Abstract
The hypothalamic paraventricular nucleus (PVN) is critically involved in elevated sympathetic output and the development of hypertension. However, changes in group I metabotropic glutamate receptors (mGluR1 and mGluR5) and their relevance to the hyperactivity of PVN presympathetic neurons in hypertension remain unclear. Here, we found that selectively blocking mGluR5 significantly reduced the basal firing activity of spinally projecting PVN neurons in spontaneously hypertensive rats (SHRs), but not in normotensive Wistar-Kyoto (WKY) rats. However, blocking mGluR1 had no effect on the firing activity of PVN neurons in either group. The mRNA and protein levels of mGluR5 in the PVN and rostral ventrolateral medulla were significantly higher in SHRs than in WKY rats. The group I mGluR selective agonist (S)-3,5-dihydroxyphenylglycine (DHPG) similarly increased the firing activity of PVN neurons in WKY rats and SHRs. In addition, blocking NMDA receptors (NMDARs) through bath application or intracellular dialysis not only decreased the basal firing in SHRs, but also eliminated DHPG-induced excitation of spinally projecting PVN neurons. DHPG significantly increased the amplitude of NMDAR currents without changing their decay kinetics. Interestingly, DHPG still increased the amplitude of NMDAR currents and caused reappearance of functional NMDAR channels after initially blocking NMDARs. In addition, protein kinase C (PKC) inhibition or intracellular dialysis with synaptosomal-associated protein of 25 kDa (SNAP-25)-blocking peptide abolished DHPG-induced increases in NMDAR currents of PVN neurons in SHRs. Our findings suggest that mGluR5 in the PVN is upregulated in hypertension and contributes to the hyperactivity of PVN presympathetic neurons through PKC- and SNAP-25-mediated surface expression of NMDARs.
Introduction
Despite advances in our understanding of the pathology and consequences of hypertension, the mechanisms responsible for the development of hypertension in the vast majority of patients remain unclear. Increased sympathetic nerve activity has been clearly implicated in the pathogenesis of essential hypertension. For example, sympathetic outflow is elevated in animal models of essential hypertension such as spontaneously hypertensive rats (SHRs; Judy et al., 1976; Allen, 2002; Li and Pan, 2007) and in hypertensive patients (Anderson et al., 1989; Grassi, 1998; Mancia et al., 1999). The paraventricular nucleus (PVN) of the hypothalamus plays important integrative roles in regulating neuroendocrine and cardiovascular homeostasis (Swanson and Sawchenko, 1983). The PVN presympathetic neurons can directly and indirectly influence the sympathetic outflow through projections to the rostral ventrolateral medulla (RVLM) and the intermediolateral cell column in the spinal cord (Ranson et al., 1998; Pyner and Coote, 2000). Although the hyperactivity of PVN presympathetic neurons has been demonstrated in hypertensive conditions (Li et al., 2008; Ye et al., 2011) and supports elevated sympathetic outflow in SHRs (Allen, 2002; Li and Pan, 2007), the underlying molecular mechanisms are not fully known.
The major excitatory neurotransmitter glutamate acts on ionotropic receptors and G-protein-coupled metabotropic glutamate receptors (mGluRs). At least eight members of the mGluR family, termed mGluR1–mGluR8, have been cloned and classified into three groups on the basis of their sequence homology and signal transduction pathways (Conn and Pin, 1997). Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11 proteins and their activation triggers multiple signaling pathways, including protein kinase C (PKC), to increase cell excitability and neurotransmitter release (O'Connor et al., 1994; Pin and Duvoisin, 1995; Conn and Pin, 1997). Conversely, group II mGluRs (mGluR2 and mGluR3) and group III mGluRs (mGluR4, mGluR6, mGluR7, and mGluR8) are coupled to Gi/o proteins to decrease neurotransmitter release (Pin and Duvoisin, 1995; Conn and Pin, 1997). Group I mGluRs are expressed in various brain regions including the PVN (Van den Pol, 1994; van den Pol et al., 1995). We have shown that glutamatergic inputs to PVN presympathetic neurons are increased and contribute to the elevated sympathetic vasomotor tone in SHRs (Li and Pan, 2007; Li et al., 2008). In addition, NMDA receptors (NMDARs) and group I mGluRs in the PVN mediate elevated sympathetic vasomotor tone in SHRs (Li and Pan, 2007; Li et al., 2008; Li and Pan, 2010). In this study, we determined changes in the functional activity of group I mGluRs and their relevance to the hyperactivity of PVN presympathetic neurons in SHRs. We also determined the role of NMDARs in mGluR5-induced hyperactivity of PVN neurons in SHRs and the signaling mechanism involved.
Materials and Methods
Animal model.
Experiments were conducted with 46 male spontaneously hypertensive rats (SHRs) and 48 age-matched Wistar-Kyoto (WKY) rats (13–15 weeks old; Taconic). SHRs were used in our study because the SHR model is the most commonly used and best characterized rat model of essential hypertension. Our surgical procedures and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas M.D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. Blood pressure was measured daily for at least 1 week before the experiments using a noninvasive tail-cuff system (IITC Life Science). The mean systolic arterial blood pressure (ABP) in 13-week-old SHRs (198.6 ± 6.5 mmHg, n = 28) was significantly higher than that of age-matched WKY rats (135.6 ± 7.5 mmHg, n = 34).
Retrograde labeling of spinally projecting PVN neurons.
We identified spinally projecting PVN neurons using retrograde labeling, as described previously (Li et al., 2008; Li et al., 2012). Briefly, rats were anesthetized with 2–3% isoflurane in O2, and laminectomy was performed to expose the spinal cord at the T2-T4 level. FluoSpheres (0.04 μm; Invitrogen) were pressure ejected bilaterally (Nanojector II; Drummond Scientific) through a glass pipette (tip diameter, 20–30 μm) placed into the intermediolateral region of the spinal cord in five or six separate 50 nl injections (Li et al., 2008). After injection, rats were treated prophylactically with an antibiotic (5 mg/kg enrofloxacin, s.c., daily for 3 d) and an analgesic (0.5 mg/kg buprenorphine, s.c., every 12 h for 3 d). The rats were then returned to their cages for 3–5 d to permit the FluoSpheres to be transported to the PVN.
Electrophysiological recordings in hypothalamic slices.
Brain slices containing the PVN were prepared from the FluoSphere-injected rats, as described previously (Li et al., 2008). Briefly, the rats were anesthetized with 2% isoflurane, decapitated, and the brain was quickly removed and placed in ice-cold artificial CSF (aCSF) saturated with a mixture of 95% O2 and 5% CO2 and containing the following (in mm): 124.0 NaCl, 3.0 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3. A tissue block containing the PVN was trimmed and glued onto the stage of a vibrating microtome (Technical Products International). Coronal slices (300 μm thick) were cut and the slices were transferred to an incubation chamber filled with aCSF continuously gassed with a mixture of 95% O2 and 5% CO2 at 34°C for at least 1 h before the electrophysiological recordings. In addition, the spinal cord was sectioned at the injected level to confirm that the injection and diffusion sites of FluoroSpheres were located in the vicinity of the intermediolateral cell column (Fig. 1A).
Figure 1.
