Abstract
Purpose
Platelet-rich plasma (PRP) contains growth factors and creates a 3D structure upon clotting; PRP or platelet lysate (PL) might be considered for annulus fibrosus (AF) repair.
Methods
Bovine AF cells were cultured with 25 % PRP, 50 % PRP, 25 % PL, 50 % PL, or 10 % FBS. After 2 and 4 days, DNA, glycosaminoglycan (GAG), and mRNA levels were analyzed. Histology was performed after injection of PRP into an AF defect in a whole disc ex vivo.
Results
By day 4, significant increases in DNA content were observed in all treatment groups. All groups also showed elevated GAG synthesis, with highest amounts at 50 % PL. Collagen I and II expression was similar between groups; aggrecan, decorin, and versican expression was highest at 25 % PL. Injection of PRP into the AF defect resulted in an increased matrix synthesis.
Conclusions
Platelet-rich preparations increased the matrix production and cell number and may therefore be considered to promote AF repair.
Keywords: Annulus fibrosus, Intervertebral disc, Platelet-rich plasma, Platelet lysate, Proteoglycans
Introduction
The intervertebral disc (IVD) has a poor self-repair capacity and undergoes progressive degeneration starting in the second decade of life. Disorganization of proteoglycan (PG) composites in the nucleus pulposus (NP) and breakdown of the organized collagen network within the annulus fibrosus (AF) result in an accelerated degenerative process. Disruption of the AF collagen causes weakening of the AF, which may ultimately lead to disc herniation [1–3].
In the early stages of the degenerative process, anabolic treatment may help to restore cell activity and enhance extracellular matrix (ECM) synthesis, leading to strengthening of the AF and preventing herniation. Several studies have shown the ability of growth factors (GFs) to enhance matrix production and accumulation and stimulate IVD cell proliferation in vitro and in vivo [4]. The application of members of bone morphogenetic proteins (BMPs) has been shown to stimulate cell proliferation and enhance matrix synthesis of AF cells in vitro [5–7]. Several studies furthermore demonstrated stimulatory effects on AF cells by application of transforming growth factor β (TGF-β) and basic fibroblast growth factor (bFGF) [8, 9].
Moreover, administration of platelet-rich plasma (PRP) with the purpose of stimulating the metabolism of degenerative IVD cells has shown positive effects in vitro [10–12] and in vivo [11–14]. Using platelets in the form of PRP offers an easily applicable way to obtain high concentrations of GFs (e.g., platelet derived GF (PDGF), insulin-like GF (IGF), epidermal GF (EGF), transforming GF (TGF), bFGF, and vascular endothelial GF (VEGF)) [15] in a small volume of plasma. PRP is an autologous plasma that has a platelet count above baseline (as compared to normal blood serum), but with a native fibrinogen concentration [16].
In orthopedic surgery, PRP is commonly used for the repair of muscle, ligament, and tendon injuries [15, 17]. It is also applied in dermatology and plastic surgery for the healing of subcutaneous tissues [18]. One of the benefits of PRP is its natural activity creating a gel-like structure, which helps to maintain the PRP within the treated defect and thus promote wound healing directly at the site of injury. For in vitro application, platelet-rich preparations are often prepared in buffered systems like phosphate buffered saline (PBS), instead of autologous plasma, to avoid gel formation in the culture dishes [19, 20]. As platelet lysates (PL) may support cell growth and proliferation in vitro similar to fetal bovine serum (FBS), the use of such autologous preparations which contain several growth factors seems advantageous [19–21]. However, the effects of PL on IVD cells have not been investigated in direct comparison to PRP preparations, which provide a 3D structure in addition to the anabolic factors. The purpose of this study was to investigate the short term response of AF cells to PRP supplementation in terms of cell proliferation and ECM production and to compare the outcome between PRP and PL preparations in vitro. Furthermore, the feasibility and initial efficacy of PRP was investigated using a whole organ culture model of the bovine IVD.
Methods
Preparation of platelet-rich plasma, platelet-poor plasma, and platelet lysate
Leucocyte depleted (< 1 × 106 leucocytes/unit) and pathogen inactivated (INTERCEPT Blood System) platelet concentrate (containing ~ 1 × 106 platelets/μL, 5 × physiological blood concentration) was obtained from the blood bank of the cantonal hospital in Chur, Switzerland. The platelet concentrate was centrifuged for 20 min at 2,000 g with weak acceleration and brake speed. The resulting supernatant (platelet-poor plasma, PPP) was removed and stored at −20 °C. For preparation of PRP, the pellet was re-suspended in 1/10th of the PPPs original volume, sonicated for 15 min and stored at −20 °C [19]. For preparation of PL, the pellet was re-suspended in PBS according to the original volume and washed twice with PBS. Thereafter, the concentrate was activated by sonication for 15 min and freezing at −20 °C [19]. No exogenous platelet activator was used. Concentrations of PDGF-AB, PDGF-BB, VEGF, and TGF-β were determined by enzyme-linked immunosorbent assays (R&D Systems, Abingdon, UK).
