Abstract
Objective:
To ascertain whether objectively measured obstructive sleep apnea (OSA) independently increases the risk of all cause death, cardiovascular disease (CVD), coronary heart disease (CHD), stroke or cancer
Design:
Community-based cohort
Setting and Participants:
400 residents of the Western Australian town of Busselton
Measures:
OSA severity was quantified via the respiratory disturbance index (RDI) as measured by a single night recording in November-December 1990 using the MESAM IV device, along with a range of other risk factors. Follow-up for deaths and hospitalizations was ascertained via record linkage to the end of 2010.
Results:
We had follow-up data in 397 people and then removed those with a previous stroke (n = 4) from the mortality/ CVD/CHD/stroke analyses and those with cancer history from the cancer analyses (n = 7). There were 77 deaths, 103 cardiovascular events (31 strokes, 59 CHD) and 125 incident cases of cancer (39 cancer fatalities) during 20 years follow-up. In fully adjusted models, moderate-severe OSA was significantly associated with all-cause mortality (HR = 4.2; 95% CI 1.9, 9.2), cancer mortality (3.4; 1.1, 10.2), incident cancer (2.5; 1.2, 5.0), and stroke (3.7; 1.2, 11.8), but not significantly with CVD (1.9; 0.75, 4.6) or CHD incidence (1.1; 0.24, 4.6). Mild sleep apnea was associated with a halving in mortality (0.5; 0.27, 0.99), but no other outcome, after control for leading risk factors.
Conclusions:
Moderate-to-severe sleep apnea is independently associated with a large increased risk of all-cause mortality, incident stroke, and cancer incidence and mortality in this community-based sample.
Commentary:
A commentary on this article appears in this issue on page 363.
Citation:
Marshall NS; Wong KK; Cullen SR; Knuiman MW; Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton health study cohort. J Clin Sleep Med 2014;10(4):355-362.
Keywords: Sleep disordered breathing, melanoma, coronary artery disease
Obstructive sleep apnea (OSA) is a common condition, affecting around 5% of the middle-aged population.1 In its severe form it appears to increase risk for premature mortality and cardiovascular diseases, including stroke,2–8 but perhaps not coronary heart disease.9 Intermittent hypoxia, repetitive arousal from sleep or the large intrathoracic pressure swings and increased sleeping blood pressure which are characteristic of OSA are generally thought to be the most likely causal pathway through which OSA causes cardiovascular disease (CVD).10–12 More recently severe sleep apnea has been linked with an increased risk of cancer.13–16
Mild sleep apnea is far more common than severe sleep apnea but it has not yet been widely confirmed that mild OSA contributes to increases in public health diseases in the way that severe OSA seems to.17 Using 20-year follow-up data from the Busselton Sleep Cohort, we investigated whether sleep apnea was associated with all-cause mortality, cardiovascular disease, coronary heart disease, stroke, and cancer (both incidence and mortality), while controlling for the leading risk factors for these outcomes and paying particular attention to whether mild sleep apnea was harmful.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea is known to increase the risk for mortality stroke and more recently cancer in studies from Spain and the USA. Further data are needed to ascertain confirm the cancer association, the significance of mild OSA and the potential long-term effects of OSA on women.
Study Impact: Sleep apnea is an important disease of public health significance that increases mortality, stroke and cancer risk in a community-based population. Mild OSA does not appear to increase mortality risk and may even protect people from the effects of other established risk factors.
METHODS
In November-December 1990 we used the MESAM IV device to assess snoring and sleep apnea in 400 residents of the rural town of Busselton in the state of Western Australia who already belonged to the ongoing Busselton Health Study. The MESAM IV (Madaus Medizen-Elektronik, Freiberg, Germany), is a 4-channel portable home-monitoring device used to quantify sleep disordered breathing via the measurement of snoring by audio recording, heart rate, oxygen saturation, and body position. The full methods for implementation and manual scoring of snoring and OSA are described in the original prevalence paper.18 From this dataset we have previously reported 3 manuscripts related to OSA and snoring outcomes,2,17,19 but have only reported on the effects of OSA out to 14 years follow-up. The parent Busselton Health Study is an ongoing representative and comprehensive survey of residents in the Shire of Busselton in the South-West region of Western Australia. The survey invites the participation of all individuals on the Commonwealth of Australia electoral roll, enrolment on which is compulsory for all Australian citizens aged over 18.
Sample Construction
Men
The two-stage sampling process has already been described elsewhere.18 Briefly, all men aged 40-65 on the Busselton Health Study Register were sent the initial sleep questionnaire (n = 758), of whom 486 responded. These 486 men were randomly telephoned until a sample of 311 had been recruited for an at-home overnight study (all available study appointments were filled—we undertook as many sleep studies as logistics would allow). Five respondents who were invited to participate in the overnight study declined the invitation. Two hundred ninety-four of these overnight study recordings were of adequate quality to score, and 293 men had matched longitudinal data.