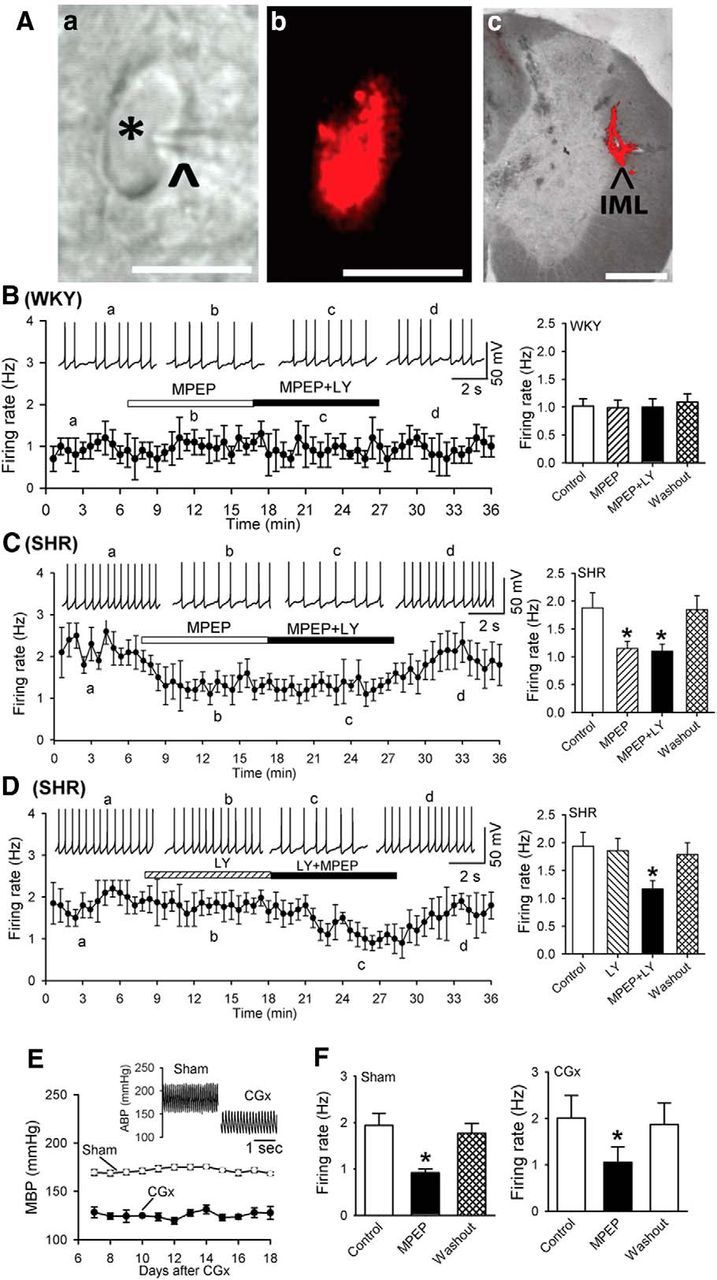
mGluR5, but not mGluR1, contributes to hyperactivity of spinally projecting PVN neurons in SHRs. A, Photomicrographs showing a FluoSphere-labeled PVN neuron (*, Aa) in the brain slice with an attached recording electrode (^). The same neuron viewed with fluorescence illumination is shown in Ab. Scale bar, 20 μm. A representative photomicrograph of the FluoSphere injection site at the intermediolateral cell column (IML) of the thoracic spinal cord in one rat is shown in Ac. The bright-field and fluorescent images were taken from the same tissue section and superimposed to show the location and diffusion of the FluoSphere (red). Scale bar, 500 μm. B, Histogram and summary data showing that MPEP or MPEP plus LY367385 had no effects on the firing activity of labeled PVN neurons in WKY rats. C, Histogram and group data showing that MPEP or MPEP plus LY367385 inhibited the firing activity of labeled PVN neurons in SHRs. D, Histogram and summary data showing that LY367385 plus MPEP, but not LY367385 alone, reduced the firing activity of labeled PVN neurons in SHRs. Inserts in B–D are representative recording traces at different time points indicated by a–d. E, Mean arterial blood pressure (MBP) measured with telemetry in SHRs subjected to sham (n = 4 rats) or CGx (n = 4 rats) surgery. Insert, Original recordings of ABP with telemetry show that CGx decreased blood pressure in SHRs. F, Summary data showing that MPEP similarly decreased the firing of labeled PVN neurons in sham and CGx groups. Data are presented as mean ± SEM. *p < 0.05 compared with the baseline control.
Whole-cell patch-clamp recordings were performed in FluoSphere-labeled PVN neurons in the hypothalamic slices. The recording chamber was continuously perfused with aCSF (saturated with 95% O2 and 5% CO2) at 3 ml/min. The temperature of the aCSF was maintained at 34°C using an inline solution heater. The labeled PVN neurons were identified with an upright microscope with a combination of epifluorescence illumination and differential interference contrast optics (Fig. 1A). The recording electrode was pulled from borosilicate capillaries using a micropipette puller. The resistance of the pipette was 3–7 MΩ when it was filled with an internal solution containing the following (in mm): 110.0 Cs2SO4, 2.0 MgCl2, 0.1 CaCl2, 10.0 HEPES, 1.1 EGTA, 0.3 Na2-GTP, and 2.0 Mg-ATP, adjusted to pH 7.25 with 1 m CsOH, 270–290 mOsm. Signals were processed with a Multiclamp 700B amplifier (Molecular Devices), filtered at 1–2 kHz, and digitized at 10 kHz using Digidata 1440 (Molecular Devices). The evoked EPSCs were elicited by electrical simulation (0.2 ms, 0.5–0.8 mA) delivered at a frequency of 0.1 Hz by a bipolar tungsten electrode connected to a stimulator. The tip of the stimulating electrode was placed on the anatomically ventral side to the recording electrode 100–150 μm away from the recorded neuron (Li et al., 2008). Evoked AMPAR-EPSCs were recorded at a holding potential of −60 mV in the presence of 20 μm bicuculline and evoked NMDAR-EPSCs were recorded at a holding potential of +40 mV in the presence of 20 μm bicuculline and 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). A sodium channel blocker, lidocaine N-ethyl bromide (QX-314, 10.0 mm), was included in the pipette solution for EPSC recordings.
The spontaneous firing activity of labeled PVN neurons was recorded using the whole-cell current-clamp technique. The recording procedures were similar to those used in voltage-clamp recording except that Cs2SO4 in the pipette solution was replaced with K+ gluconate and QX-314 was omitted. The spontaneous firing activity was recorded when it reached a steady state. To determine the possible presynaptic effect of (S)-3,5-dihydroxyphenylglycine (DHPG), two synaptic responses (A1 and A2) were evoked by a pair of stimuli given at an interval of 50 ms. The paired-pulse ratio (PPR) was expressed as the amplitude ratio of the second synaptic response to the first synaptic response (A2/A1).
All drugs were freshly prepared in aCSF before the experiments and delivered via syringe pumps at their final concentrations. DHPG, (S)-(+)-α-amino-4-carboxy-2-methylbenzene-acetic acid (LY367385), 2-methyl-6-(phenyl-ethynyl)-pyridine (MPEP), (+)-MK-801 maleate (MK-801), CNQX, (−)-bicuculline methochloride, 2-amino-5-phosphonopentanoic acid (AP5), and QX-314 were obtained from Abcam. Chelerythrine and phorbol 12-myristate 13-acetate (PMA) were purchased from Tocris Bioscience. The synaptosomal-associated protein of 25 kDa (SNAP-25) C-terminal blocking peptide was custom synthesized by Anaspec. The sequence for the blocking peptide was MEKADANKTRI and for the scrambled peptide was KANAKTDEIRM (Lau et al., 2010). Peptides were dissolved in water as 1000× stocks, aliquoted, and thawed for use on the day of the experiment.
Celiac ganglionectomy and blood pressure measurement with telemetry.
