Abstract
Purpose
Tests on animals of different species with large differences in intervertebral disc size are commonly used to investigate the therapeutic efficacy of intravenously injected solutes in the disc. We hypothesize that disc size markedly affects outcome.
Methods
Here, using a small non-metabolized molecule, glucosamine (GL) as a model solute, we calculate the influence of disc size on transport of GL into rat, rabbit, dog and human discs for 10 h post intravenous-injection. We used transient finite element models and considered an identical GL supply for all animals.
Results
Huge effects of disc size on GL concentration profiles were found. Post-injection GL concentration in the rat disc reached 70 % blood concentration within 15 min but remained below 10 % in the human disc nucleus throughout. The GL rapidly penetrated post-injection into smaller discs resulting in homogeneous concentrations. In contrast, GL concentration, albeit at much lower levels, increased with time in the human disc with a small outward flux at the annulus periphery at longer periods.
Conclusions
Changes in the disc size hugely influenced GL concentrations throughout the disc at all regions and times. Increases in administered dose can neither remedy the very low concentration levels in the disc center in larger human disc at early post-injection hours nor alter the substantial differences in concentration profiles estimated among various species. The size effect will only be exacerbated as molecular weight of the solute increases and as the endplate calcifies. Extrapolation of findings from animal to human discs on the efficacy of intravenously administered solutes must proceed with great caution.
Keywords: Intervertebral disc, Diffusion, Drug, Animal models, Pharmacokinetics
Introduction
Intervertebral discs of various species are routinely used in vivo to investigate the therapeutic efficacy of different treatment modalities following intravenous injection. Pharmacokinetics of various solutes have been examined in a wide range of species from animals as large as horses [1, 2] to sheep [3, 4], dogs [5, 6], rabbits [7–9] and finally to animals as small as rats and mice [10, 11]. Apart from inherent inter- and intra-species differences in cell type, tissue composition and mechanical properties, there is a huge difference between the disc sizes among these species. For example, the mean disc total cross-sectional area and height vary respectively from 1,727 mm2 and 11.3 mm in humans to 73.4 mm2 and 1.42 mm in rabbits and to as low as 1.19 mm2 and 0.24 mm in mice tails [12], exhibiting two to three order differences in scale.
Solutes of molecular weights up to around 1–2 kDa, encompassing solutes such as drugs, gadolinium compounds as well as nutrients and metabolites are transported from the blood supply to the disc center mainly by diffusion as shown both by experiment and by modeling [13–16]. Animal studies are typically used to test and characterize effects of such solutes on disc behavior [3, 5]. In a recent study on the transport of solutes into a human lumbar intervertebral disc after intravenous injection, despite identical serum profiles, substantial differences in concentrations across the disc regions were computed when disc geometry was altered only by ±10 and ±20 % [17]; as shown above, size differences between human and animal discs are vastly greater than 20 %. We hypothesized that substantial differences in concentration profiles would arise because of inter-species differences in disc size and this study investigates the magnitude of these differences.
Here we have carried out a computational study on glucosamine (GL), having shown previously that computations can accurately predict experimental results in animals and man [17–19]. We have chosen GL as a model solute as it is a small thus its transport through the disc is mainly by diffusion [13, 15] and it is uncharged. Its rate of uptake by the cells is also very slow [20] and thus effects of cellular metabolism on concentrations in the disc are minimal. Moreover, it is a solute of interest as it is widely taken around the world as a dietary supplement for prevention and treatment of osteoarthritis (OA) though its effects are controversial [21]. In vitro it appears to exert chondroprotective effects, upregulating proteoglycan synthesis and inhibiting activity of agents involved in tissue degradation [22]. Achievement of therapeutic levels in the disc is more of challenge than into the articular joints as the discs are considerably thicker than articular cartilages. Moreover, the discs have no free surface in contact with synovial fluid and when small molecules such as GL are injected intravenously into the blood stream, they diffuse from blood vessels at the endplates and outer annulus periphery into discs with the transport being affected by pathological alterations to these pathways. Nevertheless, there is some interest in use of GL for treatment of back pain and disc degeneration [23, 24].
Here we quantified the influence of inter-species variations in disc size on GL concentration profiles within the disc for 10 h following an intravenous injection using a transient finite element (FE) model. We calculated and compared GL penetration time-histories into the rat, rabbit, dog and human lumbar discs. The time-dependent concentration profiles at the surface of the disc, arising from the decay of GL concentration in the serum post-injection, were taken as identical for all species based on values reported in the literature [1, 5, 11, 25]. We hypothesized that substantial differences in concentration profiles would arise due to inter-species differences in disc size and that these differences would persist despite alterations in administered doses.
