Abstract
Gastric cancer (GC) is one of the most common and deadly malignancies nowadays, and inflammatory cells are closely related to tumor progression. This prospective study aims to uncover clinical significance of peripheral immune cells and build a treatment-predictive model. From July 2006 to July 2011, a total of 1131 GC patients were selected, with their general characteristics, peripheral blood and pathological parameters, and operational information obtained. The relevancies between preoperational neutrophil-lymphocyte ratio (NLR) and postsurgical pathological indexes were analyzed. SPSS 17.0 was applied in data analysis, comparing the differences of NLR between different groups using Mann-Whitney U test, contrasting the pathological differences between NLR elevated and reduced groups using Fisher test, and quantifying the correlation between post-surgical pathology and pre-operational NLR using univariate analysis. Patients were then classified into radical (applied in the training dataset) and non-radical gastrectomy (applied in the test dataset) groups, based on which we further tried to build a predictive model indicating appropriateness for radical resection using support vector machine (SVM). We found that: patients with tumor invading out of the myometrium (pT3-4) had significantly larger NLR than those with lesion limited within the myometrium (pT1-2) (P<0.05); poorly differentiated and undifferentiated malignancies were associated with higher NLR than well and moderately differentiated ones (P<0.05); there was larger NLR among patients with tumor length ≥4 cm than those <4 cm (P<0.01); preoperative NLR was significantly positively correlated with tumor TNM classification, number of metastatic lymph nodes, invasive depth and tumor size (P<0.05); larger proportion of elevated NLR was significantly associated with larger tumor size, later tumor and nodal stages, and higher TNM classification (P<0.01). We finally built a SVM model based on peripheral carcinoembryonic antigen, carbohydrate antigen 19-9, lymphocyte percentage and platelet count, effectively predicting the inappropriateness of patients undergoing curative gastrectomy when all the 4 parameters elevated with high accuracy (74.61% for the training dataset and 75.28% for the test dataset). We concluded that peripheral blood NLR indicated tumor progression, and that an efficient treatment-predictive SVM model was constructed.
Keywords: Gastric carcinoma, neutrophil-lymphocyte ratio, support vector machine, gastrectomy, tumor progression
Introduction
Gastric carcinoma (GC) is one of the most common and deadly malignancies nowadays [1,2]. The efficacy of GC treatment depends heavily on stage of the lesion and way of gastrectomy [3]. Patients receiving resection at early stages could enjoy an ideal overall 5-year survival rate of over 90%, while more than 95% of those detected at advanced stages died within 5 years post-operation [4]. However, less than 10% of GC patients could be identified at early stages due to insignificant symptoms. Surgery is the major treatment, while radical resection cannot always be guaranteed [5].
Immune cells in tumor micro-environment including granulocytes and lymphocytes, which are closely correlated with those in peripheral blood, are important components of tumor stroma, regulating carcinogenesis and metastasis [6,7]. High neutrophil and low lymphocyte percentages are considered to be linked with poor prognosis [8-10]. Better understanding of the correlation between tumor and immune system may contribute to screening effective immunotherapy strategies [11].
The progressive degree of GC is mainly obtained through post-operational pathology. If an effective treatment-predictive method is available, patients’ survival rate might be significantly improved with adequate classification-oriented treatment applied. Up till now, no study on pathological significance of peripheral neutrophil-lymphocyte ratio (NLR) has been found and no GC treatment-predictive model is available. This novel study aims to uncover clinical significance of peripheral immune cells and build a model contributing to an ideal management plan before surgery.
Materials and methods
Patients and specimens
From July 2006 to July 2011, a total of 1237 patients diagnosed with GC pathologically in the First Affiliated Hospital of Anhui Medical University were selected. Apart from 106 individuals who quitted the study half-way or were affected greatly by irrelevant factors or whose samples or data went against our standards, finally 1131 patients were included in analysis (Table 1). They were in relatively fine overall conditions (Hb>90 g/L, albumin >30 g/L) without severe dysfunction of important organs or systematic unfit like refractory ascites or dyscrasia. Besides, they were confirmed without severe mental disorders.Patients undergoing multivisceral resection or having other gastroenteric diseases were excluded from our study. The selected patients did not have a history of gastroenterological surgery. They had not received any chemo-, radio-, or interventional therapy before. We had complete data of each of them. Written informed consent was obtained from each patient, and our study was permitted by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University (reference number 2012608) and carried out according to the Helsinki Declaration.
Table 1.