Surgery was performed aseptically in SHRs anesthetized with 2% isoflurane. The celiac ganglion area was exposed through a midline laparotomy. The bifurcations of the superior mesenteric artery and celiac plexus were identified and the celiac ganglion was located within the area near the superior mesenteric artery and celiac artery. For rats undergoing celiac ganglionectomy (CGx), the celiac plexus and all visible nerves were dissected with a surgical microscope and removed as completely as possible by stripping. In sham control rats, the celiac ganglion plexus was exposed but not disturbed.
To measure blood pressure in free moving SHRs, the Millar catheter of the telemetry system (Telemetry Research) was inserted into the abdominal aorta below the bifurcation of the renal arteries when the rats were anesthetized with isoflurane. The transmitter was implanted into the abdominal cavity and attached with a suture to the peritoneum. The abdomen was closed in two layers with interrupted sutures. The rats were housed singly and buprenorphine (0.3 mg/kg, s.c.) and penicillin were given for 3 d after the surgery. The blood pressure signal was monitored in conscious rats through the receiver and the data were collected every 2 d. Two weeks after CGx or sham surgery, rats were used for brain slice recordings or Western blotting.
Western immunoblotting and quantitative PCR.
Rats were anesthetized with isoflurane and decapitated. Using the third ventricle as a reference, the PVN tissue (∼0.5 mm in diameter) spanning from 1.08 to 2.12 mm caudal to the bregma was micropunched bilaterally with a dissection microscope. In addition, the brainstem tissues were obtained bilaterally from sections containing the RVLM (eight punches from four brain slices in each animal). The RVLM was identified under the microscope as a region extending caudally 600–800 μm from the caudal pole of the facial nucleus and at the levels of 12.5–12.7 mm from the bregma. The tissue was then homogenized in ice-cold RIPA buffer (Cell Signaling Technology). The homogenate was centrifuged at 1000 × g for 15 min at 4°C to remove nuclei and large debris. For Western blotting, 20 μg of proteins were separated by a 4–12% SDS polyacrylamide gradient gel electrophoresis and transferred to a PVDF membrane (Immobilon P; Millipore). The immunoblots were probed using a rabbit anti-mGluR5 antibody (1:1500; Millipore) and GAPDH antibody (1:10,000; Cell Signaling Technology). The specificity of the anti-mGluR5 antibody has been shown previously (Romano et al., 1996; Kirschstein et al., 2007). An ECL kit was used to detect the protein bands and the band density was analyzed with ImageJ software. The amounts of mGluR5 proteins were quantified by normalizing the optical density of their protein band to that of GAPDH (used as a protein loading control). The mean values of mGluR5 protein amounts in WKY rats were considered to be 1.
To measure the mGluR5 mRNA level, total RNA was extracted from the PVN and RVLM tissues using the TRIzol Reagent RNA Purification System (Life Technologies). For each sample, 2 μg of total RNA was reverse transcribed for 60 min at 37°C with random primers and M-MLV reverse transcriptase. The cDNA was prepared with a Superscript III first-strand synthesis kit (Life Technologies). Quantitative PCR was performed with use of the iQ5 real-time PCR detection system with the SYBR Green PCR kit (Bio-Rad). All samples were run in duplicate using an annealing temperature of 60°C. The sequences of the primer pairs were as the follows: mGluR5 (NM_017012), 5′-AGCAGCCTAGTCAACTGTG-3′ (forward) and 5′-GGTACTCTTCTCATTCTGGG-3′ (reverse); GAPDH (NM_001001303), 5′-TGTGTCCGTCGTGGATCTGA-3′ (forward) and 3′-TTGCTGTTGAAGTCGCAGGAG-5′ (reverse). The housekeeping gene GAPDH was used to normalize the differences in cDNA aliquots. The mean values of the mRNA levels of mGluR5 in WKY rats were considered to be 1.
Data analysis.
Data are presented as means ± SEM. The firing rate, NMDAR-EPSCs, and AMPAR-EPSCs were analyzed offline using Clampfit (Molecular Devices). The junction potential was corrected offline according to the composition of the internal and external solutions used for the recordings. The Student's t test was used for comparisons of the two datasets. For comparisons of more than two datasets, one-way ANOVA with Dunnett's or Tukey's post hoc test or two-way ANOVA with Bonferroni's post hoc test was performed to determine the difference among experimental groups. p < 0.05 was considered statistically significant.
Results
mGluR5 contributes to the hyperactivity of spinally projecting PVN neurons in SHRs
To determine the role of mGluR1 and mGluR5 in the control of excitability of spinally projecting PVN neurons, we used the selective mGluR1 antagonist LY367385 (White et al., 2003; Werner et al., 2007) and the specific mGluR5 antagonist MPEP (Gasparini et al., 1999). Bath application of MPEP (10 μm) or MPEP plus LY367385 (50 μm) had no significant effect on the membrane potential and basal firing rate of labeled PVN neurons in WKY rats (n = 11 neurons; Fig. 1B). However, in SHRs, MPEP (10 μm) significantly hyperpolarized PVN neurons from −54.6 ± 0.6 to −58.6 ± 0.4 mV (F(3,8) = 17.14, p < 0.01) and decreased their firing rate (n = 11 neurons, F(3,10) = 32.30, p < 0.001; Fig. 1C). In the presence of MPEP, further treatment of LY367385 had no significant effect on the firing activity in SHRs (Fig. 1C). In another 9 PVN neurons in SHRs, LY367385 alone had little effect on the basal firing rate and membrane potential (−53.6 ± 1.5 vs −53.4 ± 1.1 mV, p > 0.05). However, bath application of LY367385 plus MPEP significantly hyperpolarized these 9 PVN neurons from −56.25 ± 1.2 to −59.8 ± 0.9 mV and decreased their firing rate (F(3,8) = 17.14, p < 0.001; Fig. 1D). These results indicate that increased mGluR5 activity plays a role in the hyperactivity of PVN presympathetic neurons in this animal model of essential hypertension.
To further determine whether increased mGluR5 activity in the PVN is a cause of hypertension or is an adaptive change to high blood pressure in SHRs, we examined the effects of MPEP on the firing activity of spinally projecting PVN neurons in SHRs subjected to CGx. CGx, but not sham surgery, significantly lowered the mean ABP in SHRs for at least 2 weeks (Fig. 1E). Bath application of MPEP still similarly decreased the firing rate of labeled PVN neurons in both the sham (F(2,6) = 17.40, p < 0.05) and CGx (F(2,7) = 19.76, p < 0.05; Fig. 1F) groups.
