Abstract
Study Objectives:
We sought to perform a patient-level meta-analysis using the individual patient data of the trials identified in our previous study-level meta-analysis investigating the effect of positive airway pressure (PAP) treatment for obstructive sleep apnea (OSA) on blood pressure (BP).
Design:
Patient-level meta-analysis.
Setting:
N/A.
Participants:
968 adult OSA subjects without major comorbidities drawn from eight randomized controlled trials.
Interventions:
Therapeutic PAP versus non-therapeutic control conditions (sham-PAP, pill placebo or standard care) over at least one week.
Measurements and Results:
The mean reductions in BP between PAP and non-therapeutic control arms were -2.27 mm Hg (95% CI -4.01 to -0.54) for systolic BP and -1.78 mm Hg (95% CI -2.99 to -0.58) for diastolic BP. The presence of uncontrolled hypertension at baseline was significantly associated with a reduction in systolic BP of 7.1 mm Hg and diastolic BP of 4.3 mm Hg after controlling for OSA severity (apnea-hypopnea index, Epworth Sleepiness Scale score, PAP level), patient demographics (age, gender, body mass index, use of antihypertensive medication/s), and measures of PAP efficacy (PAP adherence and treatment duration).
Conclusions:
OSA patients with uncontrolled hypertension are likely to gain the largest benefit from PAP in terms of a substantial reduction in BP, even after controlling for disease severity.
Citation:
Bakker JP; Edwards BA; Gautam SP; Montesi SB; Durán-Cantolla J; Barandiarán FA; Barbé F; Sánchezde-la-Torre M; Malhotra A. Blood pressure improvement with continuous positive airway pressure is independent of obstructive sleep apnea severity. J Clin Sleep Med 2014;10(4):365-369.
Keywords: Patient-level meta-analysis, hypertension, obstructive sleep apnea, positive airway pressure, lung
Evidence to date has established that the gold-standard treatment for obstructive sleep apnea (OSA), positive airway pressure (PAP), has beneficial effects on the cardiovascular sequelae of OSA; however, the extent to which PAP can reduce blood pressure (BP) has been difficult to discern based on individual randomized controlled trials (RCTs). More importantly, which patient subgroups likely to experience a substantial reduction in BP with PAP is unclear.
We recently published the largest systematic review and meta-analysis to date comparing the effect of PAP treatment versus a non-therapeutic control condition on BP, including 28 RCTs (representing n = 1,948 patients).1 We reported that compared to control conditions, PAP significantly reduced both systolic BP (SBP) and diastolic BP (DBP) when assessed either during the day (SBP -2.58 mm Hg and DBP -2.01 mm Hg) or night (SBP -4.09 mm Hg and DBP -1.85 mm Hg). Using meta-regression, we found that the reduction in DBP with PAP was predicted by high mean baseline DBP and hypersomnolence as measured by the Epworth Sleepiness Scale (ESS).
BRIEF SUMMARY
Current Knowledge/Study Rationale: The extent to which positive airway pressure therapy can reduce blood pressure has been difficult to discern based on individual randomized controlled trials. This investigation was performed in order to identify which patient subgroups are more likely to experience a substantial reduction in blood pressure with treatment.
Study Impact: Our findings suggest that patients with uncontrolled hypertension are likely to experience the largest reduction in blood pressure with positive airway pressure therapy, after controlling for disease severity and daytime sleepiness. This finding has direct clinical relevance, in that it suggests that even patients with mild/moderate disease who do not report hypersomnolence are likely to benefit from treatment.
Our original hypothesis that OSA severity as measured by the apnea-hypopnea index (AHI) would be a significant predictor of the change in BP with PAP was not supported; similarly, age, treatment duration, and PAP adherence were not significant predictors in meta-regression analyses. Meta-regression is conceptually similar to simple regression except that in this setting, the independent (predictor) variables are at the study level. Hence, a degree of between-study variability of the independent variable/s in question is required in order for these data to be useful predictors of the dependent variable (outcome). For example in our meta-analysis, the mean age of patients in the 28 included trials fell into the narrow range of 43-63 years, and 85% of included trials had a mean age in the range of 45-55 years, despite the fact that the age range of each individual trial was wide. The low variability in study-level independent variables therefore limited our ability to identify significant predictors of BP using meta-regression.