Isolation of annulus fibrosus cells
AF tissue was harvested from caudal discs dissected from five bovine calve tails (6–9 months old; 4–5 IVDs per tail) within 3 h of death from a local slaughterhouse. To exclude contamination by the surrounding tissue and the NP, the most outer and inner layers of the AF were discarded. Chopped AF was washed with PBS and predigested with 2 mg/mL pronase (Roche, Switzerland) in high glucose Dulbecco’s Modified Eagle Medium (DMEM, Gibco) for 60 min at 37 °C, 5 % CO2. The tissue was then washed again 3 × with PBS and digested overnight in 310 U/mL collagenase II (Worthington Biochemical Corporation, USA) in DMEM. The isolated cells were filtered through 70 and 40 μm cell strainers (BD Bioscience), washed with DMEM, counted, and seeded as described below.
Cell culture conditions
AF cells were cultured in 24-well plates at 1 × 105 cells per cm2 with the following media: (1) 25 % PRP, 75 % DMEM; (2) 50 % PRP, 50 % DMEM; (3) 25 % PL, 75 % DMEM; (4) 50 % PL, 50 % DMEM; (5) 50 % PPP, 50 % DMEM; (6) 10 % FBS, 90 % DMEM; (7) DMEM control. All media were supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (P/S; Gibco), 50 μg/mL ascorbate-2-phosphate and 1 % ITS + 1 (Sigma Aldrich). Cell morphology was analyzed with an upright light microscope Axiovert 2 (Carl Zeiss, Germany), using 4.8 Axiovision software (Carl Zeiss Imaging Solutions, Germany).
Biochemical analysis
After 2 and 4 days, whole samples were digested in 0.5 mg/mL proteinase K solution (Roche) overnight at 56 °C. DNA was measured spectrofluorometrically using Hoechst 33258 (Polysciences, Inc.) and calf thymus DNA (Invitrogen) as standard. Glycosaminoglycan (GAG) content in proteinase K digests and media was quantified using dimethylmethylene blue assay (1.9-DMMB, Sigma Aldrich) with chondroitin sulfate (chondroitin-4-sulfate sodium salt from bovine trachea, Fluka BioChemika) as standard.
Gene expression analysis
Gene expression analysis was performed for all platelet preparation groups (PRP, PL, and PPP). TRI reagent (Molecular Research Center) was used for RNA isolation according to the manufacturer’s protocol. Reverse transcription was performed using SuperScript VILO™ cDNA Synthesis Kit (Invitrogen) and 500 ng of total RNA. The StepOnePlus™ System (Applied Biosystems) was used for real-time polymerase chain reaction (PCR). Gene expression of collagen type I (COL1A2), collagen type II (COL2A1), aggrecan core protein (ACAN), elastin (ELN), versican (VCAN), biglycan (BGN), and decorin (DCN) were analyzed using primers and TaqMan™ probes (Microsynth, Switzerland) or Gene Expression Assays (Applied Biosystems) (Table 1). The comparative CT method was used to evaluate differences in mRNA expression between PRP, PPP, and PL groups. The expression was normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and to the expression of freshly isolated cells (day 0). The day of cell seeding (day 0) was chosen as reference sample, since completely serum free conditions would induce apoptotic effects in AF cells; thus 100 % DMEM was not considered as an appropriate reference sample [22, 23].
Table 1.
