Abstract
Purpose
The treatment of spinal infection remains a challenge for spinal surgeons because of the variable presentations and complicated course. The diagnostic and therapeutic value of percutaneous endoscopic lavage and drainage (PELD) has been proved in some recent studies. The purpose of this study is to evaluate the efficacy of PELD in patients with advanced infectious spondylitis which may traditionally require open surgery.
Methods
We retrospectively reviewed the medical records of 21 patients who underwent PELD to treat their advanced lumbar infectious spondylitis. Patients with severe infection resulting in significant neurological deficit and mechanical instability were excluded from the PELD procedure, which was only used on selected patients with less severe disease. The 21 patients were categorized into three groups based on their past history, clinical presentation, and imaging studies: those with paraspinal abscesses, postoperative recurrent infection, and multilevel infection. Clinical outcomes were assessed by careful physical examination, Odom’s criteria, regular serologic testing, and imaging studies to determine whether continued conservative treatment or surgical intervention was necessary.
Results
Causative bacteria were identified in 19 (90.5 %) of 21 biopsy specimens. Appropriate parenteral antibiotics for the predominant pathogen isolated from the infected tissue biopsy cultures were prescribed for the patients. All patients reported satisfactory recovery and relief of back pain, except three with multilevel infections who underwent anterior debridement and fusion within 2 weeks after treatment with PELD. The overall infection control rate was 86 %. One patient with epidural abscess and spondylolytic spondylolisthesis of the L5–S1 received instrumented fusion surgery due to mechanical instability 5 months later. No surgery-related major complications were found, except 2 patients who had transient paraesthesia in the affected lumbar segment.
Conclusions
PELD was successful in obtaining a bacteriologic diagnosis, relieving the patient’s symptoms, and assisting in eradication of lumbar infectious spondylitis. The indications of this minimally invasive procedure could be extended to treat patients suffering from spinal infections with paraspinal abscesses and postoperative recurrent infection. Patients with multilevel infection may have trivial benefits from PELD due to poor infection control and mechanical instability of the affected segments.
Keywords: Spinal infection, Minimally invasive spine surgery, Percutaneous endoscopic lavage, Percutaneous endoscopic drainage
Introduction
Conservative therapy including appropriate antibiotics and bracing is usually adequate for most patients with infectious spondylitis. Surgical intervention is typically reserved for patients with failed antibiotic therapy, intractable back pain, significant neurological deficit, large epidural abscesses, extensive vertebral body destruction, and severe kyphotic deformity or spinal instability [1–3]. However, major spinal surgery consisting of anterior debridement and accompanying bone grafting with or without supplemental instrumentation is often related to undesired postoperative complications. Delay in diagnosis and treatment of all kinds of spinal infections is common because of their early indolent courses and variable presentations, which may lead to treatment failure [4–6]. Rezai et al. [7] reported that 25 % of patients who were initially treated nonsurgically experienced failed medical therapy. Therefore, an early diagnosis plus prompt application of appropriate antibiotic therapy for cultured pathogens are crucial for successful nonsurgical treatment.
Several minimally invasive methods have been used to treat infectious spondylitis. Haaker et al. [8] treated 16 patients with spondylodiscitis using percutaneous lumbar discectomy. They concluded that it is a useful and minimally invasive technique for the conservative treatment of lumbar discitis, although the causative pathogens could be identified in only 45 % of their cases. Percutaneous suction, aspiration, drainage, and continuous irrigation with local administration of antibiotics have also been found to be effective in patients with an early-stage pyogenic spondylitis and even spinal infection accompanied by iliopsoas abscesses [9–12]. However, the continuous irrigation restrained the patients to their beds and limited their postoperative ambulation and activities. The diagnostic and therapeutic value of percutaneous endoscopic lavage and drainage (PELD) has already been proved in some recent studies [13–15]. However, most of these studies focused on the effect of PELD in early-stage infection. The purpose of this study was to evaluate the efficacy of PELD in the treatment of patients with advanced infectious spondylitis which commonly requires open surgery in current clinical practice.
