Abstract
Objective
This study was designed to identify the presence, type and origin of bacteria adjacent to the metal implant in the infected region in a canine model of pyogenic vertebral osteomyelitis treated with single-stage anterior autogenous bone grafting and instrumentation.
Methods
Dogs with pyogenic spondylodiscitis underwent one-stage debridement, autogenous bone grafting and titanium plate instrumentation and perioperative antibiotic therapy. The implants and adjacent vertebral bones were removed surgically at various postoperative time points (4, 8, 12 and 24 weeks) for bacteria detection. Bacteria were detected from retrieved spinal implants as well as surrounding bones by culture and/or pyrosequencing methods in 17 (85 %) of all 20 animals. The positive rate for bacteria presence was 45 % by culture and 80 % by pyrosequencing method.
Results
Radiological or macroscopic examination showed no signs for infection recurrence in any animal regardless of bacteria presence at the surgical site. However, organism identical with the causative bacterium for spinal infection was found in only two of nine culture-positive animals.
Conclusion
Within the confines of the study, the use of metallic implants in an infected area did not lead to a clinically relevant infection although bacteria may exist at the surgical site. The use of metallic implants in an infected area of the spine is safe. The metallic implants may not be the “culprit” for the persistence or recurrence of infection.
Keywords: Spinal infection, Animal model, Canine, Staphylococcus aureus, Titanium implant
Introduction
Despite the great evolution in imaging diagnosis, antibiotic therapy, and surgical techniques for spinal infection, management of pyogenic vertebral osteomyelitis has been posing a challenge to spine surgeons. The majority of patients with pyogenic vertebral osteomyelitis can be treated successfully by conservative methods, including antibiotic administration, bed rest or immobilization with orthotic devices. However, surgical intervention is warranted for some cases, particularly for those with intractable pain, progressive neurological deficit and significant structural instability with kyphotic deformity, when conventional therapy fails [1–8]. The incidence of pyogenic vertebral osteomyelitis has been increasing, which may be attributed to the increasing number of aged and immunocompromised population as well as diagnostic evolutions.
In the 1950s, Hodgson et al. [9] reported their techniques on the surgical management of spinal tuberculosis, which included debridement and grafting via the anterior approach. This technique has gradually been accepted by more and more surgeons, showing the advantage of immediate correction of kyphotic deformity and spinal alignment. However, the long-term outcome of this technique remains doubtful regarding issues in spinal stabilization in terms of correction loss, pseudarthrosis, and graft collapse and extrusion [10, 11]. Therefore, anterior instrumentation has become an inevitable choice in certain circumstances [12]. The advocates for spinal instrumentation argued that stabilization of the spine is very important for the suppression and eventual elimination of infections [13, 14]. Increasing evidence has also showed that instrumentation in the presence of active infection is well tolerated while spinal stability is maintained effectively with sufficient restoration of sagittal alignment of the spine and relatively shorter duration of bed rest [3, 14–25].
However, considerable doubt remains regarding whether spinal instrumentation surgery will lead to persistence or recurrence of infection or whether placing metal implants at the “focus” of infection is safe. Shad et al. [26] reported a series of five patients with cervical vertebral osteomyelitis managed with anterior or posterior debridement and instrumentation. When the implants were retrieved 6–18 months after solid spine fusion, it was noted that asymptomatic colonization of bacteria on the surface of implant was common. Interestingly, the authors found that the bacteria attached to the retrieved plates were not identical species with those preoperatively obtained in four of these five cases. The source of bacteria may be related to sampling contamination or new secondary bacterial infection. These authors claimed that removal of implants should be necessary in due time although patients are asymptomatic.
Based upon the results of study by Shad et al. and other clinical series, we hypothesized the possible presence of bacteria at the site of instrumentation for pyogenic vertebral osteomyelitis. Therefore, an animal study was designed to test this hypothesis. The objective of this study was to identify the presence, type and origin of bacteria adjacent to the metal implant in the infected region in a canine model of pyogenic vertebral osteomyelitis and determine the relation of bacteria, if present, to instrumentation or surgery and possible clinical implication; that is, whether the presence of bacteria will lead to recurrence or persistence of infection after reconstruction surgery with single-stage anterior autogenous bone grafting and instrumentation.
