Abstract
High-mobility group box 1 (HMGB1) is an important molecule for several nuclear processes. Recently, HMGB1 has gained much attention as a damage-associated molecular pattern (DAMP) and has been implicated in the pathogenesis of several (auto)-immune diseases, in particular, systemic lupus erythematosus (SLE). A main pathogenic feature in SLE is the accumulation of apoptotic cells. Since HMGB1 is released from apoptotic cells it has been hypothesized that HMGB1 might fuel the inflammatory processes, as seen in this disease, and play a fundamental role in the pathogenesis. In this review, we discuss evidence in support of the theory that HMGB1 is an important mediator in SLE and may be considered a new autoantigen.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease, characterized by the involvement of many organs and the presence of autoantibodies directed to nuclear and cytoplasmic antigens. The pathogenesis is unknown; it is assumed that a disturbed clearance of apoptotic cells plays a central role. Several lines of evidence support that accumulation of apoptotic cells is instrumental in the development of SLE. In several animal models, deficiency of proteins such as complement factor C1q and serum amyloid P component (SAP), which are involved in opsonization and thereby phagocytosis and clearance of apoptotic cells, results in spontaneous development of lupuslike features, including production of autoantibodies and proliferative glomerulonephritis (1,2). Furthermore, injection of apoptotic cells result in development of SLE-like disease (3). Also in human SLE, accumulation of apoptotic cells has been found. In peripheral blood, increased levels of apoptotic lymphocytes (4) and neutrophils (5) have been demonstrated. Moreover, in lymph nodes from lupus patients apoptotic lymphocytes are present (6). In the skin of lupus patients, after UVB irradiation, in comparison to healthy controls, apoptotic cells accumulate. Importantly, in the vicinity of these cells, inflammatory lesions occurred (7). Apoptotic cells express antigens on their cell surface that serve as targets of the autoimmune response that is typical of the disease, in part because these antigens may undergo posttranslational modifications during the process of apoptosis.
Moreover, when apoptotic cells are not cleared, these cells turn into necrotic cells that lose their membrane integrity, thereby releasing intracellular structures that can act as autoantigens. In this context, nucleosomes, bound to the nuclear protein high-mobility group box 1 (HMGB1), were shown to induce not only immune, but also inflammatory responses that are relevant for the pathogenesis of SLE (8). In this review, after a short summary of HMGB1 functions, we will discuss the (potential) contribution of HMGB1 in the different stages SLE development.
HMGB1
HMGB1 is a nonhistone nuclear protein that has several functions, depending on its location and posttranslational modifications. HMGB1 is a highly conserved protein of 215 amino acids that is ubiquitously expressed in nuclei where it binds to DNA. The protein can be divided into three separate domains: the A box, B box and acidic tail. Box A serves as a competitive antagonist for HMGB1 and inhibits HMGB1 activity. Both A and B box are important for its DNA binding functions and are positively charged, whereas the tail is negatively charged, giving the molecule a bipolar charge. HMGB1 is important in several DNA-related biological processes, reflected by the fact that HMGB1 can bend promoter regions and can interact directly with nucleosomes, thereby altering the accessibility of the DNA (9). The importance of HMGB1 was demonstrated by the fact that mice deficient for the protein die within 24 h after birth because of severe hypoglycemia. In contrast, fibroblast cell lines, derived from the same HMGB1−/− mice, can proliferate and survive, indicating that the effects might be cell specific (10).
HMGB1 can be released from the cell into the extracellular space, where it acts as a damage-associated molecular pattern (DAMP) or alarmin. Translocation from the nucleus to the cytoplasm occurs through acetylation of lysine residues, where the protein is concentrated in vesicles for secretion (11,12). The extracellular release of HMGB1 can occur either passively or actively by secretion. More importantly, the manner of release determines posttranslational modifications of released HMGB1. Passive release of HMGB1 usually results from leakage due to damaged cell membranes. Active release by secretion occurs when immune cells are exposed to, for instance, pathogen-associated molecular patterns (PAMPs), tumor necrosis factor (TNF)-α or lipopolysaccharide (LPS) via nonclassical pathways. When present in extracellular space, HMGB1 exerts several functions, including cytokine release by monocytes (13–16), cell migration (17), wound healing (18) and neovascularization (19).
INTERACTION WITH RECEPTORS: REDOX STATUS OF HMGB1
Extracellular HMGB1 can bind to several receptors, including toll-like receptor (TLR)-2, -4 and -9; receptor for advanced glycation end products (RAGE) and chemokine receptor C-X-C motif receptor 4 (CXCR4) (17,20,21). RAGE, both in soluble and membrane form, was the first receptor demonstrated to bind HMGB1. RAGE is a transmembrane, multiligand member of the immunoglobulin superfamily expressed on monocytes, macrophages and other cells (22). Binding of HMGB1 to RAGE initiates cell migration–dependent pathways via nuclear factor-κB activation (23,24). Moreover, cell migration can also occur when HMGB1 is complexed to chemokine CXCL12, and then it acts synergistically through CXCR4 (17,25).
