Abstract
It is well known that heterotrimeric G protein is composed of a Gα-subunit and a Gβγ-dimer and promotes cancer characteristics. Our recent study showed reduced G protein γ2 subunit (Gng2/GNG2) expression levels in malignant melanoma cells compared with those in benign melanocytic cells in both mice and humans. Our recent study also showed that reduced GNG2 alone augmented proliferation of malignant melanoma cells. To our knowledge, however, there is no evidence showing an effect of Gng2/GNG2 alone on metastasis of any cancers including malignant melanoma. In his study, we first prepared GNG2-overexpressed SK-Mel28 human malignant melanoma cells, in which GNG2 protein expression level was undetectably low. Migration and invasion activities of the GNG2-overexpressed malignant melanoma cells were suppressed up to 1/10th, with decreased activity of focal adhesion kinase (FAK). We then found that the expression level of GNG2 in A375M, a highly metastatic cell line, was significantly lower than that in A375P, the parental cell line of A375M. We finally showed that knockdown of GNG2 alone in A375P cells enhanced migration and invasion with increased FAK activity. Taken together, our results suggest that overexpression of GNG2 alone inhibits metastasis in human malignant melanoma cells with decreased FAK activity. Thus, GNG2 might be a candidate of molecular targets of prevention and therapy for metastasis of malignant melanoma.
Keywords: G-protein, gamma subunit, malignant melanoma, invasion
Introduction
The incidence of malignant melanoma is increasing by 3.1% per year and its incidence rate doubled over a 10-year period [1]. Malignant melanoma is the most serious skin cancer and is markedly resistant to conventional therapy [2-5]. Since malignant melanoma is known as a representative of highly invasive and metastatic cancers, control of metastasis is an important task for therapy of malignant melanoma.
Heterotrimeric G protein has been reported to be involved in various biological activities including cell proliferation, differentiation, invasion and angiogenesis [6,7]. G protein γ2 subunit (Gng2/GNG2) is one of subunits of the Gβγ-dimer composing heterotrimeric G protein with a Gα-subunit. We previously showed that Gng2 expression level in malignant melanoma was lower than that in benign melanocytic tumors in RET-transgenic mice (RET-mice), in which benign tumors and malignant melanomas spontaneously develop [8-10]. Expression levels of GNG2 in five human melanoma cell lines were also reduced compared with the level in normal human epithelial melanocytes (NHEM) [11], suggesting that GNG2 could be a novel biomarker for malignant melanoma [11]. Our previous study also showed that depleted GNG2 in human malignant melanoma cells enhanced proliferation of human malignant melanoma cells [12]. However, there has been no study showing direct evidence of the involvement of GNG2 for cancer other than the above-mentioned studies. In fact, the effect of Gng2/GNG2 alone on metastasis of any cancers including malignant melanoma remains unknown.
Previous studies showed that activities of invasion and migration are correlated with activities of metastasis [4,13]. Therefore, in this study, we examined the effect of GNG2 on migration/invasion activities in both GNG2-overexpressed and -depleted human malignant melanoma cells.
Materials and methods
Cell lines and culture conditions
SK-Mel28, A375P and A475M human malignant melanoma cells were cultured in RPMI1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin. All cells were maintained at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. A375P and A375M cells were kindly provided from Dorothy C Bennett (St. George’s, University of London, UK). SK-Mel28 cells were kindly provided from the Riken Bioresource Center Cell Bank.
Expression vectors and establishment of stable clones
Expression vector pIRES-puro3 (Invitrogen) was used for construction of the GNG2 expression vector. The human GNG2 coding region fused with a FLAG sequence was inserted into the EcoRI and BamHI sites. Empty and GNG2 expression vectors were transfected into SK-Mel28 cells with Lipofectamine LTX (Invitrogen) and stable cell clones were selected with 1 μg/ml puromycin (Wako).
Wound healing assay
Cells were seeded on 6-well plastic dishes at a density of 6 x 105 cells per well. The confluent cells were scratched with a 200 μl disposable pipette tip and allowed to migrate toward the wound for 16 hours. Images were taken with an Olympus inverted microscope coupled to a CCD-SPOT digital camera (Olympus DP71).