Patients and methods
Patients
Between January 2006 and December 2010, 21 patients with advanced infectious spondylitis, comprising 7 women and 14 men with an average age of 56.5 years (range 39–87 years), were treated with PELD procedures at our institute. Infectious spondylitis was diagnosed based on clinical examinations, including elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and radiographic and magnetic resonance imaging (MRI) findings. Patients who had infection resulting in severe structural instability or deformity, and that sustained significant neurological deficit were not enrolled in this study. The medical records of the enrolled patients including outpatient and emergency room notes, admission notes, inpatient progress and nursing notes, discharge summaries, procedure notes, surgical reports, radiology reports, pathology reports, and microbiology laboratory results were reviewed. The microbiology reports comprised microscopy and culture findings, and any specific pathogens identified by the PELD procedures. All patients in this study presented with intractable back pain requiring narcotic pain control and bed rest before PELD.
On the basis of their past history, clinical presentation, and imaging studies, these patients were divided into three groups: those with paraspinal abscesses (including epidural abscess, prevertebral abscess, and paravertebral abscess with psoas muscle extension), those with postoperative recurrent infection (including infection after instrumented fusion and recurrent infection after anterior interbody fusion with bone graft), and those with multilevel infection. Their clinical outcomes were assessed by careful physical examination, Odom’s criteria, regular serological tests, and imaging studies during admission, at 1 month after discharge, and every 3 months to determine whether continued conservative treatment was sufficient or open surgical intervention was required.
Operative procedure
The PELD procedures were performed for patients with infectious spondylitis in the lumbar region. The patients were placed prone on a radiolucent flame suitable for fluoroscopy. All procedures were performed under local anaesthesia with conscious sedation similar to that used for standard lumbar discography. Under fluoroscopic guidance, the target site was located and the entry site was marked on the skin at a point 8–12 cm from the midline. Following sterile preparation, draping, and local anaesthesia, a spinal needle was inserted directly into the centre of the targeted disc. A guide wire was introduced through the spinal needle into the central disc space and the spinal needle was then withdrawn. After creating a small stab-wound incision (about 1 cm), a dilator and a cannulated sleeve were guided over the wire and progressed sequentially into the disc centre. Fluoroscopy was repeated in two orthogonal planes to verify the correct position of the endoscope tip. The tissue dilator was then removed and the cutting tool was inserted. The cutting tool, a cylindrical sleeve with a serrated edge at its distal end, was used to harvest a core of the impacted biopsy specimen. Discectomy forceps were then inserted through the cannulated sleeve to extract additional tissue from the infected disc. Percutaneous debridement was performed piecemeal by manipulating the biopsy forceps, flexible rongeurs, and shaver into different positions to obtain as much tissue as possible under fluoroscopic monitoring. The same procedures were repeated on the other side. These two working sheaths were left on both sides for sufficient extirpation and extensive debridement of the infected intervertebral disc and even some endplate from different endoscopic direction. Approximately 35 mL of povidone-iodine was diluted with 1,000 mL normal saline to achieve a 3.5 % betadine solution ready for use during operation. After biopsy and debridement procedures, at least 10,000 mL of the dilute betadine solution was used for irrigation. One portal was connected to a lavage fluid pump for the inflow and the other portal was connected to a suction bottle for the outflow with continuous infusion. The suction function was usually kept open from the beginning to the end of the procedure for further drainage of the lavage fluid and abscess. Finally, two large-bore drainage catheters were inserted into the debrided disc space and connected to a negative-pressure Hemovac. The biopsy specimen contained disc material and parts of the vertebral endplates of adjacent vertebrae. Each biopsy specimen was examined for microorganisms and evaluated histopathologically. All tubes of the hemovac were left in place until the drainage stopped or reduced to <10 mL/day for three consecutive days.
