Abstract
Purpose
The aim of this study was to compare single posterior debridement, interbody fusion and instrumentation with one-stage anterior debridement, interbody fusion and posterior instrumentation for treating thoracic and lumbar spinal tuberculosis.
Method
From January 2006 to January 2010, we enrolled 115 spinal tuberculosis patients with obvious surgical indications. Overall, 55 patients had vertebral body destruction, accompanied by a flow injection abscess or a unilateral abscess volume greater than 500 ml. The patients underwent one-staged anterior debridement, bone grafting and posterior instrumentation (group A) or single posterior debridement, bone grafting and instrumentation (group B). Clinical and radiographic results for the two groups were analyzed and compared.
Results
Patients were followed 12–36 months (mean 21.3 months), Fusion occurred at 4–12 months (mean 7.8 months). There were significant differences between groups regarding the post-operative kyphosis angle, angle correction and angle correction rate, especially if pathology is present in thoracolumbar and lumbar regions. Operative complications affected five patients in group A, and one patient in group B. A unilateral psoas abscess was observed in three patients 12 months postoperatively. In one of them, interbody fusion did not occur, and there was fixation loosening and interbody absorption. All of them were cured by an anterior operation.
Conclusion
Anterior debridement and bone grafting with posterior instrumentation may not be the best choice for treating patients with spinal tuberculosis. Single posterior debridement/bone grafting/instrumentation for single-segment of thoracic or lumbar spine tuberculosis produced good clinical results, except in patients who had a psoas abscess.
Keywords: Spinal tuberculosis, Bone grafting, Anterior debridement, Posterior debridement, Instrumentation
Introduction
Tuberculosis, a common disease in underdeveloped countries, is increasing in developed countries because of immigration and an increasing incidence of human immunodeficiency virus infection, increasing a person’s vulnerability to infection [1]. China has the second highest burden of tuberculosis in the world, registering 17 % of all global cases [2].
Percivall Pott, in 1877, first described spinal tuberculosis was as a kyphotic deformity of the spine associated with paraplegia [3]. Although spinal tuberculosis occurs in fewer than 1 % of patients with tuberculosis, it is a disabling, life-threatening condition and deserves attention [4]. The pathophysiology involves hematogenous spread of tuberculosis into the vertebrae, decreasing vascularity, and limitating nutrient flow, with their ultimate collapse.
Chemotherapy remains the mainstay for treating spinal tuberculosis, yet it cannot prevent kyphotic degeneration. Long-term rest or rigid instrumentation has been required to prevent kyphosis progression and relieve severe back pain. A rigid stabilization system may prevent kyphosis caused by spinal instability [1, 5, 6].
The ideal surgical approach for thoracic and lumbar tuberculosis is controversial. The goals are decompression, debridement, and the maintaining/reinforcing stability to correct/prevent deformity. Traditionally, the anterior approach to the spine has been preferred because tuberculosis pathology mainly affects vertebral bodies and disc spaces. It also allows direct access to the infected focus and is convenient for debriding/reconstructing the defect [7–9]. A combined anterior and posterior approach to fixation helps overcome stability-related drawbacks with the anterior approach used alone [10–14]. Single posterior approaches have been described, wherein the anterolateral column can be reached via an extra pleural approach [1, 8, 15, 16]. However, the posterior approach does not allow complete removal of a lesion situated in front of the vertebrae. It also results in suboptimal reconstruction of the anterior spinal column.
We compared one-stage anterior debridement and fusion combined with posterior instrumentation with single posterior debridement, bone grafting, and instrumentation to treat thoracic and lumbar spinal tuberculosis.
Materials and methods
Patient population
From January 2006 to January 2010, we enrolled 115 patients with active spinal tuberculosis of the thoracic or lumbar spine (without active pulmonary tuberculosis) with indications for surgery: (1) Progressive neurological deficit; (2) Persistent pain due to instability; (3) Severe kyphosis or kyphosis likely to progress [17]; (4) Poor outcomes following conservative treatment.
Active tuberculosis was diagnosed based on clinical symptoms (weight loss, low-grade fever, night-sweats, fatigue); laboratory findings [high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)]; radiographic evidence [e.g., sequestra and abscesses confirmed by computed tomography (CT) or abnormal signal intensity of the involved vertebrae or paravertebral or psoas abscesses]. Active spinal tuberculosis was diagnosed if one of these manifestations was present and Mycobacterium tuberculosis infection was confirmed postoperatively through laboratory tests (acid-fast stain, bacterial cultures, polymerase chain reaction, positive pathology). Written informed consent was obtained from all patients. The Xiangya Hospital Ethics Committee approved the study protocol.
