Abstract
Purpose
To determine the association between glaucoma and travel away from home.
Methods
Fifty-nine glaucoma suspect controls with normal vision and 80 glaucoma subjects with bilateral visual field (VF) loss wore a cellular tracking device over 1 week of normal activity. Location data was used to evaluate the number of daily excursions away from home as well as daily time spent away from home.
Results
Control and glaucoma subjects were similar in age, race, gender, employment, driving support, cognitive ability, mood, and comorbid illness (p>0.1 for all). Better-eye VF mean deviation (MD) averaged 0.0 decibels (dB) in controls and –11.1 dB in glaucoma subjects. In multivariable models, glaucoma was associated with fewer daily excursions (β= -0.20; 95% CI=-0.38 to -0.02) and a greater likelihood of not leaving home on a given day (Odds ratio [OR]=1.82; 95% CI=1.05 to 3.06). Each 5 dB decrement in the better-eye VF MD was associated with fewer daily excursions (β= -0.06; 95% CI=-0.11 to -0.01) and a greater chance of not leaving home on a given day (OR=1.24; 95% CI=1.04 to 1.47). Time spent away from home did not significantly differ between the glaucoma subjects and suspects (p=0.18). However, each 5 dB decrement in the better-eye MD was associated with 6% less time away (95% CI=-12 to -1%).
Conclusions
Individuals with glaucoma, particularly those with greater VF loss, are more home-bound and travel away from home less than individuals with normal vision. Since being confined to the home environment may have detrimental effects on fitness and health, individuals with glaucoma should be considered for interventions such as orientation and mobility training to encourage safe travel away from home.
Keywords: glaucoma, visual field, life space, global positioning system, quality of life
Mobility is one of 2 main priorities for glaucoma patients,1,2 and individuals with glaucoma have worse balance, walk more slowly, and bump into objects more.3-6 Individuals with glaucoma also have higher fall rates,7-10 greater fear of falling,11 and engage in less physical activity as visual field (VF) loss worsens.12 Mobility complaints in glaucoma are largely focused on outdoor tasks such as difficulty walking up curbs, crossing roads, or using public transportation.2,13 However, little study has been directed towards understanding if and how these mobility complaints translate into less travel outside of the home environment.
Leaving home is a necessary component of performing certain activities of daily living that are essential to independence,14 and decreased travel away from home is strongly associated with decreased quality of life and health. Individuals who leave their home less are more likely to become frail and experience cognitive decline,15 and have higher mortality rates.16,17 Therefore, identifying and treating life-space restriction and decreased travel away from home may improve health and well-being.
Traditionally, the extent of travel away from home has been quantified by life-space questionnaires.18 However, these questionnaires rely heavily on subject recall, which is naturally suspect with aging, memory loss, and self-report biases. As an alternative, cellular technology has been recently validated as a method for accurately capturing real-world travel patterns in an objective fashion.19 In this report, we use cellular tracking technology to directly measure the time and frequency with which glaucoma patients leave their home in their normal daily routines.
Methods
The study protocol adhered to the Declaration of Helsinki and was approved by the Johns Hopkins Medicine Institutional Review Board. All participants gave written informed consent and completed the study procedures between July 2009 and June 2011.
Enrollment Criteria
Subjects were recruited from a convenience sample of Johns Hopkins Wilmer Eye Institute patients of age 60 to 80 with no history of ocular laser in the previous week, no non-ocular surgery or hospitalization in the past 2 weeks, and no eye surgery in the past 2 months. All patients meeting the enrollment criteria were approached for possible study participation on days when a research coordinator was available for recruitment.
The rationale and criteria used to define the two study groups that were enrolled (glaucoma and glaucoma suspects) have been previously described,11,12 and can be summarized as follows. Subjects with significant vision loss for reasons other than glaucoma were excluded. Glaucoma suspects had: (1) a diagnosis of suspect glaucoma based on optic nerve and VF findings as assessed by the treating physician (a Wilmer Eye Institute glaucoma faculty member), (2) presenting ETDRS acuities of at least 20/40 in both eyes, (3) VF mean deviation (MD) better than -5 decibels (dB) and a glaucoma hemifield test (GHT) result other than “outside normal limits” in both eyes, and (4) VF MD better than -3 dB in at least 1 eye. Glaucoma subjects had a physician diagnosis of primary open angle, primary angle closure, pseudoexfoliation, or pigment dispersion glaucoma based on optic nerve and VF findings (as assessed by the treating physician – a Wilmer Eye Institute glaucoma faculty member). Additionally, they had a better-eye VF MD equal to or worse than -3 dB with a GHT result of “Outside Normal Limits”, “Borderline”, or “Generalized Reduction of Sensitivity” in both eyes, and a GHT result of “Outside Normal Limits” in at least one eye.