Methods
Finite element model
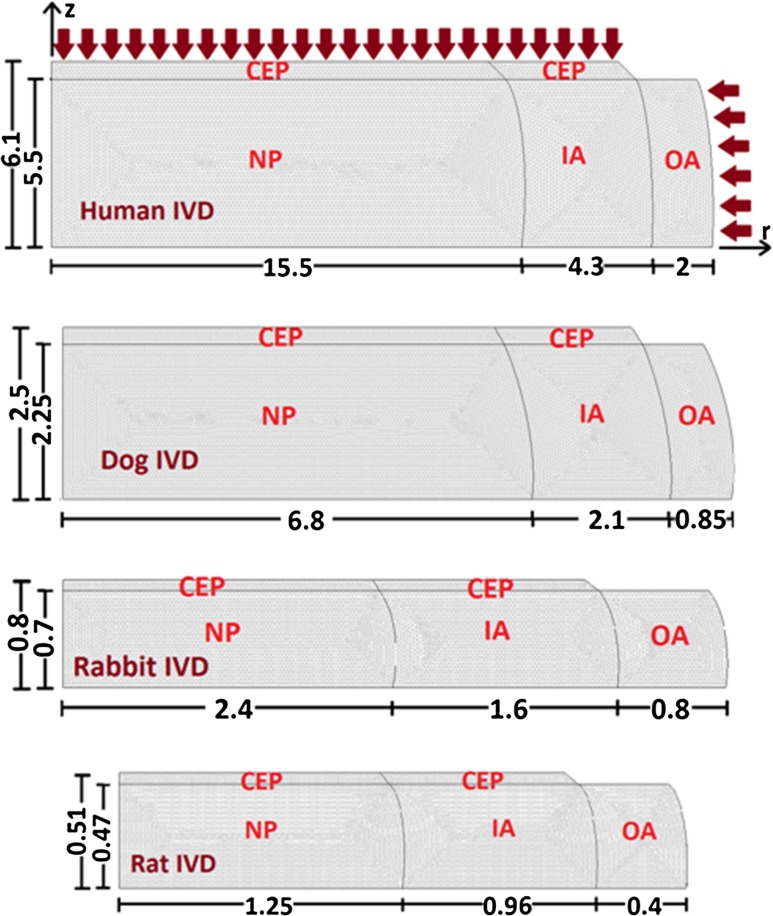
Each disc, presented as an axisymmetric model, is divided into four regions (nucleus NP, inner annulus IA, outer annulus OA and cartilaginous endplate CEP) with distinct properties. For the human disc, a L2–L3 lumbar disc is considered [26] (Fig. 1a). Dimensions for the dog, rabbit and rat discs are taken from the literature assuming similar IA/OA and relative CEP thickness values (Fig. 1b–d) [12]. In cylindrical coordinates, the species transient diffusion equation is governed by Fick’s law expressed, neglecting convection, as:
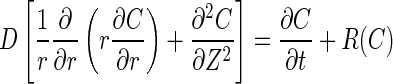 |
1 |
where R is the production or consumption rate, C is GL concentration in mg/L and D is GL diffusivity in m2/h while r and z are radial and axial coordinates. The rate of consumption of GL by cartilaginous cells, R, is very low (~10−11 mmol/h/patella in mice) [20] and hence is neglected here. The diffusion coefficient of GL, in the NP of humans was taken to be equivalent to that of glucose as these solutes have very similar molecular weights. Because of the notochordal cells and associated higher water content in NP of discs of the non-chondrodystrophoid dogs, rabbits and rats as compared to the human discs, D was calculated to be higher in these animal discs than in human discs (Table 1) [17].
Fig. 1.
Axisymmetric finite element models of species intervertebral discs, from top to bottom, human, dog, rabbit and rat consisting of 4 distinct regions with different properties: nucleus (NP), inner annulus (IA), outer annulus (OA) and cartilaginous endplate (CEP). Temporal variation of source supplies via blood vessels is prescribed at the outer annulus periphery and CEP as depicted by arrows for the human disc. The symmetry about the disc mid-horizontal plane is considered. Dimensions are all in mm
Table 1.
GL Diffusion coefficient, D for different species and disc regions
| D (e−10 m2/s) | ||
|---|---|---|
| Disc region | Human | Dog, rabbit, rat |
| NP | 2.5 | 3.75* |
| IA | 2.08 | 2.08 |
| OA | 1.7 | 1.7 |
| CEP | 1.4 | 1.4 |
* Calculated from [35] taking into account the high water content of notochordal discs of these animals
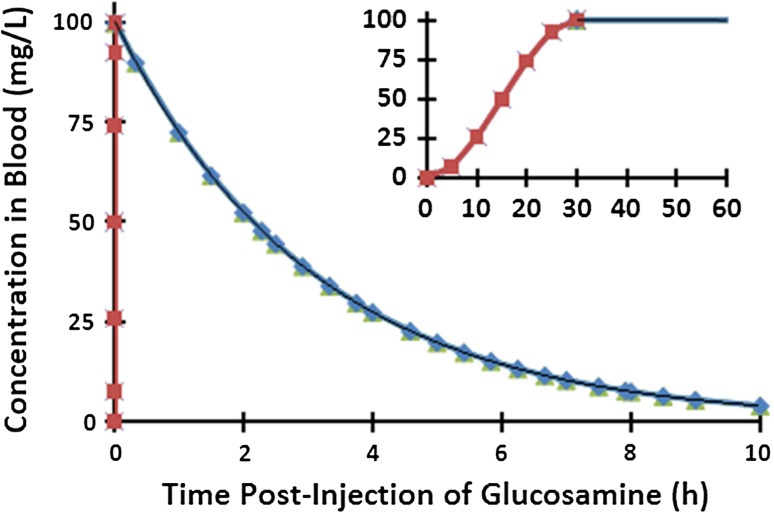
The normalized transient input supply source via the blood stream at the disc CEP and OA boundaries (Fig. 1) is taken based on measurements as identical for all discs [1, 5, 11]. The intravenous administration of GL in rats and horses at 10–20 mg/Kg dose [1, 11] and at 500 mg single dose in beagle dogs [5] resulted in nearly similar plasma GL concentration profiles assumed here. The simulated concentration profile in the current work reaches its peak of 100 mg/L at 30 s post injection and drops thereafter to 4 mg/L at 10 h (Fig. 2).