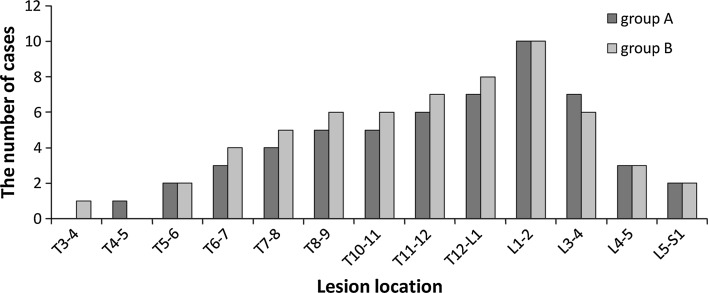
The study population consisted of 68 men and 47 women. Ages at the initial operation were 18–76 years (average ± SD 48.6 ± 12.8 years). Mean disease course was 10 months (range 3–12 months). The patients formed two groups. Group A (anterior approach) contained 55 patients who had vertebral body destruction, accompanied by a flow injection abscess or a unilateral abscess volume >500 ml. They underwent one-stage anterior debridement and fusion combined with posterior instrumentation. The others (group B: posterior approach) underwent single posterior debridement, bone grafting and instrumentation. Characteristics of each group are shown in Table 1 and Fig. 1.
Table 1.
Detailed clinical information, operation time, amount of bleeding and hospital stay of the two groups
| Sex | Pathological region | Operation time (min) | Amount of bleeding (ml) | Hospitalization day (days) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Thoracic | Thoracolumbar | Lumbar | ||||
| Group A | 32 | 23 | 15 | 28 | 12 | 231.4 ± 27.3 | 1,023.8 ± 197.9 | 18.7 ± 3.6 |
| Group B | 36 | 24 | 18 | 31 | 11 | 160.4 ± 20.5※ | 760.7 ± 146.2▲ | 13.6 ± 3.2△ |
※Student–Newman–Keuls test, compare with Group A, P = 0.026
▲Student–Newman–Keuls test, compare with Group A, P = 0.038
△Student–Newman–Keuls test, compare with Group A, P = 0.047
Fig. 1.
Lesions involved the segment of spinal
The patients presented with back pain, limited spinal mobility, and a hump-shaped deformity. 89 patients had a neurological deficit, varying in severity from unilateral or bilateral numbness and weakness of the lower extremity to walking disorders (Table 2). Pre-operative ESRs and CRP levels were abnormal (Table 3).
Table 2.
Neurologic recovery according to ASIA (Group A and Group B)
| Pre-operation | Group A/B | Final follow-up in group A* | Final follow-up in group B▲ ※ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | A | B | C | D | E | ||
| A | 0/0 | ||||||||||
| B | 4/2 | 2 | 2 | 1 | 1 | ||||||
| C | 19/17 | 6 | 13 | 7 | 10 | ||||||
| D | 21/26 | 21 | 26 | ||||||||
| E | 11/15 | 11 | 15 | ||||||||
* In group A, Wilcoxon signed rank test, compare with Pre-operation, P a = 0.0017
※In group B, Wilcoxon signed rank test, compare with Pre-operation, P a = 0.0013
▲Wilcoxon signed rank test, Group B compare with Group A, P > 0.05
Table 3.
Pre-operative, post-operative and 3-month post-operative monitoring of ESR, CRP
| ESR (mm/h) | CRP (mg/L) | |||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Pre-operative | 36.2 ± 5.6 | 37.1 ± 4.2 | 18.3 ± 7.5 | 21.5 ± 6.5 |
| Post-operative△ | 20.5 ± 4.1 | 17.4 ± 3.9 | 8.7 ± 3.8 | 6.3 ± 2.5 |
| 3-month post-operative※ | 10.2 ± 3.2 | 9.8 ± 2.3 | 3.1 ± 2.5 | 2.53 ± 1.5 |
△Student–Newman–Keuls test, compare with Pre-operative, P a = 0.032, P b = 0.029
※Student–Newman–Keuls test, compare with Post-operative, P = 0.061
Pre-operative preparation
All patients were clinically diagnosed with spinal tuberculosis without active pulmonary disease. All patients were treated with HREZ chemotherapy (isoniazid 300 mg/day, rifampicin 450 mg/day, ethambutol 750 mg/day and pyrazinamide 750 mg/day) for 2–4 weeks prior to surgery. In general, pre-operative ESRs were not higher than 40 mm/h and hemoglobin levels were not <10 g/dl.
