Abstract
Study Objectives:
The aim of this prospective controlled study was to explore the diagnostic value of repeated polysomnography (PSG) post-nasopharyngeal tube insertion in the setting of glossopharyngeal obstruction in obstructive sleep apnea hypopnea syndrome (OSAHS).
Methods:
Patients were eligible for this study if they were diagnosed as OSAHS by the first PSG and presented with moderate to severe OSAHS by repeated PSG scanning post-nasopharyngeal tube insertion (NPT-PSG). Fifty-nine patients were enrolled into this study and assigned to received either modified uvulopalatopharyngoplasty (H-UPPP; n = 25) or H-UPPP in combination with glossopharyngeal surgery (n = 34).
Results:
General data and PSG data were collected prior to and following nasopharyngeal tube insertion and were found not to be significantly different. However, both the PSG and Epworth sleepiness scale (ESS) were significantly superior in the combination group compared to the UPPP alone group post-surgery. The success rates of surgery were 82.3% and 40.0%, respectively.
Conclusion:
Patients with moderate to severe OSAHS post-nasopharyngeal tube insertion generally have glossopharyngeal obstruction. Glossopharyngeal surgery can significantly improve surgical outcome in the setting of OSAHS.
Citation:
Li S; Wu D; Bao J; Qin J. Nasopharyngeal tube: a simple and effective tool to screen patients indicated for glossopharyngeal surgery. J Clin Sleep Med 2014;10(4):385-389.
Keywords: Obstructive sleep apnea hypopnea syndrome, OSAHS, nasopharyngeal tube, glossopharyngeal, surgery
Obstructive sleep apnea hypopnea syndrome (OSAHS) is characterized by apnea and hypoventilation arising from the collapse or obstruction of the upper respiratory tract during sleep. OSAHS may be accompanied by symptoms such as snoring, disordered sleep structure, frequent decreases in blood oxygen saturation, and daytime sleepiness.1 The key feature of OSAHS is the collapse or obstruction of the upper respiratory tract during sleep. The oropharyngeal zone is most commonly obstructed, and the glossopharyngeal airway is the next most commonly obstructed.2,3 However, it is difficult to confirm or exclude the diagnosis of glossopharyngeal obstruction. Conventional physical examination, electronic endoscopy and computed tomography (CT) examination can offer some clues in the waking state of the patient,4–6 but the most accurate diagnosis depends on the airway examination during sleep. Endoscopic airway examination and imaging examination during sedative-induced sleep can be used to observe morphological changes of the glossopharyngeal area during apnea, and can be used to confirm or exclude the presence of glossopharyngeal airway obstruction.7 Whether inspection performed during induced sleep represents the actual condition of the airway during normal sleep is uncertain. Continuous airway pressure measurements or the AG200 diagnostic system require special equipment and training,8,9 thus limiting their broad use. Nasopharyngeal intubation is generally used to rescue the upper respiratory tract obstruction.10,11 In recent years, some scholars have attempted to use nasopharyngeal intubation to treat adult OSAHS, which has some demonstrable efficacy.12,13 Theoretically, nasopharyngeal intubation can keep the respiratory tract from the naris to the peak of the nasopharyngeal tube unobstructed. Hence, if nasopharyngeal intubation is ineffective, airway obstruction is likely within the respiratory tract, below the peak of the nasopharyngeal tube. Based on this, we placed the peak of the nasopharyngeal tube at the level of the free edge of the soft palate and then repeated the PSG (NPT-PSG) to confirm glossopharyngeal obstruction to guide subsequent glossopharyngeal surgical treatment.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The glossopharyngeal airway is the second most commonly obstructed site in adult obstructive sleep apnea hypopnea syndrome (OSAHS). However, the diagnosis of glossopharyngeal obstruction is challenging. The aim of this study was to explore the diagnostic value of repeated polysomnography following insertion of a nasopharyngeal tube (NPT-PSG) in the setting of glossopharyngeal obstruction in OSAHS.
Study Impact: NPT-PSG is a simple and effective tool used to screen patients who are indicated for glossopharyngeal surgery. Patients with moderate-to-severe OSAHS diagnosed by NPT-PSG generally present with glossopharyngeal airway obstruction. Glossopharyngeal surgery can significantly improve surgical outcomes in the setting of OSAHS.