Materials and methods
Animals
Twenty male mongrel dogs, weighting 12–15 kg, were used for the experimentation. They were caged separately and fed with conventional food and with water ad libitum. All experiments were done with the approval of the Animal Care and Use Committee of our institution.
Bacterial strain and preparation of inocula
A Staphylococcus aureus strain (29213; American type culture collection, ATCC) was used in the experiment. It was cultured on trypticase soy agar (TSA) 1 day before surgery and subsequently incubated at 37 °C for 18 h. The bacteria in their logarithmic growth phase were suspended in sterile phosphate buffer solution (PBS) and then centrifuged for 10 min. As the suspension was diluted in PBS to different bacterial concentrations [in colony-forming units (CFU) per millilitre] as measured by optical densities adjusted to a value corresponding to 108/ml using a spectrophotometric assay (Vitek colorimeter, bioMérieux Inc, France) standard curve. Final concentrations of bacteria were confirmed by plating the dilutions on trypticase soy agar.
Osteomyelitis model
We used a modified version of the osteomyelitis model described by Chen et al. [27]. Briefly, after animals were anaesthetized with intramuscular injection of ketamine-xylazine, an anterior retroperitoneal approach was used to get access to the lateral surface of the desired vertebrae (L2–L3). With the L2–3 disc removed partially, the endplates of the adjacent two vertebral bodies were curetted out for the bacterial inoculation. A 1-cm3 cubic gelatin sponge, loaded with 0.1 ml bacterial inoculum at a specified concentration, was inserted into the intervertebral space. Then the insertion site was sealed with bone wax. The fascia and skin layers were tied, and the skin incision was closed with a single interrupted silk suture. All surgical procedures were carried out under strict sterile conditions in a conventional operation theatre. Animals were kept in separate hutches and fed with antibiotic-free standard food before and after instrumentation surgery was made. The wounds were examined daily; weight and temperature were checked once weekly.
Surgical procedure
Instrumentation surgery was performed upon animals with vertebral pyogenic osteomyelitis 4 weeks after bacterial inoculation. Before surgery was undertaken, the presence of spinal infection were confirmed by magnetic resonance imaging (MRI) examination in all animals, as defined by characteristic changes of abnormal signal on both T1- and T2-weighted images throughout the involved disc and vertebral bodies [27, 28]. The MRI was also used for surgical planning by demonstrating the extent and severity of infection. Antibiotical therapy was begun 1 week prior to surgery with the protocol of intramuscular cefazolin (2 g twice per day) and gentamycin (80,000 units twice per day), based upon results of the preliminary study of the antibiotic sensitivities of the causative organism. The anaesthesia and surgical approach was identical to those used for the virgin spine surgery. After a thorough debridement of all infected and necrotic tissue by discectomy and corpectomy, the iliac bone graft obtained via a separate fascial incision was wedged into the defect to reconstruct the spine (Fig. 1a). Finally, anterior instrumentation was performed using a four-hole titanium plate (Fig. 1b). Postoperatively, antibiotics were applied for 4 weeks with the same regimen as before instrumentation surgery.
Fig. 1.
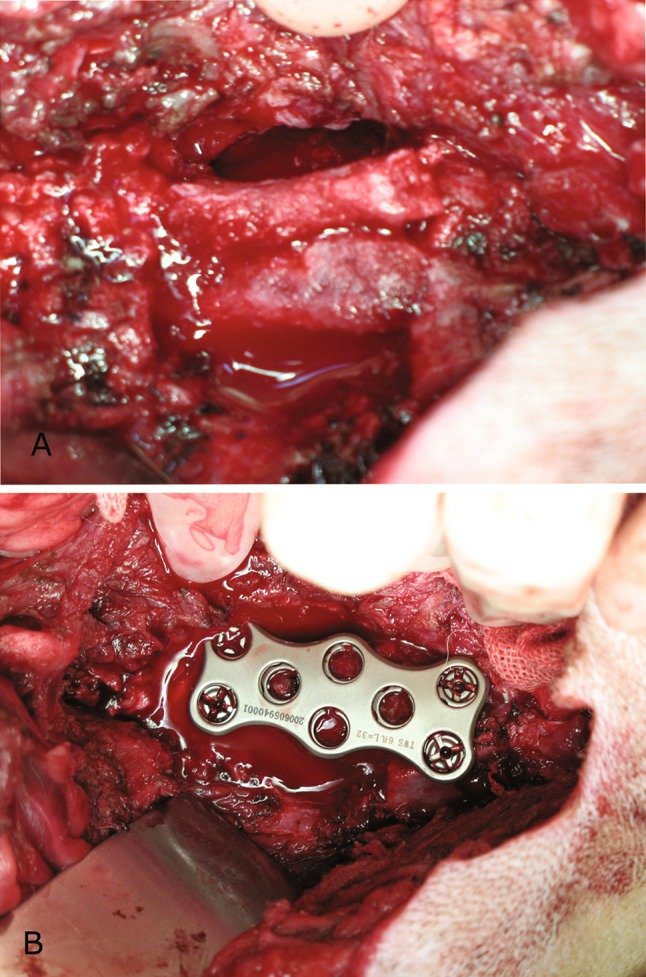