Women
All women aged 40-65 on the register (n = 810) were sent the sleep questionnaire; 537 responded. At-home overnight studies were undertaken in equal numbers from the 3 categories of snoring (never, sometimes, and almost always/always, each n = 38) so as to ensure some cases of OSA. Women were randomly telephoned until a sample of 114 had been recruited and all the available remaining study appointments had been filled. Six women who were telephoned declined participation. One hundred six of these recordings were of adequate quality to score; 104 women had matched longitudinal data. Fewer women than men were sampled for financial and logistic reasons because in 1990 sleep apnea was thought to be a relatively rare condition in women before the publication of the Wisconsin cohort in 1993.20
Outcomes: Mortality and Vitality Ascertainment, Cardiovascular and Stroke Events
The Human Research Ethics Committee of the Health Department of Western Australia gave permission to access the hospital admission, cancer registration and death records for Busselton health survey participants from 1981 to 2010. These data were accessed via record linkage to the statewide Western Australian Data Linkage System that includes the Hospital Morbidity Data System containing records of all hospital admissions from public and private hospitals in Western Australia, the Western Australian Cancer Registry that contains records from all cancer registrations in Western Australia, and the Western Australian Death register that contains all deaths among residents of Western Australia (including deaths registered in other states of Australia).21,22
Hospital admissions and cancer registrations from 1981 to 1990 were used to define the history variables for the 1990 Sleep survey cohort. History of cardiovascular disease (ICD9 390-459, ICD10 I00-I99, G45), history of coronary heart disease (ICD9 410-414, ICD10 I20-I25), and history of stroke (ICD9 430-437, ICD10 I60-I68, G45) were defined as having any hospital admission with a primary or secondary discharge diagnosis matching the ICD coding ranges during 1981 to 1990. History of cancer was defined as having any cancer registration from 1981 to 1990. Death records from 1991 to 2010 were used to define mortality outcomes time-to-death (any cause), time-to-death from cardiovascular diseases (ICD9 390-459, ICD10 I00-I99, G45), and time-to-death from cancer (ICD9 140-200, ICD10 C00-C97). Hospital admissions, cancer registrations, and death records from 1991 to 2010 were used to define the morbidity event outcome variables. Time-to-next cardiovascular event was defined as time to first hospital admission with primary diagnosis of coronary heart disease (ICD9 410-414; ICD10 I20-25), stroke (ICD9 430-437; ICD10 I60-68, G45), congestive heart failure (ICD9 428; ICD10 I50), peripheral arterial disease (ICD9 440-448; ICD10 I70-79), or death from cardiovascular disease (ICD9 390-459; ICD10 I00-99, G45). Time-to-next coronary heart disease event was defined as time to first hospital admission with primary diagnosis of coronary heart disease or death from coronary heart disease. Strokes and cancers were similarly defined (skin cancer ICD10 C43-C44). The rationale for investigating non-skin cancers was driven by the importance of melanomas in Australia. Follow-up for all time-to-event outcomes terminated upon death, departure from Western Australia (if known), or 31 December 2010.
Exposure Variables
Exposure to sleep apnea was quantified by the respiratory disturbance index (RDI), which is calculated by summing the total number of respiratory disturbances and dividing by the participant-estimated hours of sleep to give an event rate per hour. Respiratory disturbances were defined as oxygen desaturations ≥ 3% from the preceding baseline level that were accompanied by either (a) an increased heart rate ≥ 10 beats/min and/or (b) a burst of snoring associated with commencement and termination of the desaturation event (i.e., an audible apnea). Hours of sleep were estimated using lights off and lights on time.
Anthropometric variables were measured on the evening before the sleep study and included height, weight, abdominal height while supine (sagittal diameter), as well as neck, waist, and hip circumference. Blood pressure was then measured twice via an electronic sphygmomanometer (Spacelabs, Redmond, WA) with measurements ≥ 5 min apart in the supine position and after the participant had been lying quietly ≥ 10 min, which were averaged.
Smoking status was ascertained via questionnaire by asking whether the patient was a current, former, or never smoker. Former and current smokers were queried about smoking duration and use in order to calculate pack years. Alcohol consumption patterns were queried in order to calculate the approximate grams of alcohol consumption per week. Fasting blood samples were taken the morning after the overnight study for blood glucose and total and HDL cholesterol and analyzed using standard assay methods at the Western Australian State Health laboratories. History of stroke was also ascertained via questionnaire. Record linkage data on cardiovascular incidents, coronary heart disease, stroke, and cancer were used to exclude participants at baseline in the relevant models.
Data Handling and Statistical Analyses
Analyses were undertaken (by NSM) using SAS (v 9.2 SAS institute NC, USA). For sleep apnea we used the same standard clinical cut points23 we have previously used.2,17,19 No or subclinical sleep apnea served as the reference category (i.e., 0 to 4 respiratory disturbances/h), and the 2 sleep apnea categories were mild (5 to 14 RDI) and moderate-to-severe OSA (≥ 15 RDI). We were unable to examine severe OSA because only 3 participants had an RDI ≥ 30.