Evaluation of Travel Away from Home
Travel habits were assessed over 7 days of activity using a cellular network-based tracking device (pTrac Pro, Brickhouse Security, New York, NY). Subjects were instructed to clip the tracking device to their waistband during waking hours. The tracking device was set to record the unit's longitude and latitude every 15 minutes between 7am and 11pm. Each provided location was defined as “home” if the location was within one-third of a mile (the minimum device resolution) of the home location. Home location was defined empirically for each person-day as described in the Appendix A, available at [LWW insert link]. Details regarding the function and validity of the tracking device, and how location data was used to calculate excursions away from home and time spent away from home, are described in detail elsewhere.19
Ensuring and Evaluating Tracking Device Compliance
Device compliance was maximized through morning phone calls made to subjects on each of the 7 days they were scheduled to wear the device, and days of admitted non-compliance were excluded. Study days were also excluded if the time from the first to last provided location was less than 12.8 hours as a result of poor device or battery function. As part of the current study, subjects also wore a waistband accelerometer to monitor physical activity, and person-days in which detected activity spanned less than 8 hours were excluded under the assumption that neither device was worn.12
Other Measured Variables
ETDRS charts at either 4 or 1 meters were used to measure right and left eye visual acuities using patients' habitual correction. Analyses were performed using the better-eye acuity expressed as the logarithm of the minimum angle of resolution (logMAR)20 based on previous work suggesting that better-eye acuity best captures disability.21 Contrast sensitivity was evaluated under binocular conditions using Pelli-Robson charts at 1 meter distance. VF severity was summarized based on the MD of the better-seeing eye (i.e. the higher of the 2 MD values) based on the simplicity of determining these measures from clinical data and previous work showing that better-eye MD rarely differs from integrated visual field MD and predicts disability no differently than integrated visual field MD.22,23
Additional gathered information included age, race, ethnicity, employment status, use of topical glaucoma medications, and the presence of another driver in the home. Subjects describing full or part-time employment were considered employed. Topical glaucoma medications were defined as used if at least one drop daily was used in either eye. The number of reported comorbid illnesses (out of a list of 15) was recorded and summed.24 Depressive symptoms were evaluated using the Geriatric Depression Scale Short Form.25 Cognitive ability was assessed with the visually impaired version of the Mini-Mental State Exam (MMSE).26 Hand grip strength was measured using a jamar hand dynamometer (Sammons Preston, Inc.) Nuclear, cortical and posterior subcapsular lens opacity was objectively graded in each eye after pupillary dilation as previously described.11,12 Weather information was gathered through the North East Regional Climate Center (Cornell University, NY), and summarized as the percentage of study days with ≥ 0.2 inches of rainfall and the percentage of study days with an average daily temperature below 45°F (representing the 33rd percentile for average daily temperature in the study). Weather information was inferred using the weather station closest to the home location.
Statistical Methods
A sample size of 60 control and 80 glaucoma subjects was chosen to enable detection of a 20% decrease in excursions assuming roughly 7 ± 3 excursions per week in the control group. Group differences were evaluated using chi squared analyses for categorical variables, and t-test or Wilcoxon rank-sum test for continuous variables. Excursions away from home were modeled using multivariable linear regression after linear model residuals were confirmed to be normally distributed by Shapiro-Wilks testing (p=0.38). Time spent away from home was modeled using negative binomial models, which express the relationship of patient characteristics to time spent away from home as rate ratios (RR). The odds of not leaving home were analyzed for each person-day using multivariable logistic regression models employing general estimating equations. Covariates included in multivariable models included age, gender, race, variables previously demonstrated to affect travel away from home (weekend status, strength),16,19 and variables with a p<0.1 in age and gender-adjusted models. Patient characteristics which might result from decreased travel away from home (depression, cognitive ability)15 were not carried forward into multivariable models. Analyses were performed using STATA 12 (College Station, Texas).
Results
Fifty-nine glaucoma suspect controls and 80 glaucoma subjects provided data for analysis after 4 glaucoma subjects were excluded because of insufficient tracking time (3 because of poor device function and 1 because of presumed non-compliance). Included glaucoma suspects and glaucoma subjects had similar numbers of valid study days (6.2 vs. 6.1 days; p=0.48).
