Abstract
AIM: To investigate midkine (MK) and syndecan-3 protein expression in pancreatic cancer by immunohistochemistry, and to analyze their correlation with clinicopathological features, perineural invasion, and prognosis.
METHODS: Pancreatic cancer tissues (including adequately sized tumor tissue samples and tissue samples taken from areas less than 2.0 cm around the tumor) were taken from 42 patients who were undergoing a partial duodenopancreatectomy. MK and syndecan-3 proteins were detected by immunohistochemistry using a standardized streptavidin-peroxidase method, and analyzed for their correlation with clinicopathological features, perineural invasion, and prognosis. Associations of neural invasion with aggressive characteristics of pancreatic cancer and the presence of perineural invasion were assessed by two independent observers blinded to the patient status.
RESULTS: MK and syndecan-3 were found in 26 (61.9%) and 24 (57.1%) specimens, respectively. MK and syndecan-3 expression was associated with perineural invasion (P = 0.018 and 0.031, respectively). High MK expression was closely associated with advanced tumor, node and metastasis stage (P = 0.008), lymph node metastasis (P = 0.042), and decreased postoperative survival at 3 years (51.0% vs 21.8%, P = 0.001). Syndecan-3 levels were correlated with tumor size (P = 0.028). Patients who were syndecan-3 negative had a higher cumulative survival rate than those who were positive, but the difference was not significant (44.0% vs 23.0%, P > 0.05).
CONCLUSION: MK and syndecan-3 are frequently expressed in pancreatic cancer and associated with perineural invasion. High expression of MK and syndecan-3 may contribute to the highly perineural invasion and poor prognosis of human pancreatic cancer.
Keywords: Midkine, Syndecan-3, Pancreatic cancer, Neurite growth, Perineural invasion
Core tip: Midkine (MK) has diverse biological properties, including neuritis outgrowth and tumor progression. Syndecan-3 is a high-affinity receptor for MK and an essential component for neurite outgrowths. MK and its receptor may play an important role during perineural invasion. In this study, MK and syndecan-3 proteins levels were detected and analyzed for correlations with clinicopathological features, perineural invasion, and prognosis. Our results show that high expression of MK contributes to the highly perineural invasion and poor prognosis of human pancreatic cancer.
INTRODUCTION
Pancreatic cancer is one of the most aggressive and intractable human malignant cancers[1,2]. Perineural invasion extending into the pancreatic nerve plexus is a histopathologic characteristic of pancreatic cancer. However, the mechanisms contributing to the invasion of intrapancreatic nerves and spread of cancer cells along the extrapancreatic nerves during pancreatic tumorigenesis remain poorly understood. A family of proteins consisting of neurotrophic factors is of interest due to recent experimental data that showed their involvement in the neural invasion of pancreatic cancer[3,4].
Midkine (MK), a type of neurotrophic factor, is also known as a neurite growth promoting factor. MK has a molecular weight of 13 kDa[5]. MK proteins in humans and rats are identical and share a 45% amino acid similarity with pleiotrophin (PTN), which is the only other member of this family[6]. The MK family possesses diverse biological properties, including neuritis outgrowth[7,8], neuronal survival[9,10], carcinogenesis, and tumor progression[6,11]. MK is mainly expressed during early embryogenesis. In human adult tissues, MK is markedly down-regulated and present only at minimal levels in very few tissues. It is not expressed in normal pancreatic tissues, but highly expressed in pancreatic cancer[12].
Syndecan-3 is a high-affinity receptor for MK and an essential component for neurite outgrowths[13]. MK-induced neurite outgrowth can be inhibited by anti-syndecan-3 antibodies in vitro[14]. This MK receptor is mainly expressed in neural tissues by neurons and localized in neurites[15].
High expression of MK has been reported in a number of malignant tumors, including breast cancer, prostate cancer, pancreatic cancer, and neuroblastoma[16-20]. Interestingly, perineural invasion is prone to occur in these MK-positive tumors. Therefore, this suggests that as a neurite growth-promoting factor, MK and its receptor syndecan-3 may play an important role in the perineural invasion of pancreatic cancer. In previous studies, we have confirmed that another member of the MK family, PTN, and its receptor could promote neurite outgrowth[21] and perineural invasion of pancreatic cancer[22]. To elucidate the function of MK and its receptor in pancreatic cancer, we compared the expression patterns of MK and syndecan-3 by immunohistochemistry in patients with data containing clinicopathological features, perineural invasion, and overall survival.