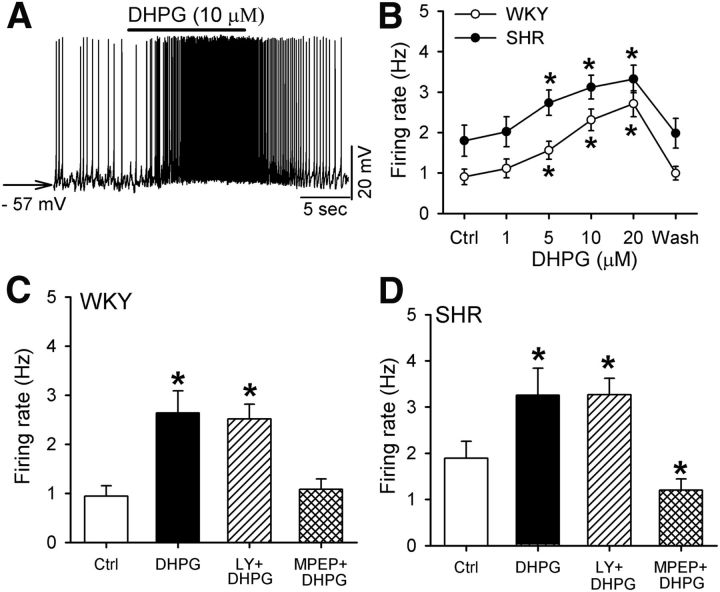
Stimulation of mGluR5 increases the firing activity of PVN neurons
Next, we determined how stimulating mGluR1 or mGluR5 affects the firing activity of spinally projecting PVN neurons in WKY rats and SHRs. Bath application of DHPG (1–20 μm), a combined mGluR1/5 agonist (Schoepp et al., 1994) depolarized the membrane potential and increased the firing rate of labeled PVN neurons in a concentration-dependent manner in both WKY rats (n = 10 neurons) and SHRs (n = 9 neurons; Fig. 2A,B). DHPG-induced increases in the firing rate were comparable in SHRs and WKY rats. Because there are no selective agonists for mGluR1 or mGluR5, we used DHPG plus LY386375 to activate mGluR5, and DHPG plus MPEP to activate mGluR1. DHPG (10 μm) plus LY367385 (50 μm) and DHPG (10 μm) alone similarly increased the firing rate of labeled PVN neurons in WKY rats and SHRs (Fig. 2C,D). However, MPEP (10 μm) plus DHPG had no significant effect on the firing rate of all PVN neurons tested in WKY rats (n = 9 neurons, p > 0.05; Fig. 2C). In SHRs, MPEP plus DHPG decreased the firing rate of labeled PVN neurons (n = 10 neurons, F(3,9) = 28.80, p < 0.05; Fig. 2D).
Figure 2.
Stimulation of mGluR5, but not mGluR1, increases the firing activity of spinally projecting PVN neurons. A, Raw traces showing that DHPG depolarized and increased the firing of a labeled PVN neuron in one SHR. B, Summary data showing that DHPG dose dependently increased the firing rate of labeled PVN neurons in WKY rats and SHRs. C, D, Summary data showing that MPEP, but not LY367385, abolished DHPG-induced increases in firing activity of PVN neurons in WKY rats and SHRs. Data are presented as mean ± SEM. *p < 0.05 compared with the baseline control.
mGluR5 does not regulate synaptic glutamate release to PVN neurons in SHRs
Because mGluR5, not mGluR1, is involved in increased firing activity of spinally projecting PVN neurons and sympathetic vasomotor tone in SHRs (Li and Pan, 2010), we investigated whether increased mGluR5 activity is involved in increased synaptic glutamate release to spinally projecting PVN neurons (Li et al., 2008). We tested the effect of MPEP on glutamatergic spontaneous EPSCs of labeled PVN neurons in SHRs. Bath application of MPEP (10 μm) did not significantly alter the frequency (from 4.16 ± 1.29 to 3.86 ± 1.18 Hz) or the amplitude (from 20.01 ± 1.55 to 19.21 ± 1.60 pA) of spontaneous EPSCs in 7 labeled PVN neurons recorded from SHRs.
mGluR5 expression level in the PVN and RVLM is increased in SHRs
To determine the mGluR5 protein level in the PVN in WKY rats and SHRs, we used Western immunoblotting to quantify the protein level of mGluR5 in micropunched PVN tissues. The anti-mGluR5 antibody detected a protein band at ∼160 kDa and another band at −300 kDa (Fig. 3A), which represent the monomer and dimer form of mGluR5, respectively (Romano et al., 1996; Kirschstein et al., 2007). Both the monomer and dimer protein levels of mGluR5 in the PVN were significantly higher in SHRs than in WKY rats (Fig. 3B). We also determined the mGluR5 mRNA levels in the PVN in WKY rats and SHRs using quantitative PCR. The mGluR5 mRNA level in the PVN was much higher in SHRs than in WKY rats (t(4) = 4.01, p < 0.01; Fig. 3C).
Figure 3.
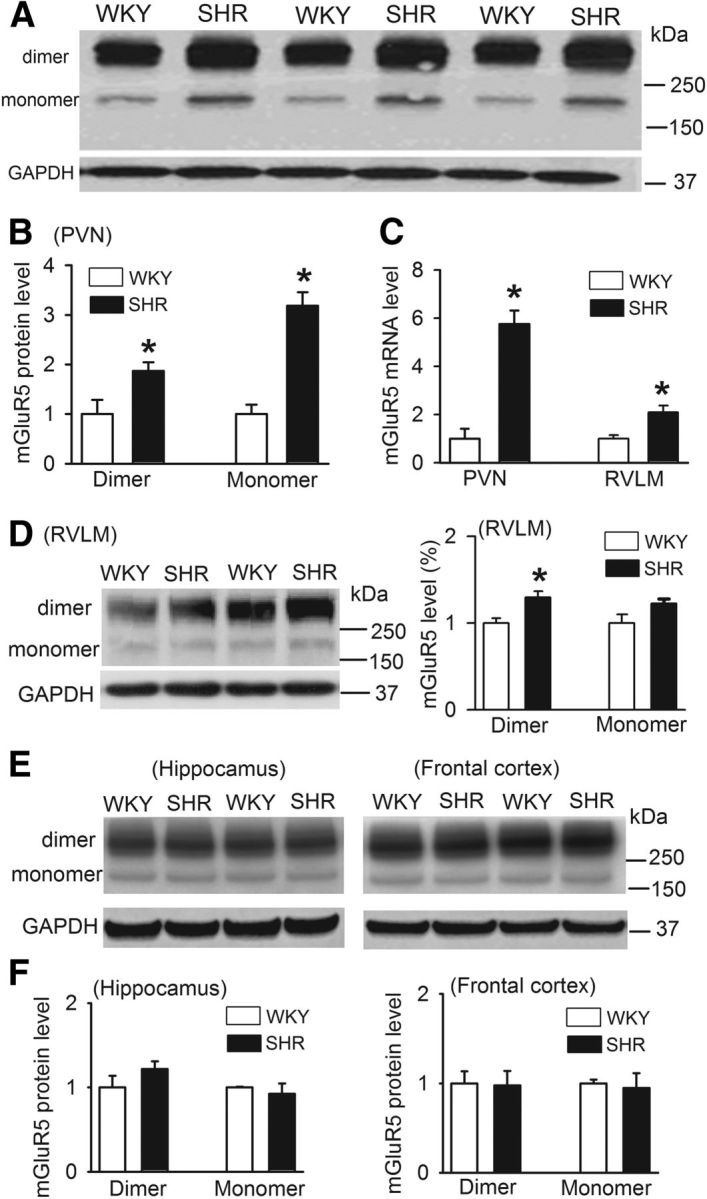
mGluR5 is upregulated in the PVN in SHRs. A, B, Original gel images and summary data showing the mGluR5 protein level in the PVN in SHRs and WKY rats (n = 4 samples in each group). Each sample contains PVN tissues from two rats and molecular weight is indicated on the right in A. C, Group data showing the difference in the mRNA level of mGluR5 in the PVN and RVLM between SHRs and WKY rats (n = 4 samples in each group). D, Gel images and summary data showing the mGluR5 protein levels in the RVLM in SHRs and WKY rats (n = 6 samples in each group). E, F, Representative gel images and group data showing the mGluR5 protein levels in the hippocampus and frontal cortex in SHRs and WKY rats (n = 4 samples in each group). Data presented as means ± SEM. *p < 0.05 compared with the WKY rats.
