survey ($15 and $20, respectively). This study was approved by the Western Institutional Review Board on July 1, 2009.
Standard Tobacco Quitline
Quitline services were funded by various state organizations and provided by Alere Wellbeing (formerly Free & Clear). Typically, state quitline provide, at no cost to the participant, a minimum of one counseling call, unlimited inbound (participant-initiated) calls for additional support, mailed quit guides, and online (web-based) support. Some quitlines also provide nicotine replacement therapy which could include from 2 to 8 weeks of patch, gum, or lozenge. For states participating in the study, one state provided a single counseling call, one provided four counseling calls, and three provided five counseling calls. All states encouraged participants to call the quitline between proactive calls or after completing treatment if they wanted extra support. Three of the states offered free nicotine replacement therapy. The phone-based counseling which follows the Tobacco Clinical Guidelines [28] was delivered by highly trained Quit Coaches. Counseling is designed to increase cessation self-efficacy and includes practical advice on how to quit tobacco, setting a quit date, developing a quit plan, obtaining social support, developing problem solving and coping skills to deal with cravings and urges, and educational information on medication use and side-effect management. Quit Coaches do not discuss weight control. However, if participants bring up the topic of weight gain, Quit Coaches are prepared to address their concerns. For example, the coach may normalize weight gain associated with cessation and/or discuss ways to cope with cravings such as sucking on straws or low calorie snacks.
Participant Measures
Baseline measures included demographics (e.g. age, gender, race, ethnicity), self-reported height and weight, tobacco use (type, amount, duration), self-efficacy in quitting and chronic disease status. Self-efficacy was assessed by asking: ‘how confident are you that you can quit smoking for good on a scale of 1 (not at all confident) to 10 (very confident)’? Chronic disease was assessed by asking participants if they have ever been diagnosed with asthma, coronary artery disease, chronic obstructive pulmonary disease, or diabetes (for each condition, response option was yes, no, or don’t know). Additional questions asked at baseline included the short form of the Patient Health Questionnaire depression scale [37] and two 6-item scales developed by Borelli and Mermelstein [38] to assess weight concerns and weight efficacy after quitting. These latter questions ask participants to rate their level of weight concerns (e.g. how likely is it that you would go back to smoking after quitting if you gained too much weight) and confidence in quitting (e.g. how confident are you that you can avoid gaining weight while staying quit?). Participants were asked to use a 10 point rating scale where 1= not at all and 10=very. Summary scores represent the average response across the six items (range 1–10) and are calculated separately for weight concerns and the weight efficacy after quitting scales.
Follow-up measures included smoking status (‘when was the last time you smoked a cigarette, even a puff’), use of nicotine replacement therapy since enrolling with the quitline, self-efficacy in quitting, weight concerns, the weight efficacy after quitting scale, and self-reported weight in pounds. Two additional questions assessed perceived change in weight (asked only at three months): ‘which statement best describes you since enrolling with the quitline: ‘my weight did not change’, ‘I gained weight’, ‘I lost weight’ and ‘how much weight did you gain/lose’. Number of counseling calls completed and shipment of nicotine replacement therapy were obtained from Alere Wellbeing’s custom database for each participating quitline.
Analyses
Chi-square or Fisher exact tests were used to compare proportions of categorical variables and ANOVA, or Kruskal-Wallis tests, to compare continuous variables across three weight groups. We used logistic or linear regression procedures for multivariate analyses.
For treatment engagement, we report mean (s.d.) number of counseling calls completed and the percent who used nicotine replacement therapy.
For cessation, we report both the responder and the intent-to-treat quit rates (non-respondents were assumed to be smokers). Logistic regression models were used to model intent-to-treat cessation rates (7-day and 30-day point prevalent abstinence) at three and six- months as a function of baseline body mass index group (normal weight, overweight, obese) adjusting for covariates.
For weight outcomes, we excluded two participants from the three-month quitters and five participants from the six-month quitters because they reported having had or were planning to have weight-loss surgery within six months of baseline. We then applied mixed-effect models to weight outcomes at baseline, three- and six-month follow-up, and set up contrasts to compare changes in weight among those who were quit for at least 30-days across baseline body mass index groups (normal weight, overweight, and obese), adjusting for other covariates. In order to provide benchmarks on the overall proportion of quitline participants who gained weight after quitting (and amount they gained), we report results of both perceived weight change and calculated weight change. Both measures provide distinct and important information about participants. We created a new variable (calculated change in weight) which was the difference between self-reported weight in pounds at baseline and follow-up. Calculated change in weight may be useful for comparisons with other studies and perceived weight gain may have a stronger effect on subsequent smoking behavior than actual weight gain. For both measures we report weight change only among abstinent smokers (at three or six months). The use of abstinent smokers is the standard method since comparing weight change for the whole sample would be confounded by smoking and thus is not an indication of post-cessation weight gain. [30, 39]
For perceived change in weight we report the categorical variable; no change, lost weight, gained weight since enrolling with the quitline. We also report perceived change in weight as a continuous variable using participants’ estimates of the amount of weight they gained or lost (mean perceived weight loss among those who quit and lost weight and mean perceived amount gained among those who quit and gained weight).
For calculated change in weight (difference in weight reported at baseline and follow-up), we compared weight groups on the mean change in weight and the proportion who lost weight or reported no change, or gained 1–4; 5–10, >10 pounds.