Oligonucleotide primers and probes and Gene Expression Assays (from Applied Biosystems) used for real-time PCR; fw, forward; rev, reverse; serial number is indicated for Gene Expression Assays
| Gene | Abbr. | Primer fw 5′–3′ |
Primer rev 5′–3′ |
Probe (5′FAM/3′TAMRA) |
|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | GGC TGC TTT TAA TTC TGG CAA A | AAT CAT ACT GGA ACA TGT AGA CCA TGT A | TGG ACA TCG TCG CCA TCA ATG ACC |
| Collagen type I | COL1A2 | TGC AGT AAC TTC GTG CCT AGC A | CGC GTG GTC CTC TAT CTC CA | CAT GCC AAT CCT TAC AAG AGG CAA CTG |
| Collagen type II | COL2A1 | AAG AAA CAC ATG TGG TTT GGA GAA A | TGG GAG CCA GGT TGT CAT | CAA CGG TGG CTT CCA CTT CAG CTA TGG |
| Aggrecan | ACAN | CCA ACG AAA CCT ATG ACG TGT ACT | GCA CTC GTT GGC TGC CTC | ATG TTG CAT AGA AGA CCT CGC CCT CCA T |
| Biglycan | BGN | Bt03244532_m1 | ||
| Decorin | DCN | Bt03230914_m1 | ||
| Elastin | ELN | Bt03216594_m1 | ||
| Versican | VCN | Bt03217632_m1 | ||
Whole disc organ culture model
Application of platelet preparation was further tested in an organ culture model. PRP was chosen for the organ culture experiment, because injection of PL could not generate reproducible results, due to repeated leakage of PL from the AF defect. Whole discs including the endplates (EPs) were isolated from tails of three bovine calves (6–9 months old). Discs were isolated using a band saw to make parallel cuts through the cranial and caudal EPs. EP surfaces were cleaned with Ringer’s balanced salt solution using a Pulsavac Wound Debridement Irrigation System (Zimmer, USA) [24]. Using a size 15 scalpel blade, a full thickness 8 mm cut was made in the AF of three discs per animal. Then, 100 μL of PRP were injected into two discs per animal, while control discs were left without PRP. Discs were cultured in DMEM containing 1 % ITS + 1, 50 μg/mL ascorbate-2-phosphate and 1 % P/S for 7 days. IVDs were fixed in 4 % buffered formalin, embedded in paraffin, sectioned transversely (5 μm), and stained with Safranin-O. The slides were viewed using 4.8 Axiovision Software in an upright light microscope Axiovert 2 (Carl Zeiss).
Statistical analysis
SPSS 21.0 software was used for statistical analysis. Data sets did not show symmetrical distribution. Biochemical values (DNA, GAG) were compared using nonparametric sample test (one-way ANOVA on Ranks, Kruskal–Wallis test). Gene expression data were normalized to the values of freshly isolated cells (day 0), and groups were compared using nonparametric sample test. For comparison between days 2 and 4 data within treatment groups, Mann–Whitney rank sum test was used.
Results
Preparation of PPP, PRP, and PL
Platelet preparations (PPP, PRP, and PL) from three donors were pooled. Pure platelet concentration was 10 × 106 platelets/μL ± 5 %. The platelet concentration procedure increased the platelet numbers in the PRP preparation on average by 10 times over blood baseline concentration. GF concentrations in platelet-rich and platelet-poor preparations are summarized in Table 2.
Table 2.
Concentrations of growth factors in platelet-rich and platelet-poor preparations as determined by enzyme-linked immunosorbent assay (ELISA)
| Growth factor | Platelet-rich preparation (PL) (ng/mL) | Platelet-poor preparation (PPP) (ng/mL) |
|---|---|---|
| PDGF-AB | 46.4 ± 17.6 | 14.5 ± 2.7 |
| PDGF-BB | 8.4 ± 3.5 | 5.3 ± 1.1 |
| VEGF | 1.4 ± 0.4 | 0.8 ± 0.6 |
| TGF-β | 68.8 ± 16.1 | 25.3 ± 3.8 |
Cell morphology
Cells cultured in the PRP containing media did not settle at the bottom of the culture dish, but rather adopted a 3D structure within the PRP medium (Fig. 1a, b). The complete clotting of the PRP group appeared after 30–45 min. The 3D environment resulted in more rounded cells which were arranged in colonies or clusters amidst the viscous medium. Cells placed in the PPP, FBS, PL, and DMEM did not experience this 3D-like structure, but rather settled at the bottom of the culture dishes (Fig. 1c, d). This monolayer culture caused a more flattened and spindle-shaped cell morphology.
Fig. 1.