Outcome measures
The culture rate, satisfactory result rate, and infection control rate of PELD in the management of spinal infection in the three groups were expressed as percentage (%). These outcomes after PELD among the three different spinal infection patterns were compared using the Chi squared test or Fisher’s exact test. A value of p < 0.05 was considered statistically significant. SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results
The 21 enrolled patients included ten with paraspinal abscess (Figs. 1, 2), six with postoperative recurrent infection (Fig. 3), and five with multilevel infection (Table 1). The most prominent clinical sign of infectious spondylitis was back pain, which was detected in all 21 patients before PELD. One week after PELD, 18 (86 %) of the 21 patients reported satisfactory relief of their back pain according to Odom’s criteria, including 2 who had excellent outcomes and 16 who had good outcomes. Of the 18 patients with satisfactory results, one with spondylolytic spondylolisthesis of the L5–S1 who underwent instrumented fusion 5 months after spinal infection had been brought under control (Fig. 4). The remaining 3 patients, including two with a fair outcome and one with a poor outcome, had persistent infection and severe back pain and underwent anterior debridement accompanied with autograft interbody fusion within 2 weeks after PELD.
Fig. 1.
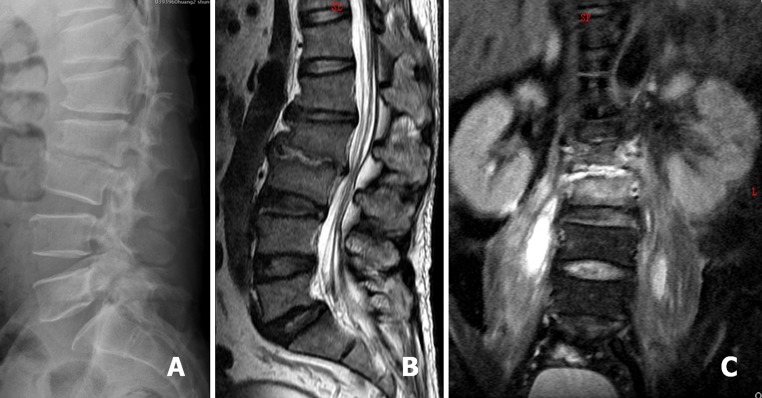
A 62-year-old man was diagnosed as L2–3 infectious spondylitis. Lateral radiograph showed L2–3 disc space collapse and endplates erosion (a). Sagittal and coronal T2-weighted magnetic resonance imaging (MRI) demonstrated a L2–3 infection source and associated bilateral psoas muscle abscess (b, c)
Fig. 2.
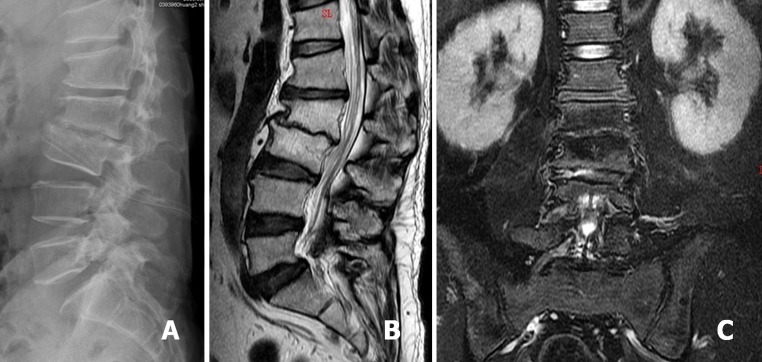
After PELD treatment, negative-pressure Hemovac with two drainage tubes was inserted into the L2–3 disc space for further continuous drainage of the offending pathogens (a). Sagittal T2-weighted MRI revealed L2–3 disc space narrowing, proceeding to spontaneous fusion (b). Coronal T2-weighted MRI demonstrated the abscess had disappeared at postoperative 8 months (c)
Fig. 3.