Glaucoma suspects and glaucoma subjects did not differ significantly with regards to age, race, gender, education level, population density of the home census tract, employment status, the presence of another driver in the home, grip strength, comorbid illness, presence of bilateral cataract/PCO, depressive symptoms, or cognitive ability (p>0.05 for all) (Table 1). Glaucoma subjects did have worse visual acuity, lower contrast sensitivity, and greater VF loss. No difference in average temperature or rainfall was noted for the subjects in the control and glaucoma groups (p>0.2 for both).
Table 1. Characteristics of tracked subjects by glaucoma status.
| Suspect glaucoma(n=58) | Glaucoma(n=79) | p value | |
|---|---|---|---|
| Vision | |||
| Visual field MD (better eye) | 0.0 (1.4) | -11.1 (7.9) | <0.001 |
| Better-eye Acuity, logMAR | 0.08 (0.11) | 0.28 (0.37) | <0.001 |
| Binocular CS, letters* | 37.7 (2.5) | 29.0 (9.0) | <0.001 |
| Cataract/PCO, both eyes (%) | 11.1 | 13.9 | 0.63 |
|
| |||
| Demographics | |||
| Age (Years) | 69.3 (5.3) | 70.3 (5.2) | 0.27 |
| African-American Race (%) | 20.7 | 35.4 | 0.06 |
| Female Gender (%) | 62.1 | 55.7 | 0.46 |
| Education (years) | 15.3 | 14.7 | 0.15 |
| Employed (%) | 39.7 | 41.8 | 0.80 |
| Other driver in home (%) | 77.6 | 77.2 | 0.96 |
| Lives alone (%) | 19.0 | 19.0 | 1.00 |
| Current driver (%) | 89.8 | 72.5 | 0.01 |
|
| |||
| Health | |||
| Comorbid illnesses (#) | 2.5 (1.6) | 2.2 (1.7) | 0.23 |
| Depressive symptoms (%) | 5.2 | 6.3 | 0.78 |
| MMSE-VI score | 20.7 (1.4) | 20.5 (1.8) | 0.68 |
| Grip Strength (kg force) | 27.3 (9.3) | 29.2 (9.9) | 0.27 |
MD = mean deviation; logMAR = logarithm of the minimum angle of resolution; CS = Contrast sensitivity; PCO = Posterior Capsular Opacification; Sig. = Significant; MMSE-VI = Mini-mental status examination for the visually impaired
Standard deviations shown in parentheses for all continuous variables.
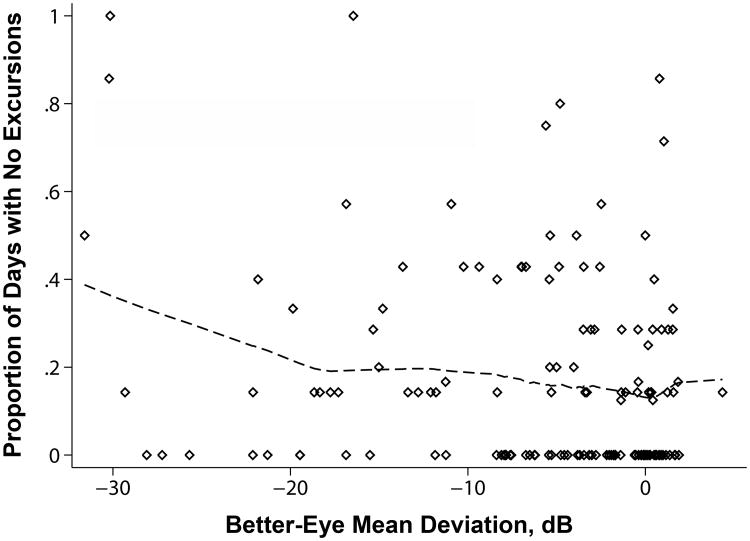
Glaucoma suspect controls made an average of 9.2 weekly excursions away from home, as compared to 8.0 weekly excursions for glaucoma subjects (p=0.05). The median control subject had no days without an excursion away from home (Interquartile range [IQR] = 0-17%), as compared to roughly 1 in 7 days without an excursion away from home for the median glaucoma subject (14%; IQR=0-40%; p=0.05) (Figure 1). The median glaucoma suspect control subject averaged 4.3 hours/day away from home (IQR=3.1 to 6.5 hours) as compared to 3.9 hours/day in glaucoma subjects (IQR=2.3 to 6.2 hours; p=0.27).
Figure 1.
Glaucoma severity and the portion of study days spent without leaving the home. Glaucoma severity defined as mild if the better-eye visual field mean deviation was better than -6 decibels (dB), moderate if between -6 dB and -12 dB, and severe if worse than -12 dB.
