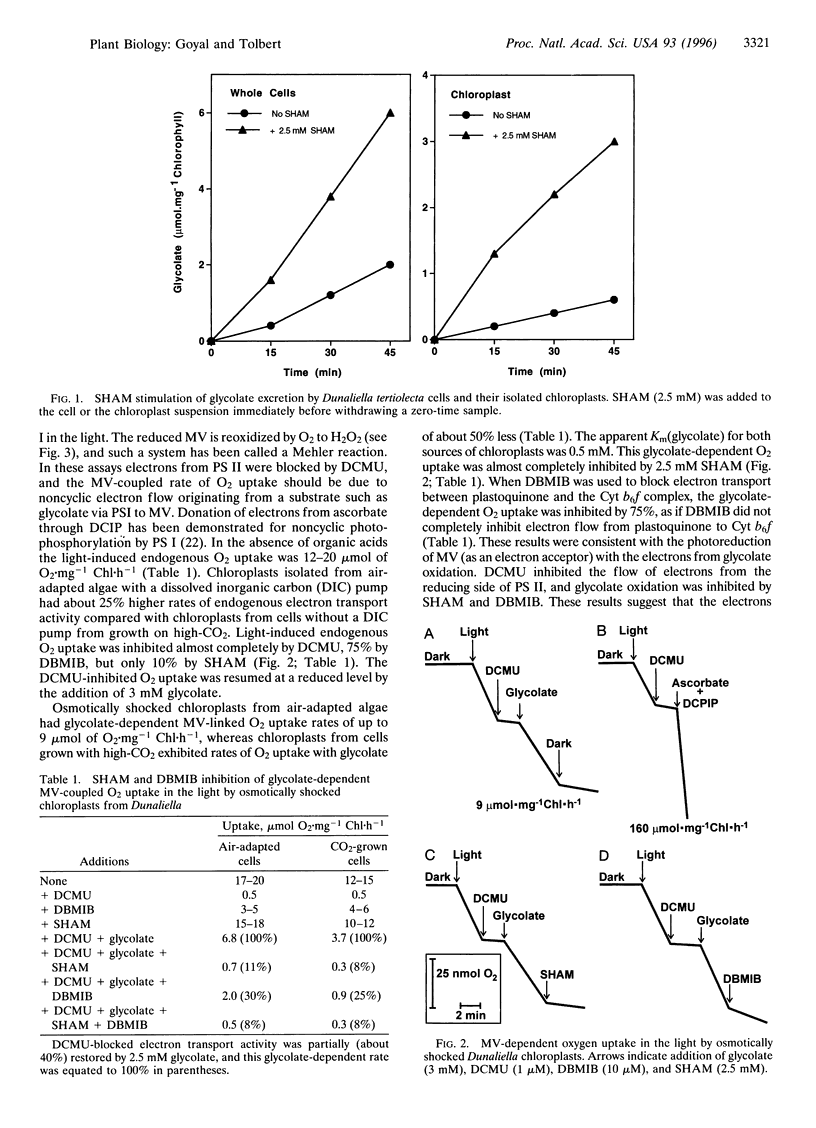
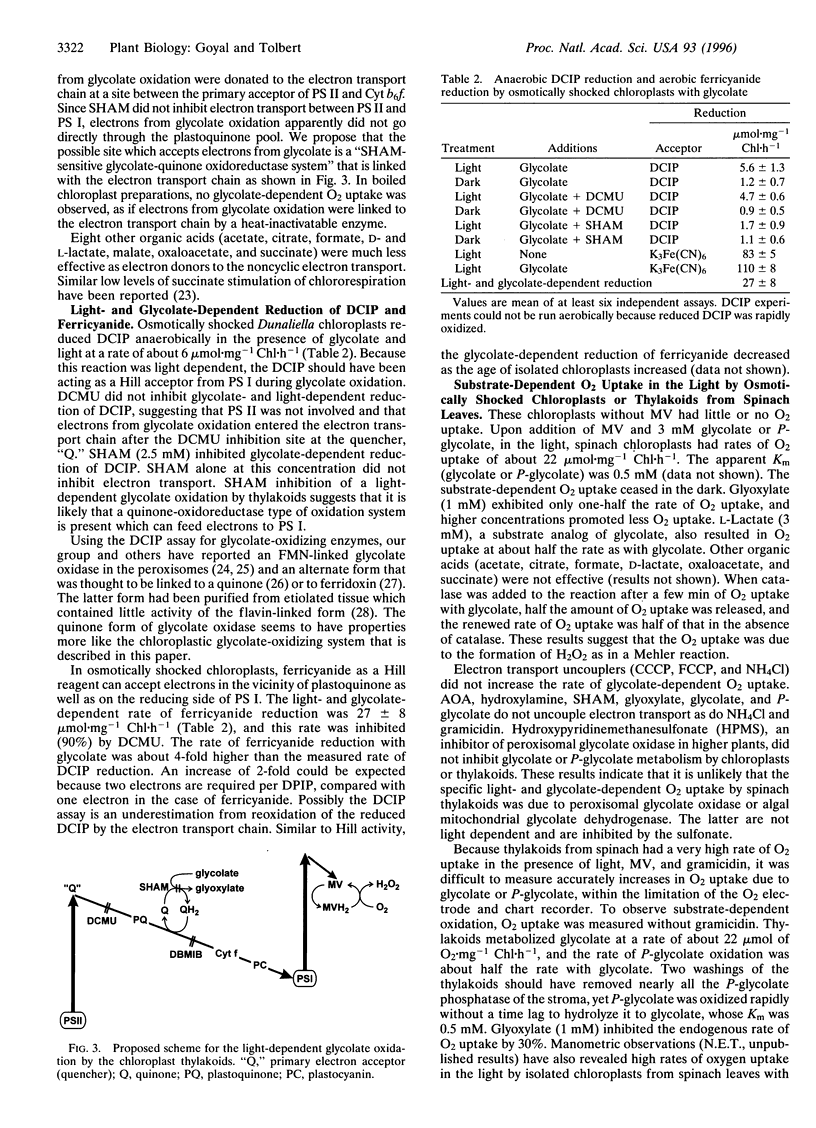
Abstract
Photosynthetic carbon metabolism is initiated by ribulose-bisphosphate carboxylase/oxygenase (Rubisco), which uses both CO2 and O2 as substrates. One 2-phosphoglycolate (P-glycolate) molecule is produced for each O2 molecule fixed. P-glycolate has been considered to be metabolized exclusively via the oxidative photosynthetic carbon cycle. This paper reports an additional pathway for P-glycolate and glycolate metabolism in the chloroplasts. Light-dependent glycolate or P-glycolate oxidation by osmotically shocked chloroplasts from the algae Dunaliella or spinach leaves was measured by three electron acceptors, methyl viologen (MV), potassium ferricyanide, or dichloroindophenol. Glycolate oxidation was assayed with 3-(3,4)-dichlorophenyl)-1,1-dimethylurea (DCMU) as oxygen uptake in the presence of MV at a rate of 9 mol per mg of chlorophyll per h. Washed thylakoids from spinach leaves oxidized glycolate at a rate of 22 mol per mg of chlorophyll per h. This light-dependent oxidation was inhibited completely by SHAM, an inhibitor of quinone oxidoreductase, and 75% by 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone (DBMIB), which inhibits electron transfer from plastoquinone to the cytochrome b6f complex. SHAM stimulated severalfold glycolate excretion by algal cells, Dunaliella or Chlamydomonas, and by isolated Dunaliella chloroplasts. Glycolate and P-glycolate were oxidized about equally well to glyoxylate and phosphate. On the basis of results of inhibitor action, the possible site which accepts electrons from glycolate or P-glycolate is a quinone after the DCMU site but before the DBMIB site. This glycolate oxidation is a light-dependent, SHAM-sensitive, glycolate-quinone oxidoreductase system that is associated with photosynthetic electron transport in the chloroplasts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beezley B. B., Gruber P. J., Frederick S. E. Cytochemical localization of glycolate dehydrogenase in mitochondria of chlamydomonas. Plant Physiol. 1976 Sep;58(3):315–319. doi: 10.1104/pp.58.