MATERIALS AND METHODS
Patient specimens
From March 2005 to May 2009, pancreatic cancer tissues (including adequately sized tumor tissue samples and tissue samples taken from areas less than 2.0 cm around the tumor) were taken from 42 patients who were undergoing a partial duodenopancreatectomy (Whipple resection) for pancreatic cancer at Department of Pancreatic Surgery (First Affiliated Hospital of Henan University of Science and Technology). Samples were fixed with 4% formalin for histological studies and analysis of perineural invasion was performed according to a previously described method[23]. In addition, 10 samples containing normal pancreatic tissues from patients who received partial pancreatectomy for benign tumors were used as normal controls.
Of the 42 patients, 26 were men and 16 were women. The median age at the time of surgery was 62.5 years (range: 40-82 years). The histological type of all 42 patients was pancreatic ductal adenocarcinoma. Tumor stage and histopathological grade were recorded based on the classification of the International Union Against Cancer[24]. There were 10 stage I, 11 stage II, 16 stage III, and 5 stage IV cancers. Histological grades were as follows: 5 grade I, 22 grade II, and 15 grade III. All patients were followed and the median duration of follow-up was 24 (2-46) mo. All studies were approved by the Human Subjects Committee of Henan University of Science and Technology, China.
Immunohistochemistry
MK and syndecan-3 proteins were detected by immunohistochemistry using a standardized streptavidin–peroxidase (SP) method. Sections (4-μm) from paraffin-embedded tissue were used for immunohistochemistry. Slides were deparaffinized in fresh xylene and rehydrated through sequential graded ethanol. Antigen retrieval was performed in citrate buffer (10 mmol/L, pH 6.0) using a pressure cooker for 2 min after air jetting. Slides were cooled for 20 min, incubated for 10 min with 0.3% (v/v) hydrogen peroxide, blocked for 20 min in 20% normal goat serum or rabbit serum, and incubated with primary antibodies overnight at 4 °C. The next day, the slides were washed in phosphate-buffered saline (PBS; pH 7.4) and incubated for 30 min with secondary antibody of biotinylated rabbit antigoat immunoglobulin G (IgG) (1:200 dilution; DAKO, Glostrup, Denmark) or biotinylated goat antirabbit IgG (1:200 dilution, DAKO). Samples were then incubated with peroxidase-conjugated streptavidin (1:200 dilution, DAKO) for 20 min at room temperature. Color was developed with 0.02% 3, 3’-diaminobenzidine (Sigma, St Louis, MO, United States) in 50 mmol/L Tris-HCl buffer (pH 7.6) for 5-7 min. The slides were washed three times in 0.25% Tween-20 in PBS for 15 min between each step, and all incubations were carried out in a humidified chamber. Finally, the sections were counterstained with hematoxylin, rinsed with water, dehydrated, cleared and cover-slipped. The following primary antibodies were applied: anti-MK (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, United States) and anti-syndecan-3 (1:100 dilution; Boster, Wuhan, China). Non-immune goat or rabbit serum were used as negative controls. The known positive breast carcinoma and neuroblastoma were used as positive controls for MK and syndecan-3, respectively. The results of MK and syndecan-3 staining were evaluated in terms of the percentage of positively stained cells, with > 10% considered positive.
Analysis of perineural invasion
Associations of neural invasion with aggressive characteristics of pancreatic cancer and the presence of perineural invasion were assessed in all pancreatic cancer specimens by two independent observers blinded to the patient status. Any differences were resolved by a joint review and consultation with a third observer. For each cancer sample, approximately 10 tissue sections from different tumor locations were checked. Perineural invasion was defined as positive if the infiltration of cancer cells into the perineurium or neural fasciculus was detected at the leading point, as reported previously[23]. The degree of perineural invasion was defined microscopically as follows: 0, perineural invasion was difficult to find, with less than one occurrence per slide; 1, perineural invasion was easy to find, with two to four occurrences per slide; and 2, perineural invasion was easy to find, with more than four occurrences per slide or intraneural invasion.