Operative technique
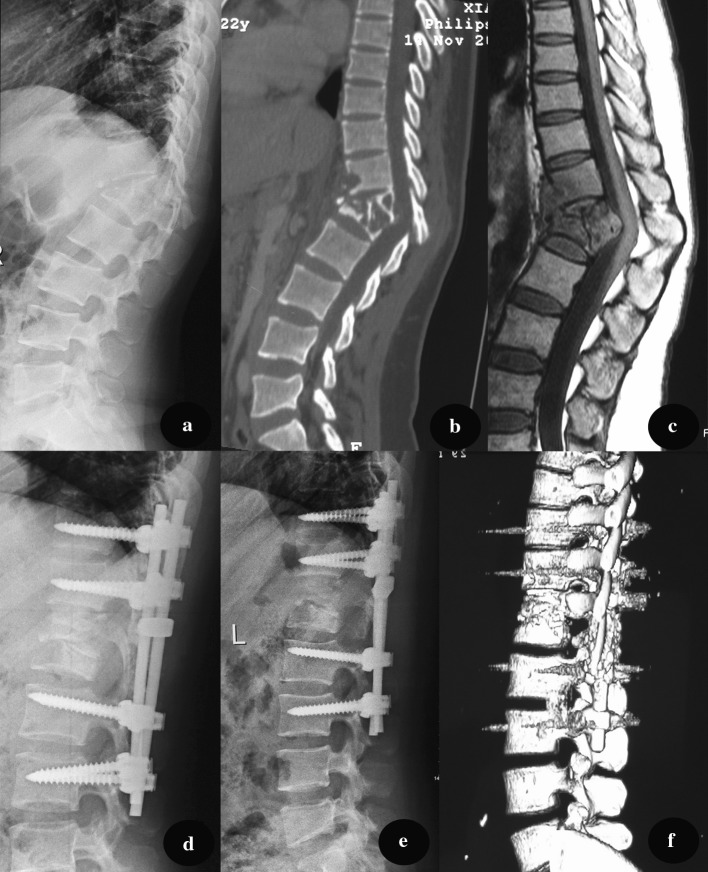
Group A patients (anterior approach) were placed in the lateral decubitus position. The extra pleural or extra peritoneal anterolateral approach was used, avoiding pleural or peritoneal injury. The tuberculosis lesion (paravertebral or psoas abscess, collapsed vertebrae, intervertebral discs) was debrided until healthy, bleeding margins were obtained. After sufficient spinal cord decompression, strut grafts were implanted to reconstruct the vertebral anterior column and restore normal height. With the patient moved to a prone position, pedicle screws were placed in two normal vertebral bodies around the lesion. Performing laminectomy depended on whether there was oppression from the spinal cord’s posterior column (Fig. 2).
Fig. 2.
A pre-operative X-ray of a 45-year-old male demonstrated the destruction of T12 and L1, with a kyphosis angle of 43°. CT and MRI (b, c) show vertebral bone destruction and paravertebral abscess formation, severed spinal cord compression. The patient underwent one-staged anterior debridement, autologous bone grafting and posterior instrumentation. d A lateral X-ray indicates that kyphosis was corrected to 6° 3 months after surgery. e, f At 18-month follow-up, fixation was in good shape, without signs of tuberculosis recurrence
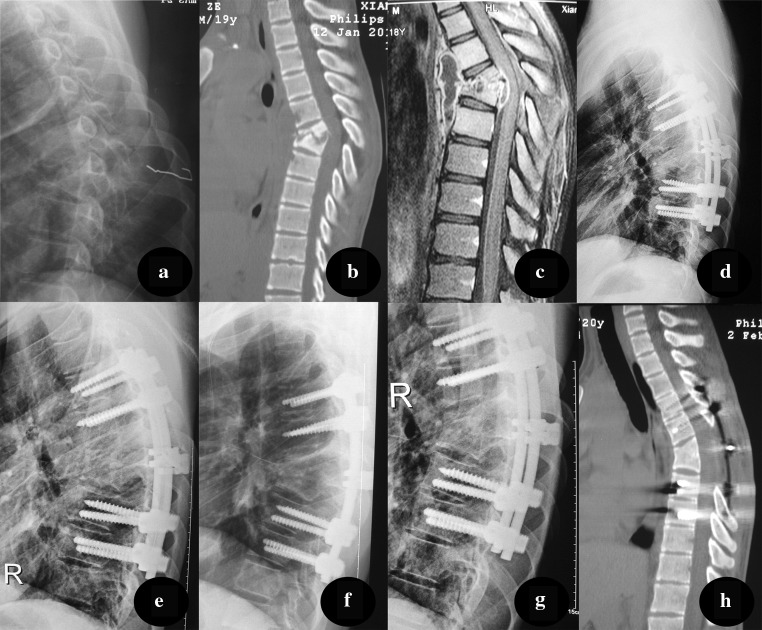
Group B patients (posterior approach) were placed in prone position. Pedicle screws were placed in the first two normal vertebral bodies around the lesion. After installing a temporary internal fixation instrument on one side, the superior and inferior articular processes of the vertebrae were bitted and exposed to the intervertebral space of the same side. Following excision of the necrotic disc, the collapsed vertebrae and paravertebral abscess, a suitable flush tube was plunged into the psoas abscess, which was washed until there was no pus outflow. Strut grafts were inserted in the bone trough to reconstruct the vertebrae and the screw tightened for internal fixation. The kyphosis slowly rectified by compressing and stretching the internal fixation instrument (Fig. 3).
Fig. 3.
A pre-operative X-ray of a 22-year-old male demonstrated the destruction of T6 and T7, with a kyphosis angle of 32°. CT and MRI (b, c) show vertebral bone destruction, paravertebral abscess formation, and spinal cord compression. d, e 3 month after surgery, a lateral X-ray indicated that kyphosis was corrected to 26°. f, h At nine-month post-operation, CT revealed successful fusion, and a kyphosis angle of 27°. g At 24-month follow-up, fixation was in good shape, without signs of tuberculosis recurrence
Resected specimens from groups A and B were sent for bacterial culture and pathological diagnosis.