MATERIALS AND METHODS
Subjects
The current study was a prospective clinically controlled study. Subjects were selected from inpatients in the Department of Otolaryngology at our hospital from January 2010 to May 2012. Patients were eligible for the study if they met criteria including: (1) confirmed diagnosis of OSAHS; (2) male; (3) no significant rhinal or nasopharyngeal obstructive diseases; (4) no craniofacial structural abnormalities such as micrognathia; (5) apnea hypopnea index (AHI) ≥ 15/h by PSG post-placement of the nasopharyngeal tube; (6) inability or unwillingness to use continuous positive airway pressure (CPAP); (7) strong desire for surgical treatment; and (8) providing signed and informed consent.
Sleep Monitoring Before and After Placement of Nasopharyngeal Tube
Sleep monitoring was conducted with the Polywin PSG system (Respironics, USA). In accordance with sleep monitoring regulations and diagnostic criteria of OSAHS set by the American Academy of Sleep Medicine,14 patients who were diagnosed OSAHS for the first time and who planned surgical treatment, were entered into the examination procedure of nasopharyngeal tube insertion. This study was approved by the local ethics committee. All patients provided written informed consent. The nasopharyngeal tube was placed as previously reported,13 with an internal diameter of 7 mm in 35 patients and 8 mm in 24 patients, respectively. Proper or successful placement of the nasopharyngeal tube should be fixed properly in the anterior naris to prevent dislocation during sleep and the inferior extremity slightly exceeded the free edge of soft palate. Then PSG was repeated (NTP-PSG), with the procedures and data collection approaches the same as with the first PSG.
Besides PSG, all patients received conventional physical and auxiliary examinations, including Friedman tongue position (FTP) grading, CT examination, conventional electronic endoscopy of the upper respiratory tract, and Muller's manoeuvre and observation.4–6
Grouping, Surgical Procedure, Follow-Up, and Efficacy Assessment
Potential rhinal and nasopharyngeal diseases had been excluded in the patients prior to enrolment into the study, and thus rhinal and nasopharyngeal surgeries were not conducted. If the AHI was ≥ 15/h after nasopharyngeal tube insertion, glossopharyngeal obstruction was considered. In combination with the above and conventional examination results, FTP grading, endoscopy, and CT results, treatment with modified UPPP15 combined with glossopharyngeal surgery was suggested. All patients were well informed of their disease condition and surgery, and it was noted that some patients agreed only to treatment with UPPP but refused glossopharyngeal surgery. Therefore, there were 2 patient groups who were comparable in terms of the collated general data. However, these patient groups received different surgical treatment schemas: modified UPPP and modified UPPP in combination with glossopharyngeal surgery. All patients were followed up > 12 months (an average of 16 months). After 12 months, both PSG and ESS scoring were repeated. Patients were dropped from the study if postoperative PSG and ESS data could not be obtained. The absolute change and improvement in the rate of AHI, lowest oxygen saturation (LaSO2), and ESS scores were calculated. The number of patients showing a reduction ≥ 50% in the AHI to levels below 20, 15, 10, and 5 events/h were counted.16 Surgical outcomes were classified as cured (AHI < 5) or markedly effective (AHI decreasing > 50% and ≤ 20) and inefficient (beyond cure and markedly effective criteria). Success was defined as cure and markedly effective outcomes, and failure as inefficient outcome.
Statistical Analysis
SPSS 13.0 software program (SPSS Inc., Chicago, IL) was used for statistical analysis. ESS and PSG data were compared (1) between the 2 groups; and (2) before and after surgery. The success rate of surgery was compared between the 2 groups. Continuous data were analyzed using the Student t-test, and categorical data were analyzed using the χ2 test.