Surgical procedure. All infected and necrotic tissue including the infected intervertebral disc, adjacent end plates and vertebral body bone were resected. a The iliac bone graft was wedged into the defect to reconstruct the spine. b Instrumentation following bone grafting was made with a titanium plate fixed with four titanium screws
Follow-up examination
The follow-up period after instrumentation surgery ranged from 4 to 24 weeks. The animals were assessed clinically for any signs of infection recurrence including swelling, redness, fistula, soft-tissue defects and pus drainage. They were euthanized 4, 8, 12, and 24 weeks after surgery (n = 5 per time point) with an intravenous overdose of pentobarbital sodium, according to the approved protocol. All dogs were examined at the corresponding time interval using MRI to determine the presence or absence of infection recurrence. MRI results were reviewed by the same investigator who was blinded to the treatment. Weight measurement was also made at each time point.
Sample acquisition and bacteria culture
After animals were killed, the instrumented spines were excised under strict aseptic conditions. The instrumented plate and bone surrounding the plate (within 1 cm of distance from the plate) were obtained from each specimen. After being evaluated for any signs of infection recurrence such as purulent exudates, abscess formation, bone resorption, and implant failure, samples were transported into separate sterile tubes for macroscopic observation or further evaluations.
The retrieved bone and plate were separated. Ten sterile swabs were passed across each of the retrieved plates with total plates amounting to 200. Then each of the plate was placed in a sterile tube with 20 mL phosphate-buffered saline and sonicated (Branson Ultrasonic Cleaner; Branson Ultrasonics, Danbury, CT, USA) at a frequency of 50 kHz for 5 min. Previous studies [29–33] have proven the efficiency of such ultrasonic treatment for removing any bacteria that attach to the plates without leading to the death of bacteria. After sonication, an aliquot of the solution was collected and submitted for further microbiologic culture (n = 200 for plates) and PCR processing. Meanwhile, ten sterile swabs were passed across each of the retrieved bone samples (n = 200 for plates). Then the removed bone samples were pulverized and homogenized in PBS solutions; finally, the suspension also were inoculated on TSA plates (n = 200 for plates) or processed of further PCR procedures. All the swabs were inoculated on separated TSA plates. Each of the aforementioned specimens was inoculated on ten TSA plates, with five plates incubated at aerobic conditions and five plates incubated at anaerobic conditions. All samples were placed in 37 °C and cultured for 1 week. At least three in five positive TSA plates were defined as positive microbiologic culture results. Finally, the colonies were processed by automatic bacteria identification system (Vitek 32, bioMérieux Inc, France) for the identification of specific species.
Ten sterile titanium plates treated with same procedures were used as the negative controls to exclude the influence of bacteria contamination during the processes.
Bacteria detection by PCR methods
Sonicates of the retrieved titanium plates were centrifuged for 15 min (10,000g). After the supernatants were discarded, the remnants were processed for water bath at 55 °C. DNA extraction and purification kit (PureLink Genomic DNA Purification Kit, Invitrogen, USA) was used for advent processing.