To determine whether the mGluR5 protein level is changed in other brain regions of SHRs, we measured the mGluR5 protein levels in the RVLM, hippocampus, and prefrontal cortex of WKY rats and SHRs. Interestingly, the mRNA and the dimer protein levels of mGluR5 in the RVLM were significantly greater in SHRs than in WKY rats (Fig. 3C,D). However, the mGluR5 protein levels in the hippocampus and frontal cortex did not differ significantly between WKY rats and SHRs (Fig. 3E,F). These data indicate that mGluR5 is upregulated in the PVN and RVLM in hypertension.
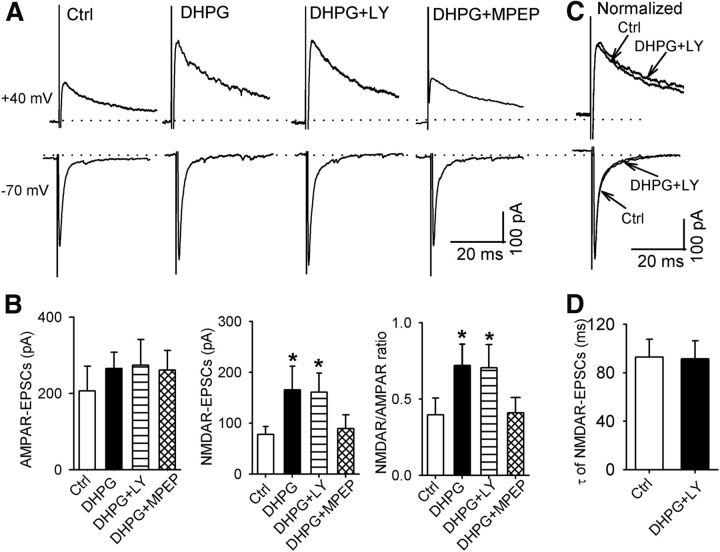
Activation of mGluR5 increases NMDAR, but not AMPAR, activity in spinally projecting PVN neurons
To determine whether activation of group I mGluRs increases ionotropic glutamate receptor activity, we tested the effect of group I mGluR activation on AMPAR- and NMDAR-EPSCs of labeled PVN neurons evoked by focal electrical stimulation. DHPG (10 μm) alone caused a small, but not significant, increase in AMPAR-EPSCs (n = 7 neurons, F(3,6) = 53.53, p < 0.01; Fig. 4A,B). In contrast, DHPG significantly increased the amplitude of NMDAR-EPSCs in these seven neurons (Fig. 4A,B). Furthermore, activation of mGluR5 with DHPG plus LY367385 also significantly increased the amplitude of NMDAR-EPSCs, but had no significant effect on the amplitude of AMPAR-EPSCs (n = 8 neurons, F(3,7) = 85.97, p < 0.01; Fig. 4A,B). However, activation of mGluR1 with DHPG plus MPEP failed to significantly alter the amplitude of either AMPAR-EPSCs or NMDAR-EPSCs (n = 7 neurons; Fig. 4A,B). In addition, DHPG or DHPG plus LY367385, but not DHPG plus MPEP, significantly increased the ratio of NMDAR-EPSCs to AMPAR-EPSCs (Fig. 4B). DHPG plus LY367385 had no significant effect on the decay time of NMDAR-EPSCs (Fig. 4C,D). These results suggest that activation of mGluR5 increases synaptic NMDAR, but not AMPAR, activity in PVN presympathetic neurons.
Figure 4.
mGluR5 activation increases NMDAR currents of spinally projecting PVN neurons. A, B, Original traces and summary data showing the effect of DHPG, DHPG plus LY367385, and DHPG plus MPEP on evoked NMDAR- and AMPAR-EPSCs in labeled PVN neurons. C, D, Representative traces and group data showing that DHPG plus LY367385 had no effect on the decay phase of evoked NMDAR-EPSCs in labeled PVN neurons. Data are presented as means ± SEM. *p < 0.05 compared with the baseline control.
Postsynaptic NMDARs mediate mGluR5-induced excitation of PVN neurons
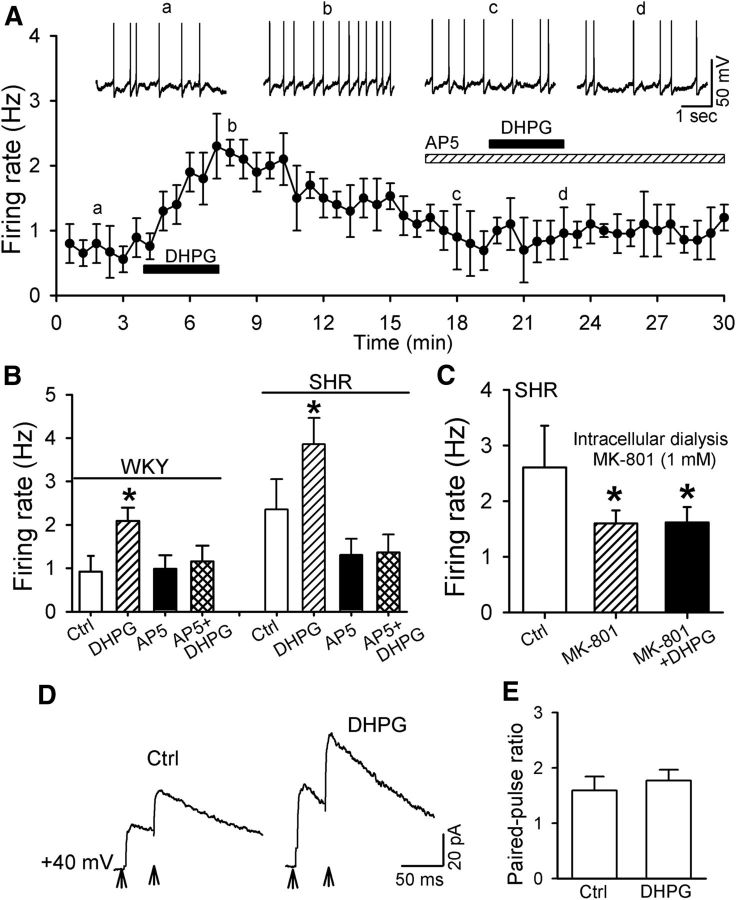
We have shown that blocking NMDARs in the PVN largely attenuates DHPG-induced sympathoexcitatory effects in vivo (Li and Pan, 2010). Therefore, we determined whether NMDARs are involved in DHPG-induced increases in the firing activity of spinally projecting PVN neurons. DHPG (10 μm) increased the firing activity of labeled PVN neurons from 0.92 ± 0.10 to 1.87 ± 0.15 Hz (n = 15 neurons, F(3,14) = 99.43, p < 0.01) in WKY rats. Repeated application of DHPG (10 μm) induced a reproducible increase in the firing activity of labeled PVN neurons (from 0.93 ± 0.15 to 1.8 ± 0.22 Hz, n = 7 neurons; p < 0.05). In the presence of the specific NMDAR antagonist AP5 (50 μm), application of DHPG had no significant effect on the firing rate of labeled PVN neurons in WKY rats (n = 8 neurons; Fig. 5A,B). In another eight labeled PVN neurons recorded from SHRs, AP5 also abolished DHPG-induced increases in their firing (Fig. 5B).
Figure 5.