Statistical analysis
Univariate analysis was used for the χ2 test. Survival rate was calculated by the Kaplan-Meier method, and differences were examined by the Log-rank test. Factors found to be significant were then chosen for a stepwise Cox multivariate proportional hazard model to determine their prognostic values. SPSS for Windows (Version 14.0; SPSS Inc., Chicago, IL, United States) was used for statistical analysis. P values less than 0.05 were considered statistically significant.
RESULTS
Expression of MK and syndecan-3 in pancreatic cancer
MK was expressed in the cytoplasm of tumor cells and found predominantly in invasive carcinoma cells. No staining was observed in normal pancreatic cells (Figure 1A-D). Interestingly, the cells in the adjacent tissue also displayed moderate staining for MK. Immunoreactions were also observed in the desmoplastic stromal cells within the tumor mass in MK-positive cases, but not in stromal cells in the areas distant from MK-positive cancer cell nests. In normal pancreatic tissues, no syndecan-3 was observed in the perineurium of nerves. Immunostaining revealed that syndecan-3 was not present in ductal and acinar cells. Pancreatic cancer cells were devoid of any syndecan-3 (Figure 1E). In tissues surrounding the tumor mass, syndecan-3 was not detected in the perineurium. However, in pancreatic cancer tissues, strong syndecan-3 immunoreactivity was present in the perineurium (Figure 1F). The perineurium infiltrated by pancreatic cancer cells was frequently severely damaged. Therefore, the intensity of the immunohistochemical signal in these regions could not be conclusively evaluated (data not shown). In total, the expression rates of MK in pancreatic cancers and tissues around the tumor mass were 61.9% (26/42) and 26.2% (11/42), respectively (P < 0.05).
Figure 1.
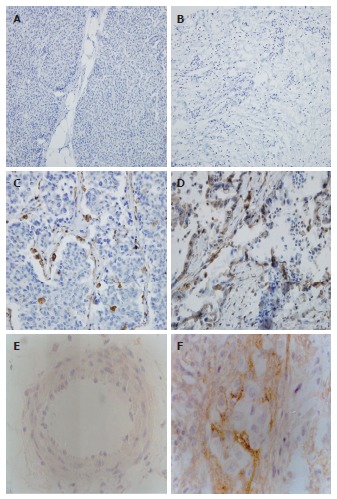
Representative immunohistochemistry results of midkine and syndecan-3 (× 200). Paraffin sections were immunostained as described in Materials and Methods. A: Normal tissue showing negative midkine (MK) expression; B: Tumor tissue showing negative MK expression; C: Tumor tissue showing moderate MK expression; D: Tumor tissue showing intense MK expression; E: Negative syndecan-3 expression in cancer; F: Positive syndecan-3 expression in the perineurium of nerves in pancreatic cancer.
Relationships of MK and syndecan-3 expression with clinicopathological features
Table 1 summarizes the associations of MK and syndecan-3 expression with clinicopathological parameters in pancreatic cancer. The MK immunostaining was positive in 9 of 21 stage I-II cases (42.9%), and the positive rate was significantly lower than those in stage III (81.3%, 13 of 16 cases) and stage IV (100%, all 5 cases) cases (P = 0.008). High MK expression was also associated with lymph nodes metastasis (P = 0.042). Syndecan-3 expression was associated with tumor size (P = 0.028). There was also a correlation between MK expression and tumor size, but it was not statistically significant (P = 0.060). We also investigated the relationship between plasma levels of the tumor marker CA19-9 in 42 patients, and MK and syndecan-3 expression. However, no relationships were observed between them (P = 0.610 and P = 0.475, respectively).
Table 1.