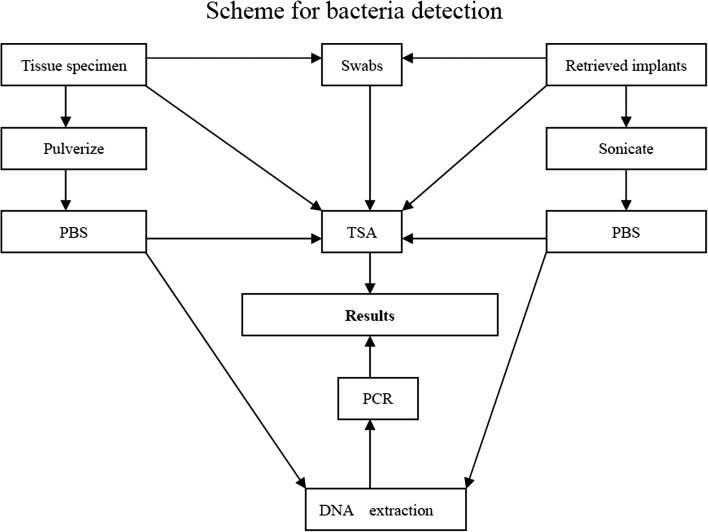
Oligonucleotide primers used were complementary to conserved regions of the 16SrRNA gene of E. coli, which possess 216 pairs of base. Specific primer sequence was named P1 (GAGGAAGGTAGGGGACTGACGT) and P2 (AGGCCCGGGAACGCTATTCTACCG) for amplification of a 216-bp fragment of the 16S rRNA gene. P1 and P2 are located at 1199–1219 and 1394–1445 gene sequence of E. coli, respectively. After being amplified for 30 cycles, PCR products were stored at −20 °C or processed for direct agarose gel electrophoresis, of which 15 μl aliquots were separated on agarose gel together with the amplification product of Staphylococcus aureus for verification of the product size, with a negative control for at least every fifth sample. Detailed scheme for bacteria detection is shown in Fig. 2.
Fig. 2.
Scheme for bacteria detection
Statistical analysis
The data were collected and analyzed using the SPSS software. The sensitivity results of bacteria detection were compared using the Chi square test with the level of statistical significance set at P < 0.05.
Results
General
There were no problems with the osteomyelitis model, the implants, the anaesthetic or the surgical procedure. Five dogs were lost before the time of instrumentation surgery. Two of them died on the third day after the first operation for unknown causes, while the other three did not exhibit signs of infection with a negative culture 4 weeks after bacterial inoculation. All these five animals were replaced in the study to remain with as balanced a design as possible. The remaining 20 animals survived without any signs of systemic infection for whole postoperative observation period. Surgical incision healed well in all animals with no open wound infection. Initial weight loss was noted, but was compensated by the end of observation. No signs of toxicity effects of systemic cefazolin or gentamycin were seen in animals treated with antibiotic drugs.
Macroscopic evaluation
No significant signs of recurrent infection such as purulent material accumulation and bone destruction were shown in any animals at all time intervals (Fig. 3). The implants were totally enveloped by soft tissues, with all screws firmly fixed when manually tested.
Fig. 3.
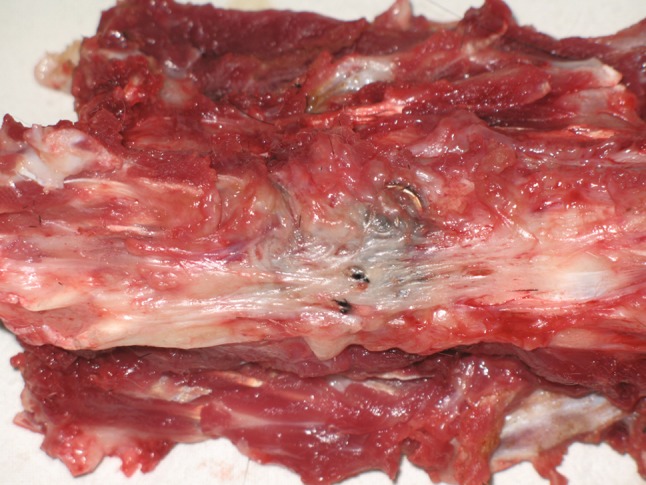
Retrieved spine column obtained at 12 weeks postoperatively. Plates were totally enveloped by normal tissues. There were no signs of recurrent infection
MRI assessment
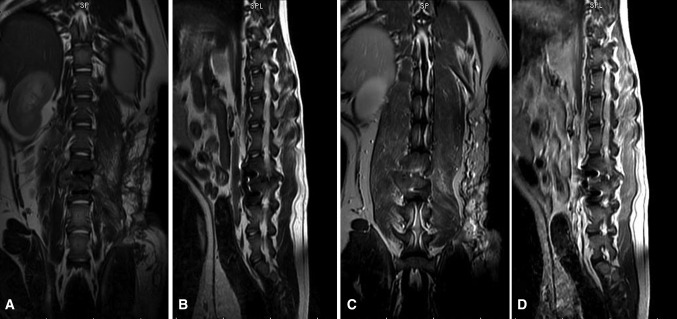
All these animals underwent MRI examination at each time interval to determine the presence or absence of infection recurrence. No suggestive lesions of recurrence of pyogenic vertebral osteomyelitis such as bone destruction or abscess formation were seen in any animals (Fig. 4).