For adjusted analyses of cessation and weight outcomes, we included covariates such as gender, race, ethnicity, education level, insurance status, chronic disease, current depressive symptoms, weight concerns, years smoking, use of nicotine replacement therapy, and number of counseling calls completed. We also conducted post-hoc analyses to explore potential moderators of treatment effects for both outcomes. We included separate interaction terms between weight group and variables known to influence outcomes; gender, chronic disease, weight concerns, number of calls completed and nicotine replacement therapy.
Results
Recruitment
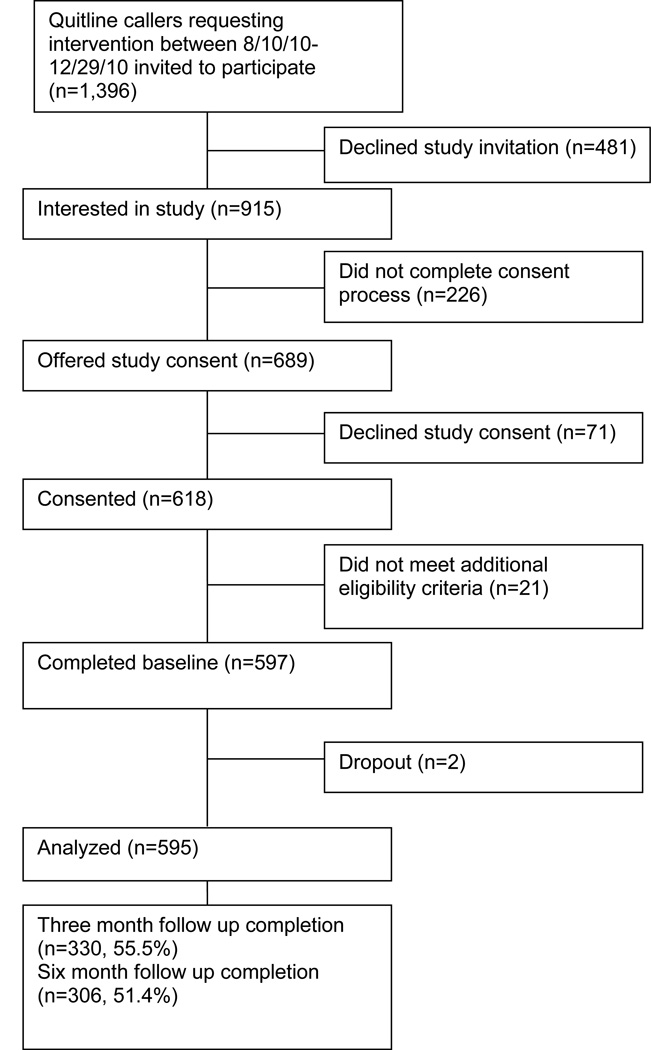
As shown in Figure 1, 1,396 adult tobacco users who called one of five state quitline were potentially eligible and asked if they were interested in a study, 915 (65.5%) were interested in hearing more about the study, 689 were eligible, 618 provided verbal consent and 597 completed the baseline. Two individuals dropped out of the study and 595 (86.4% of the 689 invited to participate) were included in the analyses. As planned, the distribution of participants was approximately proportional across weight groups; 34.6% normal weight, 30.6% overweight, 34.8% obese. The majority of participants (544/595) were from states that offered the multi-call program (170 were eligible for four calls, 374 for five calls), and 51 were eligible for 1 call. All states offered unlimited access to the quitline for support. As we previously reported, [23] weight groups were similar on most of the demographic and tobacco variables assessed, with the exception of a higher proportion of females and greater weight concerns among obese smokers. State offerings and number of calls completed did not differ significantly across weight groups.
Figure 1.
Study flow diagram among eligible Quitline callers invited to participate
Treatment engagement
The mean number of counseling calls completed based on all study participants including those only eligible for one call was 1.8 ± 1.2 and did not differ by baseline weight. Among those enrolled in the multi-call program, mean number of calls completed was 2.08 ± 1.8: normal weight: 1.98 ± 1.66, overweight: 2.21 ± 2.2, obese: 1.88 ± 1.36; p=0.19 (Table 1). Shipments of nicotine replacement therapy were marginally different by weight groups: 48.5% of normal weight, 46.2% of overweight and 57.5% of obese were mailed nicotine replacement therapy (p=.06). Self-reported use of nicotine replacement therapy at follow-up also did not differ among weight groups. At three months, 67% said they had used nicotine replacement therapy since enrolling with the quitline; 68% of normal weight, 61% of overweight and 72% of obese (p =.18). At six months, 69% said they had used nicotine replacement therapy; 71% of normal weight, 67% of overweight and 68% of obese (p=.80).
Table 1.