Post-operative care
The drainage tube was removed after 72 h unless there was an extensive paraspinal abscess or flow injection abscess, when it was left indwelling until the drainage amounted to <30 ml/24 h. Patients continued oral HREZ chemotherapy postoperatively. Pyrazinamide was discontinued at 6 months. Patients continued on 9- to 12-month regimens of HRE chemotherapy. Ambulation with a brace was allowed 6–8 weeks after surgery. Patients carried out non-weight-bearing daily activities until there was radiographic or CT evidence of fusion. Patients then returned to normal weight-bearing activity.
Evaluation standard
Operating time, amount of bleeding, and hospital stay of each patient were recorded (Table 1). The American Spinal Injury Association (ASIA) scale was used to assess pre-operative and post-operative neurological function. Tuberculosis activity was monitored with ESR and CRP tests. Bone graft fusion, loss of correction, and instrumentation failure were monitored by radiography. Fusion status was evaluated according to Lee et al. [18]. When radiographic observations were uncertain, CT was performed. The pre-operative and post-operative kyphotic angles were measured by plain lateral radiography (kyphotic angle formed by 2 lines obtained by joining the anterosuperior and posterosuperior corners of the lesion and the anteroinferior and posteroinferior corners of the vertebrae below the lesion).
Statistical analysis
All statistical analyses were performed with SPSS version 19.0 statistical software (SPSS, Inc., Chicago, IL, USA). ASIA grades preoperatively and at the last follow-up were compared using the Wilcoxon signed rank test. Repeated measurements before and after surgery and at the last follow-up were carried out to compare the degree of kyphosis deformity. Student–Newman–Keuls test was used to compare changes in various laboratory and physical parameters in the two groups. Any discrepancy in normal distribution was analyzed using the rank sum test with a significance level of 0.05.
Results
Tuberculosis was confirmed by bacterial culture or pathology for the 115 patients. Altogether, cultures were positive for Mycobacterium in 32 patients. All patients were followed postoperatively for 12–36 months (mean 21.3 months).
All grafted bones in group A ultimately fused, with fusion time ranging from 4 to 9 months (mean 7.8 months). Altogether 57 of the 60 patients obtained interbody fusion at 9.7 months (6–12 months). Fusion in the other three patients was delayed because of a psoas abscess.
Group B patients had a shorter operating time, less intra-operative bleeding and a shorter time hospital stay (P < 0.05). ESRs and CRP levels were monitored in preoperatively, immediately postoperatively, and 3 months later (Table 3).