Fig. 4.
Postoperative MR imaging findings. Coronal and sagittal T2-weighted spin-echo images obtained before (a, b) and after (c, d) the intravenous administration of gadolinium contrast agent revealed no vertebral osteomyelitis development or abscess formation, nor other signs of recurrent infection
Bacterial culture
The outcome of cultures is presented in Table 1. Nine of 20 (45 %) sampled animals had a positive culture. However, the microbiological findings showed that Staphylococcus aureus was present in only two animals. Other detected single organisms were confirmed as Escherichia coli, Staphylococcus haemolyticus, Streptococcus agalactiae, and Staphylococcus aureus, rather than the strain of Staphylococcus aureus initially innoculated.
Table 1.
Bacteria detected by culture or PCR from tissue or implants
| Euthanization time (post-surgery) | Sample no. | Bacteria isolated during surgery | Bacteria isolated at euthanization | 16S rRNA gene | ||
|---|---|---|---|---|---|---|
| Tissue | Implant | Tissue | Implant | |||
| 4w | 1 | S. aureus | N | N | N | P |
| 2 | S. aureus | N | N | N | P | |
| 3 | S. aureus | N | N | N | N | |
| 4 | S. aureus | E. coli | E. coli | N | P | |
| 5 | S. aureus | N | N | N | P | |
| 8w | 6 | S. aureus | N | N | P | N |
| 7 | S. aureus | N | E. coli | N | P | |
| 8 | S. aureus | N | E. coli | N | P | |
| 9 | S. aureus | S. aureus | S. aureus | P | P | |
| 10 | S. aureus | S. haemolyticus | N | N | P | |
| 12w | 11 | S. aureus | N | S. haemolyticus | P | P |
| 12 | S. aureus | N | N | P | N | |
| 13 | S. aureus | N | N | N | N | |
| 14 | S. aureus | N | E. coli | N | N | |
| 15 | S. aureus | N | N | N | P | |
| 24w | 16 | S. aureus | N | N | N | N |
| 17 | S. aureus | N | S. agalactiae, S. aureus | N | P | |
| 18 | S. aureus | N | N | N | P | |
| 19 | S. aureus | N | N | N | P | |
| 20 | S. aureus | N | S. haemolyticus | P | P | |
E. coli, Escherichia coli; S. haemolyticus, Staphylococcus haemolyticus; S. agalactiae, Streptococcus agalactiae; S. aureus, Staphylococcus aureus; P, positive; N, negative
PCR assay
Sixteen of 20 (80 %) samples were positive as shown by PCR. The results indicated that the use of PCR methods as opposed to culture methods significantly increases the level of detection of bacteria. The sensitivity of bacteria detection by PCR methods was significantly higher than that by bacterial culture (χ2 = 5.227, P < 0.05). There was one culture-positive, PCR-negative sample and eight culture-negative, PCR-positive samples, respectively (Table 1).
Discussion
This study is the first animal experiment to detect bacterial infection of retrieved spinal implants and surrounding bones following an anterior debridement and instrumented fusion surgery. Our study confirms the hypothesis that bacteria exist at the site of instrumentation after debridement and perioperative antibiotic administration for pyogenic vertebral osteomyelitis. Bacteria were detected from retrieved spinal implants as well as surrounding bones by bacterial culture and/or pyrosequencing methods in 17 (85 %) of all 20 animals. The positive rate for bacteria presence was 45 % by culture and 80 % by pyrosequencing method, respectively.