Previously established risk factors were analyzed with the variables transformed or parameterized to maximize model fit (using improved Akaike's information criteria; AIC) and to reduce the chance that any observed association was due to poor controlling of known risk-factors. Mean arterial pressure (2/3*Systolic+1/3*Diastolic) gave the best blood pressure fit. Body habitus was characterized in a numbers of ways: BMI classified into normal/overweight/obese (< 25, 25 to < 30, or 30 kg/m2); central obesity was calculated by waist circumference categorized into small, medium, and large for each gender grouping according to governmental recommendations (http://www.measureup.gov.au) and by sagittal diameter (abdominal height when lying down in cm). Smoking defined categorically (never, ex, current) had the best fit, whereas pack years was not significant.
Univariate associations between risk factors and time-to-event outcomes were investigated with Cox proportional hazards models using χ2 tests for significance. We also used Kaplan-Meier curves to produce the figures. All risk factors were also investigated for their association with sleep apnea using χ2, Fisher exact, ANOVA, or Kruskal-Wallis tests, where appropriate.
Cox regression modelling was used to estimate the effect of sleep apnea (as a categorical and continuous variable) on the time-to-event outcomes before (unadjusted) and after adjustment for other risk factors. Regardless of univariate association, the following risk factors were forced into the fully adjusted mortality, CVD, and CHD and stroke models because of known associations with snoring, OSA, or mortality/CVD/CHD/ stroke: age, gender, obesity, smoking status, blood pressure, total cholesterol, high density lipoprotein (HDL) cholesterol, angina, cancer history, and diabetes. Similarly, in the cancer models we forced age, gender, smoking, and obesity (using various combinations to minimize AIC). Other risk factors were examined for independent association with outcomes when they exhibited some evidence of a univariate association with either the outcomes or with sleep apnea (p < 0.1). In sensitivity analyses we stratified by gender and ignored all cases that were censored with the first 2 years of the cohort's inception. We removed from analyses the small numbers of participants with missing data in any of the fields used in the multivariable models. Proportional hazards assumptions were checked plotting Schoenfeld residuals.
RESULTS
We had sleep apnea and follow-up data in 397 participants. Those participants with data linkage or questionnaires indicating a history of stroke (n = 4) were excluded from mortality and CVD analyses leaving an analytical sample of 393 people (n = 102 females; 98% of the original cohort). In the cancer analyses we excluded 7 people with cancer history. Moderate-severe OSA was present in 18 (4.6%) people, mild OSA in 81 (20.6%) and no or subclinical OSA in 294 (74.8%) with 77 deaths (19.6%), 103 (26.2%; 17 fatalities) CVD events, 31 (7.9%) strokes, 59 CHD events, and 125 cancer events (32.1%; 39 fatalities, from n = 390). There were too few cardiovascular disease fatalities (or fatal coronary heart disease/stroke), or colorectal or prostate cancers for separate analysis (all < 20 events). The analyses had 7358 person-years of observation.
Figures 1–6 illustrate Kaplan Meier plots plus associated log rank tests of the 6 outcomes. Table 1 shows the associations of risk factors with clinical sleep apnea severities. Table 2 shows the univariate and multivariate associations between sleep apnea severity (continuous and categorical) and mortality, incident CVD, stroke, and CHD events. Table 3 does the same thing with cancer mortality and incidence and the incidence of non-skin cancers. The reason for analyzing the non-skin cancers was because melanomas might be sensitive to intermittent hypoxia,13 but that there were too few melanomas to analyze directly. So we have chosen to estimate their potential effect on overall cancer incidence by removing them to see whether the hazard ratios might appreciably change.
Figure 1. The univariate association between sleep apnea and all-cause mortality.
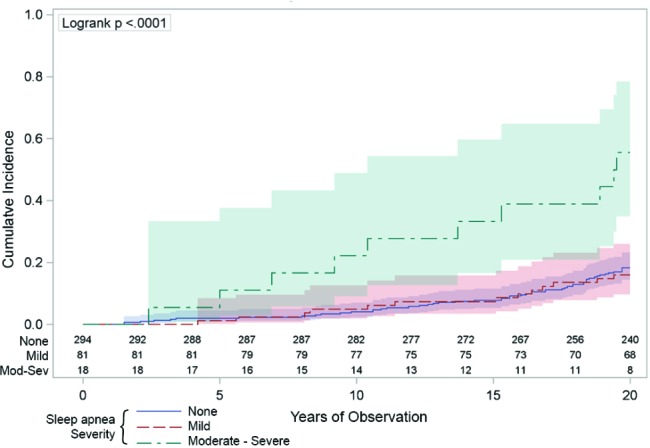
The vertical axis indicates the cumulative number of deaths was higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h: the green line) compared to those with mild sleep apnea (RDI 5-14: the red line) or those with no sleep apnea (RDI < 5: the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Figure 2. The univariate association between sleep apnea and cancer mortality.