Clinicopathological features of the patients enrolled
| Item | Value |
|---|---|
| Number of patients enrolled | 1131 |
| Gender (Male/Female) | 823/308 |
| Pathological general stage (Early/Advanced) | 115/1016 |
| Age (y) | 60.41±10.58 |
| Hemoglobin (g/L) | 113.50±25.94 |
| Leukocyte count (109/L) | 5.86±1.98 |
| Neutrophil percentage (%) | 62.25±9.74 |
| Lymphocyte percentage (%) | 27.95±8.85 |
| Monocyte percentage (%) | 6.46±3.37 |
| Eosinophil percentage (%) | 2.91±2.88 |
| Basophil percentage (%) | 0.42±0.46 |
| Platelet count (109/L) | 205.20±80.86 |
| Serum CEAa (μg/L) | 17.67±77.39 |
| Serum CA 19-9b (U/mL) | 66.77±15.44 |
| Tumor TNM classification (I/II/III/IV) | 216/234/610/71 |
| Nodal stage (N0/N1/N2/N3) | 391/253/245/242 |
| Differentiation (well and moderate/poor and undifferentiated) | 373/758 |
| Tumor length (cm) | 5.27±3.05 |
| Tumor width (cm) | 4.13±2.34 |
CEA, carcinoembryonic antigen;
CA 19-9, carbohydrate antigen 19-9.
Enrolled patients underwent either radical or non-radical gastrectomy. All resections were conducted by the same group of operators (A.M.X., L.H., L.Z. and Z.J.W.), classifying patients into radical (n=753, applied in the training dataset, including radical partial and radical total gastrectomy) and non-radical gastrectomy(n=376, applied in the test dataset, including laparotomy, palliative resection and short-circuit operation) groups.
Before operation, all eligible patients’ peripheral blood cells parameters, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) were obtained. NLR was calculated, dividing all patients into NLR elevated (n=218)and reduced groups (n=913) with a threshold of 3.5 based on the median of all data. After surgery, the removed specimens were fixed by neutral formalin, dehydrated by graded ethanol, embedded in paraffin, serially sectioned at thickness of 4 μm, dyed by hematoxylin-eosin, and observed by optical microscopy. Pathological results were in accordance to Japanese Classification of Gastric Carcinoma (3rd English edition) [12].
Statistical analyses
We applied SPSS 17.0 to analyze data. The differences of NLR between different groups were measured using Mann-Whitney U test. The pathological differences between NLR elevated and reduced groups were detected using Fisher test. The correlation between post-surgical pathology and pre-operational NLR was calculated using univariate analysis (Pearson or Spearman test according to data type) with coefficient r or rank coefficient r s calculated. Measurement data were in mean ± standard deviation. Results were considered significant with P<0.05, and very significant with P<0.01.
Construction of a treatment-predictive model
Starting from a list of 14 clinicopathologic features including patients’ general characteristics (gender and age) and peripheral blood indexes (percentages of neutrophil, lymphocyte, mononuclear, eosinophil and basophil, and leukocyte, erythrocyte and platelet counts, and hemoglobin), we applied support vector machine (SVM) to construct a classifier of the clinical outcome after treatment. We first tried to optimize the performance of SVM (the fraction of correctly classified samples divided by the total number of samples) on a training dataset of 753 patients. Here SVM with radical kernel [ISBN 0-387-98780-0] is used, because our problem at hand is highly complex and non-linear. At the beginning, we included every features in the SVM model and perform a 5-fold cross-validation to access its performance within the training dataset. By a trial on removing each single feature, we found the least informative feature among the 14 (that is, the one with best performance upon its removal) and get a reduced list of 13 clinicopathologic features. We repeated this process until only one feature left, and the SVM model with the best performance among this whole feature selection process is used and reported in this study. Finally, we also applied this trained SVM model on an additional test dataset of 376 patients. To use Receiver Operating Characteristic (ROC) curve analysis, the clinicopathologic features were dichotomized at different cutoffs.
Results
Pre-operational NLR was 2.43±1.23 (range, 0.44-10.33). There existed no significant differences in pre-operational NLR between female and male (2.28±1.31 vs 2.49±1.19, P=0.495), and individuals <65 years and those ≥65 (2.55±1.29 vs 2.36±1.19, P=0.140). Patients with tumor invading out of the myometrium (pT3-4) had significantly larger NLR than those with lesion limited within the myometrium (pT1-2) (2.51± 1.24 vs 2.19±1.15, P=0.011). Poorly differentiated and undifferentiated malignancies were associated with higher NLR than well and moderately differentiated ones (2.46±1.40 vs 2.31±1.14, P=0.020). There was larger NLR among patients with tumor length ≥4 cm than those <4 cm (2.56±1.24 vs 2.16± 1.15, P=0.000), and there also existed significant discrepancies in NLR between different tumor TNM classification (I, 2.13±1.00; II, 2.40±1.34; III, 2.53±1.25; IV, 2.60±1.07; P=0.000), and nodal stage (N0, 2.31±1.15; N1, 2.32±1.22; N2, 2.43±1.16; N3, 2.75±1.37; P=0.000) (Figure 1).