In this study, ultrasonication technique, which has been proved effective for disrupting the biofilm and dislodging bacteria from implants [29, 33, 34], was used to detect bacteria. In a study of 78 patients with aseptic failure and/or infection of prosthetic hip or knee infection [31], sonicate fluid and tissue cultures from removed implants were compared for microorganism detection. The sensitivities of sonicate fluid and periprosthetic tissue cultures were 75 and 54 %, respectively, whereas the specificities of the same specimens were 87 and 98 %, respectively. Also, direct inoculation of sonicates without using of broth enrichments might have prevented potential contamination as far as possible during the sampling procedure [33]. There was one culture-positive, PCR-negative sample and eight culture-negative, PCR-positive samples, respectively, showing much higher sensitivity of PCR assay based on the 16S rRNA gene amplification than of bacterial culture. However, further studies are necessary for the clinical implication of PCR-positive results [35].
Since most clinical series have identified S. aureus as the most frequent causative organism for pyogenic vertebral osteomyelitis [18, 21, 24], S. aureus was adopted in this study for developing the animal model of spinal infection. Two cases of infection by S. aureus were observed among nine culture-positive results. This finding is in line with the results from the study of Shad et al. [26].They found that the bacteria attached to the retrieved plates were not the same organisms with the primary infection in four of these five cases. Concerning the pathogenesis of these bacterial infections following instrumentation, one of the possible sources may be the haematogenous spread from an endogenous or cutaneous focus. Also, bacterial contamination during surgery may leave microorganisms at the surgical site and subsequently develop to a clinically relevant infection.
Despite the positive findings from the majority of experiment animals, the present study demonstrated no radiological or macroscopic signs for infection recurrence in any animal regardless of bacteria presence at the surgical site. The possible explanation for such subclinical infection status may be that microorganisms identified are most likely to be opportunists and lowly virulent strains. Whether infection recurs or not will eventually rely upon interaction between microorganisms, the implant and the host [36–38]. Weakening of the body defenses at the implant surface–tissue interface will facilitate the establishment of an infection around the implant. In this study, antibiotic administration based upon results of the preliminary study regarding the antibiotic sensitivities of the causative organism may have effectively prevented infection recurrence. Additionally, the titanium implant we used has shown the ideal biocompatibility [39, 40]. In a perspective we may expect that the use of new biomaterial could lead to a better outcome for the treatment of spinal infection.
Choice of anterior instrumentation for the treatment of pyogenic vertebral osteomyelitis is much like the strategy for the treatment of an infected hip or knee arthroplasty. In clinical settings, patients with early postoperative and acute haematogenous prosthetic joint infection are commonly treated with open debridement and prosthetic retention. However, the prostheses are not always salvaged successfully with the retention rate varying widely [41–43]. Prosthesis removal and replacement is sometimes inevitable option to definitively eradicate severe infections. In contrast, reports on the use of anterior instrumentation in the treatment of pyogenic vertebral osteomyelitis have showed great success. Such success may be attributable, to a great extent, to the ample blood supply to the vertebral bodies and adequate soft tissue coverage of the anterior spine compared with articular joints. Thus, although bacteria can be found around the implant at the surgical site, we do not recommend removal of implants that was placed in the infected region.
Conclusion
This study confirms the hypothesis that bacteria exist at the surgical site of instrumentation after debridement and perioperative antibiotic administration for pyogenic vertebral osteomyelitis. Bacteria were detected from retrieved spinal implants as well as surrounding bone by bacterial culture and/or pyrosequencing methods in the majority of animals. However, organisms were not identical with the causative bacterium for spinal infection. Radiological or macroscopic examination showed no signs for infection recurrence in any animal regardless of bacteria presence. The use of metallic implants in an infected area of the spine is safe. The metallic implants may not be the “culprit” for the persistence or recurrence of infection.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 30901513).
Conflict of interest
All authors have nothing to declare.