Rather than performing further costly clinical trials targeting narrowly defined subgroups, we aimed to address this limitation by performing a patient-level meta-analysis using the individual patient data of the trials we identified in our previous study-level meta-analysis. Our primary hypothesis was that baseline AHI would be significantly associated with a reduction in both SBP and DBP with PAP treatment. We also sought to measure a range of other potential predictors encompassing OSA severity, patient demographics, and PAP efficacy.
METHODS
Our published study-level meta-analysis1 contains details of the literature search and inclusion/exclusion criteria, which we adopted following PRISMA guidelines.2 In brief, we analyzed 28 RCTs published between 1980 and 2012 comparing PAP treatment to a non-therapeutic control condition (sham-PAP, pill placebo, or standard care) over at least one week in adult OSA patients without major comorbidity, reporting office BP and/or ambulatory BP measurements at ≥ 2 time points. For the current study, we emailed the first, second, last, and/or corresponding author of each RCT, allowing at least 8 weeks response time.
Independent Variables (Predictors)
We requested that the authors provide de-identified individual data including: descriptive information (age, gender, body mass index [BMI] at baseline, use of antihypertensive medication/s), measures associated with OSA severity (AHI at baseline, ESS at baseline, therapeutic PAP level), and measures associated with PAP efficacy (residual AHI, PAP adherence, treatment duration). A binary independent variable of “uncontrolled hypertension at baseline” was created by identifying all patients with daytime SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg regardless of medication usage. We chose to exclude patients whose BP was well controlled with antihypertensives from this group, as a substantial reduction in BP would not be expected. AHI and ESS data were categorized according to commonly accepted cutoffs (AHI ≥ 30 events/h indicating severe OSA and ESS ≥ 11/24 indicating daytime hypersomnolence).
Dependent Variables (Outcomes)
We requested all BP data available, recognizing the differing methods by which these data were collected. Our 2 dependent variables, daytime SBP and DBP, were created by using either office or daytime ambulatory measurements, with preference given to ambulatory measurements for studies that measured both. The change in SBP and DBP for each patient was calculated by subtracting the baseline value from the end-trial value, such that a negative value represented a reduction in BP. Our intention for crossover trials was to combine the data as above only when we were able to ascertain which intervention each patient received first, thereby treating these as parallel trials by ignoring the second arm of the study (in fact, no crossover studies were included in the final analyses).
Statistical Analyses
In order to determine whether the data that we received for the current analyses were representative of our previous study-level meta-analysis, we performed a standard random-effects study-level meta-analysis for daytime SBP and DBP using Review Manager (RevMan) Version 5.1 (Nordic Cochrane Center, Copenhagen, Denmark), using only trials for which individual data were obtained.3
Using individual patient data, we then evaluated associations of our 2 dependent variables using mixed linear modeling with the study as the random factor. No within-studies clustering effect was evident, so standard between-group tests and linear regression were used for all patient-level statistical analyses, using SPSS (Version 20, IBM, NY USA). The differences between the changes in SBP and DBP with PAP versus non-therapeutic controls were assessed using independent t-tests. Univariate linear regression was used to assess the association between our 10 independent variables (age, gender, BMI, antihypertensive medication/s, AHI, ESS, PAP level, PAP adherence, treatment duration, uncontrolled hypertension at baseline) and each of the 2 dependent variables (SBP, DBP) in patients randomized to PAP treatment. Three multivariate linear regression models were then constructed for each of the 2 dependent variables; all multivariate models included uncontrolled hypertension at baseline, as substantial reductions in BP would not be expected in normotensive patients. Model 1 included measures of OSA severity (AHI, ESS, PAP level); Model 2 additionally controlled for patient descriptive information (age, gender, BMI, use of antihypertensives); Model 3 additionally controlled for measures of PAP efficacy (PAP adherence and treatment duration).