Associations between midkine, syndecan-3 protein expression and clinicopathologic factors in pancreatic cancers n (%)
| Factor | Midkine positive cases | Syndecan-3 positive cases |
| Age (yr) | ||
| > 60 (n = 26) | 17 (65.4) | 17 (65.4) |
| ≤ 60 (n = 16) | 9 (56.3) | 7 (43.8) |
| Sex | ||
| Female (n = 16) | 12 (75.0) | 10 (62.5) |
| Male (n = 26) | 14 (53.8) | 14 (53.8) |
| CA19-9 (n = 42) | ||
| >37 U/mL (n = 24) | 16 (66.7) | 12 (50.0) |
| ≤ 37 U/mL (n = 18) | 12 (66.7) | 10 (55.6) |
| Histological grade | ||
| I (n = 5) | 4 (80.0) | 2 (40.0) |
| II (n = 22) | 16 (72.7) | 15 (68.2) |
| III (n = 15) | 7 (46.7) | 7 (46.7) |
| Tumor size | ||
| T ≤ 2 cm (n = 10) | 4 (40.0) | 3 (30.0)a |
| 2 cm < T ≤ 5 cm (n = 24) | 14 (58.3) | 15 (62.5)a |
| T > 5 cm (n = 8) | 8 (100.0) | 7 (87.5)a |
| Lymph node metastasis | ||
| Negative (n = 17) | 7 (41.2)a | 7 (41.2) |
| Positive (n = 25) | 19 (76.0)a | 17 (68.0) |
| Distant metastasis | ||
| Negative (n = 36) | 21 (58.3) | 19 (52.8) |
| Positive (n = 6) | 5 (83.3) | 5 (83.3) |
| TNM grade | ||
| I + II (n = 21) | 9 (42.9)b | 10 (47.6) |
| III (n = 16) | 13 (81.3)b | 10 (62.5) |
| IV (n = 5) | 5 (100.0)b | 5 (100.0) |
P < 0.05,
P < 0.01 vs negative cases.
Relationship between perineural invasion and expression of MK and syndecan-3
MK-positive cases had statistically higher levels of syndecan-3 expression (P = 0.045). High MK expression was associated with perineural invasion (P = 0.018). Syndecan-3 expression was also associated with perineural invasion (P = 0.031). MK and syndecan-3 expression levels in pancreatic cancer were significantly higher in patients with perineural invasion than in those without perineural invasion (Table 2).
Table 2.
Associations between midkine, syndecan-3 protein expression and perineural invasion in pancreatic cancer
| Protein | PNI |
P value | ||
| 0 | 1 | 2 | ||
| MK (+) (n = 26) | 3 | 10 | 13 | 0.018 |
| MK (-) (n = 16) | 7 | 7 | 2 | |
| Syndecan-3 (+) (n = 24) | 2 | 9 | 13 | 0.031 |
| Syndecan-3 (-) (n = 18) | 7 | 7 | 4 | |
Perineural invasion was scored as follows: 0, perineural invasion was difficult to find, with less than one occurrence per slide; 1, perineural invasion was easy to find, with two to four occurrences per slide; and 2, perineural invasion was easy to find, with more than four occurrences per slide or intraneural invasion. PN: Perineural invasion; MK: Midkine.
Prognostic value of MK and syndecan-3 in patients with pancreatic cancer
To determine the prognostic value of MK and syndecan-3 in pancreatic cancer, we analyzed the cumulative survival of patients based on their status (Figure 2). The cumulative survival rate in MK-negative patients (n = 16) at 3 years was 51.0% (median time 34.5 mo). In contrast, the cumulative survival rate in MK-positive patients (n = 26) was 21.8% (median time 14 mo) (P = 0.001). In addition, patients who were negative for syndecan-3 (n = 18) had a higher cumulative survival rate than those who were positive for syndecan-3 (n = 24), but this difference was not significant (44.0%, median time 31.5 mo vs 23.0%, median time 18 mo, respectively; P > 0.05).
Figure 2.
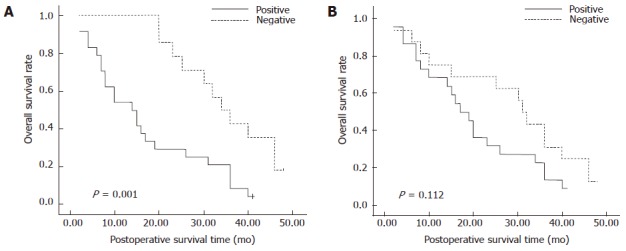
Kaplan-Meier analysis of overall postoperative survival curves in pancreatic cancer cases according to their immunohistochemical staining for midkine (A) and syndecan-3 (B).