NMDARs mediate the excitatory effect of mGluR5 activation on spinally projecting PVN neurons. A, Histogram showing that AP5 abolished DHPG-induced increases in the firing of labeled PVN neurons in WKY rats. Inserts, Representative traces at different time points indicated by a–d. B, Summary data showing that AP5 abolished DHPG-induced increases in the firing activity of labeled PVN neurons in WKY rats and SHRs. C, Group data showing that intracellular dialysis of MK-801 reduced the baseline activity and blocked DHPG-induced excitation of labeled PVN neurons in SHRs. D, Original traces (averaged six consecutive responses) of NMDAR-EPSCs evoked by paired stimulation at an interval of 50 ms during control and application of 10 μm DHPG. Stimulating artifacts are removed for clarity and are indicated by arrows. E, Summary data showing that DHPG had no effect on the PPR of NMDAR-EPSCs in labeled PVN neurons of WKY rats (n = 8). Data are presented as mean ± SEM. *p < 0.05 compared with the respective baseline control.
To determine specifically the role of postsynaptic NMDARs in DHPG-induced increases in firing activity, we blocked postsynaptic NMDARs through intracellular dialysis of an NMDAR open channel blocker, MK-801 (1 mm; Zhou et al., 2010; Ye et al., 2011). MK-801 not only significantly decreased the basal firing activity of 9 labeled PVN neurons in SHRs (F(2,8) = 14.40, p < 0.05), but also abolished DHPG-induced increases in the firing activity in these neurons (Fig. 5C).
We further determined whether DHPG augments presynaptic NMDAR activity in labeled PVN neurons. Because an alteration of the PPR represents changes in the probability of neurotransmitter release during the short-term neuroplasticity (Thomson, 2000), we examined the effect of DHPG (10 μm) on the PPR of NMDAR-EPSCs. DHPG (10 μm) had no significant effect on the PPR (A2/A1) of the evoked NMDAR-EPCSs in 8 labeled PVN neurons (Fig. 5D,E). These results suggest that mGluR5 activation excites PVN presympathetic neurons through postsynaptic NMDARs.
Signaling mechanisms underlying mGluR5-induced increases in NMDAR activity
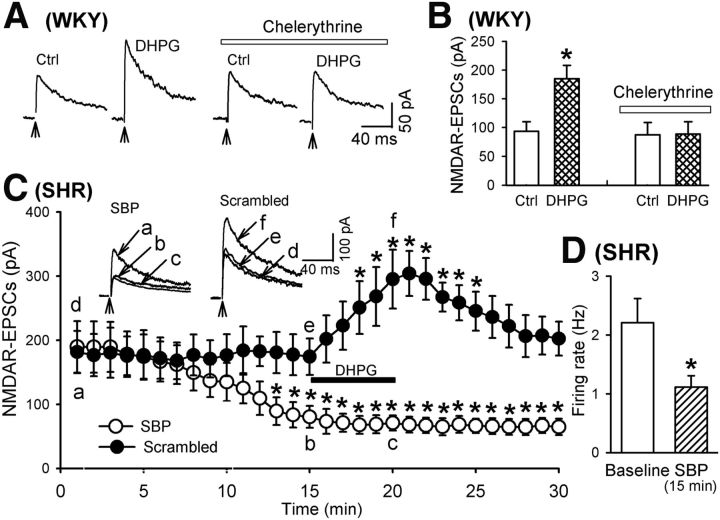
Phosphorylation is critically involved in the regulation of NMDAR activity and trafficking (Scott et al., 2003; Lau and Zukin, 2007). Activation of PKC increases NMDAR currents through rapid insertion of NMDARs to the cell membrane in hippocampal neurons and Xenopus oocytes expressing recombinant NMDARs (Lan et al., 2001b; Lau et al., 2010). Because stimulation of mGluR5 is associated with activation of PKC (Conn and Pin, 1997), we investigated whether PKC activation is involved in DHPG-induced increases in NMDAR-EPSCs of PVN neurons. The brain slices from WKY rats were pretreated with a selective PKC inhibitor, chelerythrine (1 μm), for at least 20–30 min (Huang and van den Pol, 2007). Application of DHPG had no significant effect on NMDAR-EPSCs in all eight labeled PVN neurons pretreated with chelerythrine (Fig. 6A,B).
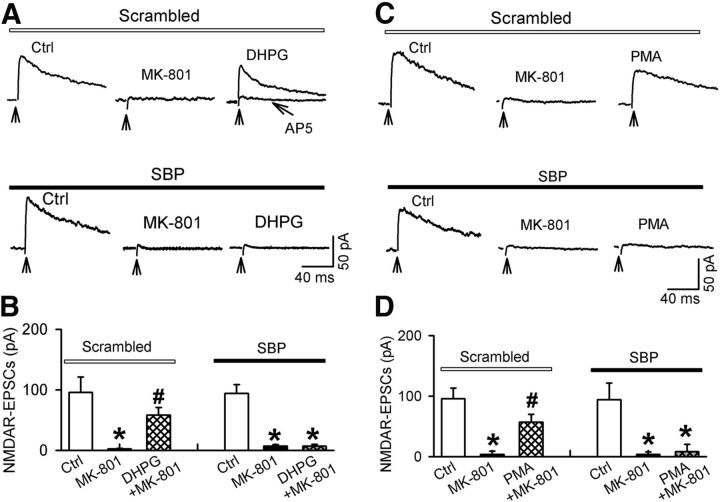
Figure 6.
Role of PKC and SNAP-25 in DHPG-induced increases in NMDAR currents of spinally projecting PVN neurons. A, B, Representative traces and summary data showing the effect of chelerythrine on DHPG-induced increases in NMDAR-EPSCs of labeled PVN neurons in WKY rats. C, Histogram showing the distinct effect of DHPG on the amplitude of NMDAR-EPSCs in labeled PVN neurons recorded with intracellular solution containing SBP (20 μm) or the scrambled control peptide in SHRs. Inserts, Representative traces of NMDAR-EPSCs recorded with SBP or the scrambled control peptide included in the pipette solution. NMDAR-EPSCs obtained at different time points are indicated by a–d. D, Summary data showing the effect of including SBP in the recording pipette solution on the basal firing activity of seven labeled PVN neurons in SHRs. Data are presented as means ± SEM. *p < 0.05 compared with respective baseline controls.
SNAP-25 is a member of the SNARE family and is a known target of PKC phosphorylation (Shimazaki et al., 1996). PKC-mediated phosphorylation of SNAP-25 is critically involved in the NMDAR trafficking (Lan et al., 2001a). To determine whether SNAP-25 is involved in DHPG-induced increases in NMDAR activity in PVN neurons, we blocked SNAP-25 signaling by dialyzing the SNAP-25-blocking peptide (SBP, 20 μm) into the recorded neuron through the recording pipette (Lau et al., 2010). Inclusion of SBP in the recording pipette solution significantly decreased the amplitude of evoked NMDAR-EPSCs of PVN neurons in SHRs (n = 9 neurons; Fig. 6C). Furthermore, bath application of DHPG (10 μm) failed to increase the amplitude of evoked NMDAR-EPSCs in PVN neurons dialyzed with SBP. However, inclusion of a scrambled SBP control peptide in the recording pipette solution did not alter the basal NMDAR-EPSCs and DHPG-induced increases in the amplitude of evoked NMDAR-EPSCs in labeled PVN neurons of SHRs (n = 7 neurons; Fig. 6C). Therefore, PKC and SNAP-25 are critically involved in mGluR5-induced potentiation of NMDAR activity in PVN presympathetic neurons. In addition, because blocking SNAP-25 signaling decreased NMDAR currents of PVN neurons in SHRs, we investigated whether SNAP-25 contributes to the hyperactivity of PVN neurons in SHRs. Inclusion of SBP in the recording pipette significantly decreased the firing activity of all 7 labeled PVN neurons examined in SHRs (t(6) = 4.75, p < 0.01; Fig. 6D).