RESULTS
Data were received from the authors of 11 original trials; however, for the 3 crossover trials we were unable to determine which intervention each patient received first,4–6 leaving us with 8 analyzable trials (all parallel designs).7–14 The authors of 12 papers were not able to send their data,15–26 and the authors of the remaining papers did not respond.27–34 Study quality was assessed by analyzing various risks of bias by 2 independent investigators (JPB and BAE); see Table 1. On the whole, study quality was high, given that we included only randomized controlled trials; the most noteworthy source of potential bias comes from the fact that 4 of the trials were not placebo-controlled and were therefore not blinded completely.
Table 1.
Authors' judgments as to risk of bias in each randomized controlled trial
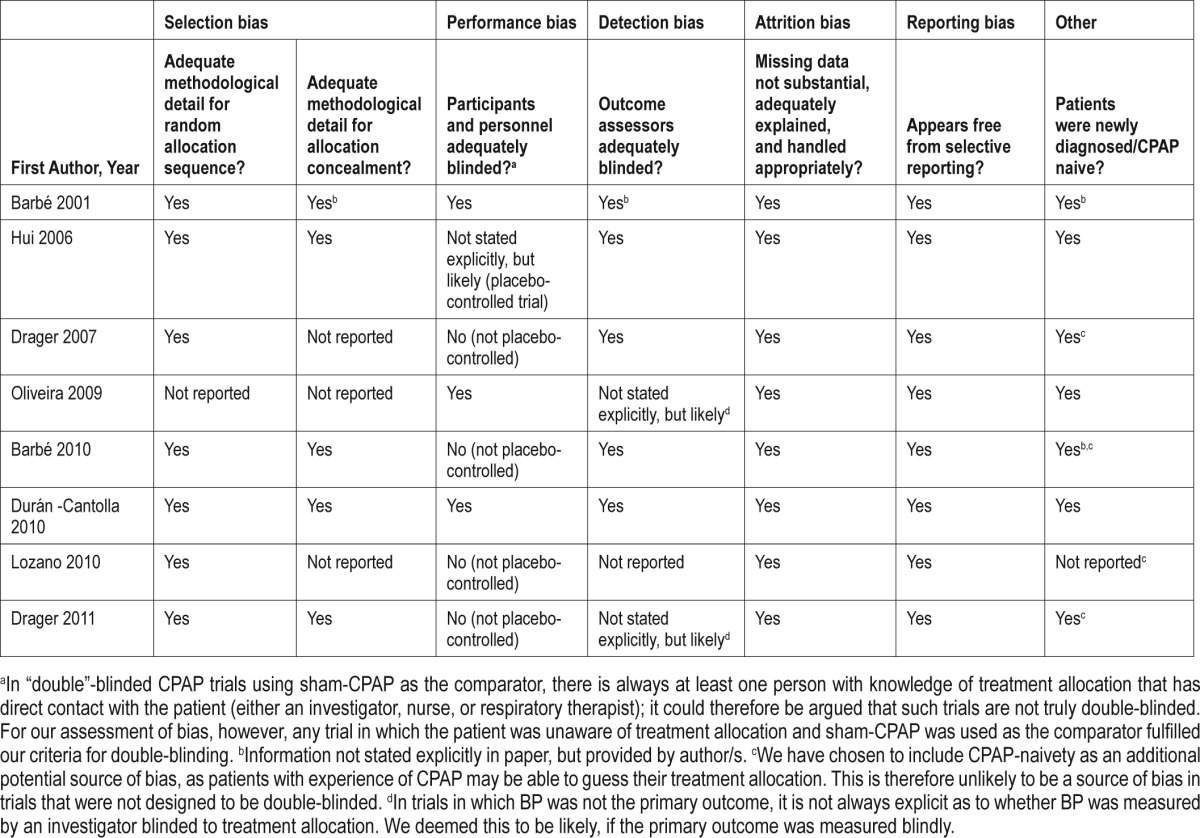
Our total sample size was n = 968; 486 patients were randomized to PAP, with the remaining 482 randomized to a non-therapeutic control condition. Six trials representing n = 564 patients8–13 used 24-h ABPM monitoring divided into diurnal/nocturnal data either by patient report or by a pre-determined clock time. The remaining 2 trials representing n = 404 patients7,14 used office BP measurements. Few studies had records pertaining to the follow-up time for individual patients; therefore, the average follow-up duration for each study as reported in the primary papers was used. Residual AHI was not available in the majority of studies due to the absence of follow-up sleep studies; this independent variable was therefore not used in any analyses.