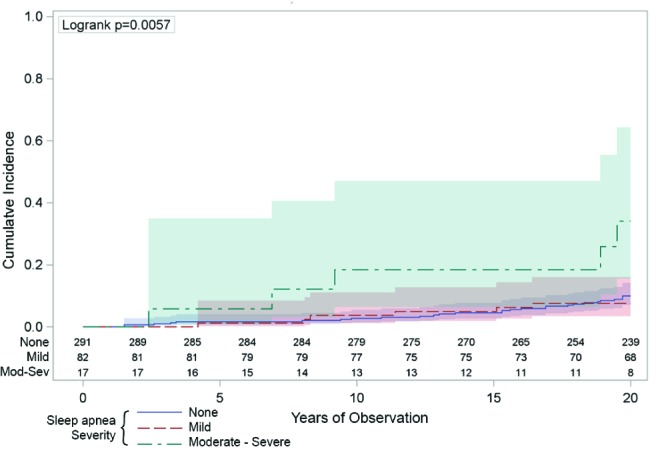
The vertical axis indicates the cumulative number of deaths attributed to cancer was higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h: the green line) compared to those with mild sleep apnea (RDI 5-14: the red line) or those with no sleep apnea (RDI < 5: the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Figure 3. The univariate association between sleep apnea and the incidence of cancer.
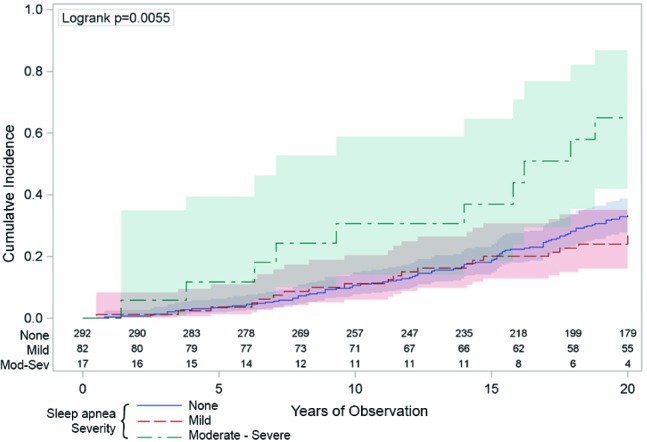
The vertical axis indicates the cumulative number cancer diagnoses was higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h: the green line) compared to those with mild sleep apnea (RDI 5-14: the red line) or those with no sleep apnea (RDI < 5: the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Figure 4. The univariate association between sleep apnea and incident stroke.
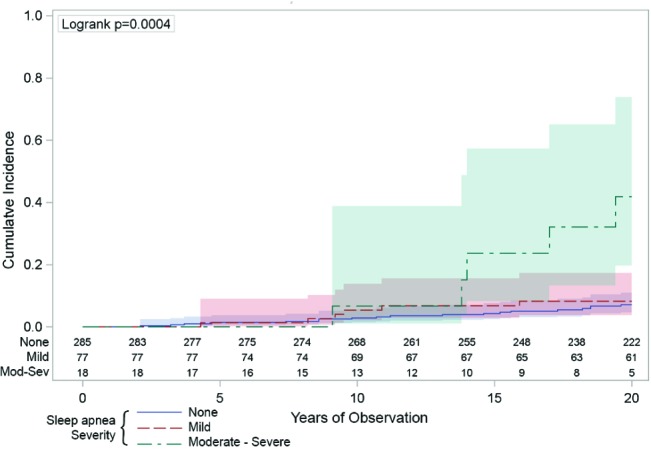
The vertical axis indicates the cumulative number hospitalizations or deaths from stroke was higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h: the green line) compared to those with mild sleep apnea (RDI 5-14: the red line) or those with no sleep apnea (RDI < 5: the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Figure 5. The univariate association between sleep apnea and incidence of cardiovascular disease.
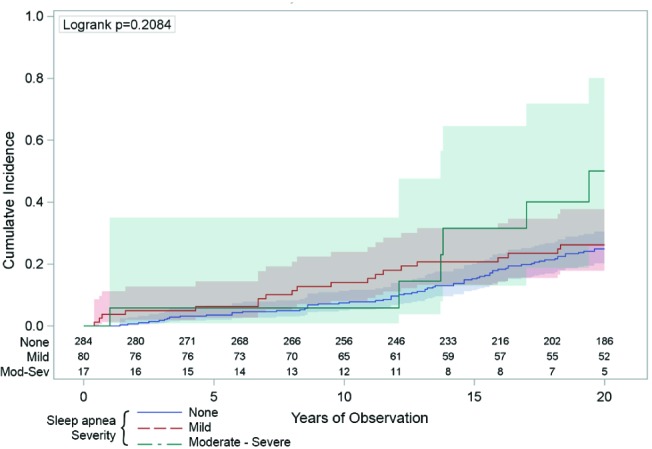
The vertical axis indicates the cumulative number of hospitalizations or deaths from coronary heart disease was not statistically higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h: the green line) compared to those with mild sleep apnea (RDI 5-14: the red line) or those with no sleep apnea (RDI < 5: the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Figure 6. The univariate association between sleep apnea and the incidence of coronary heart disease.