We next determined whether DHPG-induced increases in NMDAR activity involve recruitment of additional NMDARs to the cell membrane. We measured the reappearance of functional NMDAR channels after irreversibly blocking NMDARs using MK-801 (Huettner and Bean, 1988). Bath application of MK-801 (10 μm) to the brain slice for 3 min markedly reduced the amplitude of evoked NMDAR-EPSCs in 7 labeled PVN neurons of WKY rats (F(2,6) = 21.79, p < 0.01; Fig. 7A,B). The blocking effect of MK-801 on evoked NMDAR-EPSCs was irreversible after removing MK-801 for 30 min. However, evoked NMDAR-EPSCs reappeared after application of 10 μm DHPG for 6 min (n = 7 neurons; Fig. 7A,B). To determine whether the DHPG-induced reappearance of evoked NMDAR-EPSCs results from SNARE-dependent NMDAR trafficking, we inhibited SNAP-25 signaling through intracellular dialysis of SBP (20 μm) into the recorded neuron. SBP eliminated DHPG-induced reappearance of evoked NMDAR-EPSCs of labeled PVN neurons (n = 9 neurons; Fig. 7A,B).
Figure 7.
Activation of mGluRs or PKC promotes functional NMDAR surface expression of spinally projecting PVN neurons. A, B, Representative traces and summary data showing changes in NMDAR-EPSCs during control, MK-801 application, and DHPG application in labeled PVN neurons of WKY rats recorded with intracellular solution containing SBP or the scrambled control peptide. Note that DHPG-induced reappearance of NMDAR-EPSCs of PVN neurons recorded the scrambled control peptide included in the pipette solution, which was blocked by 50 μm AP5. C, D, Original traces and group data showing changes in NMDAR-EPSCs during control, MK-801 application, and PMA application in labeled PVN neurons of WKY rats recorded with intracellular solution containing SBP or the scrambled control peptide. Data are presented as means ± SEM. *p < 0.05 compared with the respective baseline control; #p < 0.05 compared with the value recorded with MK-801 alone.
We then investigated whether PKC activation mimics DHPG-induced reappearance of NMDAR-EPSCs in spinally projecting PVN neurons. Bath application of 10 μm MK-801 largely eliminated evoked NMDAR-EPSCs in 10 labeled PVN neurons in WKY rats (Fig. 7C,D). Bath application of a specific PKC activator, PMA (0.5 μm; Yan et al., 2011), for 5 min significantly increased the amplitude of evoked NMDAR-EPSCs in these neurons (n = 8 neurons, F(2,7) = 72.60, p < 0.001; Fig. 7C,D). Furthermore, intracellular dialysis of SBP eliminated PMA-induced reappearance of evoked NMDAR-EPSCs in labeled PVN neurons (n = 7 neurons; Fig. 7C,D). These data indicate that PKC and SNAP-25 contribute to mGluR5-activation-induced surface recruitment of NMDARs in PVN presympathetic neurons.
Discussion
The major goal of our study was to determine the contribution of group I mGluRs to the hyperactivity of PVN presympathetic neurons in SHRs, the most commonly used animal model of essential hypertension. Blocking mGluR5 in the PVN reduces elevated sympathetic vasomotor tone in SHRs (Li and Pan, 2010). We found in this study that blocking mGluR5, but not mGluR1, significantly decreased the firing activity of spinally projecting PVN neurons in SHRs. However, blocking mGluR5 or mGluR1 had no effect on the firing activity of PVN neurons in WKY rats. These data suggest that mGluR5 is tonically activated and maintains the hyperactivity of PVN presympathetic neurons in SHRs. We also found that the mRNA and protein levels of mGluR5 in the PVN were much higher in SHRs than in WKY rats, but the mGluR5 protein level in the hippocampus and frontal cortex did not differ significantly between WKY rats and SHRs. Therefore, upregulation of mGluR5 is not a global phenomenon in the brain of SHRs. Interestingly, we observed that in the RVLM, another region that is important for generating sympathetic outflow, the mRNA and protein levels of mGluR5 were also significantly higher in SHRs than in WKY rats. Because lowering blood pressure in SHRs with CGx did not significantly change the effect of blocking mGluR5 on the firing activity of PVN neurons, increased mGluR5 activity in the PVN is less likely an adaptive response to elevated blood pressure in SHRs, but probably contributes to hyperactivity of PVN presympathetic neurons in SHRs.
Activation of group I mGluRs can either enhance or depress excitatory synaptic transmission (Snyder et al., 2001; Kotecha et al., 2003; Liu et al., 2006). For example, the group I mGluR agonist DHPG reduces NMDAR surface expression and decreases NMDAR currents in cultured rat hippocampal neurons (Snyder et al., 2001). In contrast, activation of group I mGluRs induces a long-lasting enhancement of NMDAR currents in hippocampal and striatal neurons through PKC- or PKA-dependent pathways (Pisani et al., 1997; Pisani et al., 2001; Kotecha et al., 2003; Huang and van den Pol, 2007). We found that the specific NMDAR antagonist AP-5 largely attenuated the excitatory effect of DHPG on the firing activity of PVN neurons, suggesting that NMDARs are involved in the stimulatory effect of mGluR5 activation on PVN neurons. In addition, stimulation of mGluR5 with DHPG plus LY367385 significantly increased the amplitude of NMDAR-EPSCs of PVN neurons. These findings are consistent with our in vivo data showing that NMDAR mediates the sympathoexcitatory responses to stimulation of group I mGluRs in the PVN in SHRs (Li and Pan, 2010).
Activation of both presynaptic and postsynaptic NMDARs contributes to the hyperactivity of PVN presympathetic neurons in SHRs (Ye et al., 2012). We demonstrated that blocking postsynaptic NMDAR with intracellular dialysis of MK-801 eliminated the increase in the firing activity by DHPG. Although DHPG significantly increased the amplitude of NMDAR-EPSCs in PVN neurons, DHPG had no effect on the PPR of evoked NMDAR-EPSCs. We also observed that blocking mGluR5 had no significant effect on the frequency or the amplitude of spontaneous EPSCs of PVN neurons in SHRs. These data suggest that mGluR5 is unlikely present presynaptically and is not involved in the control of synaptic glutamate release to PVN presympathetic neurons in SHRs. Collectively, our findings strongly suggest that postsynaptic, but not presynaptic, NMDARs are involved in the excitatory effect of mGluR5 activation on PVN presympathetic neurons in SHRs.
Activation of mGluR5 can inhibit or augment NMDAR activity by coupling to diverse intracellular signaling pathways in different neuronal populations (Wang and Salter, 1994; Yu et al., 1997). PKC is involved in group I mGluR-mediated potentiation of NMDAR currents in the hippocampus (Aniksztejn et al., 1992; Huang and van den Pol, 2007) and can increase the NMDAR channel opening rate and promote NMDAR trafficking (Lan et al., 2001b). SNAP-25, a member of the SNARE family of vesicular fusion proteins, is a known target of PKC phosphorylation and is involved in NMDAR trafficking (Shimazaki et al., 1996; Lau et al., 2010). We found in this study that pretreatment of the brain slices of SHRs with a PKC inhibitor abolished DHPG-induced increases in NMDAR-EPSCs. In addition, DHPG or the PKC activator PMA induced a reappearance of evoked NMDAR-EPSCs after blocking NMDARs with the open channel blocker MK-801. This reappearance of NMDAR-EPSCs indicates that activation of mGluR5 recruits additional NMDARs to the plasma membrane of PVN presympathetic neurons. In addition, we found that inhibiting SNAP-25 activity by intracellular dialysis of SBP abolished DHPG-induced potentiation of NMDAR-EPSCs. Furthermore, SBP eliminated the reappearance of evoked NMDAR-EPSCs induced by PKC activation. Our findings suggest that PKC and SNAP-25 contribute to mGluR5-induced potentiation of NMDAR activity in PVN presympathetic neurons.