Study-level meta-analyses of the aforementioned 8 trials7–14 found weighted mean differences between PAP and non-therapeutic control arms of -2.12 mm Hg (95% CI -3.89 to -0.36) for SBP and -1.60 mm Hg (95% CI -3.33 to 0.13) for DBP, both favoring PAP (statistical significance for SBP only). Pooling individual patient data from the same 8 trials, the mean differences between groups were -2.27 mm Hg (95% CI -4.01 to -0.54) for SBP and -1.78 mm Hg (95% CI -2.99 to -0.58) for DBP (significantly favoring PAP for both SBP and DBP).
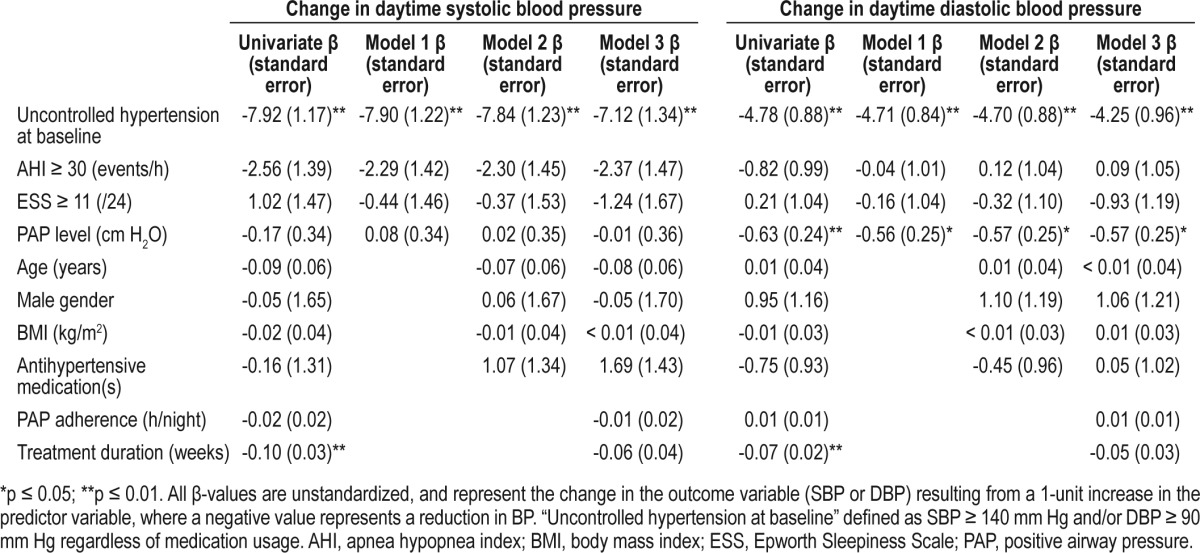
Univariate predictors of the change in SBP and DBP with PAP are shown in Table 2. Increased treatment duration and the presence of uncontrolled hypertension at baseline were both significant univariate predictors of SBP and DBP reduction; PAP level was a significant univariate predictor of the change in DBP only. Results pertaining to the 3 multivariate models are also shown in Table 2. Uncontrolled hypertension at baseline remained an independent predictor of the reduction in both SBP and DBP, after controlling for (1) measures of OSA severity, (2) patient descriptive information, and (3) measures associated with PAP efficacy. The unstandardized β-value for uncontrolled hypertension at baseline remained at ≥ 7.0 (SBP) and ≥ 4.0 (DBP) in all models (all p-values ≤ 0.01).
Table 2.