Recently, binding of HMGB1 to its receptors was shown to be related to the different redox states of HMGB1. Several studies showed that when a disulfide bridge exists between cysteines C23 and C45, and cysteine C106 is in a thiol (reduced) state, HMGB1 exerts cytokine-inducing properties via binding to TLR4 (25–27). Adding this cytokine HMGB1 to RAW 264.7 macrophages induced expression and release of TNF, whereas completely reduced HMGB1, which can be produced in the presence of dithiotreitol (DTT), did not (25–27). HMGB1 has chemoattractant properties when all three cysteines are reduced. By using a migration assay, mouse fibroblasts migrated toward increasing concentrations of all-thiol HMGB1 (17,25). Moreover, it was shown that the cytokine and chemoattractant properties of HMGB1 are mutually exclusive because of these different redox statuses of HMGB1: all-thiol HMGB1 failed to induce cytokine production, while disulfide HMGB1 did not attract mouse fibroblasts (25). When HMGB1 is released from apoptotic cells, it induces immunogenic tolerance and has no chemotactic properties, since its three cysteines are terminally oxidized by reactive oxygen species to sulfonates (11,25,27). However, when HMGB1 is released from necrotic cells, it still has inflammatory properties, as shown by the fact that necrotic HeLa HMGB1−/− cells do not induce TNF production in monocytes, whereas wild-type cells could induce TNF (12).
COMPLEXED FORMS OF HMGB1
Next to binding to CXCL12, as described before, HMGB1 forms complexes with several other molecules, including LPS, interleukin (IL)-1, —C—phosphate—G— (CpG), double-stranded DNA and nucleosomes. Hreggvidsdottir et al. (28) showed that HMGB1 forms highly immunostimulatory complexes with LPS and Pam3CSK4, thereby increasing response in macrophages up to 100-fold. These authors furthermore showed that signaling of these complexes was primarily done by TLR4 and TLR2 and not by additional signaling cascades. In SLE, formation of many immune complexes is well described, and it has been shown that HMGB1 bound in these complexes is present in plasma and serum of SLE patients (29–31). Tian et al. (29) showed that HMGB1 bound to CpG motifs induced cytokine production by interacting with RAGE and not TLR2 or TLR4. Moreover, they showed that a monoclonal antibody to HMGB1 significantly reduced interferon (IFN)-α production in peripheral blood mononucleated cells stimulated with plasma from lupus patients containing HMGB1-CpG complexes (29). HMGB1 complexes were also implicated in the pathogenesis of lupus nephritis. Qing et al. (32) showed that the pathogenic anti-DNA antibody 1A3F could bind to HMGB1 and HMGB1-DNA complexes. HMGB1 and 1A3F exhibited a synergistic proinflammatory effect in the kidney, where increased expression of HMGB1 was found in lupus patients but not in patients with other types of renal disease. They concluded that HMGB1 may act as a proinflammatory mediator in antibody-induced kidney damage in SLE. More recently, it was shown that HMGB1 has a role in autoantibody induction itself. In SLE patients, circulating DNA- containing immune complexes induced anti-dsDNA antibodies. Importantly, HMGB1 in circulating DNA-containing immunocomplexes was crucial for anti-dsDNA antibody induction (31). By using box A to block the effect of HMGB1, the production of anti-dsDNA antibodies was abrogated in a dose-dependent manner, whereas adding HMGB1 to the culture system increased production of anti-dsDNA antibodies (31). However, unlike the previous study by Tian et al. (29), here it was shown that the effect was solely mediated through TLR2 and MyD88. The data in this study are in line with the results found by Urbonaviciute et al. (30), who showed that injecting HMGB1-nucleosome complexes induced anti-dsDNA production through TLR2 in BALB/c mice. Furthermore, incubating human monocyte-derived macrophages with HMGB1-nucleosome complexes induced cytokine production, which could be blocked by adding box A. Whereas these studies focused on effects of HMGB1 on lymphocytes, Sun et al. (33) investigated the effect of HMGB1-containing immune complexes on human endothelial cells. They showed that stimulating endothelial cells with immune complexes from SLE patients increased proinflammatory cytokines such as IL-6 and IL-8. Furthermore, RAGE, vascular cell adhesion molecule (VCAM)-1 and inducible cell adhesion molecule (ICAM)-1 showed higher expression on the cell surface. These effects could be blocked by adding box A or soluble RAGE, indicating that HMGB1 plays an important role in mediating inflammation in endothelial cells. Taken together, these studies show that HMGB1 mediates several roles by acting in immune complexes, depending on the effector cells and components that are present in these complexes.