There were significant differences between the pre-operative and post-operative values in the two groups (Pa = 0.032, Pb = 0.029) but no significant differences between the immediately post-operative and 3-month post-operative values in either group (P > 0.05). ASIA grades are presented in Table 2.
Overall, 44 patients in group A and 45 in group B suffered obvious neurological deficits before the surgery. After surgery, 34 patients in group A and 36 in group B returned to normal. The rest achieved partial recovery. There was no significant difference between the two groups (P > 0.05), but there were significant differences between pre-operative and post-operative results in each group (Pa = 0.0017, Pb = 0.0013).
The lesions in the spinal kyphosis segment and the kyphosis correction loss during follow-up, by pathological regions are shown in Table 4 and Fig. 1. Preoperatively the kyphosis angles in two groups were not significantly different, whereas there was a significant difference (P < 0.05) between the two groups postoperatively in the kyphosis angles, angle correction, and angle correction rate, especially in pathological thoracolumbar and lumbar regions. During long-term follow-up, there was a loss of only 1°–2° in the kyphosis angle in group A and 1°–3° in group B. There was a significant difference in the kyphosis lost rate between two groups (P = 0.0324), but no significant difference in the kyphosis angle loss (P > 0.05). There were significant differences between the pre-operative and post-operative kyphosis angles in both groups (Pa = 0.0002, Pb = 0.0007).
Table 4.
Kyphosis correction post-operation and kyphosis lost during follow-up by pathological regions in two groups
| Pathological region | Pre-operative kyphosis angle (°)☆ | Post-operation | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|
| Kyphosis angle (°)※ | Angle correction (°)△ | Correction rate (%)▲ | Kyphosis angle (°) | Angle lost (°)★ | Lost rate (%)▽ | |||
| Group A | ||||||||
| Thoracic | 22.3 ± 13.4 | 8.7 ± 2.4 | 14.7 ± 9.1 | 66.9 ± 7.2 | 10.1 ± 2.7 | 2.1 ± 0.9 | 6.4 ± 0.7 | |
| Thoracolumbar | 47.2 ± 13.7 | 4.1 ± 1.4 | 42.7 ± 7.3 | 90.4 ± 13.6 | 5.6 ± 1.2 | 1.2 ± 0.3 | 2.8 ± 0.3 | |
| Lumbar | 28.2 ± 12.7 | 3.8 ± 1.2 | 25.1 ± 8.2 | 89.4 ± 13.2 | 4.3 ± 1.4 | 1.5 ± 0.5 | 3.4 ± 0.6 | |
| Group B | ||||||||
| Thoracic | 22.6 ± 11.2 | 8.1 ± 5.5 | 14.1 ± 6.4 | 62.4 ± 9.4 | 10.3 ± 2.1 | 1.7 ± 0.8 | 11.2 ± 1.4 | |
| Thoracolumbar | 46.4 ± 12.3 | 14.7 ± 3.2 | 32.2 ± 6.1 | 69.3 ± 6.7 | 16.6 ± 3.3 | 2.3 ± 0.3 | 9.9 ± 1.5 | |
| Lumbar | 26.8 ± 10.9 | 7.8 ± 2.1 | 19.2 ± 5.7 | 71.6 ± 6.4 | 9.7 ± 1.7 | 1.9 ± 0.7 | 6.9 ± 1.7 | |
☆Student–Newman–Keuls test, compared pre-operative kyphosis angle between two groups, P > 0.05
△Student–Newman–Keuls test, compared angle correction between two groups, P = 0.0412
▲Student–Newman–Keuls test, compared correction rate between two groups, P = 0.004
★Student–Newman–Keuls test, compared angle lost between two groups, P = 0.13
▽Student–Newman–Keuls test, compared angle lost rate between two groups, P = 0.0324
※Student–Newman–Keuls test, compared kyphosis angle with pre-operative in two groups, P a = 0.0002, P b = 0.0007
Five patients in group A experienced operative complications. One incision was superficially infected with Escherichia coli which was treated successfully with antibiotics. Two patients who suffered from sinus drainage tube formation 1-week post-operation were healed by weekly local isoniazid therapy. Two cases of pleural effusion were alleviated with in situ closed drains in situ for five days. No non-union of bone, pseudarthrosis or internal fixation loosening or rupturing was found at the last follow-up in group A.