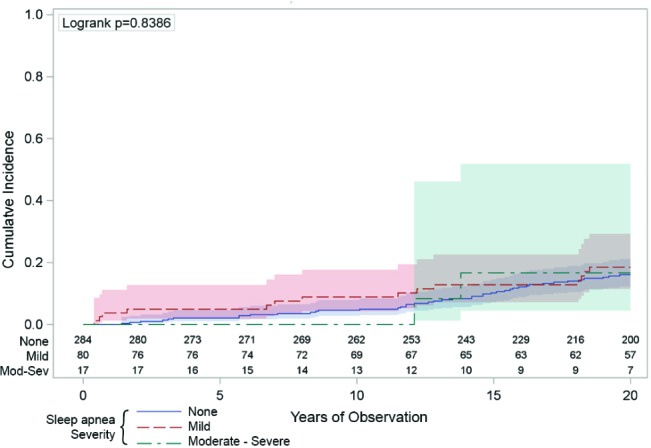
The vertical axis indicates the cumulative number of hospitalizations or deaths from coronary heart disease was not statistically higher across the 20 years (horizontal axis) in those people with moderate-severe sleep apnea (respiratory disturbance index [RDI] ≥ 15/h—the green line) compared to those with mild sleep apnea (RDI 5-14—the red line) or those with no sleep apnea (RDI < 5—the blue line). The numbers just above the horizontal axis indicate the numbers of people being observed at each 2-year time point in each of the sleep apnea groups. The shaded areas around the lines represent the 95% confidence intervals.
Table 1.
Association of sleep apnea with risk factors for cardiovascular disease and cancer
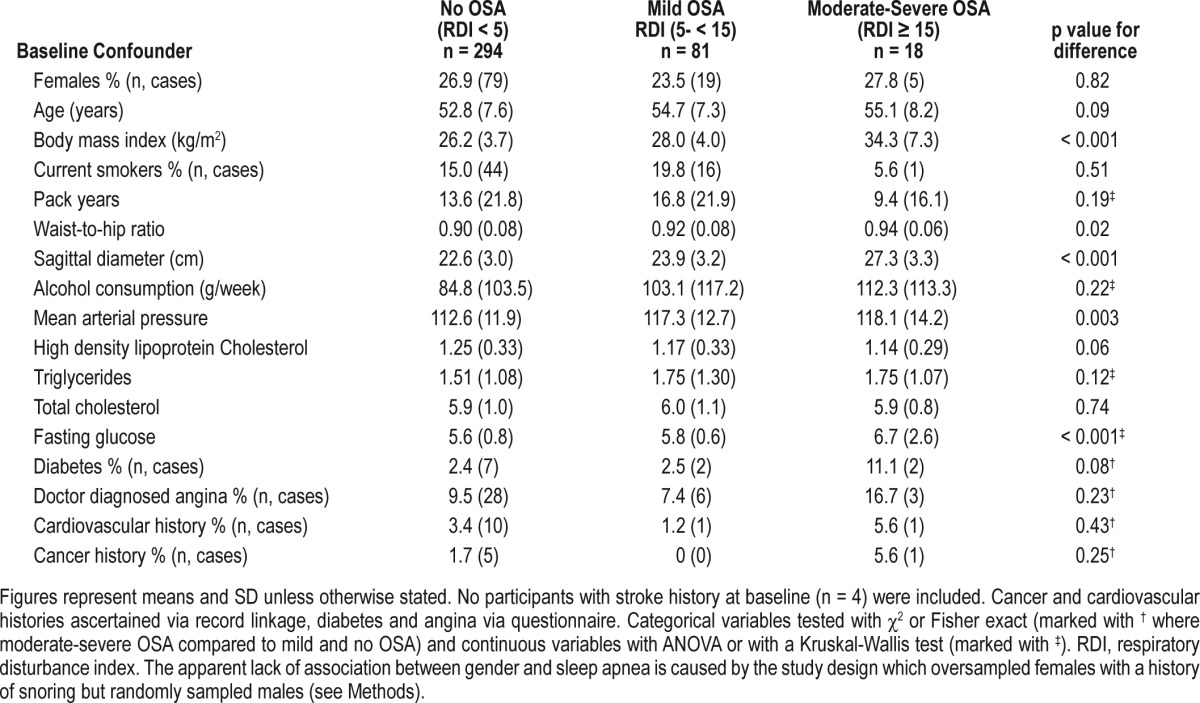
Table 2.