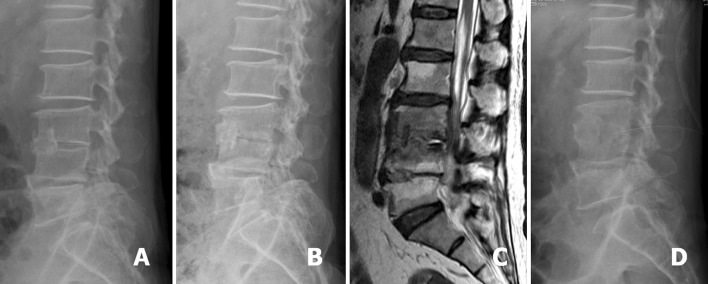
A 60-year-old man was diagnosed as having L3–4 infectious spondylitis and underwent L3–4 debridement and associated anterior interbody fusion with anterior iliac autograft. The lateral radiograph showed the bone graft located at the L3–4 anterior disc space (a). L3–4 recurrent infection developed in this patient and the follow-up lateral radiograph showed L3–4 posterior endplates erosion (b). Sagittal T2-weighted MRI revealed L3–4 infection with pus accumulation at the posterior disc space (c). The postoperative lateral radiograph showed the drainage tube inserted into the L3–4 posterior disc space for continuous drainage after PELD treatment (d)
Table 1.
Patient demographic data and clinical outcome
| Case no. | Age (years) | Gender | Infection level | Diagnosis | Odom’s criteria | Culture | Open surgery |
|---|---|---|---|---|---|---|---|
| 1 | 49 | M | L5–S1 | Epidural abscess | Good | No growth | 5 months later |
| 2 | 68 | F | L4–5, L5–S1 | Multilevel infection | Good | Candida albicans | None |
| 3 | 59 | F | L2–3 | Epidural abscess | Excellent | Enterococcus faecalis | None |
| 4 | 39 | M | L3–4 | Postoperative infection | Good | ORSA | None |
| 5 | 41 | M | L3–4 | Paraspinal abscess | Good | Pseudomonas aeruginosa | None |
| 6 | 40 | M | L5–S1 | Epidural abscess | Good | Streptococcus viridans | None |
| 7 | 63 | M | L3–4, L4–5, L5–S1 | Multilevel infection | Poor | Mycobacterium tuberculosis | 1 week later |
| 8 | 65 | F | L4–5 | Postoperative infection | Good | No growth | None |
| 9 | 87 | M | L4–5, L5–S1 | Multilevel infection | Good | ORSA | None |
| 10 | 73 | F | L3–4, L4–5 | Multilevel infection | Fair | Pseudomonas aeruginosa | 2 weeks later |
| 11 | 50 | M | L2–3 | Paraspinal abscess | Good | OSSA | None |
| 12 | 40 | F | L4–5 | Postoperative infection | Good | Streptococcus viridans | None |
| 13 | 57 | F | L5–S1 | Epidural abscess | Good | ORSA | None |
| 14 | 67 | M | L3–4 | Postoperative infection | Good | OSSA | None |
| 15 | 39 | M | L5–S1 | Postoperative infection | Good | OSSA | None |
| 16 | 57 | M | L4–5 | Epidural abscess | Good | OSSA, Escherichia coli | None |
| 17 | 71 | M | L3–4, L4–5 | Multilevel infection | Fair | Haemophilus influenza | 2 weeks later |
| 18 | 48 | M | L5–S1 | Presacral abscess | Good | ORSA | None |
| 19 | 52 | F | L3–4 | Paraspinal abscess | Good | OSSA | None |
| 20 | 62 | M | L2–3 | Paraspinal abscess | Excellent | ORSA | None |
| 21 | 60 | M | L3–4 | postoperative infection | Good | ORSA | None |
M male, F female, L lumbar spine, OSSA oxacillin sensitive Staphylococcus aureus, ORSA oxacillin resistant Staphylococcus aureus
Fig. 4.