Predictors of the change in daytime systolic and diastolic blood pressure with positive airway pressure treatment
DISCUSSION
The current study extends previous work1,35 by demonstrating that the sole predictor of a reduction in both SBP and DBP with PAP was the presence of uncontrolled hypertension at baseline. Although PAP level was also a significant independent predictor of the reduction in DBP, the magnitude of this trend was not strong (β values indicated that each 1 cm H2O increase in PAP was associated with a reduction in DBP of < 1 mm Hg). The presence of hypertension, however, was associated with a reduction in SBP of 7.1 mm Hg and DBP of 4.3 mm Hg after controlling for OSA severity, daytime sleepiness, patient demographics, and measures of PAP efficacy.
Our primary hypothesis, that baseline AHI would be significantly associated with a reduction in both SBP and DBP with PAP treatment, was not supported. Further, daytime sleepiness as measured by the ESS was not a significant predictor of the PAP-induced reduction in BP, in contrast with our previous study-level meta-analysis.1 Our previous work published in 2012 contained data that have subsequently been retracted. A major strength of the current analysis, however, is that we assembled the largest database of PAP patients measuring BP to date, and thus we were adequately powered to detect even small effect sizes, if they existed.36,37
Although the data pooled from eight trials represents only one-third of the studies included in our study-level meta-analysis, the fact that we were able to obtain data from several of the larger studies means that our sample size of n = 968 represents just over half of the available population. Given that the original trials were published over a period of 17 years, we believe that this response rate is substantial. The missing data may have led to some bias; however we believe this limitation to be minor, as (1) the point estimates for the reductions in SBP and DBP from the study-level meta-analyses performed here are in close agreement with our previously reported results,1 and (2) t-tests using individual patient data demonstrated almost identical mean differences in SBP and DBP. We are therefore confident that our data are representative of the total study population, and thus generalizable. Another limitation is that based on the type of studies included, we were limited to only assessing the effect of PAP on BP as this is the most widely reported cardiovascular measure. Similarly, very few studies reported the residual AHI and we were therefore unable to assess PAP efficacy. Approximately half the patients in our analyses had BP recorded using ABPM while the rest had BP recorded in the office setting; these methods are not necessarily comparable but we chose to combine these data. In all cases, the BP data was collected during a single 24-hour period or a single office visit, which limits reproducibility.38 Finally, we collected data from RCTs even though our regression models included only the patients randomized to PAP. We chose to do this for comparability to our previous meta-analysis, and also to limit potential publication bias resulting from the fact that non-randomized negative trials may remain unpublished whereas all RCTs should in theory be locatable.
Our findings suggest that obstructive sleep apnea patients with uncontrolled hypertension are likely to gain the largest benefit from positive airway pressure in terms of a substantial reduction in blood pressure, even after controlling for disease severity and daytime sleepiness. Whether the greater reduction in blood pressure in this group is independently associated with reductions in other cardiovascular sequelae associated with obstructive sleep apnea should be the focus of future studies.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Malhotra has consulted for Philips Respironics, Sleep HealthCenters, Sleep Group Solutions, Apnicure, Apnex, and Pfizer, but has relinquished all outside personal income since May 2012. The other authors have indicated no financial conflicts of interest. Work was performed at Brigham & Women's Hospital and Harvard Medical School, Boston, MA.
ACKNOWLEDGMENTS
We thank the authors of original trials who provided individual patient data for these analyses. This work was conducted with support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 8UL1TR000170-05 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. Dr. Bakker is supported by NIH R01HL110350. Dr. Edwards is supported by the National Health and Medical Research Council of Australia's CJ Martin Overseas Biomedical Fellowship (1035115). Dr. Malhotra is supported by NIH (R01HL110350, R01HL085188, R01HL090897, K24HL093218, P01HL095491) and AHA (0840159N) grants. Dr. Barbé is supported by Instituto de Salud Carlos III (PI 04/0165 and PI10/02763) (Fondo de Investigaciones Sanitarias, Ministerio de Sanidad y Consumo, Spain) grant. No financial support was obtained for this investigation.