Fig. 2.
Changes in glucosamine concentration with time at the disc boundary in all 4 species. The inset figure shows the initial concentration in the first 60 s post-injection
The blood-disc partition coefficient is taken in all models as 0.81 at the CEP boundary and 0.89 at the OA periphery [17, 26], reducing thus the prescribed source at the disc boundary shown in Fig. 2. The COMSOL FE package program is used to solve the present transient analyses (COMSOL, Inc., Burlington, MA, USA). After preliminary convergence studies using triangular elements with quadratic concentration field, meshes with about 33,000 elements in human disc, 22,300 in dog disc, 13,000 in rabbit disc and 16,000 in rat disc and a time increment of 1 s are used.
Since one of GL penetration routes is via the blood capillaries at the endplates, any disruption in the CEP exchange area alter its permeability and solute transport throughout the disc. In the current study, the CEP exchange area (Fig. 1) is taken either as 100 % (an idealized completely permeable interface) or 50 % (partially permeable, equivalent to that in a healthy animal [15] while that above the OA remains impermeable. The OA periphery is taken completely permeable in all cases [15].
Results
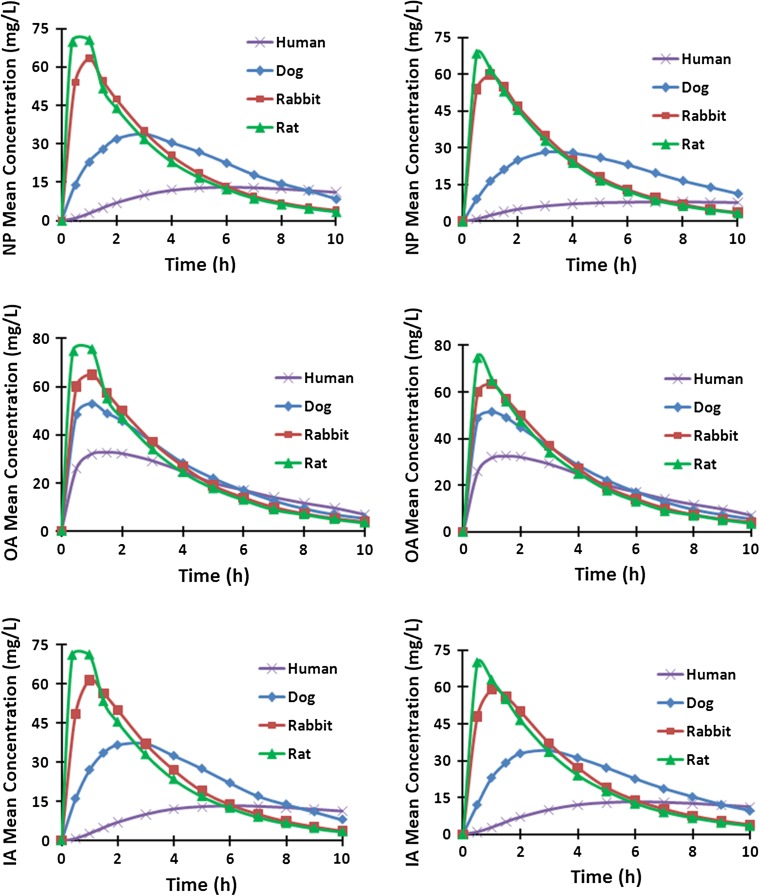
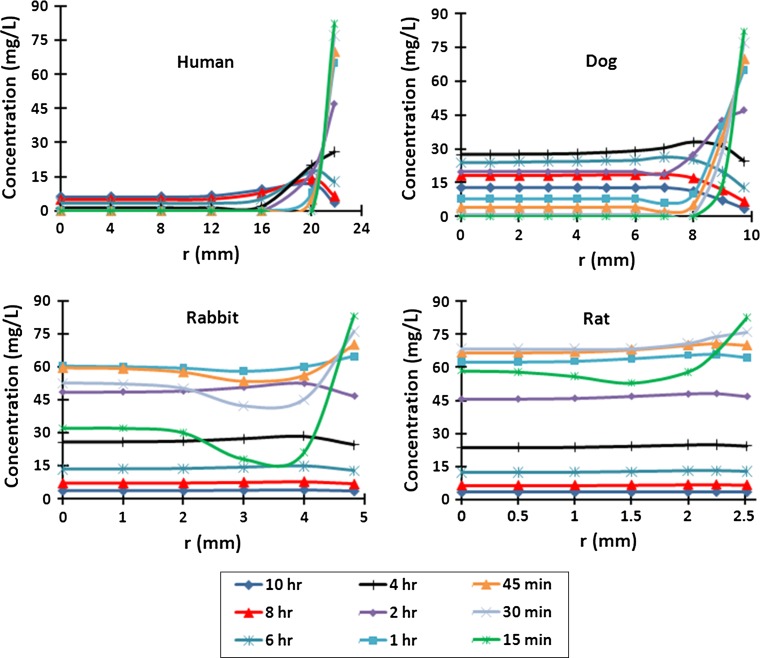
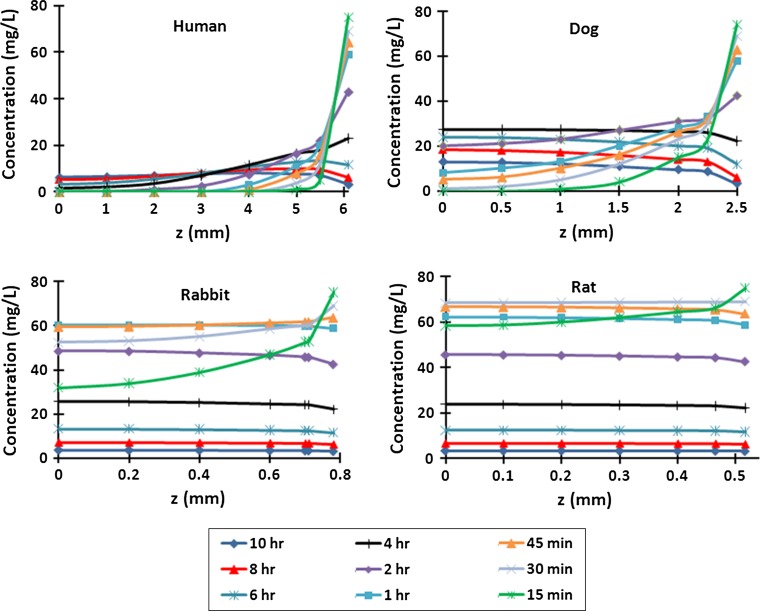
Under identical concentration decay curves at boundaries (Fig. 2), mean GL concentrations increased sharply in the rat disc in all regions post injection but then by 1–2 h began to fall up to 10 h post-injection following a trend similar to that of boundary concentrations (Fig. 3). This trend however was considerably dampened in larger discs and even disappeared in the human disc in the NP and IA (Fig. 3). The concentration profiles along the