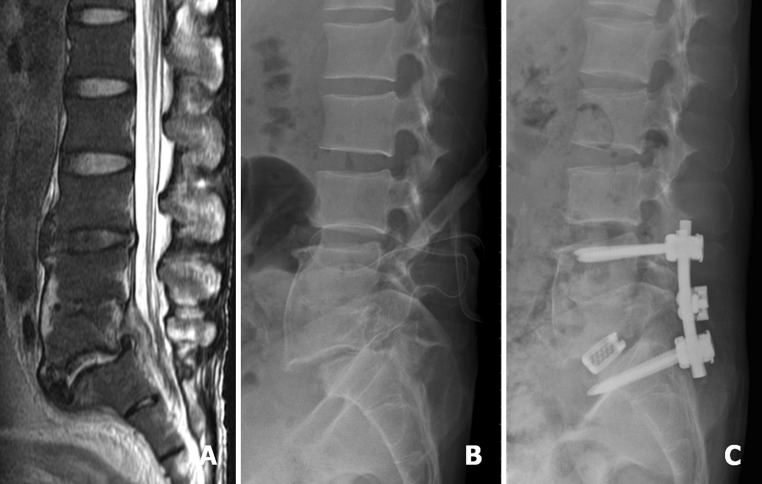
A 49-year-old man was diagnosed as having L5–S1 infectious spondylitis. Sagittal T2-weighted MRI revealed L5–S1 infection with epidural abscess (a). Postoperative lateral radiograph showed L5–S1 spondylolytic spondylolisthesis and the inserted drainage tube located at the L5–S1 disc space (b). At postoperative 5 months, after complete treatment of infection, L4–S1 instrumented fusion was performed for L5–S1 spondylolytic spondylolisthesis (c)
Causative bacteria were identified in the biopsy specimens of 19 (90 %) of 21 patients. Ten patients were infected by Staphylococcus aureus, six with the oxacillin resistant strain, and four with the oxacillin sensitive strain. Two patients had Pseudomonas aeruginosa, two had Streptococcus viridans, and other four had Candida albicans, Enterococcus faecalis, Mycobacterium tuberculosis, and Haemophilus influenzae infection, respectively. One of the 19 patients had 2 different pathogens cultured simultaneously, including oxacillin sensitive Staphylococcus aureus and Escherichia coli. Systemic antibiotics and antituberculous or antifungal chemotherapy were administered, based on the sensitivity studies for the identified pathogens. Broad-spectrum antibiotics were administered to the remaining 2 patients with negative biopsy culture results after PELD, and they recovered uneventfully.
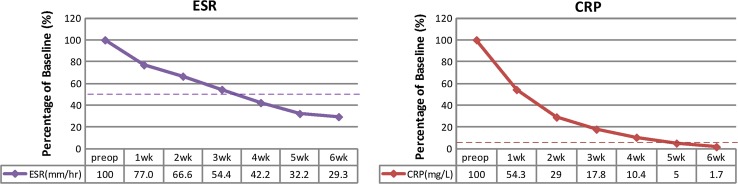
Overall, the 18 patients who had pain relief after PELD were successfully treated with at least a 6-week course of parenteral antibiotic therapy or full-course antimicrobial chemotherapy. The elevated CRP values returned to normal within a mean period of 4.7 weeks, and elevated ESR decreased irregularly to half of the original pretreatment values within a mean period of 3.6 weeks (Fig. 5). No recurrent infection was found among these patients during at least 24 months of follow-up (average 43.1 months, range 25–69 months). The remaining 3 patients underwent anterior debridement and fusion with autograft because of their persistent back pain and progressive infection. All of them originally had advanced spinal infection with multilevel involvement. Extensive osteolytic destruction of the vertebral body with spinal instability or kyphotic deformity was observed in these 3 patients. There was no significant difference in the culture rate among these three different spinal infection patterns. However, the patients who sustained multilevel infection had a worse satisfactory rate (40 %) and infection control rate (40 %) than the patients who had either paraspinal abscess or postoperative infection (100 %) (Table 2). No surgery-related major complications were noted, except in 2 patients who complained of transient paraesthesia in the affected lumbar segments.
Fig. 5.
Percentage changes in serological values, before and after PELD, in successfully treated patients
Table 2.