Migration and invasion assay
A total of 1.5 x 105 cells were seeded on a non-coated (for migration assay) or Matrigel (100 μg/ml, BD Bioscience)-coated (for invasion assay) chamber (24 wells) with 300 μl of serum-free RPMI-1640 medium, and 900 μl of 10% FBS-containing RPMI-1640 medium including chemoattractants was added to the lower chamber to induce invasion of cells. After 24 hours, the inner surface of the chamber was gently scraped to leave only cells on the outer layer. The remaining cells were subsequently fixed with 10% formaldehyde and stained with crystal violet, and the number of cells was counted. The results of three independent experiments were normalized and presented as a migration or invasion index.
Immunoblot analysis
Immunoblot analysis was performed by the method previously described [14]. Rabbit polyclonal antibodies against Gng2 (Proteintech Group) and phosphorylated tyrosine 397 in FAK (Invitrogen) and mouse monoclonal antibodies against alpha-TUBULIN (SIGMA) and FAK (Millipore) were used as first antibodies. Densitometric evaluation was performed using the software program WinROOF (MITANI Corporation) as previously reported [15].
RNA interference
Small interfering RNA (siRNA)-mediated depletion (knockdown) of GNG2 was performed with 21-nucleotide synthetic duplexes (Invitrogen). Cells were transfected with GNG2 siRNA or a 21-nucleotide control RNA (Invitrogen) using Lipofectamine RNAi MAX (Invitrogen) according to the manufacturer’s protocol. After the cells had been incubated for 48 hrs, they were used for cell migration and invasion assays.
Statistical analysis
Statistical analysis in this study was performed according to the method previously described [8]. Results from more than three independent experiments in each group were statistically analyzed by Student’s t-test. We used the SPSS (version 18) software package (SPSS Japan Inc.) for these statistical analyses, and the significance level was set at p<0.05.
Ethics statement
This study was performed in Chubu University, Japan. The study was approved by Recombination DNA Advisory Committee (approval no. 10-08) in Chubu University.
Results
GNG2 overexpression in SK-Mel28 cells repressed invasion activity
To address the role of GNG2 alone in human malignant melanomas, more than 10 GNG2-overexpressed clones were established, and 3 control and 3 GNG2-overexpressed SK-Mel28 cell clones were used in this study (Figure 1A). A wound healing assay, migration assay and invasion assay in vitro were performed to examine activities of motility and invasiveness of GNG2-overexpressed SK-Mel28 cells. In the wound healing assay, motility of GNG2-overexpressed SK-Mel28 cells was repressed (Figure 1B). In vitro cell migration (top panels of Figure 1C; left panel of Figure 1D) and invasion (bottom panels of Figure 1C; right panel of Figure 1D) assays revealed that migration and invasion activities of GNG2-overexpressed SK-Mel28 cells were suppressed to less than 1/3rd of those of control SK-Mel28 cells. The same results were obtained in comparative studies on migration and invasion using two other control cell clones (Clones 2 and 3) and one GNG2-overexpressed cell clone (Clone 5) (data not shown). These results suggest that a high expression level of GNG2 inhibits migration and invasion of human malignant melanoma cells. G-protein-related tumor invasion is associated with the activity of focal adhesion kinase (FAK) [6,7,16,17]. We thus examined the activation status of FAK in GNG2-overexpressed SK-Mel28 cells. Phosphorylated FAK levels in GNG2-overexpressed cells were significantly decreased compared with those in control cells (Figure 2). Taken together, the results suggest that overexpression of GNG2 in malignant melanoma cells suppresses motility, migration and invasion with inhibition of FAK phosphorylation.
Figure 1.