Engagement in treatment across baseline weight categories
| Group | Normal weight |
Overweight | Obese | p-value | Average |
|---|---|---|---|---|---|
| Mean (s.d.) counseling calls | 1.98 (1.66) | 2.21 (2.2) | 1.88 (1.36) | .19 | 2.08 |
| completed among those in | (1.8); | ||||
| multi-call program | n=483a | ||||
| % mailed nicotine | 48% | 46% | 57% | .06 | 50% |
| replacement therapy | n=595 | ||||
| % using nicotine | 68% | 61% | 72% | .18 | 67% |
| replacement therapy at 3 months |
n=330b | ||||
| % using nicotine | 71% | 67% | 68% | .80 | 69% |
| replacement therapy at 6 months |
n=306b |
Multi-call program includes 4 or 5 proactive calls plus unlimited in-bound calls
Used nicotine replacement therapy since enrolling among 1 call and multi-call participants who completed the follow-up survey
Follow-up survey results
All 595 participants were contacted for follow-up surveys; 330 (55.5%) completed the three-month survey and 306 (51.4%) completed the six-month survey. Those who responded to the surveys did not differ from non-responders on baseline data (e.g. age, gender, race, ethnicity, prior quit attempts, depression, weight concerns, weight efficacy after quitting or baseline weight. Three and six-month response rates were 54.4% and 48.5% for normal weight; 54.4% and 57.7% for overweight, and 61.8% and 51.7% for obese participants, (p=0.22 and 0.19, respectively).
Smoking cessation at follow-up
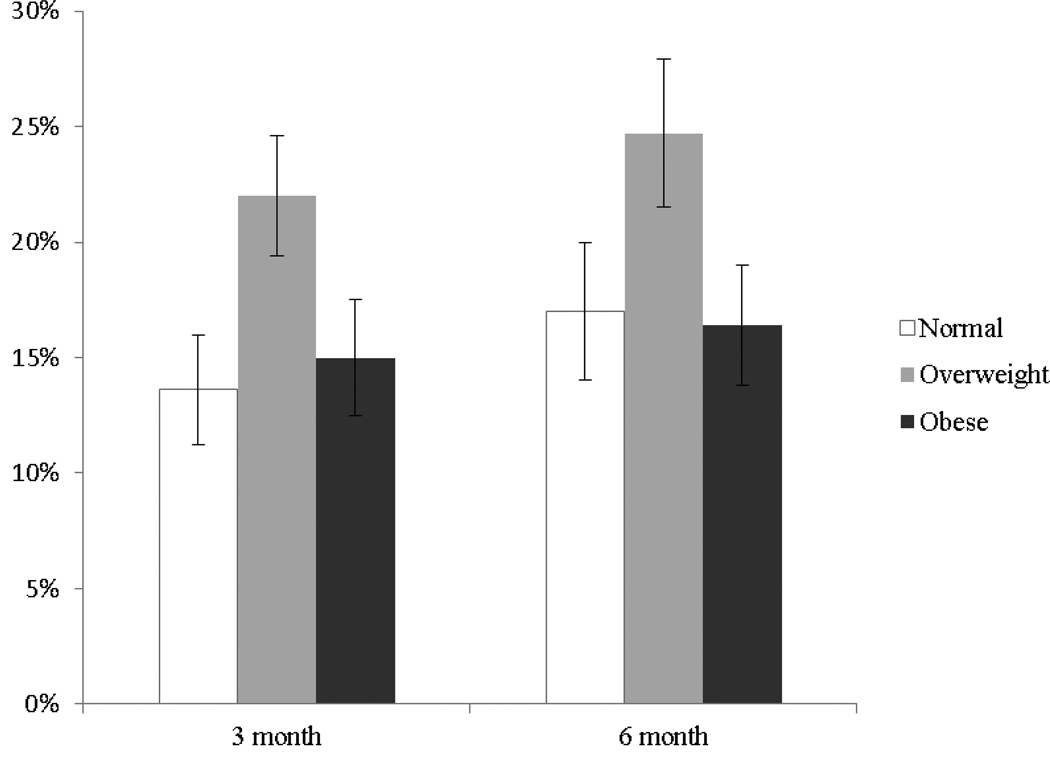
Table 2 shows respondent quit rates at three and six months for the three weight groups. At three months, those who were overweight reported a significantly higher 30-day respondent quit rate (15–16% higher) than normal weight or obese participants (p=.01). Respondent quit rates at six months were not significant across weight groups (p=.23). As shown in Figure 2, the intent-to-treat quit rates at three and six months were also higher for overweight participants. However, in adjusted analyses, baseline weight was not significant at three months (p=.12) and only marginally significant at six months (p=.06) (Table 3).
Table 2.
Unadjusted respondent 30-day quit rates at three and six months across baseline weight categories
| Normal weight |
Overweight | Obese | p-value | |
|---|---|---|---|---|
| N=206 | N=182 | N=207 | ||
|
Quit rate at three months |
||||
| 30-day respondenta | 25.0% | 40.4% | 24.2% | .01 |
|
Quit rate at six months |
||||
| 30-day respondentb | 35.0% | 42.9% | 31.8% | .23 |
N=330
N=306
Figure 2.
Unadjusted Intent To Treat 30-day quit rates at three and six months across baseline weight categoriesa
a n=595
Table 3.