HMGB1 AND PHAGOCYTOSIS
Phagocytosis (or efferocytosis) is an important mechanism that enables innate immune cells to clear cell debris and ingest microbes. Impaired phagocytosis of apoptotic debris has been implicated in SLE pathogenesis and leads to secondary cell necrosis and exposition of intracellular contents of the necrotic cells to the surrounding tissue, which in turn can induce a proinflammatory response (34). Serum factors, such as complement, are important for an effective phagocytotic response and were found to be decreased in SLE patients (35). HMGB1 has been described to be a factor that can contribute to a decrease in phagocytosis. In a series of articles by Abraham and colleagues, it was shown that HMGB1 has a negative effect on phagocytosis via different mechanisms (36–38). First, it was shown that HMGB1 can bind to phosphatidylserine (PS) present on the surface of apoptotic neutrophils. The presence of PS on the outer leaflet of cells is one of the most important “eat me” signals for macrophages. Accordingly, efferocytosis was inhibited when HMGB1 masked PS (36). Next, it was shown that when macrophages were pre-incubated with HMGB1, their ability to engulf apoptotic neutrophils and thymocytes was decreased. HMGB1 competed with the opsonin milk fat globule epidermal growth factor 8 (MFG-E8) for binding to integrin αvβ3, a receptor important in recognizing PS. MFG-E8 was shown to act as a bridge between PS and to integrin αvβ3, thereby enhancing efferocytosis (37). RAGE-deficient macrophages were shown to have a diminished capacity to phagocytose, and the inhibitory effect of HMGB1 on phagocytosis was contributed to the C-terminal tail of the protein. When the C-terminus was eliminated, the inhibitory effect of HMGB1 was greatly diminished, attributed to the fact that HMGB1 was not able to bind to RAGE in vitro and in vivo (38). Moreover, it was shown that PARPylation of HMGB1 by PARP-1 [poly(ADP)-ribose polymerase] enhanced the capacity of HMGB1 to inhibit efferocytosis, indicating that posttranslational modifications of HMGB1 play a role. PARP-1 was implicated in inflammation, and activation of PARP-1 induces migration of HMGB1 from the nucleus to the cytoplasm. It was shown that after stimulation of TLR4, PARPylated HMGB1 was secreted, which could inhibit efferocytosis more efficiently than non-PARPylated HMGB1 by enhanced binding to PS and RAGE (39). Lastly, it was shown that intracellular HMGB1 also diminishes the phagocytosis by macrophages through interactions with Src and focal adhesion kinase (FAK). Knockdown of intracellular HMGB1 by siRNA increased phagocytosis and cytoskeletal rearrangement, in which FAK plays an important role (40). As HMGB1 serum levels are increased in SLE patients (which will be discussed later on), it seems logical that serum HMGB1 can contribute to the impaired phagocytosis described in SLE patients. However, at this moment, there are no reports that address this issue.
HMGB1 AND NETS
In addition to apoptosis and necrosis, a new form of cell death was described in 2004 by Brinkmann et al. (41). They showed that neutrophils can entrap microorganisms by a programmed externalization of chromatin fibers decorated with antimicrobial proteins derived from granules. This process was called neutrophil extracellular trap (NET) formation or NETosis, and the processes involved leading to NETosis were investigated in detail (42). The first link between NETosis and autoimmunity was observed in small vessel vasculitis, where it was shown that NETs externalize the autoantigens proteinase 3 (PR3) and myeloperoxidase (MPO) and that antineutrophil cytoplasmic antibodies (ANCAs) induce NETosis (43). In 2011, enhanced NETosis in SLE patients was described (44–46). Normally, NETs are degraded by DNase-1 in the circulation, but the ability to reduce NETs is diminished in SLE patients and is associated with disease activity (47). Villanueva et al. (46) showed that low-density granulocytes spontaneously can form NETs. Low-density granulocytes are a neutrophil subset that can be found in the mononuclear cell fraction, and they have been reported to be present in all SLE patients (48). Comparing gene array profiles of low-density granulocytes to normal density neutrophils showed that low-density granulocytes significantly overexpressed mRNA of various immunostimulatory bactericidal proteins and alarmins, relative to lupus neutrophils and control neutrophils.