disc radius showed that GL penetrated through the rat and rabbit discs relatively quickly and thereafter equilibrated with concentrations in the serum; concentrations remained almost constant across the disc after the first 15 min in the rat disc and 45 min in the rabbit disc, although decreasing as concentrations in the blood decayed (Fig. 4). However, GL did not equilibrate across the larger discs within 10 h. Much lower concentrations relative to that in the serum and with greater gradients across the disc, were computed in dog (maximum 28 g/L at 4 h) and human discs (maximum 7 g/L at >7 h). Nearly similar variations were computed along the disc axis of symmetry (r = 0) (Fig. 5).
Fig. 3.
Mean Concentrations in human, dog, rabbit and rat disc regions for different CEP exchange areas; (left) 100 %, (right) 50 %
Fig. 4.
Concentration profiles along the midplane of the disc radius (z = 0) at 15 min—10 h for rat, rabbit, dog and human discs assuming 50 % CEP
Fig. 5.
Concentration profiles through the depth of the disc in the disc center (r = 0) at 15 min—10 h for rat, rabbit, dog and human discs, assuming 50 % CEP
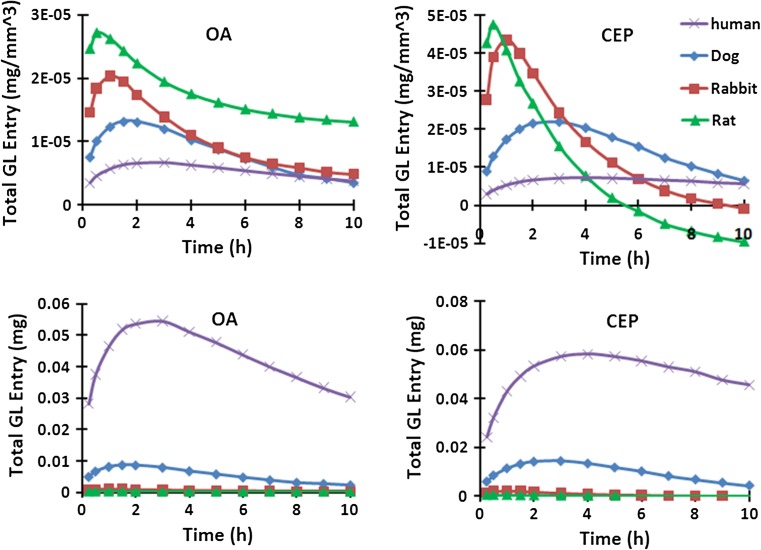
When normalized to the corresponding disc volume, the initial post-injection penetrations into the smaller discs were much larger (Fig. 6). This trend however reversed with time especially at the CEP boundary where the inward flux dropped more rapidly in smaller discs. Via the OA periphery, the balance of GL that had entered into the disc remained always positive even though the flux changed direction to become outward at about 30 min in the rat disc, 60 min in the rabbit disc, 90 min in the dog disc and 2 h in the human disc. Via the CEP, however, the total GL entry became negative after 5 h in the rat disc and 9 h in the rabbit disc.