Figure 1.
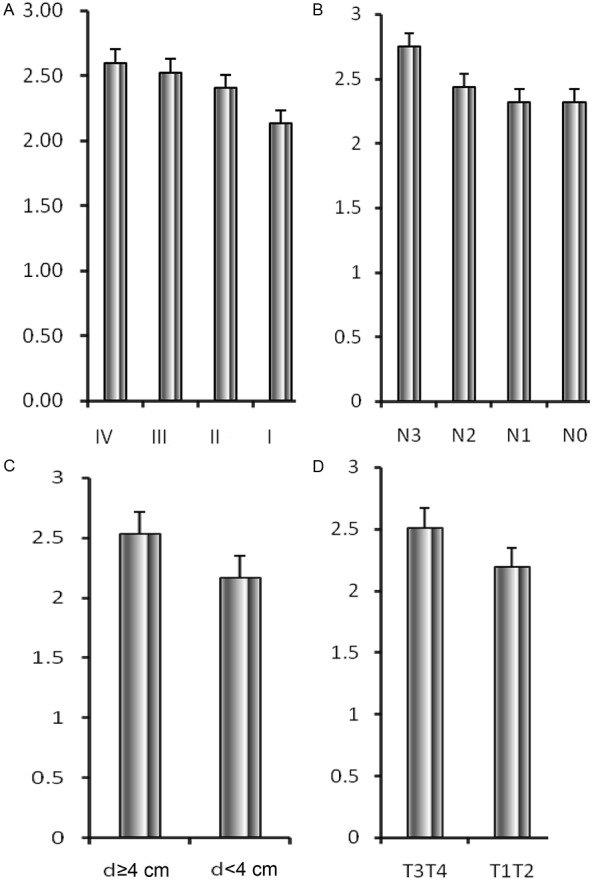
Neutrophil-lymphocyte ratio based on (A) TNM stage, (B) nodal stage [N], (C) tumor length, and (D) tumor stage [pT]. (A) There existed significant discrepancies in NLR between different tumor TNM classification (I, 2.13±1.00; II, 2.40±1.34; III, 2.53±1.25; IV, 2.60±1.07; P=0.000). (B) There existed significant differences in NLR between different tumor nodal stage (N0, 2.31±1.15; N1, 2.32±1.22; N2, 2.43±1.16; N3, 2.75±1.37; P=0.000). (C) There was larger NLR among patients with tumor length ≥4 cm than those <4 cm (2.56±1.24 vs 2.16±1.15, P=0.000). (D) Patients with tumor invading out of the myometrium (pT3-4) had significantly larger NLR than those with lesion limited within the myometrium (pT1-2) (2.51±1.24 vs 2.19±1.15, P=0.011). NLR, neutrophil-lymphocyte ratio.
Pre-operational NLR was significantly positively correlated with number of metastatic lymph nodes (r=0.091, P=0.004), depth of invasion (r=0.096, P=0.002), tumor length (r=0.154, P=0.000) and TNM classification (r s=0.112, P=0.000) according to post-surgical pathology. However, there wasn’t significant correlation between NLR and histological type (r s=0.029, P=0.368).
Patients with tumor invading out of the myometrium (pT3-4) had significantly larger proportion of those with elevated NLR than those with lesion limited within the myometrium (pT1-2) (17.2% vs 8.0%, P=0.000). There was larger percentage of elevated NLR among patients with tumor length ≥4 cm than those <4 cm (20.0% vs 6.7%, P=0.000), and there also existed significant discrepancies in proportion of elevated NLR between different tumor TNM classification (I, 5.0%; II, 14.0%; III, 17.7%; IV, 27.0%; P=0.000), and nodal stage (N0, 10.2%; N1, 13.2%; N2, 16.6%; N3, 23.6%; P=0.000). However, the differences between male and female (15.6% vs 13.4%, P=0.420), between individuals <65 years and those ≥65 (17.2% vs 13.6%, P=0.140), and between poorly differentiated/undifferentiated malignancies and well/moderately differentiated ones (16.4% vs 14.4%, P=0.441) were not statistically significant.