In age and gender-adjusted analyses, more VF loss in the better-seeing eye was associated with fewer daily away-from-home excursions (β=-0.06 excursions/day; 95% CI=-0.12 to -0.01) and less time away from home (RR=0.94; 95% CI=0.88 to 0.996). Having glaucoma (β=-0.19 excursions/day; 95% CI=-0.37 to -0.01) and reduced contrast sensitivity (β=-0.07 excursions/day/5 letter decrement; 95% CI=-0.13 to -0.02) were both associated with fewer excursions, but not with less time away from home. Additional predictors of increased daily excursions included more education, better cognitive ability, and male gender (p≤0.05 for all), while other predictors of time away from home included being actively employed, lack of depressive symptoms and younger age (p<0.01 for all). Better-eye visual acuity, race, the presence of another driver in the home, living alone, more medical comorbidities, and the percentage of weekend days in the study period were not associated with either fewer daily excursions or less time away from home (p>0.05 for all). Neither cold weather (proportion of study days with average daily temperature >45°F) nor rain (proportion of days with ≥ 0.2 inches of rainfall) was associated with less time away from home or fewer daily excursions (p>0.1 for all). The total number of glaucoma medications was also not associated with less time away from home or fewer daily excursions.
In multivariable models (Table 2), white males at the mean level of VF loss, age, education level, and cognitive ability, grip strength, and proportion of weekend study days made 1.48 excursions per day (95% CI=1.04 to 1.92). Fewer daily excursions were noted amongst glaucoma subjects (β= -0.20; 95% CI = -0.38 to -0.02). Separate multivariable models demonstrated fewer daily excursions with greater better-eye VF loss (β= -0.06 excursions/day per 5 dB decrement in better-eye VF MD; 95% CI = -0.12 to -0.01) and worse contrast sensitivity (β= -0.07 excursions/day per 5 fewer letters read; 95% CI = -0.13 to -0.01). No other variable was a significant multivariable predictor of fewer daily excursions. In multivariable models utilizing generalized estimating equations to analyze each person-day as a separate observation, glaucoma subjects were more likely to not leave their home on a given day (OR=1.82; 95% CI = 1.06 to 3.12) when compared to glaucoma suspect controls. Additionally, the odds of not leaving the home on any given day increased 1.24-fold for each 5 dB decrement in the better-eye VF (95% CI = 1.04 to 1.47). Race was an additional predictor of not leaving the house on a given day, with African-Americans significantly less likely to remain at home (OR=0.53; 95% CI =0.29 to 0.96).
Table 2.
Predictors of excursions, multivariable analysis.
| Avg daily excursions | No excursions on given day | ||||
|---|---|---|---|---|---|
| Variable | Interval | β* | 95% CI | OR** | 95% CI |
| Intercept† | 1.48 | 1.04 to 1.92 | |||
| Glaucoma | Present | -0.20 | -0.38 to -0.02 | 1.82 | 1.06 to 3.12 |
| VF MD, bettereye | 5 dB worse | -0.06 | -0.12 to -0.01 | 1.24 | 1.04 to 1.47 |
| Contrast Sensitivity | 5 letters worse | -0.07 | -0.13 to -0.01 | 1.21 | 1.01 to 1.43 |
| Age | 5 years older | 0.02 | -0.07 to 0.11 | 1.14 | 0.89 to 1.45 |
| Race | African-American | 0.02 | -0.07 to 0.35 | 0.53 | 0.29 to 0.96 |
| Gender | Female vs. Male | -0.19 | -0.47 to 0.08 | 1.39 | 0.63 to 3.03 |
| Education | 4 years more | -0.07 | -0.21 to 0.08 | 1.25 | 0.85 to 1.85 |
| MMSE-VI score | 5 points higher | -0.26 | -0.59 to 0.07 | 1.49 | 0.64 to 3.48 |
| Grip strength | 5 kg less force | 0.01 | -0.07 to 0.08 | 1.03 | 0.83 to 1.26 |
| Day of week | study day(s) on weekend+ | 0.03 | -0.03 to 0.09 | 1.22 | 0.81 to 1.84 |
Glaucoma and visual metrics were each analyzed in separate multivariable models including age, race, gender, education, MMSE-VI score, and day of week as covariates. Values for non- visual model elements were derived from a single model including better-eye VF MD and all listed non-vision variables.
β refers to the change in number of excursions/day associated with variable of interest.
OR = Odds ratio of not leaving home on a given day associated with the variable of interest. Values were derived from logistic regression models using generalized estimating equations to analyze each person-day as a separate data point.
Intercept shown for white males centered for mean deviation, age, education, MMSE score, grip strength, and proportion of weekend study days.