Adjusted logistic regression analyses testing the effects of baseline weight on abstinence reported at three and six monthsa
| Three Monthb | 30-day tobacco abstinence OR |
95% CI |
|---|---|---|
| body mass index: normal vs. overweight |
1.60 | [0.87–2.94] |
| body mass index; normal vs. obese | 0.88 | [0.47–1.68] |
| Six Monthc | ||
| body mass index: normal vs. overweight |
1.69 | [0.97–2.94] |
| body mass index: normal vs. obese | 0.90 | [0.499–1.64] |
n=595, controlling for gender, race, ethnicity, education, insurance, weight concerns, years of tobacco use, chronic disease, depression, nicotine replacement therapy shipped and number of counseling calls completed.
p value for baseline weight 0.12
p value for baseline weight 0.06
Perceived change in weight among participants who were abstinent at three months
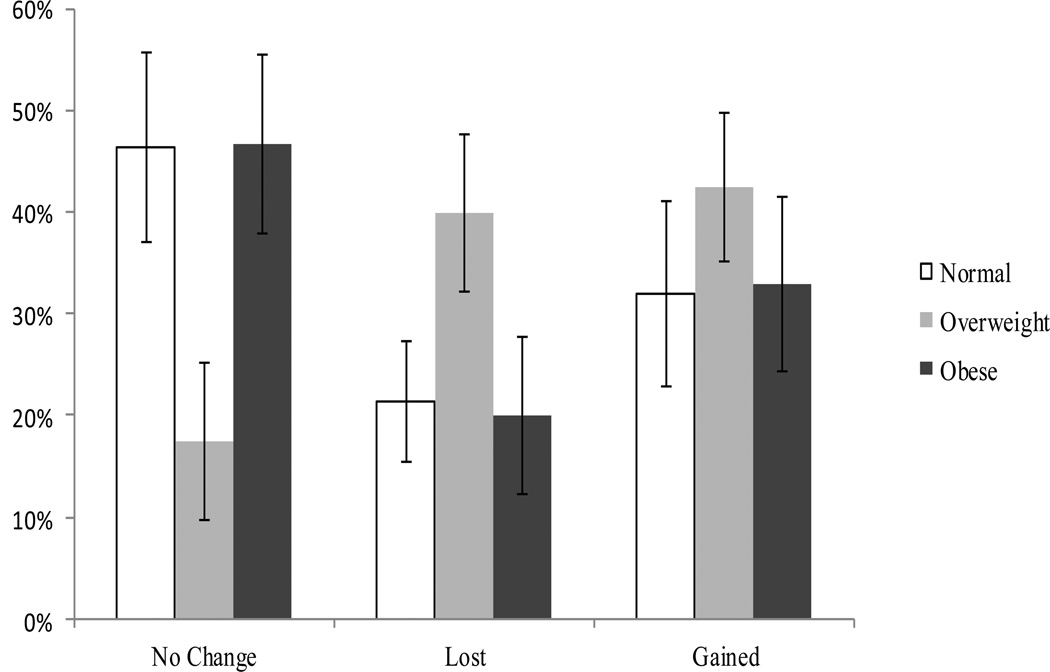
Among the 98 smokers who quit smoking for 30+ days at three months, 34.7% reported no change in weight, 28.6% lost weight and 36.7% said they gained weight (Figure 3). Overweight abstainers were more likely to report losing or gaining weight (40% and 42.5%) than were normal weight (21.4% and 32.0%) or obese (20.0% and 33.3%) abstainers (p=.05) but this was not significant after adjusting for relevant covariates. Overall, the average perceived change in weight was 2.6 ± 6.7 pounds (range = −9 to 30 pounds). Among those reporting a change in weight, the perceived amount of weight lost or gained also did not differ significantly across baseline weight groups (p=0.9).
Figure 3.
Perceived change in weight at three monthsa, showing the percentage of normal weight, overweight and obese participants who reported no change or lost or gained weight. Overall, 34.7% stayed the same; 28.6 lost weight; 36.7% gained weight; mean change=2.6±6.7 pounds*.
aamong 30 day abstainers; n=98
*p=.05
Calculated change in weight among participants who were abstinent at six months
Among the 113 who had been quit for 30+ days at six months, the mean change in weight (six-month weight minus baseline weight) was 2.7 ± 15.6 pounds (range = −81 to 75 pounds) and did not differ by baseline weight (p=.99). The proportion who lost weight, stayed the same or gained weight also did not differ significantly by baseline weight (p=.90). Among the 52 who quit and gained weight, the mean amount gained was 13.3 ± 11.9 pounds; 10% gained 1–4 pounds, 40% gained 5–10 pounds and 50% gained > 10 pounds. After adjusting for baseline characteristics, the calculated mean change in weight in quitters who gained weight was 6.8 pounds for normal weight, 5.0 pounds for overweight and 1.8 pounds for obese participants (p=0.6).
Exploratory analyses of moderators
We conducted additional analyses to explore potential moderators of treatment effects for cessation and weight outcomes as described in the analysis section. We found only one significant interaction. Compared to normal weight smokers who made an additional call to the quitline, overweight smokers who made an additional call were more likely to stay abstinent at six months (p = 0.045), and obese smokers who made an additional call did not differ from normal weight smokers with an additional call (p = 0.89).
Discussion
This is the first study conducted with state quitline participants to determine the association between baseline body mass index, engagement in quitline services and treatment outcomes for both smoking cessation and weight gain. It is also the first study to describe cessation related weight gain among smokers calling five state quitlines.