Association of sleep apnea with mortality, cardiovascular events, coronary heart disease, and strokes
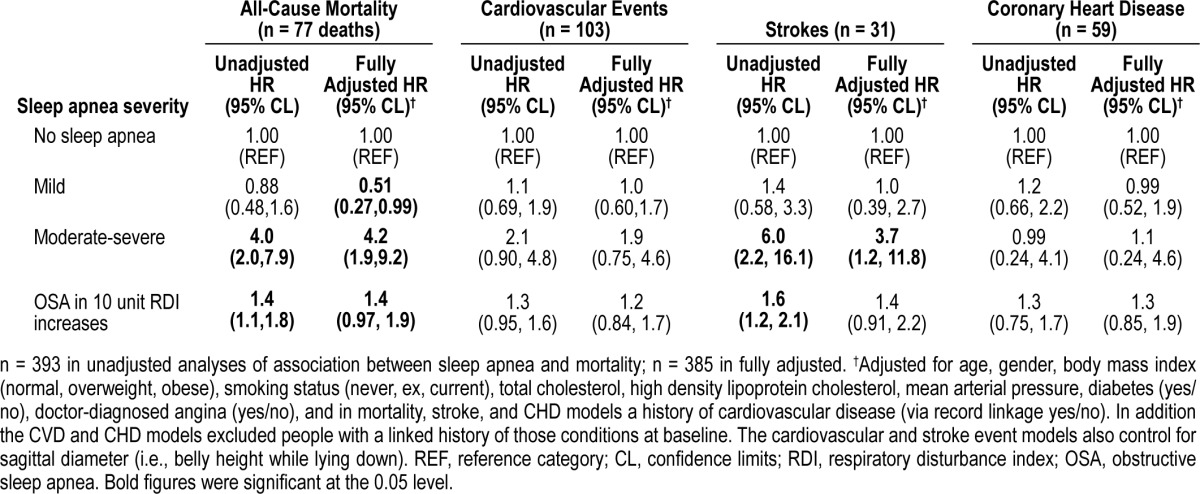
Table 3.
Association of sleep apnea with cancer mortality, incidence, and non-skin cancer incidence
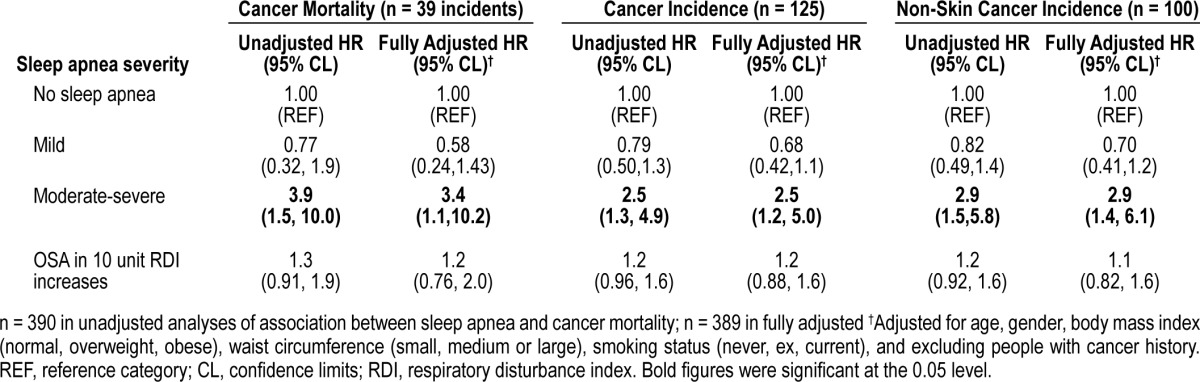
We also ran our fully adjusted models using a sex by OSA interaction and stratified the models presented in Tables 2 and 3. Interaction between gender and sleep apnea was not significant, and in the model stratified by gender, both genders were at increased risk of mortality (men HR = 3.1; 95% CI 1.2, 7.9, p = 0.02; women 25.1; 2.8, 227, p < 0.01). Hazard ratios for women were elevated in other outcome analyses but were not statistically significant, possibly due to severe under-powering. Ignoring all of the participants censored within the first 2 years did not change the size or statistical significance of any of the categorical models presented in Tables 2 or 3. We have not presented any analyses of the potential effects of OSA treatments because we believe that none of the cases were treated for their sleep apnea. The third author of the manuscript is the sleep physician for the district and set up a clinic to treat those informed of their OSA diagnosis at the baseline visit, but none presented.
DISCUSSION
People with moderate-severe sleep apnea have a marked elevated risk of mortality, cancer and stroke compared to people without sleep apnea. This excess mortality is not explained by the established leading risk factors for cardiovascular disease or cancer. Traditionally sleep apnea is thought to increase mortality primarily through cardiovascular disease. While we did observe some increased risk for incident cardiovascular disease and coronary heart disease, these were not statistically significant. However, people with moderate-severe sleep apnea are at large increased risk for stroke in the Busselton Sleep Cohort, as they are in other when they have severe OSA in other studies.4,5 We have also observed that sleep apnea is an independent risk for both incident cancer and for overall cancer mortality.13,14 The effect of sleep apnea on cancer risk might also provide an additional mechanism by which obesity reduction is associated with reduced cancer risk.24 Our estimates for cancer mortality in the moderate-to-severe range (adjHR 3.4) are consistent with those estimated from the Wisconsin Cohort recently which reported the HR for moderate OSA of 2.0 and severe OSA of 4.8.