Representative light microscopy images of AF cells in different media at day 2; scale bar 200 μm. (a), (b) AF cells treated with PRP. The images were taken at the same location at different focuses, indicating that some of the cells were attached to the bottom of the culture dish, while others were embedded in the viscous medium. Within this gel-like structure, AF cells adopted a more rounded phenotype and were arranged in colonies. (c) AF cells in PL containing medium. Whereas the cells appeared spindle-shaped like in the other monolayer culture conditions, they did form cell conglomerations. (d) AF cells in FBS containing medium. The cell conformation in PPP, FBS, and DMEM media was similar
Biochemical analysis
After 2 days, treatment of AF cells with 25 and 50 % PL resulted in a significant increase in DNA content when compared to DMEM-only treatment (Fig. 2a). After 4 days, the FBS, PL (P < 0.001), and PRP groups showed significantly increased DNA contents as compared to the DMEM treatment. With a sevenfold increase, the 50 % PL group showed the largest difference as compared to the DMEM group. GAG synthesized was higher in the 50 % PRP as compared to the DMEM group after 2 days of culture (Fig. 2b). After 4 days, total GAG content as compared to the DMEM group was significantly increased in the 50 % PL (P < 0.001), 25 % PRP, 10 % FBS, 25 % PL, and 50 % PRP treatment groups.
Fig. 2.
(a) DNA and (b) GAG content of whole samples after 2 and 4 days of cell culture with platelet-rich plasma (25 and 50 % PRP), platelet-lysate preparation (25 and 50 % PL), platelet-poor plasma (50 % PPP), fetal bovine serum (10 % FBS), and DMEM (100 % DMEM). Asterisk indicates a significant difference (P < 0.05) when compared with 100 % DMEM. Mean + SEM, n = 5, triplicates per group
Biochemical parameters were significantly up-regulated at day 4 when compared to day 2 within the treatment groups except in DMEM and PPP groups.
Gene expression analysis
Although collagens I and II and elastin expression showed no difference between the groups, the expression of aggrecan was significantly elevated at day 2 in AF cells treated with 25 % PL when compared with PPP (Fig. 3). Furthermore the expression of versican and decorin showed highest levels after treatment with 25 % PL. Significant up-regulation of versican mRNA was observed after treatment with 25 % PL at day 4, whereas decorin was increased as compared to PPP in the 25 and 50 % PL groups at day 2 and in the 25 % PL group at day 4.
Fig. 3.
Relative mRNA expression of AF cells after 2 and 4 days of culture with platelet-rich plasma (25 and 50 % PRP), platelet-lysate preparation (25 and 50 % PL) and platelet-poor plasma (50 % PPP). (a) collagen type I (COL1), (b) collagen type II (COL2), (c) aggrecan (ACAN), (d) versican (VCAN), (e) biglycan (BGN), and (f) decorin (DCN) are shown. Data were normalized to the values of freshly isolated cells (day 0). Asterisk indicates a significant difference (P < 0.05) as compared to the 50 % PPP group. Mean + SEM, n = 4, triplicates per group
Whole disc model
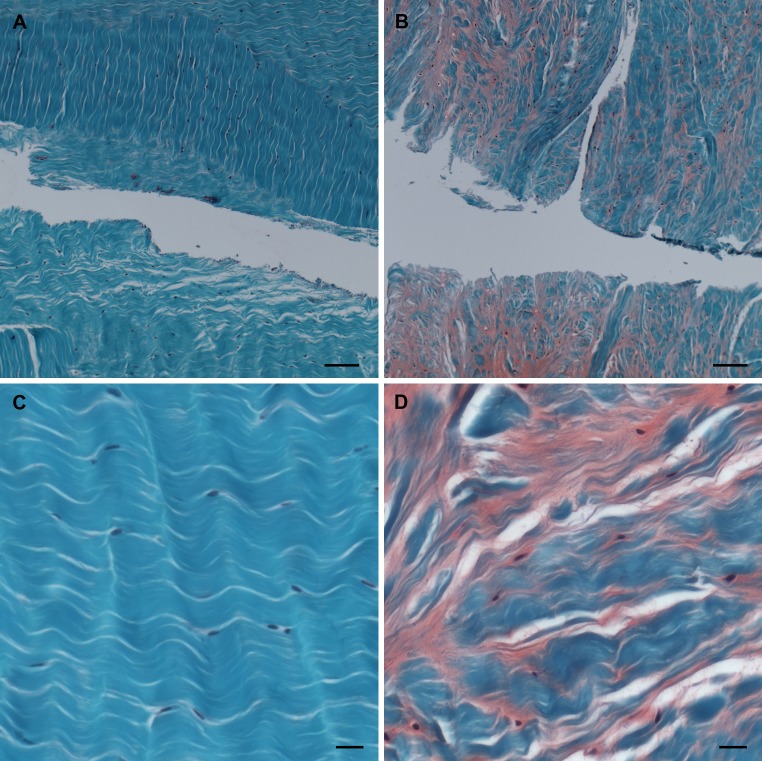
After 7 days of culture, PRP could still be seen attached to the edges of the cut made in the disc. A marked production of GAG was observed in the discs treated with PRP when compared with the disc without PRP (Fig. 4a, b). This was not only limited to the immediate area around the cut, but also within the inner two-thirds of the AF. In the discs treated with PRP, the cells adopted a more spherical morphology (Fig. 4c, d), similar to that described for the in vitro cultures (Fig. 1a, b).