Based on the radical resection group (training dataset), significant parameters included in the SVM model were preoperational peripheral blood CEA, CA 19-9, lymphocyte percentage and platelet count with treatment-predictive accuracy of 74.61% to indicate patients unsuitable for radical gastrectomy when all the above 4 parameters elevated, while the other clinicopathological data’s predictive accuracy was significantly lower than the constructed SVM model (Figure 2). With non-radical resection group as the test dataset, the accuracy of treatment prediction of non-radical gastrectomy was as high as 75.28% applying the model (Figure 3). The resulting prediction specificity and sensitivity on the clinical output, as well as those based on the SVM model, are plotted in Figures 2 and 3.
Figure 2.
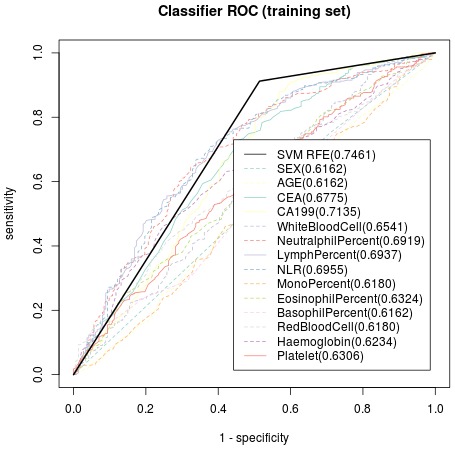
Receiver Operating Characteristic curve of the training dataset, indicating predictive accuracy, sensitivity and specificity of each potential parameter. With preoperational peripheral blood CEA, CA 19-9, lymphocyte percentage and platelet count as significant parameters included in the SVM model, the treatment-predictive accuracy was74.61%. CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9.
Figure 3.
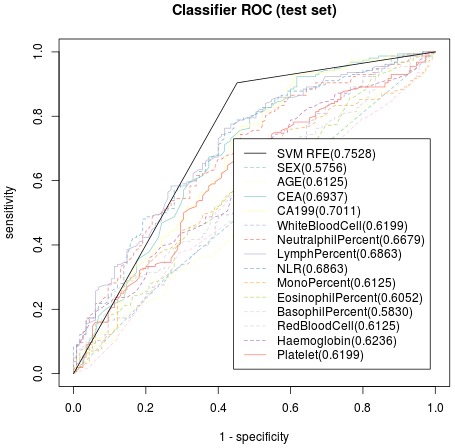
Receiver Operating Characteristic curve of the test dataset, indicating predictive accuracy, sensitivity and specificity of each parameter. The accuracy of treatment prediction was as high as 75.28%.
Discussion
Early in the 19th century, Virchow found that malignant tissue was infiltrated with many immune cells, and infered that carcinogenesis and metastasis were associated with chronic inflammatory, which gained scientific prooves later [13,14]. Researches have shown that larger amount of neutrophils in tumor stroma indicates poorer prognosis [15,16]. Neutrophils facilitate tumor progression by inducing mutation of tumor suppressor genes, secreting cytokines and enzymes to promote proliferation and metastasis, and increasing tumor angiogenesis [17-19]. NLR reveals inflammatory level, and a Japanese research based on peripheral blood shows that patients with NLR>4 suffer from poor outcomes, indicating the prognostic importance of response of immune cells to tumor [20]. Up till now, researches on pathological significance of peripheral NLR have not been found home and abroad, and there still exists no effective way to predict the appropriateness of imposing radical gastrectomy on a specific patient. In this study, we tried to bridge peripheral NLR with postsurgical pathology, and preoperational clinicopathological data with choice of gastrectomy, which appears to be innovative and may be useful for gastroenterologists to evaluate and deal with GC patients wisely based on one’s specific easily accessible clinical parameters pre-operation.
Our study showed that preoperational NLR was significantly correlated with postsurgical pathology, and that parameters indicating poor prognosis including later TNM stage, more metastatic lymph nodes, larger tumor size and deeper invasion were associated with larger NLR, suggesting that peripheral blood inflammatory cells might reveal degree of tumor progress. There are two possible mechanisms for our findings. 1. Tumor cells secrete chemokines attracting neutrophils into tumor stroma, which induces increase of peripheral neutrophils. The neutrophils in tumor microenvironment promote proliferation and metastasis of malignant cells and induce angiogenesis by secreting elastases, defensins and MMP-9 [21]. 2. There exists an obvious reduction in peripheral lymphocytes, which are suppressed by elevated neutrophils, and the reduction suggests malfunction of anti-tumor immunity, facilitating malignancy progression [22]. Thus, preoperational NLR could help to infer to tumor progression and predict prognosis as a rapid, simple, economical, clinically feasible and low-invasive method. A rise in NLR could remind gastroenterologists of more serious progression, calling for a closer investigation combined with imaging examination and tumor markers. Some of the information revealed may not be gained through pathology and imaging pre-operation, and could contribute to more precise classification of tumor stage pre-surgery and wiser decision of comprehensive management and thus may show great value to improve patients’ prognosis. In the future, treatment strategies might be based on researches into inflammatory and immune disorders during tumor progression.