Fig. 6.
Total (integrated over time) glucosamine entered from each source supply (50 % CEP condition, top: normalized over its disc volume for each case)
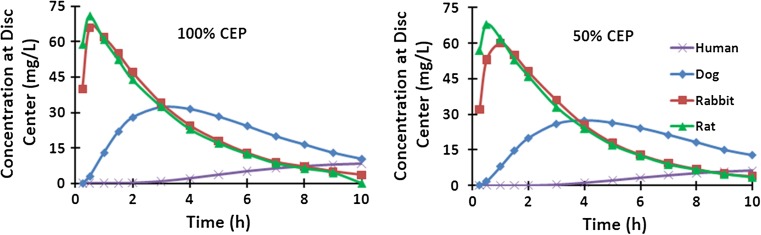
The disc center is farthest away from the source supplies at boundaries and is hence the most critical point. There was a huge difference in GL concentrations at the disc center during the early hours post-injection between smaller rat and rabbit discs and larger dog and human discs (Fig. 7). After the early peak at about 30 min, the concentration sharply dropped in rat and rabbit discs following the trend in source supplies. The GL concentration in the human disc, albeit small, increased throughout the 10 h period. Overall, the change in CEP exchange area had a small effect on results in smaller rat and rabbit discs but decreased the concentrations in the disc center in dogs and humans.
Fig. 7.
Changes in concentration with time at the disc center (r = z = 0) for rat, rabbit, dog and human discs with different endplate permeabilities (100 versus 50 % CEP)
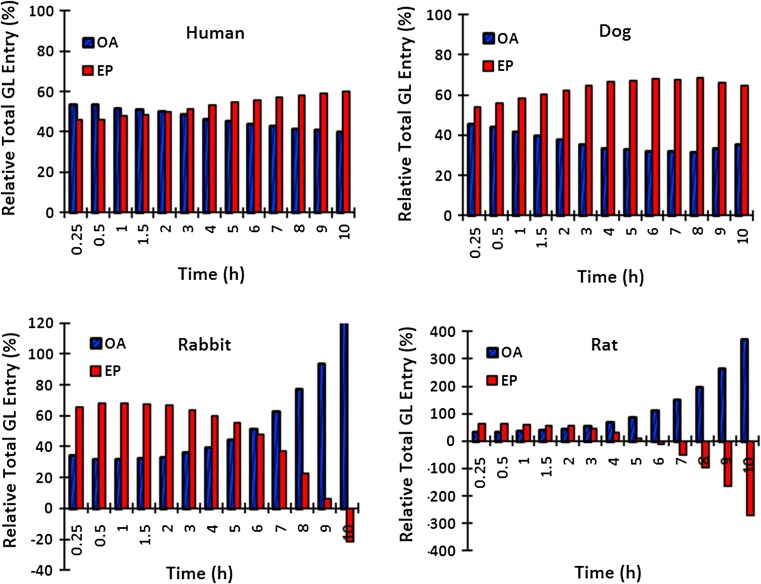
In the human disc, the CEP pathway played a more important role later in time (Fig. 8). In contrast, it was far more important in the earlier few hours in smaller rat and rabbit discs; disc is supplied and nourished primarily through the endplates early after intravenous injection in small discs. The same pathway, however, depletes the solute later on after about 8 h post injection time when the boundary sources have diminished. The endplate route played a more important role at all times in the dog disc (Fig. 8). Results here are influenced by the ratio of radius over height that is much larger in the rat and rabbit discs compared to that in the dog and human discs.
Fig. 8.
Percentage of total diffused GL via OA and CEP pathways to the total GL in the disc for the case with 50 % CEP
Due to the water content of the NP being greater in animal than in human discs, the GL diffusion coefficient was much larger in the NP of the animal discs than in the human NP (Table 1). To investigate the relative effect of this coefficient as compared to the disc size in the human disc, an analysis was performed assuming an NP diffusion coefficient of 3.75 e−10 m2/s (Table 1). The critical GL concentration in NP, IA and OA of the human disc increased however only by 10.5, 1.5 and 0.3 % suggesting that the significantly lower GL concentrations in the human disc are due primarily to the differences in the disc size rather than a lower NP diffusivity.