References
- 1.Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Goteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527–532. doi: 10.1080/00365540110026566. [DOI] [PubMed] [Google Scholar]
- 2.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. doi: 10.1302/0301-620X.79B5.8078. [DOI] [PubMed] [Google Scholar]
- 3.Carragee EJ. Instrumentation of the infected and unstable spine: a review of 17 cases from the thoracic and lumbar spine with pyogenic infections. J Spinal Disord. 1997;10:317–324. doi: 10.1097/00002517-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Chelsom J, Solberg CO. Vertebral osteomyelitis at a Norwegian university hospital 1987–97: clinical features, laboratory findings and outcome. Scand J Infect Dis. 1998;30:147–151. doi: 10.1080/003655498750003537. [DOI] [PubMed] [Google Scholar]
- 5.Dai LY, Chen WH, Jiang LS. Anterior instrumentation for the treatment of pyogenic vertebral osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2008;17:1027–1034. doi: 10.1007/s00586-008-0661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19–29. [PubMed] [Google Scholar]
- 7.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976) 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Wisneski RJ. Infectious disease of the spine. Diagnostic and treatment considerations. Orthop Clin North Am. 1991;22:491–501. [PubMed] [Google Scholar]
- 9.Hodgson AR, Stock FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266–275. doi: 10.1002/bjs.18004418508. [DOI] [PubMed] [Google Scholar]
- 10.Korovessis P, Sidiropoulos P, Piperos G, Karagiannis A. Spinal epidural abscess complicated closed vertebral fracture. A case report and review of literature. Spine (Phila Pa 1976) 1993;18:671–674. doi: 10.1097/00007632-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Safran O, Rand N, Kaplan L, Sagiv S, Floman Y. Sequential or simultaneous, same-day anterior decompression and posterior stabilization in the management of vertebral osteomyelitis of the lumbar spine. Spine (Phila Pa 1976) 1998;23:1885–1890. doi: 10.1097/00007632-199809010-00018. [DOI] [PubMed] [Google Scholar]
- 12.Chen WH, Jiang LS, Dai LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J. 2007;16:1307–1316. doi: 10.1007/s00586-006-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broner FA, Garland DE, Zigler JE. Spinal infections in the immunocompromised host. Orthop Clin North Am. 1996;27:37–46. [PubMed] [Google Scholar]
- 14.Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, Sakaeda H, Shimizu K. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine (Phila Pa 1976) 2003;28:E302–E308. doi: 10.1097/01.BRS.0000083318.40123.5E. [DOI] [PubMed] [Google Scholar]
- 15.Dimar JR, Carreon LY, Glassman SD, Campbell MJ, Hartman MJ, Johnson JR. Treatment of pyogenic vertebral osteomyelitis with anterior debridement and fusion followed by delayed posterior spinal fusion. Spine (Phila Pa 1976) 2004;29:326–332. doi: 10.1097/01.BRS.0000109410.46538.74. [DOI] [PubMed] [Google Scholar]
- 16.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15:149–156. doi: 10.1097/00024720-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. One-stage combined surgery with mesh cages for treatment of septic spondylitis. Clin Orthop Relat Res. 2006;444:51–59. doi: 10.1097/01.blo.0000203449.51769.7f. [DOI] [PubMed] [Google Scholar]
- 18.Korovessis P, Petsinis G, Koureas G, Zacharatos S. Posterior transcanal lumbar interbody fusion for septic vertebral fracture pseudarthrosis and sitting imbalance. Spine (Phila Pa 1976) 2005;30:E255–E258. doi: 10.1097/01.brs.0000160845.90903.88. [DOI] [PubMed] [Google Scholar]
- 19.Lee MC, Wang MY, Fessler RG, Liauw J, Kim DH. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004;17:E7. doi: 10.3171/foc.2004.17.6.7. [DOI] [PubMed] [Google Scholar]
- 20.Liljenqvist U, Lerner T, Bullmann V, Hackenberg L, Halm H, Winkelmann W. Titanium cages in the surgical treatment of severe vertebral osteomyelitis. Eur Spine J. 2003;12:606–612. doi: 10.1007/s00586-003-0614-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda T, Miyamoto K, Hosoe H, Sakaeda H, Tanaka M, Shimizu K. Surgical treatment with spinal instrumentation for pyogenic spondylodiscitis due to methicillin-resistant Staphylococcus aureus (MRSA): a report of five cases. Arch Orthop Trauma Surg. 2006;126:339–345. doi: 10.1007/s00402-006-0114-9. [DOI] [PubMed] [Google Scholar]