Comparison of clinical outcomes among three different spinal infection patterns treated by PELD
| Spinal infection pattern | Patient number | Culture rate | Satisfactory rate | Infection control |
|---|---|---|---|---|
| Paraspinal abscess | 10 | 90 % (9/10) | 100 % (10/10) | 100 % (10/10) |
| Postoperative infection | 6 | 83 % (5/6) | 100 % (6/6) | 100 % (6/6) |
| Multilevel infections | 5 | 100 % (5/5) | 40 % (2/5)* | 40 % (2/5)* |
| Total | 21 | 90 % (19/21) | 86 % (18/21) | 86 % (18/21) |
PELD percutaneous endoscopic lavage and drainage
* p < 0.05 with Fisher’s exact test
Discussion
The treatment of spinal infections is still a challenge for spinal surgeons because of the variable presentation and complicated course of spinal infections. Identifying the offending pathogen is critical to provide appropriate medical treatment with antimicrobial therapy. CT-guided needle biopsy has been recommended for the isolation of causative pathogens [16–18]. However, the specimen of the aspirate is often insufficient, and sometimes no organism has been cultured. Delay in appropriate treatment for spinal infections can further lead to structural instability, spinal deformity, sepsis, neurologic deficit, and even death. The efficacy of PELD in terms of its diagnostic and therapeutic value has already been reported in several studies [13–15]. Most of these studies emphasized the importance of early-stage infection control, which usually leads to satisfactory outcomes. The results of this study also demonstrate that PELD is an effective alternative to extensive open surgery for the treatment of advanced infection with paraspinal abscess and postoperative recurrent infection. The PELD technique can provide adequate biopsy specimens for pathogen isolation, relieve the patient’s symptoms, and assist in the eradication of lumbar infectious spondylitis.
In this study, we divided the patients with advanced infectious spondylitis into three groups, mainly based on both the radiographic and MRI findings. Paraspinal abscess, which included subdural abscess, presacral abscess, and perivertebral psoas muscle abscess, was the most common pathogenesis in this series. The neural element located in the lumbar spine can tolerate more mass effect from spinal canal encroachment than the neural element in the cervical or thoracic spine. Therefore, an urgent open decompression surgery is usually not required with abscesses of the lumbar spine. Furthermore, a connection generally exists between the abscesses and infected discs, which is the actual origin of spinal infection. After aggressive debridement of the infected disc and neighbouring vertebral endplates, a cavity was created, and even the sticky abscesses could be washed out by pressurized irrigation with betadine solution. With postoperative negative-pressure Hemovac suction, the pathogens in the infected tissue can be removed continuously. All patients with paraspinal abscess in this study were successfully treated by PELD. This minimally invasive technique produces less morbidity than major open surgery, and provides effective relief of the patient’s back pain by reducing the intradiscal pressure and preserving adequate stability of the spine. As such, the patients were able to ambulate with brace protection as early as possible after PELD.
The postoperative recurrent infection group included 6 patients with anterior autograft interbody fusion, four with supplemental posterior fixation and two without fixation. The culture rate was 83 % and the infection control rate was 100 %. The use of antibiotics based on the culture results of the surgery previous to PELD may explain the rather low culture rate, but satisfactory infection control rate. These patients underwent anterior open surgery with or without posterior fixation in the other hospitals. As they were suspected to have recurrent spinal infection based on their clinical symptoms and signs, they were referred to our institute. Their radiographs revealed endplate erosion at the level of the previous spinal fusion. The MRI also showed a residual and an infected disc with pus accumulation. With the assistance of biplane C-arm fluoroscopy, the endoscopic rongeurs can be precisely inserted into the infected disc to accomplish adequate debridement of the spinal infection. The results of this study showed that recurrent spinal infection can be effectively treated by PELD through a posterolateral approach at the original incision site. The difficult and complicated revision open surgery can be reserved for use in case of failure with this simple procedure.