Discussion
Here we examined the effect of disc size on transport of GL into the disc following an intravenous injection calculated to provide an identical GL supply for the human, dog, rabbit and rat discs, as achieved in vivo by dosing animals according to body weight [1, 5, 11]. Results demonstrate the huge effect of inter-species changes in the disc size on GL concentration profiles. Even though concentrations of GL at the disc boundary were identical for both rat and human for example, for the rat, the GL concentrations all across the disc reached about 70 % of maximum concentrations in the blood supply within 15 min of injection (Fig. 3), whereas in human discs, the GL concentrations were below 10 % in the NP up to 10 h post-injection. Thus while other factors which affect diffusivity such as age or matrix degeneration influence transport, disc size has an overriding effect on rates of solute transport into the disc from the external blood supply.
The time-history as well as the concentrations were very different in the discs of different sizes. GL concentrations reached their peak in different disc regions at much greater values and much earlier times in the rat and rabbit discs followed by the dog disc and finally the human disc (Fig. 3). In the disc center, the most critical location being farthest away from the GL supply (Fig. 7), the peak concentrations occurred at 30 min, 1 and 4 h in rat, rabbit and dog discs while in the human disc the concentration, albeit at much lower magnitudes, continued to increase up to 10 h post-injection. The much smaller rat and rabbit discs saturated rapidly showing constant GL concentrations throughout the disc. These homogeneous concentration fields however fell with time and followed the decay in supply. At the other extreme, the GL concentrations in human and dog discs showed large variations in IA and OA at different times. Initially they were higher in the OA than the NP, but with time and decay in supply GL concentrations in the NP exceeded those in the serum, outer annulus periphery and the CEP interface. The time at which the flux reversed was much earlier in the rat (30 min) and rabbit (45 min) discs than in the dog (2 h) and human (4 h) discs (Fig. 6).
There are factors other than size affecting transport. Reduction in endplate permeability markedly influences transport into the disc [14, 17, 19]; here we only examined the idealized case of 100 % CEP permeability and more physiological 50 % CEP permeability. Around 50 %, CEP permeability likely represents a normal young endplate in accordance with our earlier works which found no cell death with CEP permeabilities at and above 40 % permeability [19] and with experimental measurements [15]. On the other hand, the 100 % CEP condition is an idealized model and considered only to demonstrate the trends. The concentrations at the disc center decreased very little in rat (by 4 %) and rabbit (by 9 %) discs, but fall in CEP permeability to 50 % reduced GL levels by 15 and 24 %, respectively in dog and human discs (Fig. 7). The much larger fall in GL concentrations predicted in the larger dog and human discs when the CEP was lowered from 100 to 50 % highlights the crucial, more pronounced, role of CEP calcification and other endplate irregularities on penetration of solutes from the blood supply into larger discs. Deformation due to mechanical loading, not considered in this study, could also influence transport rates though to far smaller extents than those found here due to changes in disc size. Few hours of static loading have been found in vivo to inhibit transport [27]. Model studies of transport accounting for solute convection generally indicate minor effects in static compression [13, 28–31] but either negligible [29] or moderate [30, 31] effects under cyclic compression loads.
As the same concentration of a drug or agent in the serum results in very different concentration–time courses in different animals, transferring results of in vivo tests on animals to man is problematic in studies such as those on antibiotic penetration into discs. Concentrations of tobramycin and clindamycin (both positively charged) in the nucleus of rabbits dosed intravenously in vivo, exceeded 50 % serum concentrations [7] in line with results shown here (Figs. 4, 5). However, in the larger sheep discs, concentrations of cephazolin (negatively charged) were consistently below therapeutic values [3, 4]. While the charge of solutes is clearly important [15, 17] negatively charged solutes are not completely excluded from the disc, rather their concentration will be around 30 % lower than the equivalent neutral solutes [32]. The low concentrations seen in the sheep and even lower concentrations found in human NP [3] are mainly because of disc size rather than solute charge.
Transport of agents other than drugs into the disc from the blood supply is now also of interest because of the potential danger arising from injecting directly into the disc itself [26]. The results of this study thus highlight the substantial effect of species disc size on transport of solutes from the blood supply. The vastly different concentration histories could lead to substantial differences in cellular activities of bioactive molecules and, in support of earlier warnings [33], cast doubt on attempts to extrapolate findings of studies on animals with much smaller discs, to human discs.
It should be noted that size differences between human discs of different ages and at different spinal levels also influence penetration of drugs or other agents into the disc. The same concentration of drug in the serum will produce much higher concentrations in the smaller thin cervical discs than in the larger lumbar discs, or in discs of children relative to those of adults. In this regard we have shown that even moderate changes in the human lumbar disc size of ±10 and ±20 % substantially influenced the cephazolin penetration at all times everywhere in the disc [17]. Changes in the disc occurring as the result of degeneration, such as loss of endplate permeability [34] and matrix compaction as the result of aggrecan loss [29], will further exacerbate effect of disc size on concentrations of solute reaching the disc from the blood supply. In very degenerate discs, with vascular invasion [34], solute transport could be very rapid leading to very different concentration levels and time-courses than those following diffusion laws. These size and degeneration differences need to be taken into account when designing drug regimens for the disc.