Next to spontaneous NET formation in SLE patients, it was shown that autoantibodies such as anti-RNP (anti-ribonucleoprotein) or immunogenic complexes composed of neutrophil-derived antimicrobial peptides and self-DNA can induce NETosis (44,45). NETs can stimulate plasmacytoid dendritic cells to produce type I interferon (IFN-α), which has an important role in pathogenesis of SLE (44–46). NETs contain DNA and much HMGB1 and LL37, a cationic antimicrobial peptide that converts self-DNA and self-RNA into TLR activators (44). These neutrophil proteins facilitate the uptake and recognition of mammalian DNA by plasmacytoid dendritic cells. Next to being excreted by neutrophils, HMGB1 also induces NETosis in neutrophils by interaction through TLR4 but not through RAGE (49). This result has not been confirmed by other studies until now and has not been investigated in neutrophils from SLE patients.
HMGB1 IN SLE PATIENTS AND OTHER RHEUMATIC DISEASES
Different groups have shown that HMGB1 levels are elevated in serum and plasma samples from patients with SLE (50–54). In addition, it was shown that HMGB1 correlated with the SLE Disease Activity Index (SLEDAI) and anti-dsDNA, whereas there was an inverse correlation with complement C3 (50,55).
An important feature of SLE is the production of autoantibodies against several different nuclear and cytoplasmic proteins. Indeed, also antibodies to HMGB1 were found in SLE, which seems specific to the disease, since these antibodies were not found in patients with systemic vasculitis (50,56,57). Interference of these antibodies with detection of serum HMGB1 by enzyme-linked immunosorbent assay (ELISA) was noted (50,58), and it seems that the best way to detect HMGB1 in the serum of SLE patients is by immunoblot. The presence of anti-HMGB1 antibodies correlates with serum HMGB1, SLEDAI and anti-dsDNA antibodies (50), indicating that these antibodies might have a pathogenic role. In sepsis, however, a protective role of anti-HMGB1 antibodies was found (59), initiating a debate about the role of these antibodies. Epitope mapping of the anti-HMGB1 antibodies revealed that the majority of the antibodies reacted against box A (56), indicating that box A is not a treatment option for SLE. It might be expected that box A in lupus will be neutralized. In contrast, in animal models in other diseases in which anti-HMGB1 antibodies are not present, such as sepsis, administration of box A is beneficial (60).
In several other autoimmune diseases, such as Sjögren syndrome, juvenile arthritis and rheumatoid arthritis, it was documented that serum levels of HMGB1 are increased in patients compared with healthy controls, showing that increased HMGB1 levels are not a specific feature of SLE (61–65) (Table 1). Wibisono et al. (66) investigated HMGB1 levels in patients with ANCA-associated vasculitis (AAV), and they found significant increased HMGB1 levels in patients with active granulomatosus with polyangiitis (GPA), but not in patients with microscopic polyangiitis (MPA). These data were confirmed by Bruchfeld et al. (67), who also found increased HMGB1 levels in GPA patients with renal involvement, and by Henes et al. (68), who showed a correlation between HMGB1 and the burden of granulomatous inflammation. Recently, however, HMGB1 was described not to be a useful biomarker in AAV, because HMGB1 levels were not associated with an increased risk for relapse in AAV (57). In ankylosing spondylitis patients, HMGB1 levels were reported slightly elevated compared with healthy controls as well (69). Importantly, when comparing amounts of HMGB1 in serum in these different rheumatic diseases, levels are moderately increased compared with healthy controls (Table 1). In contrast, we found in 33 quiescent SLE patients (SLEDAI <4), with low amounts of anti-HMGB1, an average level of HMGB1 of 6.2 ng/mL (range 1.3–32.3) measured by ELISA (50). As mentioned before, ELISA is not suitable for measuring HMGB1 in active SLE patients, but from these data, it is suggested that active SLE patients will have much higher amounts of HMGB1 in their serum. Recently increased HMGB1 levels were reported in juvenile SLE patients (70).
Table 1.
Serum HMGB1 levels in different rheumatic diseases.