A sufficient amount of specimen for microbiological examination was obtained from different infected disc regions in the group with multilevel infection, which provided a better diagnostic accuracy. Thus, the culture rate of this group was 100 %. However, the higher culture rate did not ensure good outcomes. Three of 5 patients with multilevel infection eventually underwent open surgery for their poorly controlled infections and the considerable mechanical back pain caused by progressive destruction of the spine. The two other patients who responded to PELD treatment still complained of mild back soreness due to progressive kyphotic deformity. In addition to the problem of infection control, these patients may have suffered another problem involving mechanical instability of the spinal structure. Therefore, the effectiveness of PELD in the treatment of spinal infection in patients with multilevel infection may be limited from the viewpoint of either surgical technique or clinical prognosis.
Povidone-iodine is a widely used antiseptic and disinfectant agent. It can eradicate most pathogens including oxacillin resistant Staphylococcus aureus, and no bacterial resistance has been reported [19–23]. In an experimental study, Kaysinger et al. [24] found that the inhibitory effect of betadine on embryo chick tibia and osteoblast cells was significant at a concentration of 5 % betadine or higher. In contrast, few cytotoxic effects were observed at a lower concentration (0.5 % betadine). Goldenheim [25] reported that 1, 5, or 10 % povidone-iodine preparations do not have a deleterious effect on wound healing in animals and humans. In a clinical study, spinal surgical wounds were soaked with dilute betadine solution before wound closure, and outcomes were compared with those of irrigation with normal saline. A 10 % povidone-iodine solution was diluted to achieve a concentration of 3.5 % betadine, possessing maximal bactericidal activity and minimal cytotoxicity. No wound infection occurred in patients who received betadine irrigation during the follow-up period [26].
This study has several limitations. First, we examined only 21 cases, the results of which may not be generalized. Second, the retrospective nature of this study lacks a random assignment of subjects and does not allow for the enrolled patients to undergo different treatment methods for subsequent comparison of clinical outcomes. As the absence of a control group, it is difficult to validate that the treatment effect is not due to the antibiotics. The feasibility and benefits of PELD when using extended indications for the treatment of spinal infection with paraspinal abscess, recurrent infection, or multilevel involvement need to be rigorously evaluated in a large patient population with prospectively controlled comparison groups. Third, the clinical presentation and severity of spinal infection varied from patient to patient in this study. Some enrolled patients actually matched more than one of the three groups in terms of the initial evaluations of this study. For example, the patients may simultaneously have both epidural abscess and postoperative recurrent infection. However, the patients included in the group with postoperative recurrent infection had a definite past history of spinal infection treated with open surgery.
On the basis of the findings obtained from this study, we propose that PELD is an effective minimal invasive method for the treatment of spinal infection with paraspinal abscess and postoperative recurrent infection. Major open surgery is not always necessary in these cases. In contrast, PELD provides only trivial benefits to patients with multilevel infection because of the extensive destruction with mechanical instability of the affected segments.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine. 1989;14:284–291. [PubMed] [Google Scholar]
- 2.Hadjipavlou AG, Mader JT, Necessary JT, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. doi: 10.1097/00007632-200007010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Krodel A, Sturz H, Siebert CH. Indications for and results of operative treatment of spondylitis and spondylodiscitis. Arch Orthop Trauma Surg. 1991;110:78–82. doi: 10.1007/BF00393878. [DOI] [PubMed] [Google Scholar]
- 4.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. doi: 10.1097/01.blo.0000203452.36522.97. [DOI] [PubMed] [Google Scholar]