RESULTS
Patient Demographics
NPT-PSG was planned in 131 patients diagnosed with OSAHS. Three patients complained of nasal cavity or nasopharyngeal pain, significant discomfort, and difficulty falling asleep; thus the NPT-PSG was incomplete, and these 3 patients were excluded from the study. An additional 63 patients were removed from the study for AHI < 15 on NPT-PSG. Of the remaining 65 patients, 6 did not complete 12-month follow-up and were subsequently dropped from the study. Finally, 59 patients were enrolled into the current study, with a mean age of 39.42 ± 7.53 years (range 21 to 56 years), mean body mass index (BMI) of 28.66 ± 2.91 kg/m2 (range 20.0 to 35.7 kg/m2), mean AHI of 40.52 ± 4.77/h (range 21.2 to 96.5/h), mean LaSO2 of 0.66 ± 0.10 (range 0.41 to 0.87), and mean ESS score of 11.22 ± 5.08 (range 2 to 21). All patients had evidence of snoring, breathlessness, and apnea during sleep, daytime somnolence and fatigue, morning headache, and poor memory. Tonsil sizes of 0, I, II, III, and IV were found in 0, 14, 14, 18, and 13 cases, respectively. Friedman tongue position I, II, III, and IV were found in 5, 15, 26, and 13 cases, respectively. Friedman stage I, II, and III were found in 10, 29, and 20 cases, respectively. There were also 26 patients who presented with lingua hypertrophy and 8 patients who presented with lingual tonsil hypertrophy confirmed by endoscopic examination.
Grouping and Data Comparison between Groups
All patients were assigned according to the willingness of the patient to receive modified UPPP (n = 25) and modified UPPP in combination with glossopharyngeal surgery (n = 34). Some procedures were applied according to the causes of glossopharyngeal obstruction and included UPPP in combination with midline partial glossectomy (n = 18),17 UPPP in combination with lingual base suspension (n = 11),18 and UPPP in combination with lingual tonsil resection (n = 5). General data, ESS scores, tonsil size, Friedman stage, endoscopic results, and PSG data prior to and following nasopharyngeal tube insertion were not statistically different between the 2 groups (see Tables 1 and 2). AHI, LaSO2, and ESS post-surgery were statistically superior in the UPPP in combination with the glossopharyngeal group compared with the UPPP alone group (Table 3).
Table 1.
Preoperative clinical data comparison of both groups (mean ± SD or the number of cases)

Table 2.
Preoperative PSG data comparison of both groups (mean ± SD)

Table 3.
Postoperative group comparison

Data Comparison Before and After Operation in Same Group
In the UPPP alone group, AHI, LaSO2, and ESS significantly differed before and after surgery (Figure 1), with an average absolute changes of 17.04, 0.08, and 4.88, and improvement rates of 43.5%, 12.3%, and 43.0%, respectively. The results suggested that surgery was effective. In the UPPP in combination with the glossopharyngeal surgical group, measurement of AHI, LaSO2, and ESS also showed significant differences prior to and post-surgery (Figure 1), with an average absolute change of 29.52, 0.12, and 6.68, and an improvement rate of 75.3%, 17.9%, and 60.1%, respectively. This also suggested that surgery was effective. The average absolute change and improvement rates of the 3 indices were significantly higher in the combined treatment group than in the UPPP alone group.
Figure 1.
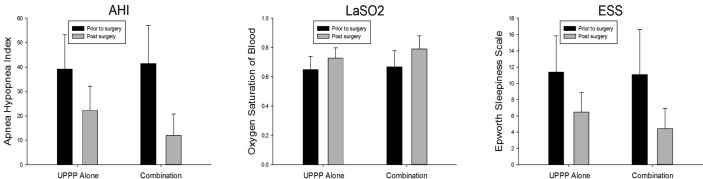
Both in the UPPP alone and the combination groups, AHI, LaSO2, and ESS were significantly different before and after surgery. The improvement rates of the three indices were significantly higher in the combination treatment group than in the group treated with UPPP alone.
Efficacy
Both PSG and ESS scoring were repeated in the postoperative 12-month period (Table 4). Results showed that the curing, markedly effective, and ineffective rates were significantly different between the 2 groups (χ2 = 11.92, p = 0.003), and the successful rate of surgery was significantly higher in the combination treatment group (82.3%) than in the UPPP alone group (40.0%) (χ2 = 11.273, p = 0.001).
Table 4.