We have analyzed all of these big public health outcomes together in one report to give an overall effect of sleep apnea. Mortality and morbidity in OSA seem to be driven by a combination of cancer and cardiovascular disease (particularly stroke). This overall elevation in mortality from a range of causes may be making it difficult to confirm statistically the cardiovascular component here, specifically. This may also be partly due to the Busselton having lower CVD rates than surrounding areas and Western Australia in general.25 But the lack of association between sleep apnea and coronary heart disease was also reported by the Sleep Heart Health Study.9 Regardless, the hazard for overall mortality is large (4.2, 95% CI 1.9, 9.2), unexplained by traditional risk factors, and sleep apnea is a common condition making it a disease of significant public health impact. The hazard ratios we report tend to be a little higher than those reported by other cohorts, probably because we do not think that the sleep apnea we observed was ever treated despite an invitation to see a sleep physician. Effective sleep apnea treatment could reduce risk and treatment of the population would bias towards a null finding.
Community-based studies have so far not been able to confirm that sleep apnea is bad for women. In the fully adjusted mortality model we found a statistically elevated mortality risk in women with moderate-severe OSA (HR 25.1; 95% CI 2.8, 227, p < 0.01). However this analysis is based on a small number of people and a very small number of people with moderate-severe OSA and should be interpreted with caution. We were not able to show a statistically significant increase for women in any of the other models. Our results are consilient with those from the Spanish multicenter clinical cohort26 who have recently reported that CPAP treatment in women reduced CVD mortality, although we have been unable to confirm that mortality is CVD-related. Quantification of the effects of OSA in women may require a multi-cohort approach or more time in the larger cohorts to confirm.
Mild sleep apnea (stopping breathing 5 to 15 times per hour of sleep) was associated with a halving of the risk for all-cause mortality (but no other outcome). However, it only did this in fully adjusted models (see Table 2 and also see lack of risk in Figure 1). We also indirectly tested the potential for mild sleep apnea to be helpful or harmful in the linear models presented at the bottoms of Tables 2 and 3; these were either nonsignificant or less predictive than separating the severities of sleep apnea into independent categories. We tentatively suggest this might mean that mild sleep apnea protects people against the effects of their other clustered cardiometabolic risk factors. So a person with multiple traditional risk factors has a lower chance of death if they also have a case of mild sleep apnea than if they did not have any sleep apnea. Mild sleep apnea has been observed to protect against death previously and might be biologically plausible if a mild case of sleep apnea has beneficial effects in the same way that exercise while awake does.27 Another potential mechanism might be ischemic preconditioning. People with sleep apnea who survive a myocardial infarction appear to be protected against some of the damage.28 However, this fails to explain why mild OSA was nonsignificantly associated with protection against cancer (see Table 3).
Potential limitations of the study include the measurement of sleep apnea using the MESAM IV device, rather than full polysomnography. However, this device had very close agreement with standard polysomnography in terms of quantifying sleep apnea (ICC = 0.98) in the original prevalence study, as well as being validated by other research groups.18,29,30 Underpowering is probably the biggest limitation to the Busselton Sleep Cohort, particularly with only 18 people in the highest exposure category. Some of the estimates we provide, like those for mortality in women, have very large confidence intervals. Our ability to control for some cancer risk factors was limited in this study, due to the historical focus of the cohort on respiratory health and we are unable to control for a number of known carcinogens and protective factors, particularly occupational confounders. Our results could be sensitive to the misclassification of hospital diagnoses and causes of death. Hospital morbidity codes for CHD31 and stroke32 have been previously validated, as have vascular deaths.33 Death certificate data in Australia rarely misclassifies alive people as having died, so the ascertainment of mortality is unlikely to be inaccurate, but people who die overseas may be missing.22 Finally, the external validity of these findings might be questionable because all of the data arise from people living in one town in Western Australia.
CONCLUSIONS
Moderate-severe OSA is an independent risk factor for all-cause mortality, cancer mortality, cancer incidence, and stroke. Sleep apnea is a relatively common condition similar in prevalence to diabetes and with large effects on overall mortality, making it an important disease of public health significance. The emerging but strong association with cancer13,14 is a key area for further investigation aimed at determining causality.
DISCLOSURE STATEMENT
This was not an industry supported study. Research was supported by Australian NHMRC grants to RRG 264598, 202916 & 571421. Dr. Marshall has received in-kind research support from Teva Cephalon for a clinical trial for sleep apnea. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of Dr. Helen Bearpark who collected and processed the baseline exposure data presented here with the expressed intention of longitudinal study. She was tragically killed in a road accident in December 1996.
The authors would like to thank Mark Divitini for programming assistance and the staff at the Western Australian Data Linkage Branch, Department of Health Inpatient Data Collection, Cancer Registry and Registrar General's Office for the provision of linked hospital admission, cancer registration and mortality data. The Busselton Population Medical Research Foundation gave us access to the study data and we would also like to thank the community of Busselton for their long-standing support. We would also like to thank the Marburg Sleep group (Wilma Althaus and Hartmut Schneider), Lyn Davies, and Jan Hedner for their pivotal role in baseline data collection and Brendon Funnell for graphical design assistance.