Fig. 4.
Representative images of the whole disc model after Safranin-O staining; scale bar 200 μm. (a) Cut through the AF without addition of PRP (control). (b) Injured AF and injection of PRP. (c) The area adjacent to the cut; sample without PRP. (d) The area adjacent to the cut, after PRP injection
Discussion
By concentrating platelets as in PRP preparations, it is expected that the interplay between diverse GFs shows synergistic effects. In order to evaluate whether the stimulatory effects are influenced by the 3D structure, we added to the AF cells either PRP or PL. This treatment, with all the factors released from the platelets, but without adopting a 3D structure, interestingly increased the cell numbers and GAG concentrations to an extent similar to the treatment with PRP. The gel formation did not significantly reduce the cell growth, although there was a trend towards lower cell numbers in the 50 % PRP group compared to the PL formulation. The PL treated cells showed furthermore higher mRNA expression for specific ECM molecules, notably the AF proteoglycans decorin and versican [25], as compared to PRP cultures. Facilitated binding to the soluble GFs might be one reason for the more pronounced effects of the PL preparations on the gene expression levels.
Although there has been abundant work testing the efficacy of PRP on soft tissue healing, only few studies have evaluated the impact of platelet-rich preparations on the IVD. PRP effectively stimulated cell proliferation, proteoglycan, and collagen synthesis rates, notably in AF cells cultured in alginate [10]. Obata et al. [12] investigated the reparative effects of PRP-releasate after annular puncture in New Zealand White Rabbits. Although reparative effects were demonstrated, PPP showed similar effects in histological analysis, especially in the posterior inner AF and NP. PPP has also been shown to affect cell proliferation and ECM homeostasis similar to FBS in vitro [12]. In our study, 50 % PPP enhanced cell proliferation and ECM synthesis, although to a markedly lower extent than PRP and PL.
In an ex vivo degenerative whole organ culture model using chymopapain, PRP treatment enhanced chondrogenic differentiation and NP tissue formation similar to the normal control [11]. Using a bovine AF defect model, the present study demonstrates reparative effects of PRP in a similar setting. PRP could be delivered directly into the site of the generated defect and jellified within a short time, thus increasing its local effects.
One aspect to consider when comparing different studies is whether exogenous platelet stimulators were used for PRP activation, which markedly affects the release of GFs. A concern is the risk of developing antibodies against exogenously added thrombin and its alternatives [26]. Type I collagen is known to be as effective as thrombin in stimulating the release of GFs and stabilizing the PRP clot [26]. Endogenous activation of platelets is moreover suggested to result in a slower aggregation and more natural release of GFs [27]. Furthermore, activation through freeze–thawing of platelet suspensions is suggested to induce platelet degranulation by hypo-osmotic shock [21]. In the current study, no exogenous activator was used. Platelet activation was accomplished through sonication, freezing and thawing of the preparations [19, 20], and potentially through the endogenous collagen of the AF cell matrix.
Under in vitro culture conditions, AF cells are fragile and tend to lose their specific markers when expanded and passaged [28]. Using freshly isolated cells, we observed a down-regulation in matrix gene expression within all groups during culture. It is likely that during culture the AF cells reduced the expression of their native phenotype when compared with the freshly isolated cells (day 0). Culture under more specific conditions including mechanical load and low oxygen tension may be necessary to fully maintain the phenotype of the disc cells in vitro. Furthermore, there was no significant effect of PRP/PL on the regulation of the collagen gene expression. This finding is consistent with an in vitro study on tendon cells, which also showed no significant differences in collagen gene expression between cells treated with platelet-rich and platelet-poor clot releasates [29]. One reason might be that specific gene expression responses of the AF cells to the platelet preparation may depend on the concentration of the factors present in the formulation. It has been shown previously that only high concentrations of PRP (100 %) up-regulated the gene expression of collagens I and III in short-term tendon explant cultures [30]. Dose-dependent effects of platelet-rich preparations have been reported [15, 27]. Excessive amounts of platelets may, on the other hand, lead to apoptosis, GF receptor down-regulation and desensitization, ultimately resulting in paradoxical inhibitory effects [27]. With our method, we were able to increase the platelet count within the undiluted PRP by 10 times. Even though the in vitro application showed increased cell proliferation rates and ECM molecule synthesis in all PRP and PL groups, gene expression of several matrix molecules was notably highest in the 25 % PL group, which confirms a dose-dependent response.