With advancement in diagnostic techniques, most GC can be diagnosed correctly with preoperational pathology. However, auxiliary examinations cannot tell accurate condition of progression. Some patients with negative presurgical test results are only found to be accompanied with widespread metastases during operation, which could cause systematic inflammatory response, leading to rise in neutrophils, which aggravate tumor development and proliferation. Thus it’s very important to judge effectively whether a patient could undergo radical gastrectomy pre-operation.
The SVM was first introduced by Vapnik [ISBN 0-387-98780-0] and is widely used in data classification now [23]. The application of SVM in biomedical studies exhibit particularly active development, such as in tumor classification, and prediction of prognosis and survival [24]. With the versatile computer-aided SVM, we successfully built a model based on CEA, CA19-9, lymphocyte percentage and platelet count which are routinely monitored before surgery, effectively indicating whether a radical resection is appropriate for a specific patient. CEA and CA 19-9 are commonly detected parameters assisting diagnosis and reflecting tumor progression. The later stage a patient is in, the lower possibility a radical gastrectomy could be conducted. Through comparison, efficiency of the SVM model built by us was superior to CEA, CA 19-9 and other potential indexes alone in our test set, suggesting greater value in prediction. Thus in our clinical practice, according to specific characteristics of every patient, we could wisely make a decision on the comprehensive treatment, potentially benefitting a lot to our patients. This serves as a useful novel supplement to preoperational imaging techniques when screening potential patients suitable to undergo radical operation.
In conclusion, peripheral blood NLR indicates tumor progression, and an efficient treatment-predictive SVM model is constructed.
Acknowledgements
We thank Department of Clinical Laboratory and the Information Center in the First Affiliated Hospital of Anhui Medical University for their contributions to our study. This work was supported by Foundation of Anhui Science and Technology Agency (No. 12070403061). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
All authors declare no conflict of interest.
Abbreviations
- GC
gastric carcinoma
- NLR
neutrophil-lymphocyte ratio
- SVM
support vector machine
- CEA
carcinoembryonic antigen
- CA 19-9
carbohydrate antigen 19-9
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Kilic L, Ordu C, Ekenel M, Yildiz I, Keskin S, Sen F, Gural Z, Asoglu O, Kizir A, Aykan F. Comparison of two different adjuvant treatment modalities for pN3 gastric cancer patients after D2 lymph node dissection: can we avoid radiotherapy in a subgroup of patients? Med Oncol. 2013;30:660. doi: 10.1007/s12032-013-0660-2. [DOI] [PubMed] [Google Scholar]
- 4.Fidan E, Mentese A, Ozdemir F, Deger O, Kavgaci H, Caner Karahan S, Aydin F. Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Med Oncol. 2013;30:540. doi: 10.1007/s12032-013-0540-9. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Xu A, Li T, Han W, Wu S, Wang Y. Detection of perioperative cancer antigen 72-4 in gastric juice pre- and post-distal gastrectomy and its significances. Med Oncol. 2013;30:651. doi: 10.1007/s12032-013-0651-3. [DOI] [PubMed] [Google Scholar]
- 6.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 7.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 10.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 14.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:1997–2005. doi: 10.1200/JCO.2005.03.9594. [DOI] [PubMed] [Google Scholar]
- 17.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer. 2010;13:170–176. doi: 10.1007/s10120-010-0554-3. [DOI] [PubMed] [Google Scholar]
- 21.Depuydt B, van Loo G, Vandenabeele P, Declercq W. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J Cell Sci. 2005;118:497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- 22.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 23.Zheng CH, Ng TY, Zhang L, Shiu CK, Wang HQ. Tumor classification based on non-negative matrix factorization using gene expression data. IEEE Trans Nanobioscience. 2011;10:86–93. doi: 10.1109/TNB.2011.2144998. [DOI] [PubMed] [Google Scholar]
- 24.Brown MP, Grundy WN, Lin D, Cristianini N, Sugnet CW, Furey TS, Ares M Jr, Haussler D. Knowledge-based analysis of microarray gene expression data by using support vector machines. Proc Natl Acad Sci U S A. 2000;97:262–267. doi: 10.1073/pnas.97.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]