Efficacy of treatment: comparison of both groups

DISCUSSION
Obstructive sleep apnea hypopnea syndrome (OSAHS) results from collapse or obstruction of the upper respiratory tract during sleep.1 The first choice for treating OSAHS is continuous positive airway pressure (CPAP) without the need for either AG200 or sedated endoscopic examination. However, in some patients unwilling or unable to accept CPAP, surgery is an alternative approach, and the obstructive site of the upper airway must then be diagnosed. The oropharyngeal zone is the most common site involved, closely followed by the glossopharyngeal zone.2,3 However, glossopharyngeal obstruction is difficult to diagnose. Common methods include conventional physical examination, electronic endoscopy, CT scanning in the waking state, upper airway endoscopy, and imaging examinations in sedative-induced short sleep, and continuous airway pressure measurement and AG200 localization diagnosis in whole night sleep.4–9
However, these methods have some disadvantages, such as being poorly accurate, expensive, and requiring specific equipment and devices. In recent years, some investigators have tried to use the nasopharyngeal tube to treat adult OSAHS and have obtained some efficacious outcomes.12,13 Theoretically, the nasopharyngeal tube can keep the respiratory tract from naris to the peak of nasopharyngeal tube unobstructed. In this situation, if patients still have OSAHS by PSG, the airway below the peak of the nasopharyngeal tube is confirmed as being obstructed.13 Based on this, we placed the peak of the nasopharyngeal tube at the level of the free edge of the soft palate and then repeated PSG to get important information on the obstruction of the glossopharyngeal airway and below this zone. In the current study, an AHI ≥ 15 post-nasopharyngeal tube placement was the key criterion for treatment with glossopharyngeal surgery.
Potential rhinal and nasopharyngeal diseases had been excluded prior to surgery, and thus rhinal and nasopharyngeal surgeries were unnecessary under these situations. All patients were well informed of their disease condition. According to the willingness of the patients, 25 patients agreed to UPPP and refused glossopharyngeal surgery; the other 34 patients received both surgical procedures. The two groups were comparable in terms of general data, ESS scores, and PSG results prior to and following nasopharyngeal tube. However, postoperative indices were superior in the combination group compared with the UPPP group alone, with success rates of 82.3% vs. 40.0%. In the same group, AHI and ESS were greatly decreased and LaSO2 was significantly increased post-surgery. However, the average absolute change and the improvement rates of the three indices were significantly much greater in the combination group than was found in the UPPP alone group; this might have contributed to the different outcomes between the two groups. These results illustrate that patients with an AHI ≥ 15/h post-nasopharyngeal tube do indeed have glossopharyngeal obstruction and can greatly improve with intervention by proper glossopharyngeal surgery.
It is of note that of the 25 patients undergoing UPPP alone, three recovered completely and seven had effective outcomes, yielding a success rate of 10/25. Of these 10 patients, one had an AHI of 67.7/h in the first PSG and 16.4/h post-nasopharyngeal intubation, suggesting that glossopharyngeal obstruction existed but was not dominant. UPPP improves dominant oropharyngeal obstruction, and an AHI of 18.6/h promoted a successful surgery. The other nine patients had greater than grade III hypertrophy of the palatine tonsilla (9/10). Only four of the remaining 15 patients undergoing UPPP alone showed poor surgical outcomes and presented with greater than grade III palatine tonsilla (4/15). The inferior extremity of the nasopharyngeal tube only slightly exceeded the free edge of the soft palate; therefore, hypertrophy of the palatine tonsilla might induce an AHI that is too high. In UPPP, the tonsil is resected. Consequently, UPPP alone is successful.