| Disease | Number of patients | Disease state | HMGB1 level | Detection method | Reference |
|---|---|---|---|---|---|
| SLE | |||||
| 20 | Lupus nephritis | 108 ng/mL (19.8–202.4) | WB | Zickert et al. (54) | |
| HC: 13 ± 10 ng/mL | |||||
| 33 | Inactive | 6.2 ng/mL (1.3–32.3) | ELISA (Shino/IBL) | Abdulahad et al. (50) | |
| HC: 2.9 ng/mL (0–7.7) | |||||
| 51 AU (28–121) | WB | ||||
| 37 | Active | 135 AU (81–496) | WB | ||
| HC: 43 AU (7–85) | |||||
| 11 | Inactive | 4.12 ± 1.97 ng/mL | ELISA (Shino/IBL) | Ma et al. (53) | |
| 26 | Active | 6.44 ± 4.18 ng/mL | |||
| HC: 4.45 ± 1.59 | |||||
| Juvenile SLE | |||||
| 26 | Inactive | 30 ± 5.28 ng/mL | ELISA (Amsbio) | Kanakoudi-Tsakalidou et al. (70) | |
| Active | 17.98 ± 3.2 ng/mL | ||||
| HC: 1.38 ± 0.23 ng/mL | |||||
| Vasculitis | |||||
| 46 | GPA inactive | 11.63 ± 8.8 ng/mL | ELISA (Shino/IBL) | Wibisono (66) | |
| active | 4.89 ± 3.37 ng/mL | ||||
| 44 | MPA inactive | 2.61 ± 2.5 ng/mL | |||
| active | 2.61 ± 2.7 ng/mL | ||||
| HC: 3.0 ± 2.8 ng/mL | |||||
| 169 | GPA | 4.68 ± 3.66 ng/mL | ELISA (Shino/IBL) | Henes et al. (68) | |
| HC: 2.34 ± 2.01 ng/mL | |||||
| 30 | GPA inactive | 78 ± 46 ng/mL | WB | Bruchfeld et al. (67) | |
| active | 120 ± 48 ng/mL | ||||
| HC: approx. 10 ng/mL | |||||
| 33 | GPA | 2.66 1.83 ng/mL | ELISA (Shino/IBL) | de Souza et al. (57) | |
| 11 | MPA | 3.11 ± 1.91 ng/mL | |||
| 8 | Renal limited vasculitis | 1.92 ± 1.48 ng/mL | |||
| HC: 2.39 ± 1.09 ng/mL | |||||
| Rheumatoid arthritis | |||||
| 30 | 1.5 ± 4.2 ng/mL | WB | Taniguchi et al. (65) | ||
| No HC | |||||
| 13 | 71 ng/mL (45–99) | WB | Goldstein (62) | ||
| HC: 18 ng/mL (0–40) | |||||
| 81 | Postmenopausal | 0.887 ng/mL (0–1.592) | ELISA (Shino/IBL) | Pullerits et al. (63) | |
| No HC | |||||
| Juvenile idiopathic arthritis | |||||
| 23 | Equal to HC | ELISA (Shino/IBL) | Schierbeck et al. (64) | ||
| Sjögren syndrome | |||||
| 101 | 1.79 ng/mL (0.25–3.35) | ELISA (unsp) | Dupire et al. (61) | ||
| HC: 1.46 ng/mL (0.94–1.74) | |||||
| Ankylosing spondylitis | |||||
| 30 | 0.86 ± 0.37 ng/mL | ELISA (USCN) | Oktayoglu et al. (69) | ||
| HC: 0.65 ± 0.39 |
Data are means ± standard deviation or median (range). AU, arbitrary units; HC, healthy controls; WB, Western blot.
As mentioned above, redox status plays an important role in HMGB1 functionality. Until now, one report has investigated the redox status of HMGB1 in serum of SLE patients. It was shown that oxidized HMGB1 is present in these serum samples. However, this study included only a small number of patients with severe disease activity (71). More research is needed in larger patient groups with different disease activity to investigate which isoforms of HMGB1 are present in serum of SLE patients.
HMGB1 AND LUPUS NEPHRITIS
In patients with renal involvement, higher HMGB1 levels were observed in serum compared with patients without renal involvement (50,52). In kidney biopsies of patients with lupus nephritis, cytoplasmic and extracellular HMGB1 was found (54,55), which was not present in control tissue. Furthermore, HMGB1 was detectable in urine of patients with lupus nephritis, but not in healthy controls, and correlated positively with serum HMGB1, proteinuria and SLEDAI and inversely with complement C3 levels. Strong expression levels of the receptors for HMGB1 (RAGE, TLR2 and TLR4) were seen in kidney tissue from patients with active lupus nephritis, indicating that HMGB1 can exert it functions (55). These studies suggest that HMGB1 might serve as a marker of disease activity in SLE patients, especially in those with renal involvement.
HMGB1 AND CUTANEOUS LUPUS
Research on the effects of ultraviolet irradiation on the skin of lupus patients showed that exposure to ultraviolet light induces apoptosis of keratinocytes and the release of proinflammatory cytokines (72). In susceptible patients, the presence or even accumulation of apoptotic cells after ultraviolet exposure results in the induction of characteristic inflammatory skin lesions, which might be due to a delayed and proinflammatory clearance of these apoptotic cells. A few studies analyzed the presence of HMGB1 in the skin of patients with cutaneous lupus. A higher expression of cytoplasmic and extracellular HMGB1 was observed in lesional skin of lupus patients. Extracellular HMGB1 was only observed in lesional skin and not in healthy subjects (73). Moreover, in experimentally photo-provoked lesions, the number of HMGB1-negative cells increased, and cytoplasmic HMGB1 was significantly more present in active lesions (74). These two studies, however, only used skin of patients who were photosensitive, whereas the study by our group also investigated the role of HMGB1 in skin of lupus patients, irrespective of having a history of photosensitivity (75). After irradiation, the number of negative nuclei indicating translocation of HMGB1 to the cytoplasm significantly increased in patients compared with healthy controls. In patients, there was also a significant correlation between the presence of apoptotic keratinocytes, also called sunburn cells, and HMGB1-negative nuclei. Moreover, in patients, there was a significantly higher influx of inflammatory cells compared with healthy controls, but there was no correlation with number of negative nuclei, indicating released HMGB1. Together, these studies show that HMGB1 plays a proinflammatory role in cutaneous lupus.