Another salient finding of our study is that disrupting SNARE activity decreased the basal NMDAR-EPSCs in spinally projecting PVN neurons in SHRs, but not in WKY rats. Therefore, SNAP-25 contributes critically to increased NMDAR activity in PVN presympathetic neurons in SHRs. It is possible that increased PKC activity leads to phosphorylation of SNAP-25 and consequently results in enhanced NMDAR activity in PVN neurons in SHRs. It has been suggested that DHPG may act as a co-agonist at the glycine site of NMDARs and thus potentiate NMDAR responses (Contractor et al., 1998) or it may act via a G-protein-independent pathway (Benquet et al., 2002). However, it is less likely that either of these mechanisms is involved in DHPG-induced potentiation of NMDAR in PVN neurons because the PKC inhibitor or the mGluR5 antagonist completely blocked the effect of DHPG on NMDAR-EPSCs in the PVN presympathetic neurons.
In summary, our findings provide new information about the molecular and signaling mechanisms involved in increased glutamatergic input and the hyperactivity of PVN presympathetic neurons in SHRs. Potentiation of NMDARs is ultimately involved in mGluR5-induced hyperactivity of PVN presympathetic neurons in SHRs. Activation of mGluR5 leads to recruitment of additional NMDARs to the plasma membrane through the PKC-SNAP-25 signaling pathway. By potentiating NMDAR trafficking, mGluR5 is involved in the hyperactivity of PVN presympathetic neurons in hypertension. Excessive glutamate release in the PVN in SHRs can activate both NMDARs and mGluRs, including mGluR5 and group II mGluRs. However, stimulation of mGluR5 and group II mGluRs has opposing actions in the PVN. Activation of group II mGluRs primarily reduces synaptic glutamate release and may restrain the firing of PVN presympathetic neurons in SHRs (Ye et al., 2013). We have shown that CK2 also contributes to increased NMDAR activity in the PVN in SHRs possibly through phosphorylation of NMDARs and/or their interacting proteins (Ye et al., 2011; Ye et al., 2012). It remains unclear whether upregulation of mGluR5 may serve as an upstream mechanism of increased CK2 activity to cooperatively potentiate NMDAR activity of PVN presympathetic neurons in SHRs. Therefore, mGluR5 may represent a potentially new target to reduce sympathetic outflow in hypertension.
Footnotes
This work was supported by the National Institutes of Health (Grants HL077400 and MH096086) and the N.G. and Helen T. Hawkins endowment (to H.-L.P.).
The authors declare no competing financial interests.
References
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.HYP.14.2.177. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L, Otani S, Ben-Ari Y. Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. Eur J Neurosci. 1992;4:500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Contractor A, Gereau RW, 4th, Green T, Heinemann SF. Direct effects of metabotropic glutamate receptor compounds on native and recombinant N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1998;95:8969–8974. doi: 10.1073/pnas.95.15.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/S0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Huang H, van den Pol AN. Rapid direct excitation and long-lasting enhancement of NMDA response by group I metabotropic glutamate receptor activation of hypothalamic melanin-concentrating hormone neurons. J Neurosci. 2007;27:11560–11572. doi: 10.1523/JNEUROSCI.2147-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy WV, Watanabe AM, Henry DP, Besch HR, Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976;38:21–29. doi: 10.1161/01.RES.38.6.21. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Bauer M, Müller L, Rüschenschmidt C, Reitze M, Becker AJ, Schoch S, Beck H. Loss of metabotropic glutamate receptor-dependent long-term depression via downregulation of mGluR5 after status epilepticus. J Neurosci. 2007;27:7696–7704. doi: 10.1523/JNEUROSCI.4572-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Zheng X, Bennett MV, Zukin RS. Activation of metabotropic glutamate receptor 1 accelerates NMDA receptor trafficking. J Neurosci. 2001a;21:6058–6068. doi: 10.1523/JNEUROSCI.21-16-06058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001b;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;299:R552–R561. doi: 10.1152/ajpregu.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci. 2012;32:372–380. doi: 10.1523/JNEUROSCI.3222-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Zhang G, Hornby G, Vasylyev D, Bowlby M, Park K, Gilbert A, Marquis K, Andree TH. The effect of mGlu5 receptor positive allosteric modulators on signaling molecules in brain slices. Eur J Pharmacol. 2006;536:262–268. doi: 10.1016/j.ejphar.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999;34:724–728. doi: 10.1161/01.HYP.34.4.724. [DOI] [PubMed] [Google Scholar]
- O'Connor JJ, Rowan MJ, Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994;367:557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-G. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/S0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/S0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem. 1996;271:28612–28616. doi: 10.1074/jbc.271.45.28612. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/S0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y, Nishiki T, Omori A, Sekiguchi M, Kamata Y, Kozaki S, Takahashi M. Phosphorylation of 25-kDa synaptosome-associated protein. Possible involvement in protein kinase C-mediated regulation of neurotransmitter release. J Biol Chem. 1996;271:14548–14553. doi: 10.1074/jbc.271.24.14548. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/S0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. Metabotropic glutamate receptor mGluR1 distribution and ultrastructural localization in hypothalamus. J Comp Neurol. 1994;349:615–632. doi: 10.1002/cne.903490409. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362:134–150. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Werner CG, Scartabelli T, Pancani T, Landucci E, Moroni F, Pellegrini-Giampietro DE. Differential role of mGlu1 and mGlu5 receptors in rat hippocampal slice models of ischemic tolerance. Eur J Neurosci. 2007;25:3597–3604. doi: 10.1111/j.1460-9568.2007.05614.x. [DOI] [PubMed] [Google Scholar]
- White AM, Kylänpää RA, Christie LA, McIntosh SJ, Irving AJ, Platt B. Presynaptic group I metabotropic glutamate receptors modulate synaptic transmission in the rat superior colliculus via 4-AP sensitive K(+) channels. Br J Pharmacol. 2003;140:1421–1433. doi: 10.1038/sj.bjp.0705570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JZ, Xu Z, Ren SQ, Hu B, Yao W, Wang SH, Liu SY, Lu W. Protein kinase C promotes N-methyl-D-aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin-dependent protein kinase II (CaMKII) autophosphorylation. J Biol Chem. 2011;286:25187–25200. doi: 10.1074/jbc.M110.192708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci. 2011;31:8271–8279. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li L, Li DP, Pan HL. Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-D-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem. 2012;287:17438–17446. doi: 10.1074/jbc.M111.331165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Pan HL. Regulation of hypothalamic presympathetic neurons and sympathetic outflow by group II metabotropic glutamate receptors in spontaneously hypertensive rats. Hypertension. 2013;62:255–262. doi: 10.1161/HYPERTENSIONAHA.113.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SP, Sensi SL, Canzoniero LM, Buisson A, Choi DW. Membrane-delimited modulation of NMDA currents by metabotropic glutamate receptor subtypes 1/5 in cultured mouse cortical neurons. J Physiol. 1997;499:721–732. doi: 10.1113/jphysiol.1997.sp021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H, Pan HL. Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 2010;30:4460–4466. doi: 10.1523/JNEUROSCI.5857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]