In conclusion, repeated PSG post-placement of nasopharyngeal tube requires no specific devices and can be used as a simple and effective method to diagnose glossopharyngeal obstruction. Glossopharyngeal surgery should be recommended to patients with an AHI ≥ 15/h post-placement of the nasopharyngeal tube and with no obvious hypertrophy of the tonsils.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Isono S, Remmers JE. Anatomy and physiology of upper airway obstruction. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2nd ed. Philadelphia: Saunders; 1994. [Google Scholar]
- 2.Soares D, Sinawe H, Folbe AJ, et al. Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope. 2012;122:473–9. doi: 10.1002/lary.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin HS, Rowley JA, Badr MS, et al. Transoral robotic surgery for treatment of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2013;123:1811–6. doi: 10.1002/lary.23913. [DOI] [PubMed] [Google Scholar]
- 4.Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope. 2004;114:454–9. doi: 10.1097/00005537-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Bachar G, Nageris B, Feinmesser R, et al. Novel grading system for quantifying upper-airway obstruction on sleep endoscopy. Lung. 2012;190:313–8. doi: 10.1007/s00408-011-9367-3. [DOI] [PubMed] [Google Scholar]
- 6.Tang XL, Yi HL, Luo HP, et al. The application of CT to localize the upper airway obstruction plane in patients with OSAHS. Otolaryngol Head Neck Surg. 2012;147:1148–51. doi: 10.1177/0194599812459461. [DOI] [PubMed] [Google Scholar]
- 7.Soares D, Folbe AJ, Yoo G, Badr MS, Rowley JA, Lin HS. Drug-induced sleep endoscopy vs awake Muller's maneuver in the diagnosis of severe upper airway obstruction. Otolaryngol Head Neck Surg. 2013;148:151–6. doi: 10.1177/0194599812460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A, Al-Reefy H, Hewitt R, Kotecha B. Evaluation of ApneaGraph in the diagnosis of sleep-related breathing disorders. Eur Arch Otorhinolaryngol. 2008;265:1489–94. doi: 10.1007/s00405-008-0685-x. [DOI] [PubMed] [Google Scholar]
- 9.Demin H, Jingying Y, Jun W, Qingwen Y, Yuhua L, Jiangyong W. Determining the site of airway obstruction in obstructive sleep apnea with airway pressure measurements during sleep. Laryngoscope. 2002;112:2081–5. doi: 10.1097/00005537-200211000-00032. [DOI] [PubMed] [Google Scholar]
- 10.Chang AB, Masters IB, Williams GR, Harris M, O'Neil MC. A modified nasopharyngeal tube to relieve high upper airway obstruction. Pediatr Pulmonol. 2000;29:299–306. doi: 10.1002/(sici)1099-0496(200004)29:4<299::aid-ppul10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Tweedie DJ, Skilbeck CJ, Lloyd-Thomas AR, Albert DM. The nasopharyngeal prong airway: an effective postoperative adjunct after adenotonsillectomy for obstructive sleep apnoea in children. Int J Pediatr Otorhinolaryngol. 2007;71:563–9. doi: 10.1016/j.ijporl.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Nahmias JS, Karetzky MS. Treatment of the obstructive sleep apnea syndrome using a nasopharyngeal tube. Chest. 1988;94:1142–7. doi: 10.1378/chest.94.6.1142. [DOI] [PubMed] [Google Scholar]
- 13.Huo H, Li WY, Shen P, Liu JH. One night treatment of obstructive sleep apnea and hypopnea syndrome with nasopharyngeal airway. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:382–6. [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. [Google Scholar]
- 15.Han D, Ye J, Lin Z, Wang J, Wang J, Zhang Y. Revised uvulopalatopharyngoplasty with uvula preservation and its clinical study. ORL J Otorhinolaryngol Relat Spec. 2005;67:213–9. doi: 10.1159/000087390. [DOI] [PubMed] [Google Scholar]
- 16.Kezirian EJ, Weaver EM, Criswell MA, Vries N, Woodson T, Piccirill JF. Reporting results of obstructive sleep apnea syndrome surgery trials. Otolaryngol Head Neck Surg. 2011;144:496–9. doi: 10.1177/0194599810396791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Shi H. Lingual artery CTA-guided midline partial glossectomy for treatment of obstructive sleep apnea hypopnea syndrome. Acta Otolaryngol. 2013;133:749–54. doi: 10.3109/00016489.2013.765968. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wu D, Shi H. Treatment of obstructive sleep apnea hypopnea syndrome caused by glossoptosis with tongue-base suspension. Eur Arch Otorhinolaryngol. 2013;270:2915–20. doi: 10.1007/s00405-013-2536-7. [DOI] [PubMed] [Google Scholar]