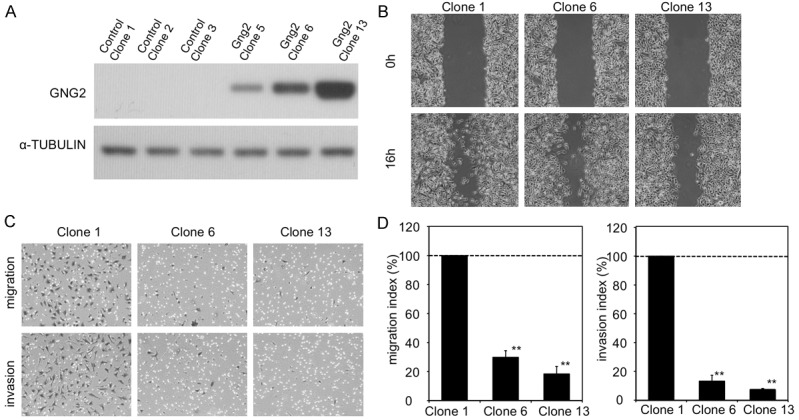
GNG2 inhibits migration and invasion of SK-Mel28 cells. (A) Levels of GNG2 protein expression in stable clones of SK-Mel28 cells with empty (Clones 1-3) or GNG2 expression (Clones 5, 6 and 13) vectors are presented. Two stable clones (Clones 6 and 13) were used in subsequent experiments. (B) Wound healing assay was performed with control (Clone 1) and GNG2-overexpressed (Clones 6 and 13) SK-Mel28 cells. Cultured cells were scratched with a sterile pipette tip and evaluated after incubation for 16 hrs. (C, D) Cell migration (top panels in C; left panel in D) and invasion (bottom panels in C; right panel in D) in vitro were examined by the Boyden chamber assay and Matrigel invasion assay, respectively. Morphology (C) and index as percentages (D; mean ± SE) of migrating and invading cells after incubation for 16 hrs are shown. **, Significantly different (**, p<0.01) from the control (Clone 1) by Student’s t-test.
Figure 2.
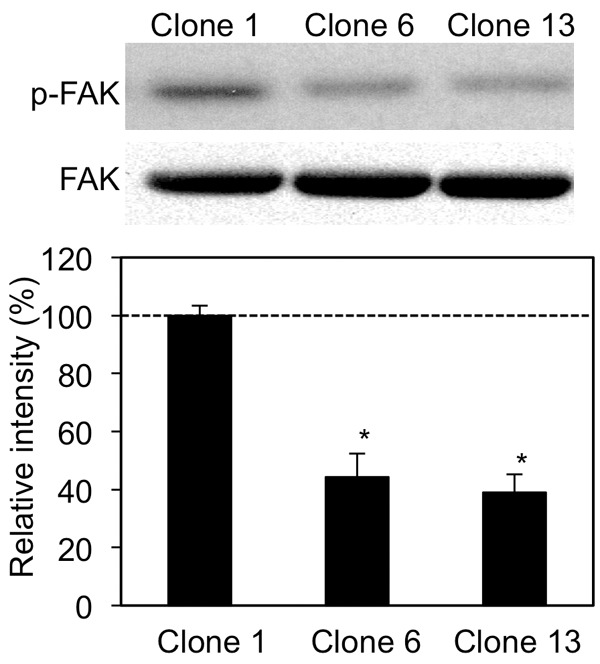
FAK activities in GNG2-overexpressed SK-Mel28 cells. Phosphorylation and/or protein expression levels of FAK are presented. Intensities of bands are presented as percentages (mean ± SD; n=3) relative to the control (Clone 1). *, Significantly different (*, p<0.05) by Student’s t-test.
GNG2 depletion in A375P cells enhanced invasion activity
Previous studies revealed that two sub-lines of A375 represented the parental cell line (A375P) and a highly metastatic sub-line (A375M) derived by serial passage of the tumor through tail vein injections and isolation of pulmonary metastases [18]. To elucidate the relationship between expression level of GNG2 and invasiveness, we assayed expression levels of GNG2 in A375P and A375M cells. In this assay, expression level of GNG2 in A375P cells was significantly higher than that in A375M cells, suggesting a potential correlation between Gng2 and invasiveness (Figure 3A). We further examined the effects of decreased GNG2 expression on activities of migration and invasion in GNG2-deleted A375P human malignant melanoma cells through siRNA transfection (Figures 3B and 4). As expected, migration (top panels of Figure 4A and left graph of Figure 4B) and invasion (bottom panels of Figure 4A and right graph of Figure 4B) activities were enhanced in GNG2-depleted A375P cells. In accordance with the cellular physiological results, phosphorylation levels of FAK were significantly increased in GNG2-depleted A375P cells (Figure 4C). These results suggest that depletion of GNG2 enhances the promotion of migration and invasion through FAK activation.