A multivariate analysis revealed that lymph node metastasis (P = 0.010) and tumor size (P = 0.035) were independent prognostic factors for overall survival in patients with pancreatic cancer. The expression of MK or syndecan-3 was not an independent prognostic factor for overall survival (P > 0.05). Perineural invasion and other clinical parameters were also found not to be independent prognostic factors.
DISCUSSION
Perineural invasion is defined as the presence of cancer cells along nerves and/or within the epineurial, perineurial, and endoneurial spaces of the neuronal sheath[25]. Perineural invasion is a multifactorial process that involves various signaling molecules from different signaling pathways[26-28]. These signaling molecules include NGFs, neurotrophic factors, chemokines, and cell-surface ligands, and receptors[29,30]. Pancreatic ductal adenocarcinoma cells respond strongly to neurotrophic factors. Since the pancreas is in close proximity to several neural plexuses, this might partially explain the high incidence of perineural invasion in pancreatic cancer[31]. It is likely that as a neurite growth-promoting factor, MK acts synergistically to promote the development of perineural invasion.
Our present study suggests that high MK expression is closely correlated with advanced tumor, node and metastasis stage, lymph node metastasis, and perineural invasion. In contrast, although tissue samples reveal moderate to intense MK-immunoreactive staining in patients with distant metastasis, univariate analysis did not indicate a positive correlation between intense MK expression and distant metastasis. This may be due to the small sample size and because only six patients with distant metastasis had received operations and were entered into our cohort. Surprisingly, positive MK expression is also observed in normal tissue surrounding the tumor. We believe that MK upregulation in normal cells surrounding the tumor might be due to the binding and uptake of MK produced by nearby tumor cells.
Perineural invasion extending into the pancreatic nerve plexus is a histopathological characteristic of pancreatic cancer[32]. Perineural invasion occurs not only in the extrapancreatic nerve, but also in the intrapancreatic nerve in pancreatic carcinoma[33]. Previous studies have demonstrated that syndecan-3 acts as a receptor or a co-receptor in MK-induced neurite outgrowth in perinatal rat brain neurons[34,35]. It is possible that MK-syndecan interactions play key roles in neurite outgrowth and development of perineural invasion in human pancreatic cancer. In this study, MK and syndecan-3 protein levels were markedly increased in pancreatic cancer samples compared to normal pancreas samples by immunostaining analysis. MK was highly expressed in pancreatic cancer cells, whereas syndecan-3 was primarily found in neuritis and the perineurium of pancreatic nerves and absent in pancreatic cancer cells. Raulo also showed that syndecan-3 is localized on the surface of neuritis and growth cones in rat primary neurons found on MK-coated matrix in vitro[14]. The statistical analysis in our study demonstrated that high MK expression was associated with perineural invasion (P = 0.018). Syndecan-3 expression was also associated with perineural invasion (P = 0.031). MK and syndecan-3 expression levels in pancreatic cancer were significantly higher in patients with perineural invasion than in those without perineural invasion. Therefore, these observations suggest that the MK/syndecan-3 pathway may have an important role in perineural invasion.
Whether high MK expression is a prognostic factor for pancreatic cancer is unknown. In our study, patients with negative MK expression had a higher 3-year survival rate and a longer established survival time than those who were positive for MK expression (survival rate 51.0% vs 21.8%, P = 0.001 and survival time 34.5 mo vs 14 mo, respectively). This indicates that the MK negative expression correlated with a better survival outcome. We believe that the coexpression of syndecan-3 and MK was associated with perineural invasion of pancreatic cancer, and that invasion is significantly associated with the prognosis[36]. Furthermore, patients with negative MK expression were early-staged (stages Iand II) and accounted for almost half the number of patients who received a pancreaticoduodenectomy or radical operation. Patients who were positive for MK expression had advanced tumors without an option for undergoing radical surgery. These findings also demonstrated that MK activation appeared to be a crucially progressive step for tumor invasion and metastasis.
In conclusion, the present data suggest that MK may act as a useful biomarker for aggressive pancreatic cancer. MK and its receptor protein are frequently expressed in human pancreatic cancer. Their co-expression may suggest their coordination in the tumor perineural invasion and prognosis of pancreatic cancer. In future studies, we will determine the functional role of MK in the progression and neural invasion of pancreatic cancer by in vitro and in vivo loss-of-function studies.