INTERVENTION/FUTURE
HMGB1, as has been shown in this review, plays an important role as an inflammatory mediator in SLE and can act alone or in complex with several other molecules. In Figure 1 the role of HMGB1 in SLE is depicted. Currently, most studies have focused on observations that HMGB1 is increased in lupus patients, either in serum, urine, kidney or skin. To our knowledge, there are no intervention studies blocking the effects of HMGB1 in lupus animal models or in SLE patients. Several different animal models of (autoimmune) disease use blocking agents, such as box A or neutralizing antibodies against HMGB1 to alleviate symptoms of disease (60). Because of the increase of HMGB1 in serum of SLE patients, this seems to be an appealing option. However, because anti-HMGB1 antibodies are present in sera from SLE patients that recognize box A, these two treatments do not seem like a feasible option for SLE. In animal models, other interventions have been tested, such as small interference RNA (siRNA), metformin (an antidiabetic drug) and glycyrrhizin, a small molecule that can bind directly to HMGB1 (60). Most work has been done in LPS-mediated mice models or sepsis models, and more research is needed to translate these findings to autoimmunity and SLE specifically.
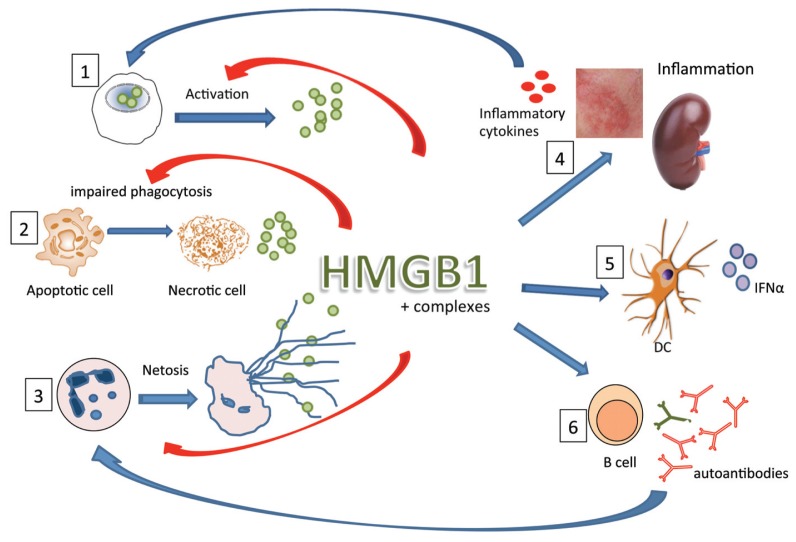
Figure 1.
Role of HMGB1 in pathogenesis of SLE. In SLE, release of HMGB1 can occur via different routes: first, HMGB1 can be released from activated cells in an inflammatory environment; second, in SLE, apoptotic cells are not cleared properly because of impaired phagocytosis, which can lead to necrotic cells, which in turn release HMGB1; third, in SLE, low-density granulocytes are present that can spontaneously form NET (neutrophil extracellular traps), a process that leads to externalization of cellular content including HMGB1. In circulation or at inflammatory sites, HMGB1 can form complexes with DNA, nucleosomes, LPS or cytokines. HMGB1 alone or in complex can induce inflammation, for instance, in the kidney or in the skin of lupus patients (4), which in turn can lead to production of inflammatory cytokines. Also, complexed HMGB1 can activate plasmacytoid dendritic cells to produce IFN-α (5), a driving cytokine in lupus pathogenesis. Lastly, complexed HMGB1 can stimulate B-cells to produce autoantibodies (6). These activations lead to an inflammatory loop in which cytokines activate other cells, autoantibodies play a role in NETosis and HMGB1 itself can also induce NETosis and can play a role in the impaired phagocytosis.
 , HMGB1;
, HMGB1;
 , IFN-α;
, IFN-α;
 , inflammatory cytokines.
, inflammatory cytokines.