In group B only one patient had an operative complications refractory intercostal neuralgia, which was relieved by non-steroidal anti-inflammatory drugs. A unilateral psoas abscess was observed in each of three group A patients at the 12-month follow-up. None of the three had achieved interbody fusion. Each was cured by anterior debridement. Fixation loosening and interbody absorption appeared in one, who underwent anterior debridement and interbody fusion combined with long segment posterior instrumentation. No severe neurological complications were observed in either group.
Discussion
Since the first description of tuberculosis in 1877 [3], numerous therapeutic methods have been proposed. The combination of anterior debridement, strut grafting fusion and fixation, has become the gold standard surgical treatment for spinal tuberculosis [7]. Kim et al. [19] reporting on 140 patients who underwent radical anterior surgery, obtained 51.0 % initial correction of kyphosis, but the rate of correction dropped to 7.5 % 2 years later. Schulitz et al. [20], reported mild kyphosis associated with long-term loss of correction, although the problem could be somewhat alleviated with anterior instrumentation [21]. The disadvantages of the anterior-alone approach include insufficient kyphosis correction and great loss of correction postoperatively.
With the introduction of a screw and rod fixation system, one-stage anterior debridement/bone grafting/posterior instrumentation and single posterior debridement/bone grafting/instrumentation became new ways to treat active spinal tuberculosis. Laheri et al. [12] reported that 28 patients with kyphosis deformity averaging 64.3° obtained a 62.5 % reduced kyphosis angle after operation. Deng et al. [22] reported 34 spinal tuberculosis patients with an angulated kyphotic deformity who underwent posterior en bloc spondylectomy. They had 90 % correction of the kyphosis angle returned to normal activity after 4–8 weeks. All of our patients achieved at least 60 % correction of the kyphosis angle, allowing ambulation in a brace 6–8 weeks after surgery.
Posterior instrumentation, providing long-segmental and three-column fixation, successfully solves any correction lost postoperatively. However, the stability of the spine is disrupted because the posterior column is excised, which may lead to a large loss of correction. Although the kyphosis angle loss rate was significantly different between our groups, the correction was satisfactorily reserved with only 1°–2° kyphosis angle loss in group A and 1°–3° loss in group B. These results are similar to those of Louw et al. (3.3°) [15] and Moon et al. (3.0°) [14] but significantly better than the case reported by Sundararaj et al. (4.64°) [23].
In addition to increasing the stability of the spinal column, instrumentation can promote neurological recovery and accelerate treatment of tuberculosis [24]. Our results confirm that neurological deficits clinically diminished at least one ASIA grade in both groups after surgery, and 70 patients returned to normal function. The ESRs and CRP levels decreased postoperatively.
There are many differences between the anterior and posterior approaches described herein. The traditional anterior approach allows direct access to the disease pathology and decompression under orthophoria conditions. Thus, for active spinal tuberculosis, anterior debridement and strut graft fusion are imperative and have been, advocated by many scholars [7–9]. Also, anterior debridement and strut grafts fusion can remove the psoas abscess and paravertebral abscess completely, reconstructing the anterior column, and restoring normal sagittal alignment, especially in patients with a severely damaged anterior column, associated with multiple-level tuberculosis lesions (especially flow injection abscesses). Zhang et al. [25] reported 23 patients who underwent one-stage anterior debridement, strut auto-grafting, and posterior instrumentation, which these authors found therapeutically effective in adults. Huang et al. [26] reported one-stage surgical management for children with spinal tuberculosis using anterior decompression, bone grafting, posterior instrumentation, and fusion. All of their patients were cured.
The intervertebral space, pedicle, and extrapleural and extraperitoneal spaces provide operational space for posterior debridement, and bone grafting. The posterior approach has been successfully applied to treat spinal tuberculosis. Gokce et al. [27] reported that 12 patients with angular kyphotic deformity who underwent posterior decompression and closing wedge osteotomy with instrumentation had good clinical outcomes. Equally, Lee et al. [1] reported that transpedicular instrumentation provided rapid relief of instability and excellent prevention of late angular deformity in patients with limited spinal bone destruction. Although in our study we may not have eliminated the infected focus, the result of an abscess remnant and possible recurrence of tuberculosis, most of the group B patients were cured using this approach.