A recent study also suggests that a polymer of polyglycolic acid combined with hyaluronan is favorable for AF cell re-differentiation [31]. Oriented scaffolding structures have been designed with properties similar to native tissue with the aim to mimic the AF cell environment and therefore support the AF-specific phenotype [3]. An attractive approach for AF repair would consist in the combination of an appropriate scaffold providing a structure and mechanical support and infiltrated PRP or PL to stimulate cell proliferation and new matrix formation. For larger AF defects and to prevent loss or leakage of PRP under load, an effective annulus closure method will be necessary to support and supplement the PRP application ([3] for review). Furthermore, NP replacement or regeneration techniques have been used to facilitate the restoration of the disc’s biomechanical function after discectomy. However, these techniques will only be successful if the AF concurrently maintains its function to withstand the intradiscal pressure [32]. Different attempts have been described to close an AF defect, including sutures with and without sealants, such as fibrin and cyanoacrylate glues [33, 34]. Furthermore, various implants have been used to seal and reinforce the AF defect either by suturing them to the remaining AF or by anchoring them into the adjacent vertebral bone. However, their safety and efficacy remain controversial. Effective AF repair strategies need to withstand the high tensile hoop stresses originating from NP pressurization and tensile and compressive stresses resulting from spinal motion. Recent data have demonstrated the potential and limitations of in vitro biomechanical tests for evaluation of AF closure devices [35], and it is suggested that purely mechanical repair may often not be sufficient. Currently, novel biomaterial-based approaches for AF repair are under investigation in in vitro, organ culture and biomechanical studies [3, 36–38]. Thus, combination of a biomaterial-based annular repair system or annular closure device with an anabolic stimulus, such as PRP or PL may represent a promising strategy for more effective AF repair.
This study demonstrates the feasibility of PRP/PL delivery in vitro and in a short-term organ culture. Immediate effects of PRP or PL have previously been described in alginate cultures of IVD cells [10], in fibroblasts and osteoblasts [39], tenocytes [30], and co-cultures of endothelial progenitor cells and mesenchymal stem cells [40]. Taken together, these results suggest a positive influence of PRP/PL on the initial healing response of an AF defect. Nevertheless, the data do not allow drawing conclusions with respect to a sustained repair effect. Long-term experiments, carried out in a bioreactor system under relevant mechanical loading conditions [41], will reveal continued effects of PRP/PL on AF regeneration. Ultimately, while in vitro and organ culture experiments provide important insight into the principal effects of PRP and PL preparations, large animal models with biomechanical properties similar to the human will be required to assess their performance in a preclinical situation. Annular defect studies have been described in sheep [42–44], mini-pig [45, 46], and goat [47] models. Such investigations will be supportive to evaluate the effectiveness of platelet preparations, and their combinations with annular closure procedures, for sustained AF restoration and prevention of disc herniation.
Conclusions
The present findings indicate that both PRP and PL have proliferative effects on AF cells and are able to increase ECM production in vitro. PRP supplementation created a gel-like structure, which affected the morphology of the AF cells, but had no major influence on the cell phenotype in short time culture. Platelet-rich preparations may therefore be considered to promote AF repair. For direct delivery to the defect site, the immediate formation of a stable gel is a clear advantage of PRP compared to PL, minimizing the risk of leakage from the defect site. PL may be considered for AF repair in combination with an AF closure device or material. Long term and preclinical studies will be needed to assess the therapeutic potential of platelet preparations to restore functional AF tissue.
Conflict of interest
None.