REFERENCES
- 1.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 3.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 4.Comondore VR, Cheema R, Fox J, et al. The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung. 2009;187:17–22. doi: 10.1007/s00408-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 5.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–64. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 6.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:773–80. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira W, Campos O, Cintra F, et al. Impact of continuous positive airway pressure treatment on left atrial volume and function in patients with obstructive sleep apnoea assessed by real-time three-dimensional echocardiography. Heart. 2009;95:1872–8. doi: 10.1136/hrt.2009.173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 9.Drager LF, Pedrosa RP, Diniz PM, et al. The effects of continuous positive airway pressure on prehypertension and masked hypertension in men with severe obstructive sleep apnea. Hypertension. 2011;57:549–55. doi: 10.1161/HYPERTENSIONAHA.110.165969. [DOI] [PubMed] [Google Scholar]
- 10.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–12. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 11.Hui DS, To KW, Ko FW, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. 2006;61:1083–90. doi: 10.1136/thx.2006.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duran-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 13.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Barbe F, Duran-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718–26. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 15.Kohler M, Stoewhas AC, Ayers L, et al. Effects of CPAP therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2011;184:1192–9. doi: 10.1164/rccm.201106-0964OC. [DOI] [PubMed] [Google Scholar]
- 16.Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32:1488–96. doi: 10.1183/09031936.00026608. [DOI] [PubMed] [Google Scholar]
- 17.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 18.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 19.Dimsdale JE, Loredo JS, Profant J. Effect of continuous positive airway pressure on blood pressure: a placebo trial. Hypertension. 2000;35:144–7. doi: 10.1161/01.hyp.35.1.144. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64:581–6. doi: 10.1136/thx.2008.100537. [DOI] [PubMed] [Google Scholar]
- 21.Cross MD, Mills NL, Al-Abri M, et al. Continuous positive airway pressure improves vascular function in obstructive sleep apnoea/hypopnoea syndrome: a randomised controlled trial. Thorax. 2008;63:578–83. doi: 10.1136/thx.2007.081877. [DOI] [PubMed] [Google Scholar]
- 22.Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. 2007;29:720–7. doi: 10.1183/09031936.00043306. [DOI] [PubMed] [Google Scholar]
- 23.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27:1229–35. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 24.Alonso-Fernandez A, Garcia-Rio F, Arias MA, et al. Obstructive sleep apnoeahypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J. 2006;27:207–15. doi: 10.1093/eurheartj/ehi621. [DOI] [PubMed] [Google Scholar]
- 25.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–8. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 26.Engleman HM, Gough K, Martin SE, Kingshott RN, Padfield PL, Douglas NJ. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in “non-dippers”. Sleep. 1996;19:378–81. doi: 10.1093/sleep/19.5.378. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson. 2010;12:50. doi: 10.1186/1532-429X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam JC, Lam B, Yao TJ, et al. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J. 2010;35:138–45. doi: 10.1183/09031936.00047709. [DOI] [PubMed] [Google Scholar]
- 29.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129:1459–67. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 31.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 32.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 33.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 34.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;164:939–43. doi: 10.1164/ajrccm.164.6.2008010. [DOI] [PubMed] [Google Scholar]
- 35.Middleton S, Vermeulen W, Byth K, Sullivan CE, Middleton PG. Treatment of obstructive sleep apnoea in Samoa progressively reduces daytime blood pressure over 6 months. Respirology. 2009;14:404–10. doi: 10.1111/j.1440-1843.2009.01510.x. [DOI] [PubMed] [Google Scholar]
- 36.Miles J, Shevlin M. London: Sage; 2001. Applying Regression and Correlation: A Guide for Students and Researchers. [Google Scholar]
- 37.Green S. How many subjects does it take to do a regression analysis? Multivariate Behav Res. 1991;26:499–510. doi: 10.1207/s15327906mbr2603_7. [DOI] [PubMed] [Google Scholar]
- 38.Palatini P, Mormino P, Canali C, et al. Factors affecting ambulatory blood pressure reproducibility. Results of the HARVEST Trial. Hypertension and Ambulatory Recording Venetia Study. Hypertension. 1994;23:211–6. doi: 10.1161/01.hyp.23.2.211. [DOI] [PubMed] [Google Scholar]