Exploring the mechanisms behind HMGB1-induced inflammation in SLE is needed, since there is still much unknown. Much knowledge about HMGB1 biology has already been amassed by in vitro studies that investigate the pathways, receptors and posttranslational modifications; however, most of the studies have not been translated to disease models. Future studies, in lupus animal models and in SLE patients, should focus on delineating the kinetics, cellular location and redox status of HMGB1.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Bickerstaff MC, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–7. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 2.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 3.Sanford AN, Dietzmann K, Sullivan KE. Apoptotic cells, autoantibodies, and the role of HMGB1 in the subcellular localization of an autoantigen. J Autoimmun. 2005;25:264–71. doi: 10.1016/j.jaut.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7:113–8. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- 5.Courtney PA, et al. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58:309–14. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann I, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Reefman E, et al. Is disturbed clearance of apoptotic keratinocytes responsible for UVB-induced inflammatory skin lesions in systemic lupus erythematosus? Arthritis Res Ther. 2006;8:R156. doi: 10.1186/ar2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbonaviciute V, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev. 2005;15:496–506. doi: 10.1016/j.gde.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Calogero S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–80. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- 11.Kazama H, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Andersson U, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn JH, Oh YJ, Kim ES, Choi JE, Shin J. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-α production in human monocytes. 2008;180:5067–74. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Hreggvidsdottir HS, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–62. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 17.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straino S, et al. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J Invest Dermatol. 2008;128:1545–53. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 19.Mitola S, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Van Zoelen MAD, et al. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–4. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JS, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 22.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo R, et al. Src family kinases are necessary for cell migration induced by extracellular HMGB1. J Leukoc Biol. 2009;86:617–23. doi: 10.1189/jlb.0908581. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo R, et al. Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF-kappaB activation. J Cell Biol. 2007;179:33–40. doi: 10.1083/jcb.200704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, et al. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–9. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Hreggvidsdottir HS, et al. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med. 2012;18:224–30. doi: 10.2119/molmed.2011.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 30.Urbonaviciute V, et al. HMGB1-containing nucleosomes released from secondary necrotic cells cause activation and cytokine release from monocytes/macrophages and dendritic cells: implications for the immunopathogenesis of SLE. Ann Rheum Dis. 2007;66:A11. [Google Scholar]
- 31.Wen Z, et al. Autoantibody induction by DNA-containing immune complexes requires HMGB1 with the TLR2/microRNA-155 pathway. J Immunol. 2013;190:5411–22. doi: 10.4049/jimmunol.1203301. [DOI] [PubMed] [Google Scholar]
- 32.Qing X, et al. Pathogenic anti-DNA antibodies modulate gene expression in mesangial cells: involvement of HMGB1 in anti-DNA antibody-induced renal injury. Immunol Lett. 2008;121:61–73. doi: 10.1016/j.imlet.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun W, et al. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab Invest. 2013;93:626–38. doi: 10.1038/labinvest.2013.61. [DOI] [PubMed] [Google Scholar]
- 34.Munoz LE, et al. Apoptosis in the pathogenesis of systemic lupus erythematosus. Lupus. 2008;17:371–5. doi: 10.1177/0961203308089990. [DOI] [PubMed] [Google Scholar]
- 35.Bijl M, Reefman E, Horst G, Limburg PC, Kallenberg CG. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann Rheum Dis. 2006;65:57–63. doi: 10.1136/ard.2005.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008;181:4240–6. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friggeri A, et al. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am J Physiol Cell Physiol. 2010;299:C1267–76. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol. 2010;88:973–9. doi: 10.1189/jlb.0510262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis K, et al. Poly(ADP-ribosyl)ation of high mobility group box 1 (HMGB1) protein enhances inhibition of efferocytosis. Mol Med. 2012;18:359–69. doi: 10.2119/molmed.2011.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee S, et al. Intracellular HMGB1 negatively regulates efferocytosis. J Immunol. 2011;187:4686–94. doi: 10.4049/jimmunol.1101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 42.Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J. Vis. Exp. 2010;(36):e1724. doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–5. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leffler J, et al. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R84. doi: 10.1186/ar4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–63. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tadie JM, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013;304:L342–9. doi: 10.1152/ajplung.00151.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdulahad DA, et al. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:727–8. doi: 10.1136/ard.2007.074484. [DOI] [PubMed] [Google Scholar]