Proportion of study days occurring on weekends
VF – visual field; MD – mean deviation; dB – decibels; MMSE-VI – Mini-mental state exam for the visually impaired; PCO – Posterior capsule opacification
Fewer excursions and a lower likelihood of leaving home remained associated with glaucoma when cataract/PCO and employment status was added to models (β=-0.22 excursions/day; 95% CI=-0.40 to -0.03). When visual acuity was added to models, the association between glaucoma and daily excursions became borderline significant with a similar regression coefficient (β= -0.18; 95% CI=-0.38 to 0.006), while the likelihood of not leaving the home on a given day remained unchanged (OR=1.81; 95% CI=1.06 to 3.09). When VF MD was added to models testing the association between glaucoma status and excursions, glaucoma was no longer associated with either the number of daily excursions or the likelihood of not leaving home on a given day (p>0.35 for both). Finally, when driving cessation was added to multivariable models, the association between glaucoma and daily excursions became borderline significant with minimal change in the regression coefficient (β= -0.17; 95% CI=-0.17 to 0.02), as did the likelihood of leaving home on a given day (OR=1.67; 95% CI=0.96 to 2.93).
Multivariable analyses demonstrated a non-statistically significant 12% decrease in time away from home amongst all glaucoma subjects as compared to glaucoma suspect controls (95% CI = -27 to +6%) (Table 3). However, time away from home was 6% lower for each 5 dB decrement in the better-eye VF MD (95% CI = -12 to -1%) and each 5 letter decrement in contrast sensitivity (95% CI= -12 to -0.3%). Other variables predicting greater time away from home included employment status (73% increase in time away; 95% CI=+43 to +109%).
Table 3.
Predictors of time outside the home, multivariable analysis.
| Daily time outside home | |||
|---|---|---|---|
| Variable | Interval | RR | 95% CI |
| Glaucoma | Present | 0.88 | 0.73 – 1.06 |
| VF MD, bettereye | 5 dB worse | 0.94 | 0.88 to 0.99 |
| Contrast Sensitivity | 5 letters worse | 0.94 | 0.88 to 0.997 |
| Age | 5 years older | 0.93 | 0.84 to 1.01 |
| Race | African-American | 1.08 | 0.73 to 1.29 |
| Gender | Female vs. Male | 0.97 | 0.73 to 1.29 |
| Employed | Yes | 1.73 | 1.43 to 2.09 |
| Grip strength | 5 kg less force | 0.99 | 0.92 to 1.06 |
| Day of week | % study days on weekend | 1.06 | 0.99 to 1.14 |
Visual predictors were each analyzed in separate multivariable models including age, race, gender, employment status, MMSE-VI score and day of week as covariates. Values for non-visual model elements were derived from a single model including better-eye VF MD and all listed non-vision variables.
RR = Rate ratio – Refers to the ratio of time away from home/day in people with the variable of interest as compared to people without the variable of interest. A RR less than 1 Indicates less time away from home.
Subjects with a better eye VF MD worse than -12 dB.
CI – confidence interval; VF – visual field; MD – mean deviation; dB – decibels; MMSE-VI – Mini-mental state exam for the visually impaired.
The role of individual topical glaucoma medicines as a potential cause of restricted travel was investigated through additional multivariable models incorporating individual medication use, better-eye VF MD, and the covariates described in Table 3. Topical alpha agonist use was associated with fewer daily excursions (β=-0.36; 95% CI= -0.66 to -0.07) and a greater likelihood of not leaving the home on a given day (OR=4.42, 95% CI=1.88 to 10.41), but not with less time spent outside the home (p=0.97). No other medication class usage predicted any travel outcome (Table 4). In models accounting for medication use, severity of VF loss remained an independent predictor of not leaving the home on a given day and time outside the home, while its association with the number of daily excursions fell outside criteria for statistical significance (p=0.11).
Table 4. Influence of glaucoma medications on travel metrics, multivariable analysis.
| Variable | Δ Daily Excursions | No Excursions on Given Day (OR) | Time Outside Home (RR) |
|---|---|---|---|
| Class of Topical Medication Used | |||
| Prostaglandin | -0.02 (-0.18 – +0.21) | 0.60 (0.32 – 1.13) | 1.07 (0.85 – 1.35) |
| Beta blocker | +0.10 (-0.16 – +0.36) | 0.76 (0.35 – 1.65) | 1.08 (0.79 – 1.47) |
| Carbonic anhydrase inhibitor | -0.12 (-0.39 – +0.15) | 0.82 (0.29 – 2.30) | 0.91 (0.66 – 1.27) |
| Alpha agonist | -0.36 (-0.66 – -0.07) | 4.42 (1.88 – 10.41) | 1.00 (0.70 – 1.40) |
|
| |||
| Better-eye VF MD (5 dB decrement) | -0.05 (-0.11 – +0.01) | 1.25 (1.04 – 1.51) | 0.92 (0.86 – 0.99) |
95% CIs shown in parentheses.