First, regarding treatment engagement, we found that baseline weight was not associated with calls completed or use of nicotine replacement therapy. It is important to note that among those eligible for 4–5 counseling calls, participants completed an average of two calls. This is consistent with data from other state quitlines [34,40,41] and prior research indicating that individuals often complete less than half of scheduled counseling sessions. [42] Efforts to boost treatment engagement may be needed since non-adherence to medications and counseling can reduce treatment effectiveness. [19,42–44]
Second, regarding cessation outcomes, we found that overweight smokers reported significantly higher quit rates than normal weight or obese smokers but only in unadjusted analyses at three months. This association was marginally significant at six months after controlling for other baseline characteristics (p=.06). Evaluation of potential moderators of effects showed that completing more counseling calls was significantly associated with better quit rates for overweight smokers but not for normal weight or obese smokers and only at six months. There is no clear scientific or clinical explanation for this finding. Notably, this result came from one of ten additional analyses of interactions and could be a statistical artifact. Future research is needed to verify these findings. If subsequent studies confirm that overweight smokers have greater success at quitting, then other possible mechanisms need to be explored to understand this finding. For example, group differences in dieting, prior quit attempts, use of prescription medications, other attitudes and beliefs (e.g. body image) and other interactions could have a differential impact on treatment outcomes by baseline weight. Nonetheless, our findings are relevant to data reported in prior publications. Two studies showed that a higher baseline weight was associated with lower quit rates. [19,45] Another study found that higher baseline weight was associated with higher quit rates. [26] Apparent contradictory findings could be due to variation in study designs, case mix, the type and duration of treatments received, measures used to assess abstinence and weight and when the outcomes were measured. [11,18–19,46]
Third, regarding the association between baseline weight and weight outcomes, although unadjusted analyses revealed that overweight smokers were significantly more likely to perceive that they gained weight after quitting (at three months), the proportion who gained weight and the amount gained after quitting by baseline weight was not significant at six months and not significant in multivariate analyses at three or six months. Analyses of potential moderators of treatment effects were also not significantly related to change in weight. This lack of an association between weight prior to quitting and subsequent weight gain post quit is consistent with one other study.[47] In contrast, the findings in this study are not consistent with other studies showing a strong relationship between baseline weight and cessation related weight gain. [12,18,48–49] For example, Kasteridis [49] showed that being older, being female, and having higher body mass index prior to quitting were each associated with an increase in weight that exceeded 8% (e.g. about 13+ pounds) 10 years post quit. Lycette [12] reported that high and very low baseline weight was associated with greater weight gain in those continuously abstinent at eight years. For example, a baseline body mass index of 36 was associated with a weight gain of 19.4 kilograms and a body mass index of 29 was associated with a 10 kilogram gain compared with 7.8 kilograms for those with lower body mass index. [12]
Overall, across all body mass index groups, we found that more than one third of respondents to the three or six month surveys said they gained weight with a mean change in weight ranging from one to three pounds. Kasteridis and team also found that quitting smoking resulted in minimal weight gain but the magnitude of weight gain varied by age and gender with an increase in body mass index ranging from 3–5%. [49] Note that we had included these variables in our multivariate analyses as covariates and moderators. Another study reported that 80% of smokers gained weight after quitting averaging 5 kilograms in the first year and 6–7 kilograms overall.[6] Similarly, Lycette [12] evaluated weight gain among those who had quit from three months post treatment through year 8 and found that abstinent participants gained about 8.79 kilograms (equivalent to 19.3 pounds). In fact, 83% of quitters gained more than 2.5 kilograms over eight years.
As with other research, a high degree of variability was observed in post cessation change in weight.[5–6,18,48,50] Some participants lost or gained in excess of 30 pounds, and a significant number of quitters gained more than 10 pounds, placing them at increased risk for diabetes [16,51] and hypertension.[11,52] Notably, greater variability was found in weight change among those who were classified as overweight based on perceived weight change. At three months, overweight quitters were more likely to believe that they lost or gained weight (83%), compared with normal weight or obese participants; 52%–54% reported they lost or gained weight. Sample size may have been a contributing factor since there were more quitters in the overweight group. However, based on calculated change in weight, the mean, standard deviation and range of weight change at six months was similar for all weight groups.
Again, issues regarding study methods must be considered when comparing such data within and across research studies. Measurement issues, sample selection and analytic strategies must be taken into consideration. For example, the amount of weight gained or lost at follow up among abstinent participants may vary depending on how it was assessed (e.g. perceived change or calculated change), who was included in the analyses (e.g. only those who gained weight or also including those who lost weight or reported no change in weight); the starting point for measuring outcomes (e.g. baseline, quit date or end of treatment); when the outcomes were obtained (e.g. three, six, 12 months following starting point); whether researchers selected quitters based on point prevalence or continuous abstinence and whether analyses controlled for covariates. Future research is warranted to explore other potential factors that could have a differential effect on outcomes by body mass index (e.g. group differences in attitudes and beliefs, accuracy and consistency in reporting weight, history of dieting).
While this study provides new and important data on cessation related weight gain in quitline settings, potential limitations of the study must be considered. For example, the population comes from smokers seeking cessation treatment through state quitlines and although quitlines have been shown to reach a broad and representative population, the findings may not generalize to those who receive cessation treatment in health care settings, through their employers, or those who are recruited for research and evaluation studies. In addition, the lack of consistency in services offered across the five quitlines in this study might have had an impact on the results. However, no significant differences were found across weight groups in number of calls provided in the individuals’ state, the amount of nicotine replacement therapy offered and the actual use of these services. In addition, all of the quitlines provided unlimited access to call the quitline regardless of number of proactive calls offered thus allowing individuals in the one-call states to receive multiple counseling calls. Since we found no association between baseline weight and state services provided, we did not control for ‘state’ in the analyses.