Footnotes
T. N. Pirvu and J. E. Schroeder contributed equally to this work.
References
- 1.Videman T, Nurminen M (2004) The occurrence of annular tears and their relation to lifetime back pain history: a cadaveric study using barium sulfate discography. Spine (Phila Pa 1976) 29:2668–2676 [DOI] [PubMed]
- 2.Loreto C, Musumeci G, Castorina A, Loreto C, Martinez G. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin-positive cells and cell death. Ann Anat. 2011;193:156–162. doi: 10.1016/j.aanat.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Guterl CC, See EY, Blanquer SB, Pandit A, Ferguson SJ, Benneker LM, Grijpma DW, Sakai D, Eglin D, Alini M, Iatridis JC, Grad S. Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013;25:1–21. doi: 10.22203/ecm.v025a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(Suppl 4):441–451. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–456. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Lee JU, Moon SH, Kim HC, Kwon UH, Seol NH, Kim HJ, Park JO, Chun HJ, Kwon IK, Lee HM (2009) Zonal responsiveness of the human intervertebral disc to bone morphogenetic protein-2. Spine (Phila Pa 1976) 34:1834–1838 [DOI] [PubMed]
- 7.Takegami K, An HS, Kumano F, Chiba K, Thonar EJ, Singh K, Masuda K. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced in vitro chemonucleolysis. Spine J. 2005;5:231–238. doi: 10.1016/j.spinee.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 9.Hegewald AA, Zouhair S, Endres M, Cabraja M, Woiciechowsky C, Thome C, Kaps C. Towards biological annulus repair: TGF-beta3, FGF-2 and human serum support matrix formation by human annulus fibrosus cells. Tissue Cell. 2013;45:68–76. doi: 10.1016/j.tice.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K (2006) Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and annulus fibrosus cells cultured in alginate beads. Spine (Phila Pa 1976) 31:959–966 [DOI] [PubMed]
- 11.Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ, Deng WP. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30:5523–5533. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Obata S, Akeda K, Imanishi T, Masuda K, Bae W, Morimoto R, Asanuma Y, Kasai Y, Uchida A, Sudo A. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit annular puncture model: a preclinical study. Arthritis Res Ther. 2012;14:R241. doi: 10.1186/ar4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagae M, Ikeda T, Mikami Y, Hase H, Ozawa H, Matsuda K, Sakamoto H, Tabata Y, Kawata M, Kubo T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007;13:147–158. doi: 10.1089/ten.2006.0042. [DOI] [PubMed] [Google Scholar]
- 14.Sawamura K, Ikeda T, Nagae M, Okamoto S, Mikami Y, Hase H, Ikoma K, Yamada T, Sakamoto H, Matsuda K, Tabata Y, Kawata M, Kubo T. Characterization of in vivo effects of platelet-rich plasma and biodegradable gelatin hydrogel microspheres on degenerated intervertebral discs. Tissue Eng Part A. 2009;15:3719–3727. doi: 10.1089/ten.tea.2008.0697. [DOI] [PubMed] [Google Scholar]
- 15.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269–278. doi: 10.1016/j.arthro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Paoloni J, De Vos RJ, Hamilton B, Murrell GA, Orchard J. Platelet-rich plasma treatment for ligament and tendon injuries. Clin J Sport Med. 2011;21:37–45. doi: 10.1097/JSM.0b013e31820758c7. [DOI] [PubMed] [Google Scholar]
- 18.Cieslik-Bielecka A, Choukroun J, Odin G, Dohan Ehrenfest DM. L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr Pharm Biotechnol. 2012;13:1266–1277. doi: 10.2174/138920112800624463. [DOI] [PubMed] [Google Scholar]
- 19.Verrier S, Meury TR, Kupcsik L, Heini P, Stoll T, Alini M. Platelet-released supernatant induces osteoblastic differentiation of human mesenchymal stem cells: potential role of BMP-2. Eur Cell Mater. 2010;20:403–414. doi: 10.22203/ecm.v020a33. [DOI] [PubMed] [Google Scholar]
- 20.Lippross S, Loibl M, Hoppe S, Meury T, Benneker L, Alini M, Verrier S. Platelet released growth factors boost expansion of bone marrow derived CD34(+) and CD133(+) endothelial progenitor cells for autologous grafting. Platelets. 2011;22:422–432. doi: 10.3109/09537104.2011.559559. [DOI] [PubMed] [Google Scholar]
- 21.Rauch C, Feifel E, Amann EM, Spotl HP, Schennach H, Pfaller W, Gstraunthaler G. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE, Norton HJ, Hanley EN Jr (2000) Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine (Phila Pa 1976) 25:2153–2157 [DOI] [PubMed]
- 23.Zhao CQ, Liu D, Li H, Jiang LS, Dai LY. Interleukin-1beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis. 2007;12:2155–2161. doi: 10.1007/s10495-007-0137-x. [DOI] [PubMed] [Google Scholar]
