Abstract
Phytochrome is consistently observed in pellets centrifuged from homogenates of etiolated, 5-day-old oat seedlings. The majority of pigment associated with the pellet cannot be removed by buffer washes, nor can appreciable quantities of additional phytochrome be adsorbed onto the sedimented material. Over 70% of phytochrome in the pellet is released by 1% Triton X-100.
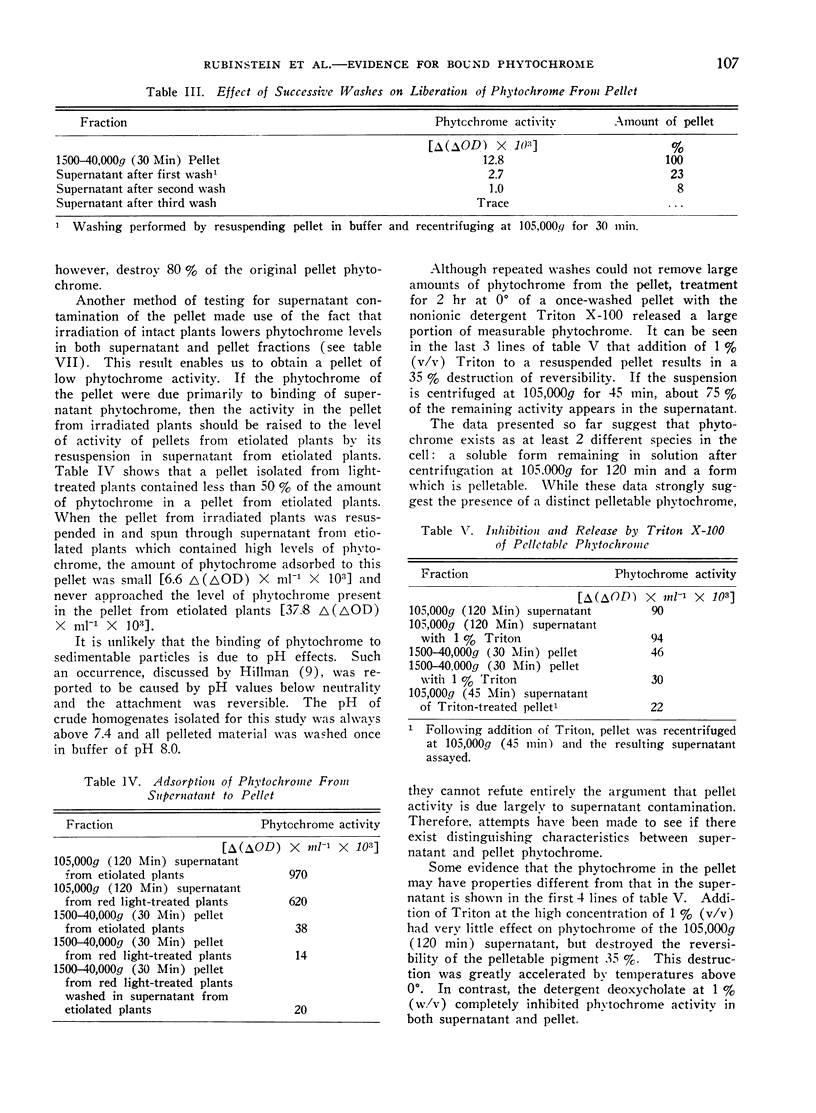
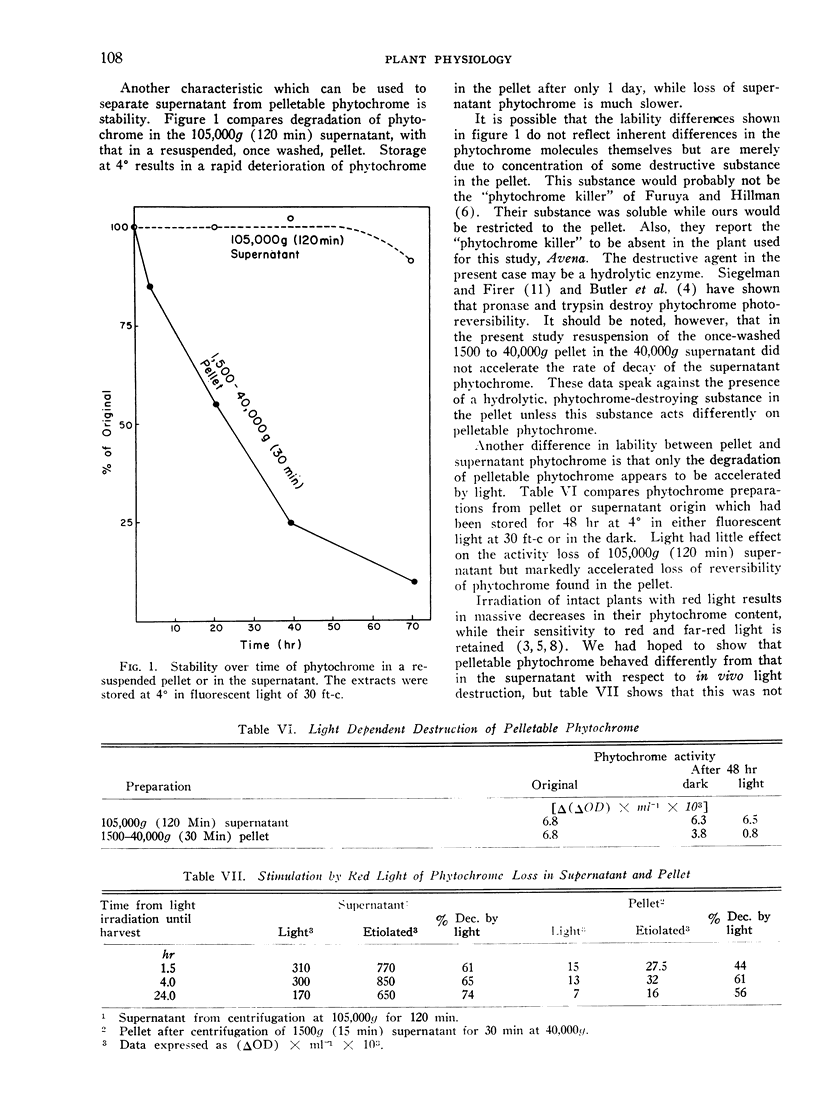
Storage at 0°, irradiation by white light, and Triton treatment all cause much greater loss of photoreversibility in pelleted phytochrome than in supernatant phytochrome. We conclude that the phytochrome in the 1500 to 40,000g (30 min) pellet is distinct from the soluble phytochrome in the supernatant.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Chon H. P. The physiological versus the spectrophotometric status of phytochrome in corn coleoptiles. Plant Physiol. 1966 Sep;41(7):1159–1166. doi: 10.1104/pp.41.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. R., Hillman W. S. Response of tissue with different phytochrome contents to various initial photostationary States. Plant Physiol. 1968 May;43(5):823–826. doi: 10.1104/pp.43.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hillman W. S. Rapid Destruction of the P(FS) Form of Phytochrome by a Substance in Extracts of Pisum Tissue. Plant Physiol. 1966 Sep;41(7):1242–1244. doi: 10.1104/pp.41.7.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks S. B., Borthwick H. A. The function of phytochrome in regulation of plant growth. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2125–2130. doi: 10.1073/pnas.58.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGELMAN H. W., FIRER E. M. PURIFICATION OF PHYTOCHROME FROM OAT SEEDLINGS. Biochemistry. 1964 Mar;3:418–423. doi: 10.1021/bi00891a019. [DOI] [PubMed] [Google Scholar]
- Spruit C. J. Photoreversible pigment transformations in etiolated plants. Biochim Biophys Acta. 1966 Jan 4;112(1):186–188. doi: 10.1016/s0926-6585(96)90025-4. [DOI] [PubMed] [Google Scholar]
- Taylor A. O. In vitro phytochrome dark reversion process. Plant Physiol. 1968 May;43(5):767–774. doi: 10.1104/pp.43.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. B. Red Light and the Geotropic Response of the Avena Coleoptile. Plant Physiol. 1965 Jan;40(1):24–34. doi: 10.1104/pp.40.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]