Another consideration is that results may not generalize to smokers with low self-efficacy or those with more severe weight concerns. As recently reported, [23] study participants’ pre-treatment confidence in quitting and confidence in quitting without weight gain were fairly high across weight groups and gender; averaging 8.0 on the 10 point confidence in quitting scale and over 6.0 on the 10 point scale measuring weight related self-efficacy and the average rating of weight concerns was less than 5.0 on the 10 point scale. While a previous study showed that the majority of smokers enrolled in a quit smoking program were unconcerned about weight gain, [53] we have previously reported that two thirds of smokers calling the Oklahoma Quitline were considered to have significant weight concerns. [21]
Another potential limitation common in phone-based studies includes use of self-report measures of smoking. Although biochemical validation of smoking is ideal, using self-reported smoking status is consistent with standard measures of telephone-based interventions. Research shows that false reporting of smoking status is minimal for low-intensity interventions with no face-to-face contact or population based studies. [54–57]
Similarly, regarding self-reported weight, the literature indicates that people generally underestimate their weight across time points and underestimation is disproportionately greater among those who are overweight or obese. [58–60] To minimize these potential problems, we asked participants their current weight at baseline and at follow-up, and then calculated their change in weight. So, if a particular person consistently underestimates their weight by ten pounds at baseline, three months and six months, their calculated change in weight over time should provide a good estimate of change in weight since the biases cancel. Moreover, studies have shown strong correlations between measured and self-reported weight indicating that self-reported weight is an excellent approximation of actual weight across a population. [61–63]
Another potential limitation relates to response rates to the follow-up surveys (51–55%) which are lower than those observed in efficacy trials but similar to, if not higher than, what others have found when conducting effectiveness trials with smokers. [35,64–66] Moreover, we report intent-to-treat quit rates as well as responder quit rates. Since response rates to follow-up did not differ by weight groups, we do not expect different conclusions had we used other methods to account for missing data (e.g. multiple imputations).
Finally, limitations in study measures must also be considered. Our measure of cessation related weight gain was restricted to point prevalence measures of cessation (30-day) and thus may underestimate true weight gain associated with quitting. [11] Measuring weight change among those who were continuously abstinent would require a much larger sample size, more frequent assessments, and a longer follow-up period to ascertain quit trajectories and when weight gain occurs following cessation, when it stabilizes and if a specific amount of weight gain predicts relapse to smoking.
Despite potential limitations, the strengths of this study include the significance of the population being studied, the relatively large sample size recruited from five states and situating the study within a telephone quitline setting which increases the likelihood that findings will be informative for population-based settings. Data reported here also provides the first ever benchmarks of quit rates and weight gain overall and by weight groups for comparison to other quitline studies. Obese smokers are considered a high risk, high cost population, but this group has received only limited study and virtually none in an effectiveness context such as a quitline.
In summary, our results indicate that among a quitline population, baseline weight was not consistently associated with treatment engagement or outcomes. In contrast to prior research, we found that obese smokers did not have worse quit outcomes than normal weight smokers and were not more likely to gain weight after quitting. Also, unadjusted analyses revealed that overweight smokers had better quit rates but also gained more weight after quitting than normal weight and obese smokers. However, this trend was not significant at six months. While the largest health and economic benefits to quitting may be for obese smokers, [67] overweight smokers are also considered high risk for obesity if they gain even a moderate amount of weight after quitting. Thus, both groups (overweight and obese) may benefit from more intensive or different forms of cessation treatment. Given the research showing that weight gain associated with smoking cessation can increase one’s risk for diabetes [16] and new evidence showing that women who gain more than 5 kilograms are at increased risk for developing diabetes regardless of their baseline weight, [51] cessation interventions that include some form of weight management may be worthy of further investigation. While we conclude that quitlines are effective across the body mass index spectrum for male and female adults, research is needed to identify effective strategies for increasing acceptance and use of all available treatment (counseling and pharmacotherapy) and to identify optimal combinations of treatments both before and during attempts to quit smoking in order to increase quitline efficacy and effectiveness for both cessation and weight.
Acknowledgements
The project described was supported by Grant Number R21DA026580 from the National Institute On Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Drug Abuse or the National Institutes of Health.
Footnotes
Conflict of interest
The authors had no conflict of interest with the exception that five authors were employed by Alere Wellbeing (service provider for the five state quitlines).