It must be noted that a unilateral psoas abscess was observed in three patients (group A) at 12-months postoperatively, even with anti-tuberculosis therapy. Non-union was found in one of them a year later. Uncured abscesses may be the result of a thick abscess wall and poor local blood supply, which leads to the concentration of anti-tuberculosis drug is low in abscess internal and unable to effective bactericidal. Incomplete debridement provides a poor micro-environment for graft fusion. Long-term non-fusion of the bone graft resulting in its absorption, and internal fixation fatigue can lead to failure. There is a potential risk of tuberculosis spreading to healthy regions with posterior debridement, resulting in infection, and fistulas. None of our patients experienced such spread.
Many authors believe that the anterior approach is associated with a longer operating time, more blood loss, a longer hospital stay than are seen in the posterior approach. The anterior approach also easily leads to nerve and vascular injuries, increasing surgical complications [16, 23, 27–29], (confirmed in the current study). There were five operative complications in group A (anterior approach), as described earlier, whereas there was only one operative complication in group B (posterior approach). Advantages of the posterior approach are that it relieves spinal nerve compression, corrects the spinal deformity, reconstructs a stable spine, reduces trauma for patients, and improves patients’ quality of life. Other advantages are a shorter time in surgery, less bleeding, and a shorter hospital stay.
Conclusion
Single posterior debridement/bone grafting/instrumentation can effectively correct kyphosis and reconstruct the anterior column, when the patient does not have a psoas abscess. The authors believe that single posterior debridement/bone grafting/instrumentation may be the best way to treat single-segment thoracic and lumbar spinal tuberculosis.
Acknowledgments
This publication was funded in part by the National Natural Science Foundation of China (81171736).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Xiyang Wang, Phone: +86-0731-89753001, FAX: +86-0731-89753001, Email: wqlwqwxy@163.com.
Xiaoyang Pang, Email: xiaoyangpang@163.com.
References
- 1.Lee TC, Lu K, Yang LC, Huang HY, Liang CL. Transpedicular instrumentation as an adjunct in the treatment of thoracolumbar and lumbar spine tuberculosis with early stage bone destruction. J Neurosurg. 1999;91(2 Suppl):163–169. doi: 10.3171/spi.1999.91.2.0163. [DOI] [PubMed] [Google Scholar]
- 2.Global tuberculosis control. Switzerland: WHO Report; 2004. p. 22. [Google Scholar]
- 3.Slucky AV, Eismont FJ. Spinal infections. In: Bridwell KH, Dewald RL, editors. The textbook of spinal surgery. Philadelphia: Lippincott Raven; 1997. pp. 2141–2183. [Google Scholar]
- 4.Turgut M. Spinal tuberculosis (Pott’s disease): its clinical presentation, surgical management, and outcome. A survey study on 694 patients. Neurosurg Rev. 2001;24(1):8–13. doi: 10.1007/PL00011973. [DOI] [PubMed] [Google Scholar]
- 5.Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. Anterior surgery with insertion of titanium mesh cage and posterior instrumented fusion performed sequentially on the same day under one anesthesia for septic spondylitis of thoracolumbar spine: is the use of titanium mesh cages safe? Spine. 2006;31(9):1014–1019. doi: 10.1097/01.brs.0000215049.08622.9d. [DOI] [PubMed] [Google Scholar]
- 6.Shipley JA, Craig JB. Spinal tuberculosis with translational instability. Spine. 1993;18(3):397–401. doi: 10.1097/00007632-199303000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Benli IT, Kaya A, Acaroglu E. Anterior instrumentation in tuberculous spondylitis: is it effective and safe? Clin Orthop Relat Res. 2007;460:108–116. doi: 10.1097/BLO.0b013e318065b70d. [DOI] [PubMed] [Google Scholar]
- 8.Jain AK, Dhammi IK, Prashad B, Sinha S, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone Joint Surg Br. 2008;90(11):1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172–178. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 10.Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002;15(2):149–156. doi: 10.1097/00024720-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Fukuta S, Miyamoto K, Masuda T, Hosoe H, Kodama H, Nishimoto H, Sakaeda H, Shimizu K. Two-stage (posterior and anterior) surgical treatment using posterior spinal instrumentation for pyogenic and tuberculotic spondylitis. Spine. 2003;28(15):E302–E308. doi: 10.1097/01.BRS.0000083318.40123.5E. [DOI] [PubMed] [Google Scholar]