OR = Odds Ratio; RR = Rate ratio; VF = Visual Field; MD = Mean Deviation; dB = decibels.
All models run with age, race, gender, cognitive ability, education, weekend/weekday status, and grip strength as additional covariates. Influence of better-eye VF MD characterized in models including these covariates as well as use of each of the medicines shown.
Discussion
Our results demonstrate that greater severity of VF loss from glaucoma is associated with less travel outside the home, as evidenced by fewer daily excursions, less time outside the home, and more days in which the home is not left. Similar findings were observed when comparing these metrics between the group of glaucoma suspect controls and the group of glaucoma patients, though differences in time outside the home were not different at a statistically significant level. This combination of fewer daily excursions but less significant differences in time away from home may reflect longer excursions outside the home in some glaucoma subjects, possibly as a result of slower movement and/or a desire to accomplish more during each excursion. Our findings represent the first characterization of life space in patients with glaucoma and, to our knowledge, are the first objective characterization of travel away from home as it occurs in the full normal routine of older individuals.
Restriction of travel outside the home in individuals with glaucomatous VF loss is consistent with prior research demonstrating a broad range of mobility deficits in individuals with VF loss including worse balance, more bumping into objects, driving restriction, falls, fear of falling, decreased physical activity and greater self-reported mobility difficulty.1-12,27-30 We suspected driving cessation would largely explain the decreased travel amongst glaucoma subjects, but found that our outcomes changed only slightly when driving status was incorporated into multivariable models. We also investigated glaucoma medication use as a possible explanation of decreased travel, and did indeed find that use of topical alpha agonists appeared to partially explain the association between glaucomatous VF loss and decreased travel outside the home. However, it should be noted that alpha agonists were the least used class of glaucoma medications in the studied population, and their use may have been saved for poor surgical candidates. Therefore, further research is required to determine whether alpha agonists truly impact real-world functional measures such as travel outside the home, or whether they simply tend to be used more in individuals with poor health and restricted travel. Further research is also required to determine to what extent balance deficits, fear of falling, or decreased motivation to leave the home because of social deficits (i.e. recognizing friends and family)31 or other functional deficits (difficulty reading labels, navigating stores)32 contribute to travel restriction.
Less travel away from home is an objective measure of life space constriction, and previous studies have documented adverse health consequences with a more constricted life space. Individuals with less travel outside the home have a higher incident mortality,16,17 are more likely to become frail,16 and have higher incident rates of Alzheimer's dementia and cognitive decline even after adjusting for numerous other factors.15 These studies strongly support the common-sense notion that getting out of the home is an important aspect of happy and healthy living, and highlight the importance of establishing methods, i.e. orientation and mobility training or fall prevention techniques such as tai-chi or exercise, to encourage safe travel outside the home in individuals with significant VF loss from glaucoma. Of note, previous studies have not found an association between glaucoma and mortality rate,33,34 though other studies have found associations between visual impairment and mortality,35 suggesting that mortality problems may manifest only in a subset of glaucoma patients with more considerable visual impairment. No non-visual variables consistently predicted restriction of travel across the metrics employed in this analysis, highlighting the relative importance of vision with regards to travel restriction.
The current study differs from previous assessments of travel away from the home in that travel was measured directly with a cellular tracking device instead of relying on patient recall which may be biased by knowledge of one's disease and may not be accurate in groups such as the elderly. A further advantage of the cellular tracking data is that it also provides specifics about where people went outside the home, which will allow additional analyses about precisely where individuals with and without glaucoma went. Disadvantages of cellular tracking include the inability to detect trips to the porch or very short walks. Device compliance is also difficult to assess, though we attempted to maximize compliance through daily phone calls and by only using data when a second study device, an accelerometer, was worn by subjects on the same day.12
The paucity of previous research directly quantifying excursions outside the home also presents challenges. Given the limited research in this area, the factors predicting travel outside the home are not well-characterized, and it is possible that unmeasured covariates may have contributed to the observed differences across group and severity of vision loss. Moreover, while the current study suggests that glaucoma is associated with less travel outside the home, it is not clear to what extent this restriction is the result of visual impairment, diagnosis awareness, and treatment side-effects. However, fewer excursions were observed at greater levels of VF loss, and the association between glaucoma and daily excursions was no longer significant when severity of VF loss was included in regression models, suggesting that visual impairment is at least one significant driver of travel restriction.