Figure 3.
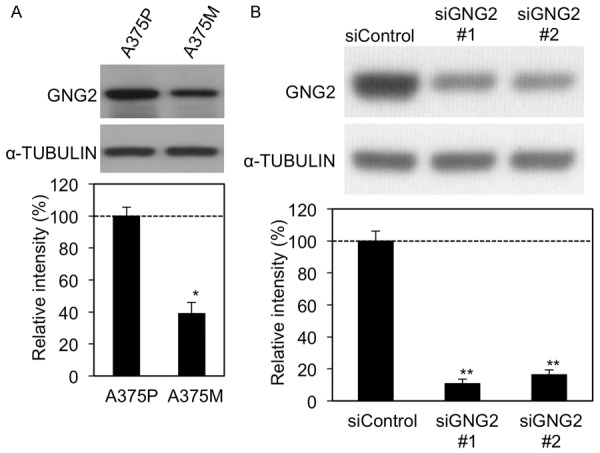
Expression levels of GNG2 protein in A375P and A375M human melanoma cell lines. (A) Levels of GNG2 protein expression in A375P and A375M cells. (B) Levels of GNG2 protein expression in control (siControl) and GNG2-depleted (siGNG2 #1 and #2) A375P cells. Intensities of bands are presented as percentages (mean ± SD; n=3) relative to A375P (A) or siControl (B). * and **, Significantly different (*, p<0.05; **, p<0.01) by Student’s t-test.
Figure 4.
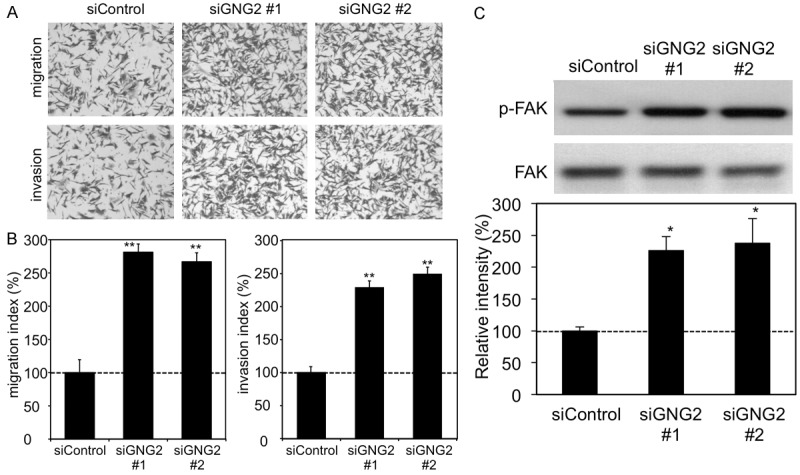
Effect of GNG2 depletion on characteristics of A375P cells. (A) Cell migration (top panels) and invasion (bottom panels) of control (siControl) and GNG2-depleted (siGNG2 #1 and #2) A375P cells were examined by the Boyden chamber assay and Matrigel invasion assay, respectively. (B) Indexes of cell migration and invasion after incubation for 24 hrs are shown as percentages (mean ± SD; n=3) relative to siControl. (C) Phosphorylation and/or protein expression levels of FAK in control (siControl) and GNG2-depleted (siGNG2 #1 and #2) A375P cells are presented. Intensities of bands are presented as percentages (mean ± SD; n=3) relative to siControl. * and **, Significantly different (*, p<0.05; **, p<0.01) by Student’s t-test.
Discussion
In this study, we first demonstrated that cell migration and invasion were strongly inhibited in stable GNG2-overexpressed SK-Mel28 human malignant melanoma cells with inhibition of FAK phosphorylation. We then demonstrated that GNG2 expression level in the highly invasive A375M human malignant melanoma cell line was significantly lower than that in the weakly invasive A375P cell line, which is a parental cell line of A375M. We finally demonstrated that migration and invasion of A375P cells with GNG2 depletion by siRNA knockdown were enhanced with increased FAK activity.