- 12.Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39(8):429–436. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 13.Rowe SM, Chung JY, Moon ES, Song EK. Bent plate fixation in combined intertrochanteric and subtrochanteric fractures of the femur. Orthopedics. 1991;14(10):1123–1128. doi: 10.3928/0147-7447-19911001-11. [DOI] [PubMed] [Google Scholar]
- 14.Moon MS, Woo YK, Lee KS, Ha KY, Kim SS, Sun DH. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20(17):1910–1916. doi: 10.1097/00007632-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Louw JA. Spinal tuberculosis with neurological deficit. Treatment with anterior vascularised rib grafts, posterior osteotomies and fusion. J Bone Joint Surg Br. 1990;72(4):686–693. doi: 10.1302/0301-620X.72B4.2380228. [DOI] [PubMed] [Google Scholar]
- 16.Jain AK, Aggarwal A, Dhammi IK, Aggarwal PK, Singh S. Extrapleural anterolateral decompression in tuberculosis of the dorsal spine. J Bone Joint Surg Br. 2004;86(7):1027–1031. doi: 10.1302/0301-620X.86B7.14546. [DOI] [PubMed] [Google Scholar]
- 17.Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br. 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 18.Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements. The results of disc excision and posterior lumbar interbody fusion. Spine. 1995;20(3):356–361. doi: 10.1097/00007632-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, Ko HS, Lim Y, et al. The clinical study of tuberculous spondylitis. J Korean Orthop Assoc. 1993;28:2221–2232. [Google Scholar]
- 20.Schulitz KP, Kothe R, Leong JC, Wehling P. Growth changes of solidly fused kyphotic bloc after surgery for tuberculosis. Comparison of four procedures. Spine. 1997;22(10):1150–1155. doi: 10.1097/00007632-199705150-00016. [DOI] [PubMed] [Google Scholar]
- 21.Dai LY, Jiang LS, Wang W, Cui YM. Single-stage anterior autogenous bone grafting and instrumentation in the surgical management of spinal tuberculosis. Spine. 2005;30(20):2342–2349. doi: 10.1097/01.brs.0000182109.36973.93. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y, Lv G, An HS. En bloc spondylectomy for the treatment of spinal tuberculosis with fixed and sharply angulated kyphotic deformity. Spine. 2009;34(20):2140–2146. doi: 10.1097/BRS.0b013e3181b34ce7. [DOI] [PubMed] [Google Scholar]
- 23.Sundararaj GD, Behera S, Ravi V, Venkatesh K, Cherian VM, Lee V. Role of posterior stabilisation in the management of tuberculosis of the dorsal and lumbar spine. J Bone Joint Surg Br. 2003;85(1):100–106. doi: 10.1302/0301-620X.85B1.13300. [DOI] [PubMed] [Google Scholar]
- 24.Klockner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine. 2003;28(10):1036–1042. doi: 10.1097/01.BRS.0000061991.11489.7F. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HQ, Guo CF, Xiao XG, Long WR, Deng ZS, Chen J. One-stage surgical management for multilevel tuberculous spondylitis of the upper thoracic region by anterior decompression, strut autografting, posterior instrumentation, and fusion. J Spinal Disord Tech. 2007;20(4):263–267. doi: 10.1097/01.bsd.0000211281.68400.1b. [DOI] [PubMed] [Google Scholar]
- 26.Huang QS, Zheng C, Hu Y, Yin X, Xu H, Zhang G, Wang Q. One-stage surgical management for children with spinal tuberculosis by anterior decompression and posterior instrumentation. Int Orthop. 2009;33(5):1385–1390. doi: 10.1007/s00264-009-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gokce A, Ozturkmen Y, Mutlu S, Caniklioglu M. Spinal osteotomy: correcting sagittal balance in tuberculous spondylitis. J Spinal Disord Tech. 2008;21(7):484–488. doi: 10.1097/BSD.0b013e3181586023. [DOI] [PubMed] [Google Scholar]
- 28.Garg B, Kandwal P, Nagaraja UB, Goswami A, Jayaswal A. Anterior versus posterior procedure for surgical treatment of thoracolumbar tuberculosis: a retrospective analysis. Indian J Orthop. 2012;46(2):165–170. doi: 10.4103/0019-5413.93682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang X, Shen X, Wu P, Luo C, Xu Z, Wang X. Thoracolumbar spinal tuberculosis with psoas abscesses treated by one-stage posterior transforaminal lumbar debridement, interbody fusion, posterior instrumentation, and postural drainage. Arch Orthop Trauma Surg. 2013 doi: 10.1007/s00402-013-1722-9. [DOI] [PubMed] [Google Scholar]