COMMENTS
Background
Pancreatic cancer is still one of the most aggressive and intractable human malignant tumors. Perineural invasion extending into the pancreatic nerve plexus is a histopathological characteristic of pancreatic cancer. However, the mechanisms contributing to the invasion of intrapancreatic nerves and spread of cancer cells along the extrapancreatic nerves during pancreatic cancer progression are still poorly understood. As a neurite growth-promoting factor, midkine (MK) and its receptor syndecan-3 may play an important role in the perineural invasion and prognosis of human pancreatic cancer. Therefore, in this study, MK and syndecan-3 proteins levels in pancreatic cancer were detected by immunohistochemistry and analyzed for correlations with clinicopathological features, perineural invasion, and prognosis.
Research frontiers
Perineural invasion extending into the pancreatic nerve plexus is a histopathological characteristic of pancreatic cancer. The neural invasion of pancreatic cancer leads to local recurrence, metastasis and poor prognosis, which have brought difficulty for diagnosing and treating pancreatic cancer.
Innovations and breakthroughs
In recent years, studies on pancreatic cancer have focused on biological characteristics, especially perineural invasion. This study found that high expression of MK and syndecan-3 may contribute to the highly perineural invasion and poor prognosis of human pancreatic cancer, which might reveal the functional mechanism of neural invasion.
Applications
The study indicates that MK and syndecan-3 might be an attractive target for gene therapy of nerve infiltration of pancreatic cancer.
Terminology
MK is a type of neurotrophic factor and is also known as a neurite growth promoting factor. Syndecan-3 is a transmembrane protein and a high-affinity receptor for MK. MK and syndecan-3 are important in promoting neurite outgrowth. High expression of MK and its receptor may contribute to the highly perineural invasion and poor prognosis of human pancreatic cancer.
Peer review
The authors provided data on the relevance between expression of midkine and its receptor syndecan-3 and some clinical features in pancreatic cancer patients. These findings contribute to the highly perineural invasion and poor prognosis of ductal adenocarcinoma of the pancreas. The number of cases included is small but the evidence of such expression of neurite growth-promoting factors in pancreatic cancer is interesting.
Footnotes
Supported by National Natural Science Foundation of China, No. U1204819; the Health Science and Technology Innovation Talents Program of Henan Province, No. 4203
P- Reviewers: Chowdhury P, Gu DS, Sperti C S- Editor: Zhai HH L- Editor: Wang TQ E- Editor: Wu HL
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer. 2005;5:459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 3.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Büchler MW, Friess H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veit C, Genze F, Menke A, Hoeffert S, Gress TM, Gierschik P, Giehl K. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 2004;64:5291–5300. doi: 10.1158/0008-5472.CAN-04-1112. [DOI] [PubMed] [Google Scholar]
- 5.Tomomura M, Kadomatsu K, Matsubara S, Muramatsu T. A retinoic acid-responsive gene, MK, found in the teratocarcinoma system. Heterogeneity of the transcript and the nature of the translation product. J Biol Chem. 1990;265:10765–10770. [PubMed] [Google Scholar]
- 6.Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 7.Rauvala H, Peng HB. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog Neurobiol. 1997;52:127–144. doi: 10.1016/s0301-0082(97)00007-5. [DOI] [PubMed] [Google Scholar]
- 8.Kurosawa N, Chen GY, Kadomatsu K, Ikematsu S, Sakuma S, Muramatsu T. Glypican-2 binds to midkine: the role of glypican-2 in neuronal cell adhesion and neurite outgrowth. Glycoconj J. 2001;18:499–507. doi: 10.1023/a:1016042303253. [DOI] [PubMed] [Google Scholar]