- 52.Li J, et al. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J Rheumatol. 2010;37:766–75. doi: 10.3899/jrheum.090663. [DOI] [PubMed] [Google Scholar]
- 53.Ma C, et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-alpha and TNF-alpha in systemic lupus erythematosus. Rheumatol Int. 2012;32:395–402. doi: 10.1007/s00296-010-1636-6. [DOI] [PubMed] [Google Scholar]
- 54.Zickert A, et al. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdulahad DA, et al. Urine levels of HMGB1 in systemic lupus erythematosus patients with and without renal manifestations. Arthritis Res Ther. 2012;14:R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayashi A, et al. Lupus antibodies to the HMGB1 chromosomal protein: epitope mapping and association with disease activity. Mod Rheumatol. 2009;19:283–92. doi: 10.1007/s10165-009-0151-7. [DOI] [PubMed] [Google Scholar]
- 57.de Souza AW, et al. Is serum HMGB1 a biomarker in ANCA-associated vasculitis? Arthritis Res Ther. 2013;15:R104. doi: 10.1186/ar4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urbonaviciute V, et al. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 59.Barnay-Verdier S, et al. Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 2011;37:957–62. doi: 10.1007/s00134-011-2192-6. [DOI] [PubMed] [Google Scholar]
- 60.Schaper F, Heeringa P, Bijl M, Westra J. Inhibition of high-mobility group box 1 as therapeutic option in autoimmune disease: lessons from animal models. Curr Opin Rheumatol. 2013;25:254–9. doi: 10.1097/BOR.0b013e32835cee2d. [DOI] [PubMed] [Google Scholar]
- 61.Dupire G, Nicaise C, Gangji V, Soyfoo MS. Increased serum levels of high-mobility group box 1 (HMGB1) in primary Sjögren’s syndrome. Scand J Rheumatol. 2012;41:120–3. doi: 10.3109/03009742.2011.633099. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein RS, et al. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–5. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pullerits R, Urbonaviciute V, Voll RE, Forsblad-D’Elia H, Carlsten H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J Rheumatol. 2011;38:1523–5. doi: 10.3899/jrheum.110091. [DOI] [PubMed] [Google Scholar]
- 64.Schierbeck H, et al. HMGB1 levels are increased in patients with juvenile idiopathic arthritis, correlate with early onset of disease, and are independent of disease duration. J Rheumatol. 2013;40:1604–13. doi: 10.3899/jrheum.120987. [DOI] [PubMed] [Google Scholar]
- 65.Taniguchi N, et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003;48:971–81. doi: 10.1002/art.10859. [DOI] [PubMed] [Google Scholar]
- 66.Wibisono D, et al. Serum HMGB1 levels are increased in active Wegener’s granulomatosis and differentiate between active forms of ANCA-associated vasculitis. Ann Rheum Dis. 2010;69:1888–9. doi: 10.1136/ard.2009.119172. [DOI] [PubMed] [Google Scholar]
- 67.Bruchfeld A, et al. High-mobility group box-1 protein (HMGB1) is increased in antineutrophilic cytoplasmatic antibody (ANCA)- associated vasculitis with renal manifestations. Mol Med. 2011;17:29–35. doi: 10.2119/molmed.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henes FO, et al. Correlation of serum level of high mobility group box 1 with the burden of granulomatous inflammation in granulomatosis with polyangiitis (Wegener’s) Ann Rheum Dis. 2011;70:1926–9. doi: 10.1136/ard.2010.146456. [DOI] [PubMed] [Google Scholar]
- 69.Oktayoglu P, et al. Elevated serum levels of high mobility group box protein 1 (HMGB1) in patients with ankylosing spondylitis and its association with disease activity and quality of life. Rheumatol Int. 2013;33:1327–31. doi: 10.1007/s00296-012-2578-y. [DOI] [PubMed] [Google Scholar]
- 70.Kanakoudi-Tsakalidou F, et al. Simultaneous changes in serum HMGB1 and IFN-α levels and in LAIR-1 expression on plasmatoid dendritic cells of patients with juvenile SLE. New therapeutic options? Lupus. 2014;23:305–12. doi: 10.1177/0961203313519157. [DOI] [PubMed] [Google Scholar]
- 71.Urbonaviciute V, et al. Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis. Autoimmunity. 2009;42:305–7. doi: 10.1080/08916930902831803. [DOI] [PubMed] [Google Scholar]
- 72.Kuhn A, Bijl M. Pathogenesis of cutaneous lupus erythematosus. Lupus. 2008;17:389–93. doi: 10.1177/0961203308090019. [DOI] [PubMed] [Google Scholar]
- 73.Popovic K, et al. Increased expression of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in skin lesions of patients with lupus erythematosus. Arthritis Rheum. 2005;52:3639–45. doi: 10.1002/art.21398. [DOI] [PubMed] [Google Scholar]
- 74.Barkauskaite V, et al. Translocation of the novel cytokine HMGB1 to the cytoplasm and extracellular space coincides with the peak of clinical activity in experimentally UV-induced lesions of cutaneous lupus erythematosus. Lupus. 2007;16:794–802. doi: 10.1177/0961203307081895. [DOI] [PubMed] [Google Scholar]
- 75.Abdulahad DA, et al. High mobility group box 1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) Lupus. 2013;22:597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]