We used identical source supply at EP and outer AF boundaries for the human, dog, rabbit and rat discs (Fig. 2) in accordance with post-intravenous injection measurement of GL concentration in plasma in studies using doses proportional to body weight [1, 5, 11]. Due to the linear relationship between the source input and concentration values, any changes in administered dose are expected to proportionally shift up or down the computed concentrations at all times. The temporal trends would nevertheless remain unchanged irrespective of the dose. In other words, any increase in the GL dose in larger human disc will not alter the concentration profiles to look alike those in smaller rat discs. As an example, huge increase in dose is needed to yield even noticeable concentrations in the early post-injection hours at the NP center in the human disc (Fig. 7). For this reason, changes in the administered dose by itself cannot remedy the very low concentrations found in larger discs.
In summary, we computed the effects of disc size on penetration of GL from the blood stream into the disc. We ignored some factors such as mechanical stress, as the aim was to compare effects of disc size only, under identical boundary conditions. Our calculations demonstrate that changes in the disc size hugely influenced the GL concentrations throughout the disc at all regions and times. Increases in administered dose can neither remedy the very low concentration levels computed in the disc NP in larger human disc especially at early post-injection hours nor alter the substantial differences in concentration profiles estimated among various species. While we only examined the influence of size on a small molecule, GL, the size effect will only be exacerbated as molecular weight of the solute increases and as the endplate calcifies. These results call for caution when attempting to extrapolate findings on discs of different animals to the human discs.
Acknowledgments
The current study was supported in part by the NSERC-Canada and the European Community’s Seventh Framework Programme (FP7, 2007-2013) under Grant agreement no. HEALTH-F2-2008-201626.
Conflict of interest
None.
References
- 1.Laverty S, Sandy JD, Celeste C, Vachon P, Marier JF, Plaas AH. Synovial fluid levels and serum pharmacokinetics in a large animal model following treatment with oral glucosamine at clinically relevant doses. Arthr Rheum. 2005;52:181–191. doi: 10.1002/art.20762. [DOI] [PubMed] [Google Scholar]
- 2.Pinto N, Schumacher J, Taintor J, Degraves F, Duran S, Boothe D. Pharmacokinetics of amikacin in plasma and selected body fluids of healthy horses after a single intravenous dose. Equine Vet J. 2011;43:112–116. doi: 10.1111/j.2042-3306.2010.00144.x. [DOI] [PubMed] [Google Scholar]
- 3.Walters R, Rahmat R, Fraser R, Moore R. Preventing and treating discitis: cephazolin penetration in ovine lumbar intervertebral disc. Eur Spine J. 2006;15:1397–1403. doi: 10.1007/s00586-006-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters R, Moore R, Fraser R. Penetration of cephazolin in human lumbar intervertebral disc. Spine. 2006;31:567–570. doi: 10.1097/01.brs.0000201244.24003.2d. [DOI] [PubMed] [Google Scholar]
- 5.Adebowale A, Du J, Liang Z, Leslie JL, Eddington ND. The bioavailability and pharmacokinetics of glucosamine hydrochloride and low molecular weight chondroitin sulfate after single and multiple doses to beagle dogs. Biopharm Drug Dispos. 2002;23:217–225. doi: 10.1002/bdd.315. [DOI] [PubMed] [Google Scholar]
- 6.Freeman AC, Platt SR, Kent M, Howerth E, Holmes SP. Magnetic resonance imaging enhancement of intervertebral disc disease in 30 dogs following chemical fat saturation. J Small Anim Pract. 2012;53(2):120–125. doi: 10.1111/j.1748-5827.2011.01174.x. [DOI] [PubMed] [Google Scholar]
- 7.Eismont FJ, Wiesel SW, Brighton CT, Rothman RH (1987) Antibiotic penetration into rabbit nucleus pulposus. Spine (Phila Pa 1976) 12:254–256 [DOI] [PubMed]
- 8.Pastorini E, Vecchiotti S, Colliva C, Persiani S, Rotini R, Roatti G, Zaccarilli L, Rovati LC, Roda A. Identification and quantification of glucosamine in rabbite cartilage and correlation with plasma levels by high performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Chim Acta. 2011;695:77–83. doi: 10.1016/j.aca.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Perlewitz TJ, Haughton VM, Riley LHI, Nguyen-Minh C, George V (1997) Effect of molecular weight on the diffusion of contrast media into cartilage. Spine 22(23):2707–2710 [DOI] [PubMed]
- 10.Deng M, Griffith JF, Zhu XM, Poon WS, Ahuja AT, Wang Y-XJ (2012) Effect of ovariectomy on contrast agent diffusion into lumbar intervertebral disc: a dynamic contrast-enhanced MRI study in female rats. Magn Reson Imaging 30(5):683–688 [DOI] [PubMed]
- 11.Ibrahim A, Gilzad-Kohan MH, Aghazadeh-Habashi A, Jamili F. Absorption and bioavailability of glucosamine in the rat. J Pharm Sci. 2012;101:2574–2583. doi: 10.1002/jps.23145. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animal used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson SJ, Ito K, Nolte LP. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech. 2004;37:213–221. doi: 10.1016/S0021-9290(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 14.Grunhagen T, Shirazi-Adl A, Fairbank JC, Urban JP. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin N Am. 2011;42:465–477. doi: 10.1016/j.ocl.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 16.Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clin Orthop Relat Res. 1982;170:296–302. [PubMed] [Google Scholar]