- 9.Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, Michikawa M, Ikematsu S, Sakuma S, Muramatsu T. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci Res. 2003;45:219–224. doi: 10.1016/s0168-0102(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 10.Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T, Michikawa M. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- 11.Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- 12.Maeda S, Shinchi H, Kurahara H, Mataki Y, Noma H, Maemura K, Aridome K, Yokomine T, Natsugoe S, Aikou T, et al. Clinical significance of midkine expression in pancreatic head carcinoma. Br J Cancer. 2007;97:405–411. doi: 10.1038/sj.bjc.6603879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnunen A, Kinnunen T, Kaksonen M, Nolo R, Panula P, Rauvala H. N-syndecan and HB-GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur J Neurosci. 1998;10:635–648. doi: 10.1046/j.1460-9568.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 14.Raulo E, Chernousov MA, Carey DJ, Nolo R, Rauvala H. Isolation of a neuronal cell surface receptor of heparin binding growth-associated molecule (HB-GAM). Identification as N-syndecan (syndecan-3) J Biol Chem. 1994;269:12999–13004. [PubMed] [Google Scholar]
- 15.Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- 16.Ohhashi S, Ohuchida K, Mizumoto K, Egami T, Yu J, Cui L, Toma H, Takahata S, Nabae T, Tanaka M. Midkine mRNA is overexpressed in pancreatic cancer. Dig Dis Sci. 2009;54:811–815. doi: 10.1007/s10620-008-0434-4. [DOI] [PubMed] [Google Scholar]
- 17.Ibusuki M, Fujimori H, Yamamoto Y, Ota K, Ueda M, Shinriki S, Taketomi M, Sakuma S, Shinohara M, Iwase H, et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009;100:1735–1739. doi: 10.1111/j.1349-7006.2009.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nordin A, Wang W, Welén K, Damber JE. Midkine is associated with neuroendocrine differentiation in castration-resistant prostate cancer. Prostate. 2013;73:657–667. doi: 10.1002/pros.22607. [DOI] [PubMed] [Google Scholar]
- 19.Ikematsu S, Nakagawara A, Nakamura Y, Ohira M, Shinjo M, Kishida S, Kadomatsu K. Plasma midkine level is a prognostic factor for human neuroblastoma. Cancer Sci. 2008;99:2070–2074. doi: 10.1111/j.1349-7006.2008.00957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishida S, Mu P, Miyakawa S, Fujiwara M, Abe T, Sakamoto K, Onishi A, Nakamura Y, Kadomatsu K. Midkine promotes neuroblastoma through Notch2 signaling. Cancer Res. 2013;73:1318–1327. doi: 10.1158/0008-5472.CAN-12-3070. [DOI] [PubMed] [Google Scholar]
- 21.Yao J, Zhang M, Ma QY, Wang Z, Wang LC, Zhang D. PAd-shRNA-PTN reduces pleiotrophin of pancreatic cancer cells and inhibits neurite outgrowth of DRG. World J Gastroenterol. 2011;17:2667–2673. doi: 10.3748/wjg.v17.i21.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Ma Q, Wang L, Zhang M. Pleiotrophin expression in human pancreatic cancer and its correlation with clinicopathological features, perineural invasion, and prognosis. Dig Dis Sci. 2009;54:895–901. doi: 10.1007/s10620-008-0433-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, Büchler MW. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 24.Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- 25.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 26.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 27.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Müller MW, Giese NA, Friess H, Schäfer KH. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374:442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, Carlson DL, Shah JP, Fong Y, Wong RJ. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J Gastroenterol Hepatol. 2008;23:1852–1859. doi: 10.1111/j.1440-1746.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 30.Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 31.Ceyhan GO, Schäfer KH, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, Böhm C, Müller MW, Büchler MW, Giese NA, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann Surg. 2010;251:923–931. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- 32.Hirai I, Kimura W, Ozawa K, Kudo S, Suto K, Kuzu H, Fuse A. Perineural invasion in pancreatic cancer. Pancreas. 2002;24:15–25. doi: 10.1097/00006676-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225–229. doi: 10.1097/00006676-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kinnunen T, Raulo E, Nolo R, Maccarana M, Lindahl U, Rauvala H. Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J Biol Chem. 1996;271:2243–2248. doi: 10.1074/jbc.271.4.2243. [DOI] [PubMed] [Google Scholar]
- 35.Nolo R, Kaksonen M, Raulo E, Rauvala H. Co-expression of heparin-binding growth-associated molecule (HB-GAM) and N-syndecan (syndecan-3) in developing rat brain. Neurosci Lett. 1995;191:39–42. doi: 10.1016/0304-3940(94)11551-1. [DOI] [PubMed] [Google Scholar]
- 36.Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Nakagohri T, Ochiai A. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]