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerović Z. G., Plesnicar M. An improved procedure for the isolation of intact chloroplasts of high photosynthetic capacity. Biochem J. 1984 Oct 15;223(2):543–545. doi: 10.1042/bj2230543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick S. E., Gruber P. J., Tolbert N. E. The occurrence of glycolate dehydrogenase and glycolate oxidase in green plants: an evolutionary survey. Plant Physiol. 1973 Oct;52(4):318–323. doi: 10.1104/pp.52.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A., Betsche T., Tolbert N. E. Isolation of Intact Chloroplasts from Dunaliella tertiolecta. Plant Physiol. 1988 Nov;88(3):543–546. doi: 10.1104/pp.88.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Pan R. L. Photosystem I electron transport and phosphorylation supported by electron donation to the plastoquinone region. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1171–1177. doi: 10.1016/0006-291x(78)91518-8. [DOI] [PubMed] [Google Scholar]
- Kuczmak M., Tolbert N. E. Glycolic acid oxidase formation in greening leaves. Plant Physiol. 1962 Nov;37(6):729–734. doi: 10.1104/pp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., BURRIS R. H. Light activation of the plant enzyme which oxidizes glycolic acid. J Biol Chem. 1950 Oct;186(2):791–804. [PubMed] [Google Scholar]
- Takabe T., Akazawa T. Mechanism of glycolate transport in spinach leaf chloroplasts. Plant Physiol. 1981 Nov;68(5):1093–1097. doi: 10.1104/pp.68.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Harrison M., Selph N. Aminooxyacetate stimulation of glycolate formation and excretion by chlamydomonas. Plant Physiol. 1983 Aug;72(4):1075–1083. doi: 10.1104/pp.72.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Willeford K. O., Gombos Z., Gibbs M. Evidence for Chloroplastic Succinate Dehydrogenase Participating in the Chloroplastic Respiratory and Photosynthetic Electron Transport Chains of Chlamydomonas reinhardtii. Plant Physiol. 1989 Jul;90(3):1084–1087. doi: 10.1104/pp.90.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I., GOTTO A. M. Properties of a new glyoxylate reductase from leaves. Biochem J. 1962 Sep;84:541–546. doi: 10.1042/bj0840541. [DOI] [PMC free article] [PubMed] [Google Scholar]