References
- 1.Freedman DM, Sigurdson AJ, Rajaraman P, Doody MM, Linet MS, Ron E. The mortality risk of smoking and obesity combined. American Journal of Preventive Medicine. 2006;31:355–362. doi: 10.1016/j.amepre.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 4.Healton CG, Vallone D, McCausland KL, Xiao H, Green MP. Smoking, obesity, and their co-occurrence in the United States: Cross sectional analysis. British Medical Journal. 2006;333:25–26. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: Meta-analysis. British Medical Journal. 2012;345:e4439. doi: 10.1136/bmj.e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aveyard P, Lycett D, Farley A. Managing smoking cessation-related weight gain. Polskie Archiwum Medycyny Wewnętrznej. 2012;122:494–498. [PubMed] [Google Scholar]
- 7.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Smoking cessation for weight-concerned women: Group vs. individually tailored, dietary, and weight-control follow-up sessions. Addictive Behaviors. 2006;31:115–127. doi: 10.1016/j.addbeh.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Cropsey KL, McClure LA, Jackson DO, Villalobos GC, Weaver MF, Stitzer ML. The impact of quitting smoking on weight among women prisoners participating in a smoking cessation intervention. American Journal of Public Health. 2010;100:1442–1448. doi: 10.2105/AJPH.2009.172783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. Journal of Family Practice. 1998;46:460–464. [PubMed] [Google Scholar]
- 10.Klesges RC, Meyers AW, Klesges LM, LaVasque ME. Smoking, body weight, and their effects on smoking behavior: A comprehension review of the literature. Psychology Bulletin. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 11.Klesges RC, Winders S, Meyers A, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. Journal of Consulting and Clinical Psychology. 1997;65:286–291. doi: 10.1037//0022-006x.65.2.286. [DOI] [PubMed] [Google Scholar]
- 12.Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2010;106:188–196. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- 13.Nides M, Rand C, Dolce J, et al. Weight gain as a function of smoking cessation and 2-mg nicotine gum use among middle-aged smokers with mild lung impairment in the first 2 years of the Lung Health Study. Health Psychology. 1994;13:354–361. doi: 10.1037//0278-6133.13.4.354. [DOI] [PubMed] [Google Scholar]
- 14.Perkins KA, Marcus M, Levine M. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. Journal of Consulting and Clinical Psychology. 2001;69:604–613. [PubMed] [Google Scholar]
- 15.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 16.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus. Annals of Internal Medicine. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg D, Quinn BC. Estimating the effect of smoking cessation on weight gain: An instrumental variable approach. Health Services Research. 2006;41:2255–2267. doi: 10.1111/j.1475-6773.2006.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swan GE, Jack LM, WARD MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–218. [PubMed] [Google Scholar]
- 19.Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee TA. Heterogeneity in 12-month outcome among female and male smokers. Addiction. 2004;99:237–250. doi: 10.1111/j.1360-0443.2003.00629.x. [DOI] [PubMed] [Google Scholar]
- 20.Levine MD, Kalarchian MA, Courcoulas AP, Wisinski MSC, Marcus MD. History of smoking and postcessation weight gain among weight loss surgery candidates. Addictive Behaviors. 2007;32:2365–2371. doi: 10.1016/j.addbeh.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush TM, Levine MD, Deprey TM, et al. Prevalence of weight concerns and obesity among smokers calling a quitline. Journal of Smoking Cessation. 2009;4:74–78. doi: 10.1375/jsc.4.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins BN, Nair U, Hovell MF, Audrain-McGovern J. Smoking-related weight concerns among underserved, black maternal smokers. American Journal of Health Behaviors. 2009;33:699–709. doi: 10.5993/ajhb.33.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine MD, Bush T, Magnusson B, Cheng Y, Chen X. Smoking-related weight concerns and obesity: Differences among normal weight, overweight, and obese smokers using a telephone tobacco quitline. Nicotine and Tobacco Research. 2013;15(6):1136–1140. doi: 10.1093/ntr/nts226. Epub 2012 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: The Inter99 study. Preventive Medicine. 2007;44:283–289. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Rigotti NA. Treatment options for the weight-conscious smoker. Archives of Internal Medicine. 1999;159:1169–1171. doi: 10.1001/archinte.159.11.1169. [DOI] [PubMed] [Google Scholar]
- 26.Twardella D, Loew M, Rothenbacher D, Stegmaier C, Ziegler H, Brenner H. The impact of body weight on smoking cessation in German adults. Preventive Medicine. 2006;42:109–113. doi: 10.1016/j.ypmed.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology. 2001;69:511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- 28.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use And Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. U.S. Public Health Service; 2008. [Google Scholar]
- 29.Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Systematic Reviews. 2009;1:CD006219. doi: 10.1002/14651858.CD006219.pub2. CD006219 pub2. [DOI] [PubMed] [Google Scholar]
- 30.Spring B, Howe D, Berendsen M, et al. Behavioral intervention to promote smoking cessation and prevent weight gain: A systematic review and meta-analysis. Addiction. 2009;104:1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush T, Levine MD, Zbikowski SM, et al. Weight gain after quitting: Attitudes, beliefs and counselling strategies of cessation counsellors. Journal of Smoking Cessation. 2009;3:124–132. doi: 10.1375/jsc.3.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orleans CT, Schoenbach VJ, Wagner EH, et al. Self-help quit smoking interventions: Effects of self-help materials, social support instructions, and telephone counseling. Journal of Consulting and Clinical Psychology. 1991;59:439–448. doi: 10.1037//0022-006x.59.3.439. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S, Anderson CM, Tedeschi GJ, et al. Evidence of real-world effectiveness of a telephone quitline for smokers. New England Journal of Medicine. 2002;347:1087–1093. doi: 10.1056/NEJMsa020660. [DOI] [PubMed] [Google Scholar]