- 5.Bonfiglio M, Lange TA, Kim YM. The classic: pyogenic vertebral osteomyelitis: disk space infections. Clin Orthop Relat Res. 2006;444:4–8. doi: 10.1097/01.blo.0000201172.64764.bb. [DOI] [PubMed] [Google Scholar]
- 6.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. doi: 10.1302/0301-620X.79B5.8078. [DOI] [PubMed] [Google Scholar]
- 7.Rezai AR, Woo HH, Errico TJ, et al. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018–1025. doi: 10.1097/00006123-199905000-00047. [DOI] [PubMed] [Google Scholar]
- 8.Haaker RG, Senkal M, Kielich T, et al. Percutaneous lumbar discectomy in the treatment of lumbar discitis. Eur Spine J. 1997;6:98–101. doi: 10.1007/BF01358740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata K, Ohashi T, Ariyoshi M, et al. Percutaneous suction aspiration and drainage for pyogenic spondylitis. Spine. 1998;15:1600–1606. doi: 10.1097/00007632-199807150-00018. [DOI] [PubMed] [Google Scholar]
- 10.Hadjipavlou AG, Katonis PK, Gaitanis IN, et al. Percutaneous transpedicular discectomy and drainage in pyogenic spondylodiscitis. Eur Spine J. 2004;13:707–713. doi: 10.1007/s00586-004-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanaoka N, Kawasaki Y, Sakai T, et al. Percutaneous drainage and continuous irrigation in patients with severe pyogenic spondylitis, abscess formation, and marked bone destruction. J Neurosurg Spine. 2006;4:374–379. doi: 10.3171/spi.2006.4.5.374. [DOI] [PubMed] [Google Scholar]
- 12.Tofuku K, Koga H, Yone K, et al. Continuous irrigation in pyogenic spondylitis accompanied by iliopsoas abscess. Spine. 2007;32:E382–E387. doi: 10.1097/BRS.0b013e318067e35e. [DOI] [PubMed] [Google Scholar]
- 13.Ito M, Abumi K, Kotani Y, et al. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine. 2007;32:200–206. doi: 10.1097/01.brs.0000251645.58076.96. [DOI] [PubMed] [Google Scholar]
- 14.Yang SC, Fu TS, Chen LH, et al. Percutaneous endoscopic discectomy and drainage for infectious spondylitis. Int Orthop. 2007;31:367–373. doi: 10.1007/s00264-006-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu TS, Yang SC, Tsai TT, et al. Percutaneous endoscopic debridement and drainage in immunocompromised patients with complicated infectious spondylitis. Minim Invasive Ther Allied Technol. 2010;19:42–47. doi: 10.3109/13645700903384450. [DOI] [PubMed] [Google Scholar]
- 16.Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;18:211–214. doi: 10.1148/radiology.218.1.r01ja06211. [DOI] [PubMed] [Google Scholar]
- 17.Rankine JJ, Barron DA, Robinson P, et al. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80:607–609. doi: 10.1136/pgmj.2003.017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang SC, Fu TS, Chen LH, et al. Identify pathogen of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop Relat Res. 2008;466:3086–3092. doi: 10.1007/s11999-008-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill RL, Casewell MW. The in vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions. J Hosp Infect. 2000;45:198–205. doi: 10.1053/jhin.2000.0733. [DOI] [PubMed] [Google Scholar]
- 20.McLure AR, Gordon J. In-vitro evaluation of povidone-iodine and chlorhexidine against methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1992;21:291–299. doi: 10.1016/0195-6701(92)90139-D. [DOI] [PubMed] [Google Scholar]
- 21.Reimer K, Wichelhaus TA, Schäfer V, et al. Antimicrobial effectiveness of povidone-iodine and consequences for new application areas. Dermatology. 2002;204(Suppl 1):S114–S120. doi: 10.1159/000057738. [DOI] [PubMed] [Google Scholar]
- 22.Block C, Robenshtok E, Simhon A, et al. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect. 2000;46:147–152. doi: 10.1053/jhin.2000.0805. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda T, Yoshimura S, Katsuno Y, et al. Comparison of bactericidal activities of various disinfectants against methicillin-sensitive Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Postgrad Med J. 1993;69(Suppl 3):S66–S69. [PubMed] [Google Scholar]
- 24.Kaysinger KK, Nicholson NC, Ramp WK, et al. Toxic effects of wound irrigation solutions on cultured tibiae and osteoblasts. J Orthop Trauma. 1995;9:303–311. doi: 10.1097/00005131-199509040-00006. [DOI] [PubMed] [Google Scholar]
- 25.Goldenheim PD. An appraisal of povidone-iodine and wound healing. Postgrad Med J. 1993;69(Suppl 3):S97–S105. [PubMed] [Google Scholar]
- 26.Cheng MT, Chang MC, Wang ST, et al. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine. 2005;30:1689–1693. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]