- 17.Motaghinasab S, Shirazi-Adl A, Urban JP, Parnianpour M. Computational pharmacokinetics of solute penetration into human intervertebral discs—effects of endplate permeability, solute molecular weight and disc size. J Biomech. 2012;45:2195–2202. doi: 10.1016/j.jbiomech.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Motaghinasab S, Shirazi-Adl A, Urban J, Parnianpour M, Hoviattalab M (2011) Computational investigation of sulphate diffusion into the dog disc. In: Proceedings, 18th Iranian conference on biomedical engineering, IEEE conference publications (BME4:1569505611), Tehran, pp 161–164
- 19.Shirazi-Adl A, Taheri M, Urban JPG. Analysis of cell viability in intervertebral disc: effect of endplate permeability on cell population. J Biomech. 2010;43:1330–1336. doi: 10.1016/j.jbiomech.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Van der Kraan PM, de Vries BJ, Vitters EL, Van den Berg WB, Van de Putte LBA. The effect of low sulfate concentrations on the glycosaminoglycan synthesis in anatomically intact articular cartilage of the mouse. J Orthop Res. 1989;7:645–653. doi: 10.1002/jor.1100070504. [DOI] [PubMed] [Google Scholar]
- 21.Henrotin Y, Mobasheri A, Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthr Res Ther. 2012;14:201. doi: 10.1186/ar3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavrogonatou E, Kletsas D. The effect of glucosamine sulfate on the proliferative potential and glycosaminoglycan synthesis of nucleus pulposus intervertebral disc cells. Spine. 2013;38:308–314. doi: 10.1097/BRS.0b013e31826a0a8d. [DOI] [PubMed] [Google Scholar]
- 23.van Blitterswijk WJ, van de Nes JC, Wuisman PI. Glucosamine and chondroitin sulfate supplementation to treat symptomatic disc degeneration: biochemical rationale and case report. BMC Complement Altern Med. 2003;3:2. doi: 10.1186/1472-6882-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkens P, Storheim K, Scheel I, Berg L, Espeland A. No effect of 6-month intake of glucosamine sulfate on Modic changes or high intensity zones in the lumbar spine: sub-group analysis of a randomized controlled trial. J Negat Results Biomed. 2012;11:13. doi: 10.1186/1477-5751-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persiani S, Roda E, Rovati LC, Locatelli M, Giacovelli G, Roda A. Glucosamine oral bioavaiability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthr Cartil. 2005;13:1041–1049. doi: 10.1016/j.joca.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Mokhbi Sukane D, Mokhbi Soukane D, Shirazi-Adl A, Urban JPG. Computation of coupled diffusion of oxygen, glucose and lactic acid in an intervertebral disc. J Biomech. 2007;40:2645–2654. doi: 10.1016/j.jbiomech.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Arun R, Freeman BJC, Scammell BE, Mcnally DS, Cox E, Gowland P. What influence does sustained mechanical load have on diffusion in the human intervertebral disc?: an in vivo study using serial postcontrast magnetic resonance imaging. Spine. 2009;34:2324–2337. doi: 10.1097/BRS.0b013e3181b4df92. [DOI] [PubMed] [Google Scholar]
- 28.Jackson AR, Yuan T-Y, Huang C-Y, Brown MD, Gu WY. Nutrient transport in human annulus fibrosus is affected by compressive strain and anisotropy. Ann Biomed Eng. 2012;40:2551–2558. doi: 10.1007/s10439-012-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malandrino A, Noailly J, Lacroix D. The effect of sustained compression on oxygen metabolic transport in the intervertebral disc decreases with degenerative changes. PLoS Comp Biol. 2011;7(8):1–12. doi: 10.1371/journal.pcbi.1002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C-Y, Gu WY. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech. 2008;41:1184–1196. doi: 10.1016/j.jbiomech.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson AR, Huang C-YC, Brown MD, Gu WY. 3D finite element analysis of nutrient distribution and cell viability in the intervertebral disc: effects of deformation and degeneration. J Biomech Eng. 2011;133:1–7. doi: 10.1115/1.4004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maroudas A (1979) Physico-chemical properties of articular cartilagein adult articular cartilage. In: Freeman M (ed) Pitman Medical, 2 edn. London, pp 215–290
- 33.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2007;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajasekaran S, Babu JN, Arun R, Armstrong BRW, Shetty AP, Murugan S. A study of diffusion in human lumbar discs: a serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine. 2004;29:2654–2667. doi: 10.1097/01.brs.0000148014.15210.64. [DOI] [PubMed] [Google Scholar]
- 35.Mackie J, Meares P. The diffusion of electrolytes in a cation-exchange resin membrane theoretical. Proc R Soc Lond Ser A. 1955;232:498–509. doi: 10.1098/rspa.1955.0234. [DOI] [Google Scholar]