- 24.Illien-Junger S, Pattappa G, Peroglio M, Benneker LM, Stoddart MJ, Sakai D, Mochida J, Grad S, Alini M (2012) Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine (Phila Pa 1976) 37:1865–1873 [DOI] [PubMed]
- 25.Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28:998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 28.Chou AI, Reza AT, Nicoll SB. Distinct intervertebral disc cell populations adopt similar phenotypes in three-dimensional culture. Tissue Eng Part A. 2008;14:2079–2087. doi: 10.1089/ten.tea.2007.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 30.Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 31.Cabraja M, Endres M, Hegewald AA, Vetterlein S, Thome C, Woiciechowsky C, Kaps C. A 3D environment for annulus fibrosus regeneration. J Neurosurg Spine. 2012;17:177–183. doi: 10.3171/2012.4.SPINE111095. [DOI] [PubMed] [Google Scholar]
- 32.Veres SP, Robertson PA, Broom ND (2008) ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine (Phila Pa 1976) 33:2711–2720 [DOI] [PubMed]
- 33.Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP (2000) Effect of annular repair on the healing strength of the intervertebral disc: a sheep model. Spine (Phila Pa 1976) 25:2165–2170 [DOI] [PubMed]
- 34.Heuer F, Ulrich S, Claes L, Wilke HJ. Biomechanical evaluation of conventional annulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J Neurosurg Spine. 2008;9:307–313. doi: 10.3171/SPI/2008/9/9/307. [DOI] [PubMed] [Google Scholar]
- 35.Bron JL, van der Veen AJ, Helder MN, van Royen BJ, Smit TH. Biomechanical and in vivo evaluation of experimental closure devices of the annulus fibrosus designed for a goat nucleus replacement model. Eur Spine J. 2010;19:1347–1355. doi: 10.1007/s00586-010-1384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wismer N, Grad S, Fortunato G, Ferguson SJ, Alini M, Eglin D (2013) Biodegradable electrospun scaffolds for annulus fibrosus tissue engineering: effect of scaffold structure and composition on annulus fibrosus cells in vitro. Tissue Eng Part A [DOI] [PubMed]
- 37.Nerurkar NL, Baker BM, Sen S, Wible EE, Elliott DM, Mauck RL. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat Mater. 2009;8:986–992. doi: 10.1038/nmat2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bron JL, Helder MN, Meisel HJ, van Royen BJ, Smit TH. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18:301–313. doi: 10.1007/s00586-008-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res. 2006;17:212–219. doi: 10.1111/j.1600-0501.2005.01203.x. [DOI] [PubMed] [Google Scholar]
- 40.Duttenhoefer F, de Lara FR, Meury T, Loibl M, Benneker LM, Richards RG, Alini M, Verrier S. 3D scaffolds co-seeded with human endothelial progenitor and mesenchymal stem cells: evidence of prevascularisation within 7 days. Eur Cell Mater. 2013;26:49–64. doi: 10.22203/ecm.v026a04. [DOI] [PubMed] [Google Scholar]
- 41.Illien-Junger S, Gantenbein-Ritter B, Grad S, Lezuo P, Ferguson SJ, Alini M, Ito K (2010) The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 35:1744–1752 [DOI] [PubMed]
- 42.Ledet EH, Jeshuran W, Glennon JC, Shaffrey C, De DP, Belden C, Kallakury B, Carl AL (2009) Small intestinal submucosa for annular defect closure: long-term response in an in vivo sheep model. Spine (Phila Pa 1976) 34:1457–1463 [DOI] [PubMed]
- 43.Osti OL, Vernon-Roberts B, Fraser RD (1990) 1990 Volvo Award in experimental studies. Annulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 15:762–767 [DOI] [PubMed]
- 44.Melrose J, Smith SM, Little CB, Moore RJ, Vernon-Roberts B, Fraser RD. Recent advances in annular pathobiology provide insights into rim-lesion mediated intervertebral disc degeneration and potential new approaches to annular repair strategies. Eur Spine J. 2008;17:1131–1148. doi: 10.1007/s00586-008-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YH, Kuo TF, Wang JL. The implantation of non-cell-based materials to prevent the recurrent disc herniation: an in vivo porcine model using quantitative discomanometry examination. Eur Spine J. 2007;16:1021–1027. doi: 10.1007/s00586-007-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon SH, Miyazaki M, Hong SW, Tow B, Morishita Y, Hu M, Ahn SJ, Wang JC. A porcine model of intervertebral disc degeneration induced by annular injury characterized with magnetic resonance imaging and histopathological findings. Laboratory investigation. J Neurosurg Spine. 2008;8:450–457. doi: 10.3171/SPI/2008/8/5/450. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Drapeau S, An HS, Markova D, Lenart BA, Anderson DG (2011) Histological features of the degenerating intervertebral disc in a goat disc-injury model. Spine (Phila Pa 1976) 36:1519–1527 [DOI] [PMC free article] [PubMed]