- 34.Bush T, Levine MD, Beebe LA, et al. Addressing weight gain in smoking cessation treatment: A randomized controlled trial. American Journal of Health Promotion. 2012;27:94–102. doi: 10.4278/ajhp.110603-QUAN-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Love SJ, Sheffer CE, Bursac Z, Prewitt TE, Krukowski RA, West DS. Offer of a weight management program to overweight and obese weight-concerned smokers improves tobacco dependence treatment outcomes. American Journal of Addiction. 2011;20:1–8. doi: 10.1111/j.1521-0391.2010.00091.x. [DOI] [PubMed] [Google Scholar]
- 36.Stunkard AJ, Wadden TA. Obesity: Theory. 2nd ed. New York: Raven Press; 1993. [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 38.Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addictive Behaviors. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 39.Levine MD, Perkins KA, Kalarchian MA, et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bush T, Zbikowski S, Mahoney L, Deprey M, Mowery PD, Magnusson B. The 2009 US federal cigarette tax increase and quitline utilization in 16 states. Journal of Environmental and Public Health. 2012;2012:314740. doi: 10.1155/2012/314740. Epub 2012 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu S, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: Effects of single-session and multiple session interventions. Journal of Consulting and Clinical Psychology. 1996;64:202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 42.Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine. 2009;169:2148–2155. doi: 10.1001/archinternmed.2009.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns EK, Levinson AH, Deaton EA. Factors in nonadherence to quitline services: Smoker characteristics explain little. Health Education & Behavior. 2012;39:596–602. doi: 10.1177/1090198111425186. [DOI] [PubMed] [Google Scholar]
- 44.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clinical Therapeutics. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Oster G, Colditz GA, Kelly NL. The economic costs of smoking and benefits of quitting for individual smokers. Preventive Medicine. 1984;13:377–389. doi: 10.1016/0091-7435(84)90029-x. [DOI] [PubMed] [Google Scholar]
- 46.Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychology. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- 47.Rabkin S. Relationship between weight change and the reduction or cessation of cigarette smoking. International Journal of Obesity. 1984;8:665–673. [PubMed] [Google Scholar]
- 48.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. American Journal of Clinical Nutrition. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 49.Kasteridis P, Yen ST. Smoking cessation and body weight: evidence from the Behavioral Risk Factor Surveillance Survey. Health Services Research. 2012;47:1580–1602. doi: 10.1111/j.1475-6773.2012.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obesity Review. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 51.Luo J, Rossouw J, Tong E, et al. Smoking cessation, weight gain, and risk of type 2 diabetes mellitus among postmenopausal women. Archives of Internal Medicine. 2012;172:438–440. doi: 10.1001/archinternmed.2012.24. [DOI] [PubMed] [Google Scholar]
- 52.Gerace TA, Hollis J, Ockene JK, Svendsen K. Smoking cessation and change in diastolic blood pressure, body weight, and plasma lipids. Preventive Medicine. 1991;20:602–620. doi: 10.1016/0091-7435(91)90058-c. [DOI] [PubMed] [Google Scholar]
- 53.Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. Journal of Consulting and Clinical Psychology. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- 54.Glasgow RE, Mullooly JP, Vogt TM, et al. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addictive Behaviors. 1993;18:511–527. doi: 10.1016/0306-4603(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 55.Gross R, Bentur N, Elhayany A, Sherf M, Epstein L. The validity of self-reports on chronic disease: Characteristics of underreporters and implications for the planning of services. Public Health Reviews. 1996;24:167–182. [PubMed] [Google Scholar]
- 56.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 58.Niedhammer I, Bugel I, Bonenfant S, Goldberg M, Leclerc A. Validity of self-reported weight and height in the French GAZEL cohort. International Journal of Obesity and Related Metabolic Disorders. 2000;24:1111–1118. doi: 10.1038/sj.ijo.0801375. [DOI] [PubMed] [Google Scholar]
- 59.Rowland ML. Self -reported weight and height. American Journal of Clinical Nutrition. 1990;52:1125–1133. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]
- 60.Stunkard AJ, Albaum JM. The accuracy of self-reported weights. American Journal of Clinical Nutrition. 1981;34:1593–1599. doi: 10.1093/ajcn/34.8.1593. [DOI] [PubMed] [Google Scholar]
- 61.Jeffery RW. Bias in reported body weight as a function of education, occupation, health and weight concern. Addictive Behaviors. 1996;21:217–222. doi: 10.1016/0306-4603(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 62.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured body mass index as correlates of disease markers in U.S. adults. Obesity (Silver Spring) 2007 Jan;15:188–196. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 63.Nyholm M, Gullberg B, Merlo J, Lundqvist-Persson C, Rastam L, Lindblad U. The validity of obesity based on self-reported weight and height: Implications for population studies. Obesity (Silver Spring) 2007 Jan;15:197–208. doi: 10.1038/oby.2007.536. [DOI] [PubMed] [Google Scholar]
- 64.Cummins SE, Bailey L, Campbell S, Koon-Kirby C, Zhu SH. Tobacco cessation quitline in North America: A descriptive study. Tobacco Control. 2007;(16 Suppl 1):i9–i15. doi: 10.1136/tc.2007.020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoile EC, Free C, Edwards PJ, Felix LM. Methods to increase response rates for data collected by telephone. Cochrane Database of Systematic Reviews. 2009;3:MR000029. doi: 10.1002/14651858.MR000008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.North American Quitline Consortium. Measuring quit rates. Quality improvement initiative. Phoenix, AZ; 2009. http://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/docs/naqc_issuepaper_measuringqui.pdf. [Google Scholar]
- 67.Koster A, Leitzmann MF, Schatzkin A, et al. The combined relations of adiposity and smoking on mortality. American Journal of Clinical Nutrition. 2008;88:1206–1212. doi: 10.3945/ajcn.2008.26298. [DOI] [PMC free article] [PubMed] [Google Scholar]