Results from the current cohort may not generalize to all glaucoma patients, as subjects were selected from individuals able to visit an urban tertiary care center and may exclude individuals with the greatest life space restriction. Patients with the greatest life space restriction may also have been less likely to participate in the study given the need for an additional study visit, though participation was encouraged by allowing individuals to complete the study testing on the same day as a clinical visit. Glaucoma suspects were chosen as our comparison group instead of normally-sighted controls as recruitment of “normals” from groups such as spouses/volunteers is more likely to exclude individuals preferring to stay at home because of poor mobility, thus creating a bias towards a positive finding. Moreover, our group of glaucoma suspects had similar better and worse-eye VF MDs when compared to subjects without glaucoma assessed during the Salisbury Eye Evaluation (0.0 vs. -0.9 dB and -1.6 vs. -3.5 dB respectively), suggesting that this group was indeed visually normal. A final limitation is that we did not investigate the impact of VF loss in particular locations or VF loss outside the central 24°, which may have led to different conclusions. We also did not evaluate the impact of integrated VF loss on travel outside the home, though recent evidence suggests that integrated VF loss does rarely differs from, and does not predict disability differently than, measures of better-eye VF loss.22,23
Glaucoma is associated with less travel away from home, including a nearly 2-fold increase in the likelihood of not leaving home on a given day. The strong associations between travel restriction and health/quality of life suggest that individuals with glaucoma may also be at risk for adverse health outcomes associated with constriction of life space. Further investigation is warranted to prospectively explore the effects of glaucoma medication on mobility, and to develop and standardize methods that facilitate and encourage safe travel away from home in persons with more advanced glaucoma.
Supplementary Material
Acknowledgments
This work was supported by the Dennis W. Jahnigen Memorial Award, NIH grant number EY018595 and Research to Prevent Blindness. The funding organizations had no role in the design or conduct of the study.
Footnotes
None of the authors has any competing interests with regards to the materials discussed within this manuscript.
Appendix: Appendix A is available online at [LWW insert link].
References
- 1.Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1907–15. doi: 10.1167/iovs.07-0559. [DOI] [PubMed] [Google Scholar]
- 2.Nelson P, Aspinall P, O'Brien C. Patients' perception of visual impairment in glaucoma: a pilot study. Br J Ophthalmol. 1999;83:546–52. doi: 10.1136/bjo.83.5.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman DS, Freeman E, Munoz B, Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114:2232–7. doi: 10.1016/j.ophtha.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Shabana N, Cornilleau-Peres V, Droulez J, Goh JC, Lee GS, Chew PT. Postural stability in primary open angle glaucoma. Clin Experiment Ophthalmol. 2005;33:264–73. doi: 10.1111/j.1442-9071.2005.01003.x. [DOI] [PubMed] [Google Scholar]
- 5.Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–97. doi: 10.1097/OPX.0b013e31817882db. [DOI] [PubMed] [Google Scholar]
- 6.Popescu ML, Boisjoly H, Schmaltz H, Kergoat MJ, Rousseau J, Moghadaszadeh S, Djafari F, Freeman EE. Age-related eye disease and mobility limitations in older adults. Invest Ophthalmol Vis Sci. 2011;52:7168–74. doi: 10.1167/iovs.11-7564. [DOI] [PubMed] [Google Scholar]
- 7.Black AA, Wood JM, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011;88:1275–82. doi: 10.1097/OPX.0b013e31822f4d6a. [DOI] [PubMed] [Google Scholar]
- 8.Haymes SA, Leblanc RP, Nicolela MT, Chiasson LA, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1149–55. doi: 10.1167/iovs.06-0886. [DOI] [PubMed] [Google Scholar]
- 9.Lamoureux EL, Chong E, Wang JJ, Saw SM, Aung T, Mitchell P, Wong TY. Visual impairment, causes of vision loss, and falls: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2008;49:528–33. doi: 10.1167/iovs.07-1036. [DOI] [PubMed] [Google Scholar]
- 10.Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue Mountains Eye Study. J Am Geriatr Soc. 1998;46:58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramulu PY, van Landingham SW, Massof RW, Chan ES, Ferrucci L, Friedman DS. Fear of falling and visual field loss from glaucoma. Ophthalmology. 2012;119:1352–8. doi: 10.1016/j.ophtha.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramulu PY, Maul E, Hochberg C, Chan ES, Ferrucci L, Friedman DS. Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology. 2012;119:1159–66. doi: 10.1016/j.ophtha.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheong AM, Geruschat DR, Congdon N. Traffic gap judgment in people with significant peripheral field loss. Optom Vis Sci. 2008;85:26–36. doi: 10.1097/OPX.0b013e31815ed6fd. [DOI] [PubMed] [Google Scholar]