- 22.Nakase H, Tamaki R, Matsuda R, Tei R, Park YS, Sakaki T. Delayed reconstruction by titanium mesh-bone graft composite in pyogenic spinal infection: a long-term follow-up study. J Spinal Disord Tech. 2006;19:48–54. doi: 10.1097/01.bsd.0000179134.53997.2a. [DOI] [PubMed] [Google Scholar]
- 23.Nather A, David V, Hee HT, Thambiah J. Pyogenic vertebral osteomyelitis: a review of 14 cases. J Orthop Surg (Hong Kong) 2005;13:240–244. doi: 10.1177/230949900501300305. [DOI] [PubMed] [Google Scholar]
- 24.Ogden AT, Kaiser MG. Single-stage debridement and instrumentation for pyogenic spinal infections. Neurosurg Focus. 2004;17:E5. doi: 10.3171/foc.2004.17.6.5. [DOI] [PubMed] [Google Scholar]
- 25.Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg. 2001;94:1–7. doi: 10.3171/jns.2001.94.1.0001. [DOI] [PubMed] [Google Scholar]
- 26.Shad A, Shariff S, Fairbank J, Byren I, Teddy PJ, Cadoux-Hudson TA. Internal fixation for osteomyelitis of cervical spine: the issue of persistence of culture positive infection around the implants. Acta Neurochir Wien. 2003;145:957–960. doi: 10.1007/s00701-003-0129-8. [DOI] [PubMed] [Google Scholar]
- 27.Chen WH, Jiang LS, Dai LY. A novel canine model of acute pyogenic spondylodiscitis. Neurosurg Rev. 2009;32:485–490. doi: 10.1007/s10143-009-0209-1. [DOI] [PubMed] [Google Scholar]
- 28.Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, Rehm S. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–166. doi: 10.1148/radiology.157.1.3875878. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N, Bauer TW, Tuohy MJ, Fujishiro T, Procop GW. Brief ultrasonication improves detection of biofilm-formative bacteria around a metal implant. Clin Orthop Relat Res. 2007;457:210–213. doi: 10.1097/BLO.0b013e3180312042. [DOI] [PubMed] [Google Scholar]
- 30.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 31.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 32.Tunney MM, Patrick S, Curran MD, Ramage G, Anderson N, Davis RI, Gorman SP, Nixon JR. Detection of prosthetic joint biofilm infection using immunological and molecular techniques. Methods Enzymol. 1999;310:566–576. doi: 10.1016/S0076-6879(99)10044-2. [DOI] [PubMed] [Google Scholar]
- 33.Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37:3281–3290. doi: 10.1128/jcm.37.10.3281-3290.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R (2003) Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthop Relat Res 69–88. doi:10.1097/01.blo.0000087324.60612.93 [DOI] [PubMed]
- 35.Kobayashi N, Procop GW, Krebs V, Kobayashi H, Bauer TW. Molecular identification of bacteria from aseptically loose implants. Clin Orthop Relat Res. 2008;466:1716–1725. doi: 10.1007/s11999-008-0263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barth E, Myrvik QM, Wagner W, Gristina AG. In vitro and in vivo comparative colonization of Staphylococcus aureus and Staphylococcus epidermidis on orthopaedic implant materials. Biomaterials. 1989;10:325–328. doi: 10.1016/0142-9612(89)90073-2. [DOI] [PubMed] [Google Scholar]
- 37.Chen WH, Jiang LS, Dai LY. Influence of bacteria on spinal implant-centered infection: an in vitro and in vivo experimental comparison between Staphylococcus aureus and Mycobacterium tuberculosis. Spine (Phila Pa 1976) 2011;36:103–108. doi: 10.1097/BRS.0b013e3181cb46ba. [DOI] [PubMed] [Google Scholar]
- 38.Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clinical and biologic study. Spine (Phila Pa 1976) 1993;18:1890–1894. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Arens S, Schlegel U, Printzen G, Ziegler WJ, Perren SM, Hansis M. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br. 1996;78:647–651. [PubMed] [Google Scholar]
- 40.Tillander J, Hagberg K, Hagberg L, Branemark R. Osseointegrated titanium implants for limb prostheses attachments: infectious complications. Clin Orthop Relat Res. 2010;468:2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azzam KA, Seeley M, Ghanem E, Austin MS, Purtill JJ, Parvizi J. Irrigation and debridement in the management of prosthetic joint infection: traditional indications revisited. J Arthroplasty. 2010;25:1022–1027. doi: 10.1016/j.arth.2010.01.104. [DOI] [PubMed] [Google Scholar]
- 42.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty. 2009;24:101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 43.Van Kleunen JP, Knox D, Garino JP, Lee GC. Irrigation and debridement and prosthesis retention for treating acute periprosthetic infections. Clin Orthop Relat Res. 2010;468:2024–2028. doi: 10.1007/s11999-010-1291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]