In previous studies, overexpression of the Gβγ-dimer promoted invasion with activation of FAK [7,16], while reduction of the Gβγ-dimer suppressed cell invasion [19]. However, our results showed that overexpression of GNG2 alone in SK-Mel28 human melanoma cells inhibited cell motility, migration and invasion with decreased FAK activity and that reduction of GNG2 alone enhanced migration and invasion of A375M human melanoma cells with increased FAK activity. Previous studies showed that expression of human G protein gamma 7 subunit (GNG7) was downregulated in esophageal cancer cells [20] and that loss of the expression of GNG7 alone increased the depth of tumor invasion and aggressiveness [21]. Thus, modulation of the expression level of G protein gamma subunit alone such as GNG2 and GNG7 may exhibit different biological functions compared with that of Gβγ-dimer. However, it remains unknown how GNG2 might function as a tumor suppressor in malignant melanoma. For example, dopamine receptor-interacting protein 78 (DRiP78), one of the modulators for Gβγ assembly, interacts with the Gγ subunit but not with Gß and Gα subunits. DRiP78 also competes with the Gß subunit to interact with the Gγ subunit and prevents degradation of Gγ until provision of Gß [22]. Overexpression of GNG2 alone may affect the function of regulators for Gßγ such as DRiP78 and modulate normal Gßγ assembly, resulting in a suppressive effect on malignant melanoma. In addition, depletion of Gng2 alone in zebrafish development blocked the normal angiogenic process by modulating Vegf signaling [6], suggesting that the function of GNG2 might be associated with angiogenesis to trigger invasion of malignant melanoma. In any case, further investigation of the roles of GNG2 alone in malignant melanoma cells is needed.
In summary, this study showed that modulation of GNG2 protein expression level affects the invasion and migration activities of malignant melanoma cells with modulation of FAK activity. Our results suggest that GNG2 could be a new potential molecular target for prevention and therapy of metastasis in malignant melanoma.
Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research (B) (24390157 and 24406002), and (C) (25340052), Research Fellow of Japan Society for the Promotion of Science (25-40080), Grant-in-Aid for Challenging Exploratory Research (23650241) and Grant-in-Aid for Scientific Research on Innovative Areas (24108001) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), the Uehara Memorial Foundation, the Naito Foundation, the Cosmetology Research Foundation, TOYOAKI Scholarship Foundation, the Aichi Health Promotion Foundation and the Aichi Cancer Research Foundation.
Disclosure of conflict of interest
None.
References
- 1.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149–2182. doi: 10.1101/gad.1437206. [DOI] [PubMed] [Google Scholar]
- 3.Yajima I, Kumasaka MY, Thang ND, Goto Y, Takeda K, Iida M, Ohgami N, Tamura H, Yamanoshita O, Kawamoto Y, Furukawa K, Kato M. Molecular Network Associated with MITF in Skin Melanoma Development and Progression. J Skin Cancer. 2011;2011:730170. doi: 10.1155/2011/730170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yajima I, Kumasaka MY, Thang ND, Goto Y, Takeda K, Yamanoshita O, Iida M, Ohgami N, Tamura H, Kawamoto Y, Kato M. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT Signaling in Malignant Melanoma Progression and Therapy. Dermatol Res Pract. 2012;2012:354191. doi: 10.1155/2012/354191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagishita T, Yajima I, Kumasaka M, Kawamoto Y, Tsuzuki T, Matsumoto Y, Watanabe D, Kato M. Actin-binding Protein, Espin: a Novel Metastatic Regulator for Melanoma. Mol Cancer Res. 2013 doi: 10.1158/1541-7786.MCR-13-0468-T. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Leung T, Chen H, Stauffer AM, Giger KE, Sinha S, Horstick EJ, Humbert JE, Hansen CA, Robishaw JD. Zebrafish G protein gamma2 is required for VEGF signaling during angiogenesis. Blood. 2006;108:160–166. doi: 10.1182/blood-2005-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- 8.Kato M, Hattori T, Ito H, Kageyama M, Yamashita T, Nitta Y, Nakashima I. Serum-soluble Fas levels as a marker to distinguish allergic and nonallergic rhinitis. J Allergy Clin Immunol. 1999;103:1213–1214. doi: 10.1016/s0091-6749(99)70202-2. [DOI] [PubMed] [Google Scholar]
- 9.Kato M, Takeda K, Kawamoto Y, Tsuzuki T, Hossain K, Tamakoshi A, Kunisada T, Kambayashi Y, Ogino K, Suzuki H, Takahashi M, Nakashima I. c-Kit-targeting immunotherapy for hereditary melanoma in a mouse model. Cancer Res. 2004;64:801–806. doi: 10.1158/0008-5472.can-03-2532. [DOI] [PubMed] [Google Scholar]
- 10.Kumasaka MY, Yajima I, Hossain K, Iida M, Tsuzuki T, Ohno T, Takahashi M, Yanagisawa M, Kato M. A novel mouse model for de novo Melanoma. Cancer Res. 2010;70:24–29. doi: 10.1158/0008-5472.CAN-09-2838. [DOI] [PubMed] [Google Scholar]
- 11.Yajima I, Kumasaka MY, Naito Y, Yoshikawa T, Takahashi H, Funasaka Y, Suzuki T, Kato M. Reduced GNG2 expression levels in mouse malignant melanomas and human melanoma cell lines. Am J Cancer Res. 2012;2:322–329. [PMC free article] [PubMed] [Google Scholar]
- 12.Yajima I, Kumasaka MY, Tamura H, Ohgami N, Kato M. Functional analysis of GNG2 in human malignant melanoma cells. J Dermatol Sci. 2012;68:172–178. doi: 10.1016/j.jdermsci.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Thang ND, Yajima I, Kumasaka MY, Ohnuma S, Yanagishita T, Hayashi R, Shekhar HU, Watanabe D, Kato M. Barium promotes anchorage independent growth and invasion of human HaCaT keratinocytes via activation of c-SRC kinase. PLoS One. 2011;6:e25636. doi: 10.1371/journal.pone.0025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato M, Wickner W. Vam10p defines a Sec18p-independent step of priming that allows yeast vacuole tethering. Proc Natl Acad Sci U S A. 2003;100:6398–6403. doi: 10.1073/pnas.1132162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohgami N, Ida-Eto M, Shimotake T, Sakashita N, Sone M, Nakashima T, Tabuchi K, Hoshino T, Shimada A, Tsuzuki T, Yamamoto M, Sobue G, Jijiwa M, Asai N, Hara A, Takahashi M, Kato M. c-Ret-mediated hearing loss in mice with Hirschsprung disease. Proc Natl Acad Sci U S A. 2010;107:13051–13056. doi: 10.1073/pnas.1004520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 18.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 19.Faivre S, Regnauld K, Bruyneel E, Nguyen QD, Mareel M, Emami S, Gespach C. Suppression of cellular invasion by activated G-protein subunits Galphao, Galphai1, Galphai2, and Galphai3 and sequestration of Gbetagamma. Mol Pharmacol. 2001;60:363–372. doi: 10.1124/mol.60.2.363. [DOI] [PubMed] [Google Scholar]
- 20.Shibata K, Mori M, Tanaka S, Kitano S, Akiyoshi T. Identification and cloning of human G-protein gamma 7, down-regulated in pancreatic cancer. Biochem Biophys Res Commun. 1998;246:205–209. doi: 10.1006/bbrc.1998.8581. [DOI] [PubMed] [Google Scholar]
- 21.Ohta M, Mimori K, Fukuyoshi Y, Kita Y, Motoyama K, Yamashita K, Ishii H, Inoue H, Mori M. Clinical significance of the reduced expression of G protein gamma 7 (GNG7) in oesophageal cancer. Br J Cancer. 2008;98:410–417. doi: 10.1038/sj.bjc.6604124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupre DJ, Robitaille M, Richer M, Ethier N, Mamarbachi AM, Hebert TE. Dopamine receptor interacting protein 78 acts as a molecular chaperone for Ggamma subunits before assembly with Gbeta. J Biol Chem. 2007;282:13703–13715. doi: 10.1074/jbc.M608846200. [DOI] [PubMed] [Google Scholar]