- 14.Shimada H, Ishizaki T, Kato M, Morimoto A, Tamate A, Uchiyama Y, Yasumura S. How often and how far do frail elderly people need to go outdoors to maintain functional capacity? Arch Gerontol Geriatr. 2010;50:140–6. doi: 10.1016/j.archger.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 15.James BD, Boyle PA, Buchman AS, Barnes LL, Bennett DA. Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry. 2011;19:961–9. doi: 10.1097/JGP.0b013e318211c219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue QL, Fried LP, Glass TA, Laffan A, Chaves PH. Life-space constriction, development of frailty, and the competing risk of mortality: the Women's Health And Aging Study I. Am J Epidemiol. 2008;167:240–8. doi: 10.1093/aje/kwm270. [DOI] [PubMed] [Google Scholar]
- 17.Boyle PA, Buchman AS, Barnes LL, James BD, Bennett DA. Association between life space and risk of mortality in advanced age. J Am Geriatr Soc. 2010;58:1925–30. doi: 10.1111/j.1532-5415.2010.03058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stalvey BT, Owsley C, Sloane ME, Ball K. The life space questionnnaire: a measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:460–78. [Google Scholar]
- 19.Ramulu PY, Chan ES, Loyd TL, Ferrucci L, Friedman DS. Comparison of home and away-from-home physical activity using accelerometers and cellular network-based tracking devices. J Phys Act Health. 2012;9:809–17. doi: 10.1123/jpah.9.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–32. [PubMed] [Google Scholar]
- 21.Richman J, Lorenzana LL, Lankaranian D, Dugar J, Mayer J, Wizov SS, Spaeth GL. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Arch Ophthalmol. 2010;128:1576–82. doi: 10.1001/archophthalmol.2010.275. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni KM, Mayer JR, Lorenzana LL, Myers JS, Spaeth GL. Visual field staging systems in glaucoma and the activities of daily living. Am J Ophthalmol. 2012;154:445–51. doi: 10.1016/j.ajo.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 23.Arora KS, Boland MV, Friedman DS, Jefferys JL, West SK, Ramulu PY. The relationship between better-eye and integrated visual field mean deviation and visual disability. Ophthalmology 2013. 2013 Aug 30; doi: 10.1016/j.ophtha.2013.07.020. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turano KA, Broman AT, Bandeen-Roche K, Munoz B, Rubin GS, West S. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004;81:298–307. doi: 10.1097/01.opx.0000134903.13651.8e. [DOI] [PubMed] [Google Scholar]
- 25.Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–12. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- 26.Busse A, Sonntag A, Bischkopf J, Matschinger H, Angermeyer MC. Adaptation of dementia screening for vision-impaired older persons: administration of the Mini-Mental State Examination (MMSE) J Clin Epidemiol. 2002;55:909–15. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 27.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20:92–8. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramulu PY, West SK, Munoz B, Jampel HD, Friedman DS. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009;116:1846–53. doi: 10.1016/j.ophtha.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altangerel U, Spaeth GL, Steinmann WC. Assessment of function related to vision (AFREV) Ophthalmic Epidemiol. 2006;13:67–80. doi: 10.1080/09286580500428500. [DOI] [PubMed] [Google Scholar]
- 30.van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology. 2012;119:2486–92. doi: 10.1016/j.ophtha.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Glen FC, Crabb DP, Smith ND, Burton R, Garway-Heath DF. Do patients with glaucoma have difficulty recognizing faces? Invest Ophthalmol Vis Sci. 2012;53:3629–37. doi: 10.1167/iovs.11-8538. [DOI] [PubMed] [Google Scholar]
- 32.Hochberg C, Maul E, Chan ES, Van Landingham S, Ferrucci L, Friedman DS, Ramulu PY. Association of vision loss in glaucoma and age-related macular degeneration with IADL disability. Invest Ophthalmol Vis Sci. 2012;53:3201–6. doi: 10.1167/iovs.12-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grodum K, Heijl A, Bengtsson B. Glaucoma and mortality. Graefes Arch Clin Exp Ophthalmol. 2004;242:397–401. doi: 10.1007/s00417-004-0858-2. [DOI] [PubMed] [Google Scholar]
- 34.Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–9. doi: 10.1001/archopht.124.2.243. [DOI] [PubMed] [Google Scholar]
- 35.Lee DJ, Gomez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002;120:1544–50. doi: 10.1001/archopht.120.11.1544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.