Drs. Marshall, Wong, Knuiman, and Grunstein were responsible for the study concept and design. Dr. Knuiman and Grunstein obtained funding. Dr. Cullen, Knuiman and Grunstein acquired the data, as did the late Dr Helen Bearpark and colleagues listed in the Acknowledgements. Dr. Marshall analysed the data and was aided in interpretation by Drs. Wong, Cullen, Knuiman, and Grunstein. Dr. Marshall drafted the manuscript, and all authors revised it for important intellectual content. Drs. Marshall, Knuiman, and Grunstein are joint guarantors.
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients- a multivariate analysis of risk factors. Sleep. 1995;18:149–57. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 8.Marin J, Carrizo S, Vicente E, Agusti A. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: An outcome-based definition of hypopneas. Am J Respir Crit Care Med. 2008;177:1150–5. doi: 10.1164/rccm.200712-1884OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 12.Durán-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: Randomised controlled trial. BMJ. 2010:341. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 13.Nieto FJ, Peppard PE, Young T, et al. Sleep disordered breathing and cancer mortality: Results from the Wisconsin sleep cohort study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105. doi: 10.1164/rccm.201209-1671OC. [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Quan SF. Sleep apnea: A common mechanism for the deadly triad— cardiovascular disease, diabetes, and cancer? Am J Respir Crit Care Med. 2012;186:123–4. doi: 10.1164/rccm.201204-0657ED. [DOI] [PubMed] [Google Scholar]
- 16.Almendros I, Montserrat JM, Torres M, et al. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012;13:1254–60. doi: 10.1016/j.sleep.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Marshall NS, Wong KKH, Cullen S, Knuiman MW, Grunstein RR. Snoring is not associated with all-cause mortality, incident cardiovascular disease or stroke in the Busselton Health Study. Sleep. 2012;35:1235–40. doi: 10.5665/sleep.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bearpark H, Elliot L, Grunstein R, et al. Snoring and sleep apnea: A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 19.Marshall NS, Wong KK, Phillips CL, et al. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 21.Holman CD, Bass AJ, Rouse IL, Hobbs MS. Population-based linkage of health records in Western Australia: Development of a health services research linked database. Aust N Z J Public Health. 1999;23:453–9. doi: 10.1111/j.1467-842x.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 22.Magliano D, Liew D, Pater H, et al. Accuracy of the australian national death index: Comparison with adjudicated fatal outcomes among australian participants in the long-term intervention with pravastatin in ischaemic disease (lipid) study. Aust N Z J Public Health. 2003;27:649–53. doi: 10.1111/j.1467-842x.2003.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine Taskforce. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in sweden (swedish obese subjects study): A prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 25.Knuiman MW, Clarkson JP, Bulsara M, Bartholomew HC. Evaluating the impact of repeated community-wide health surveys on cardiovascular morbidity and mortality in the busselton population. Aust N Z J Public Health. 2004;28:267–72. doi: 10.1111/j.1467-842x.2004.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 26.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment. Ann Intern Med. 2012;156:115–22. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 27.Lavie P, Lavie L. Unexpected survival advantage in elderly people with moderate sleep apnoea. J Sleep Res. 2009;18:397–403. doi: 10.1111/j.1365-2869.2009.00754.x. [DOI] [PubMed] [Google Scholar]
- 28.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: Ischemic preconditioning? Sleep Breath. 2013;17:819–26. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 29.Esnaola S, Duran J, Infante-Rivard C, Rubio R, Fernandez A. Diagnostic accuracy of a portable recording device (MESAM IV) in suspected obstructive sleep apnoea. Eur Respir J. 1996;9:2597–605. doi: 10.1183/09031936.96.09122597. [DOI] [PubMed] [Google Scholar]
- 30.Stoohs R, Guilleminault C. Mesam 4: An ambulatory device for the detection of patients at risk for obstructive sleep apnea syndrome (OSAS) Chest. 1992;101:1221–7. doi: 10.1378/chest.101.5.1221. [DOI] [PubMed] [Google Scholar]
- 31.Sanfilippo F, Hobbs M, Knuiman M, et al. Can we monitor heart attack in the troponin era: Evidence from a population-based cohort study. BMC Cardiovasc Disord. 2011;11:35. doi: 10.1186/1471-2261-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamrozik K, Dobson A, Hobbs M, et al. Monitoring the incidence of cardiovascular disease in Australia. Canberra: Australian Institute of Health and Welfare (AIHW); 2001. [Google Scholar]
- 33.Norman PE, Semmens JB, Lawrence-Brown MM, Holman CD. Long term relative survival after surgery for abdominal aortic aneurysm in western australia: Population based study. BMJ. 1998;317:852–6. doi: 10.1136/bmj.317.7162.852. [DOI] [PMC free article] [PubMed] [Google Scholar]


